Technology of Orodispersible Polymer Films with Micronized Loratadine—Influence of Different Drug Loadings on Film Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Casting Suspension and ODFs
2.3. Homogeneity of the Casting Suspension and ODFs
2.4. Morphological Properties
2.5. Viscosity of the Casting Mass
2.6. Water Content
2.7. Disintegration Time
2.8. Viscoelastic Properties
2.9. Folding Endurance
2.10. Uniformity of Content
2.11. Statistical Analysis
3. Results
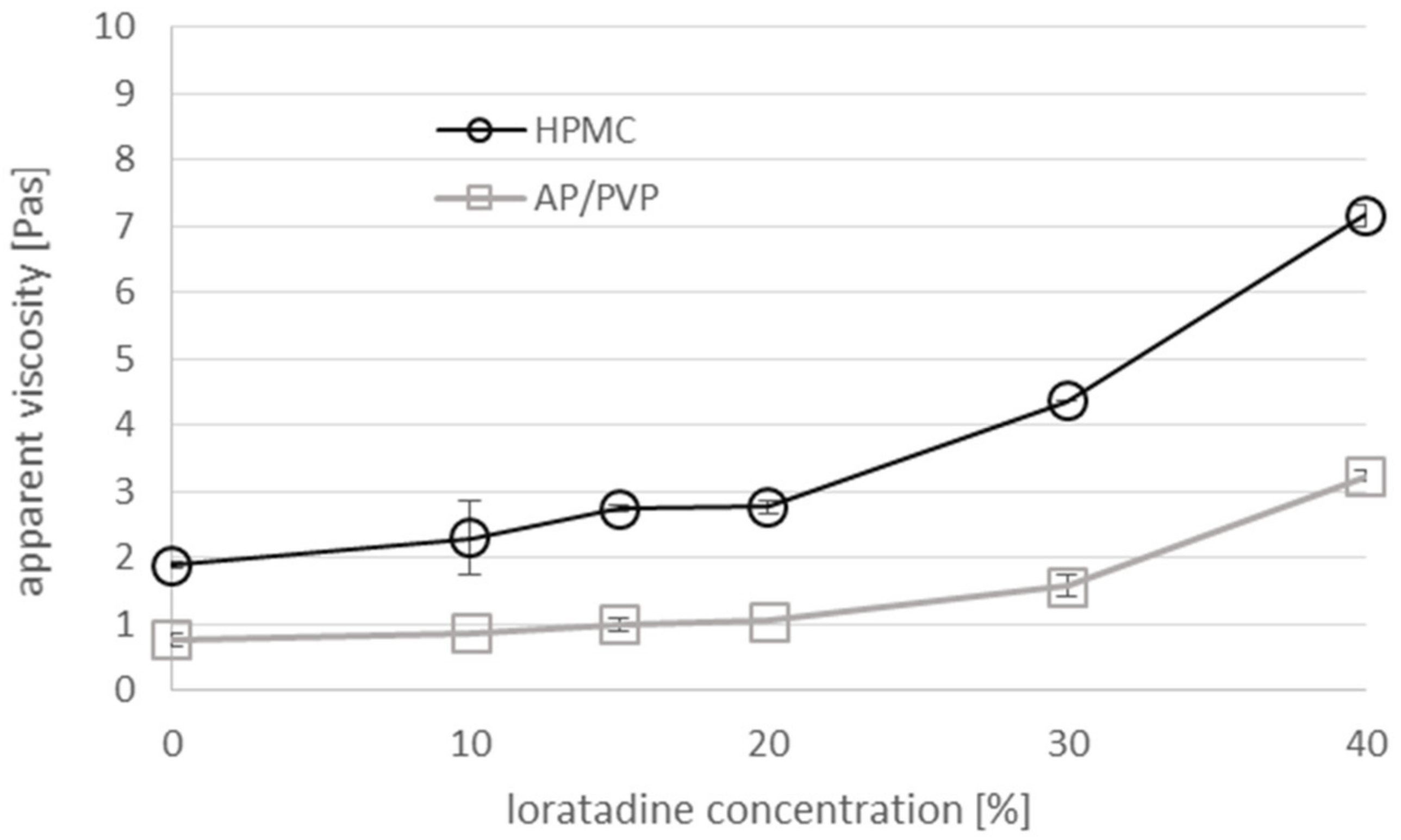
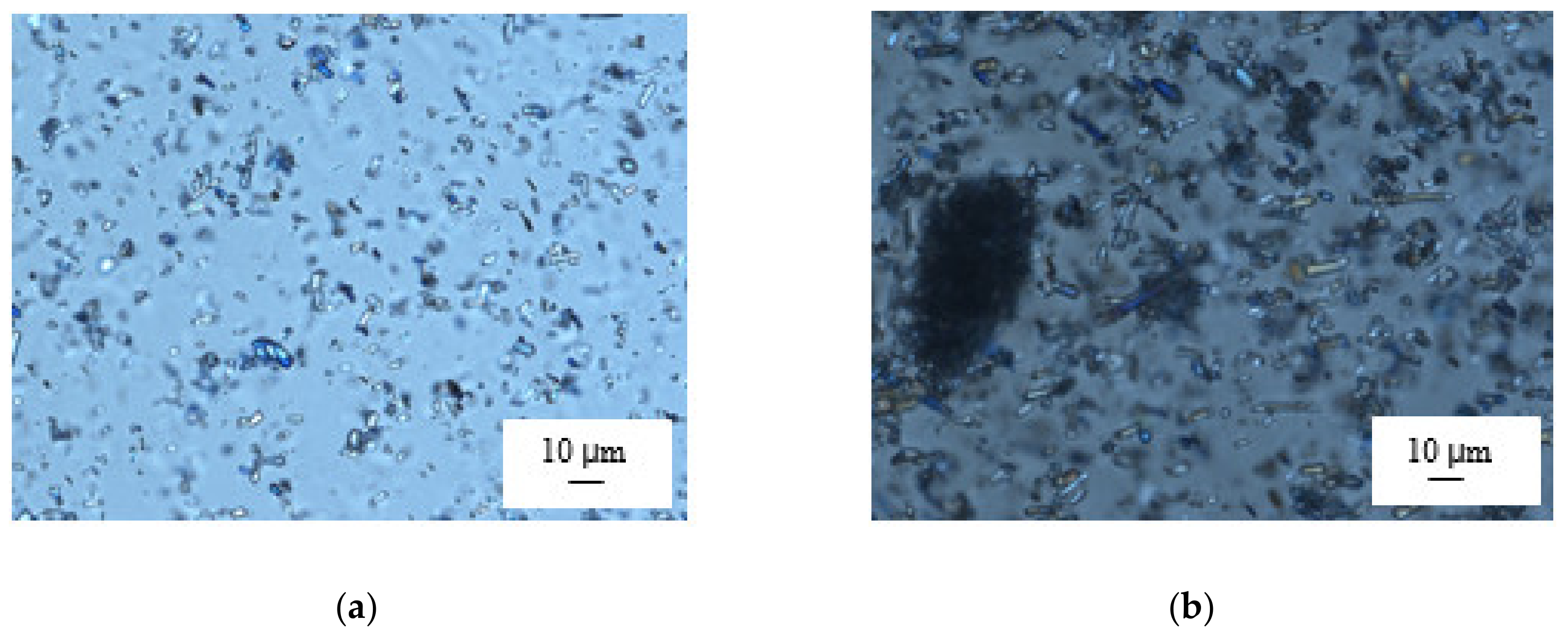
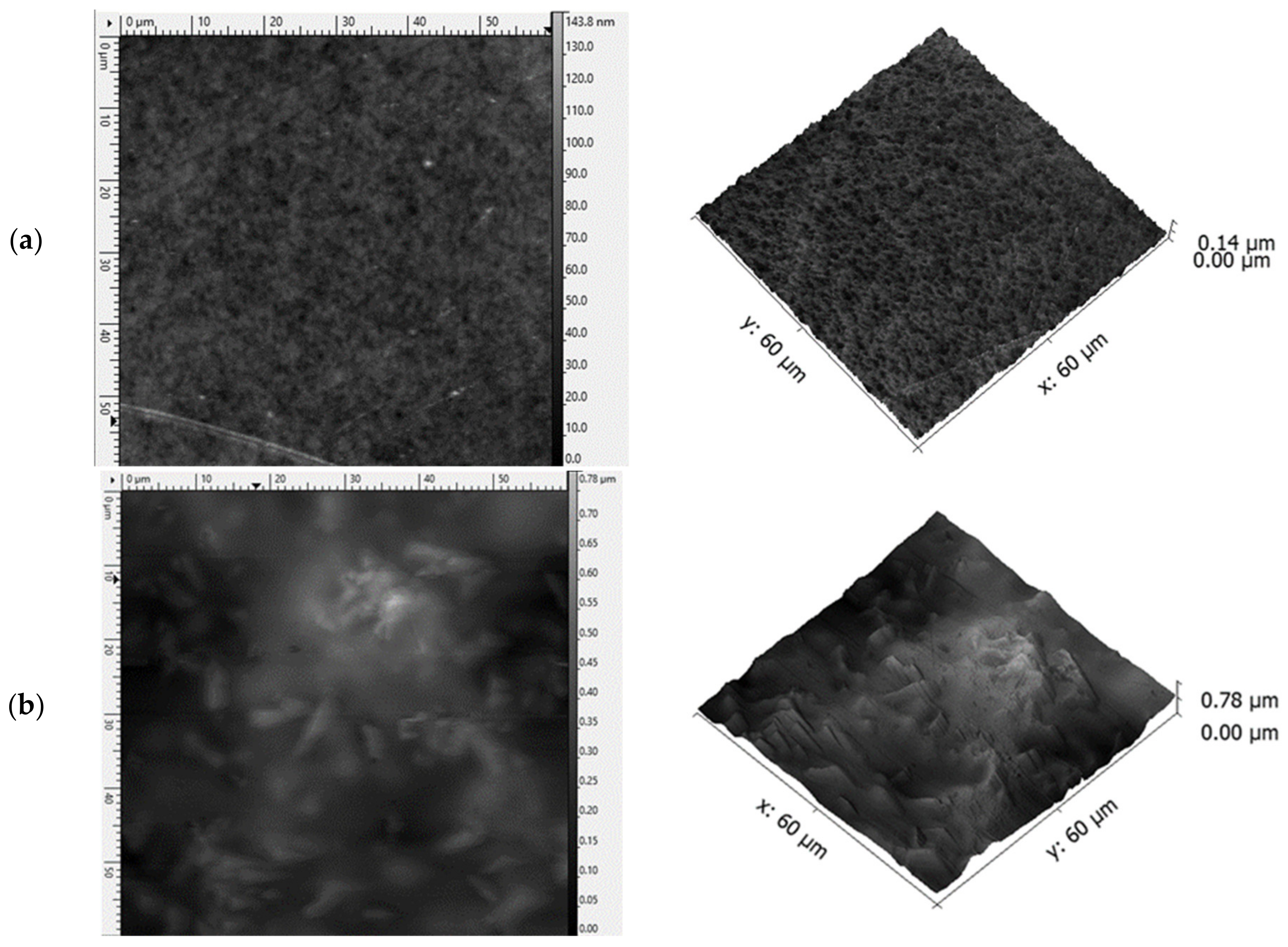
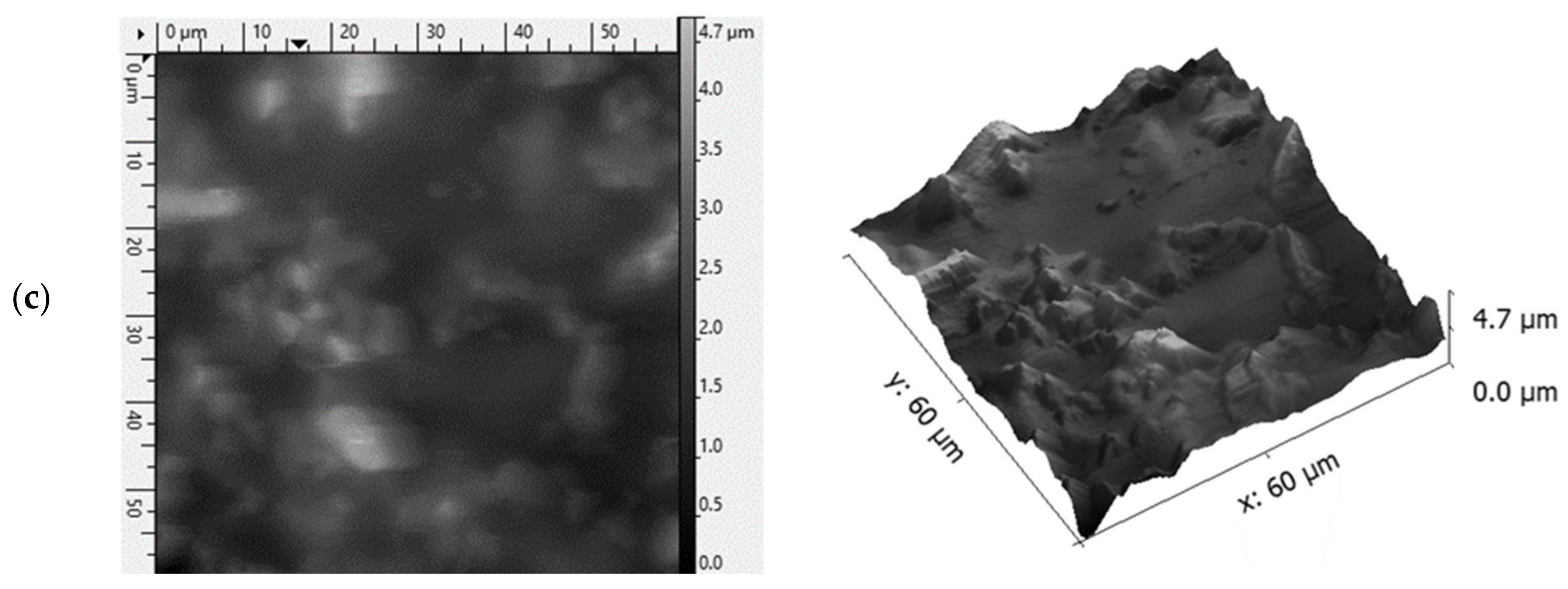
3.1. Viscosity of the Casting Mass and Morphology of ODFs
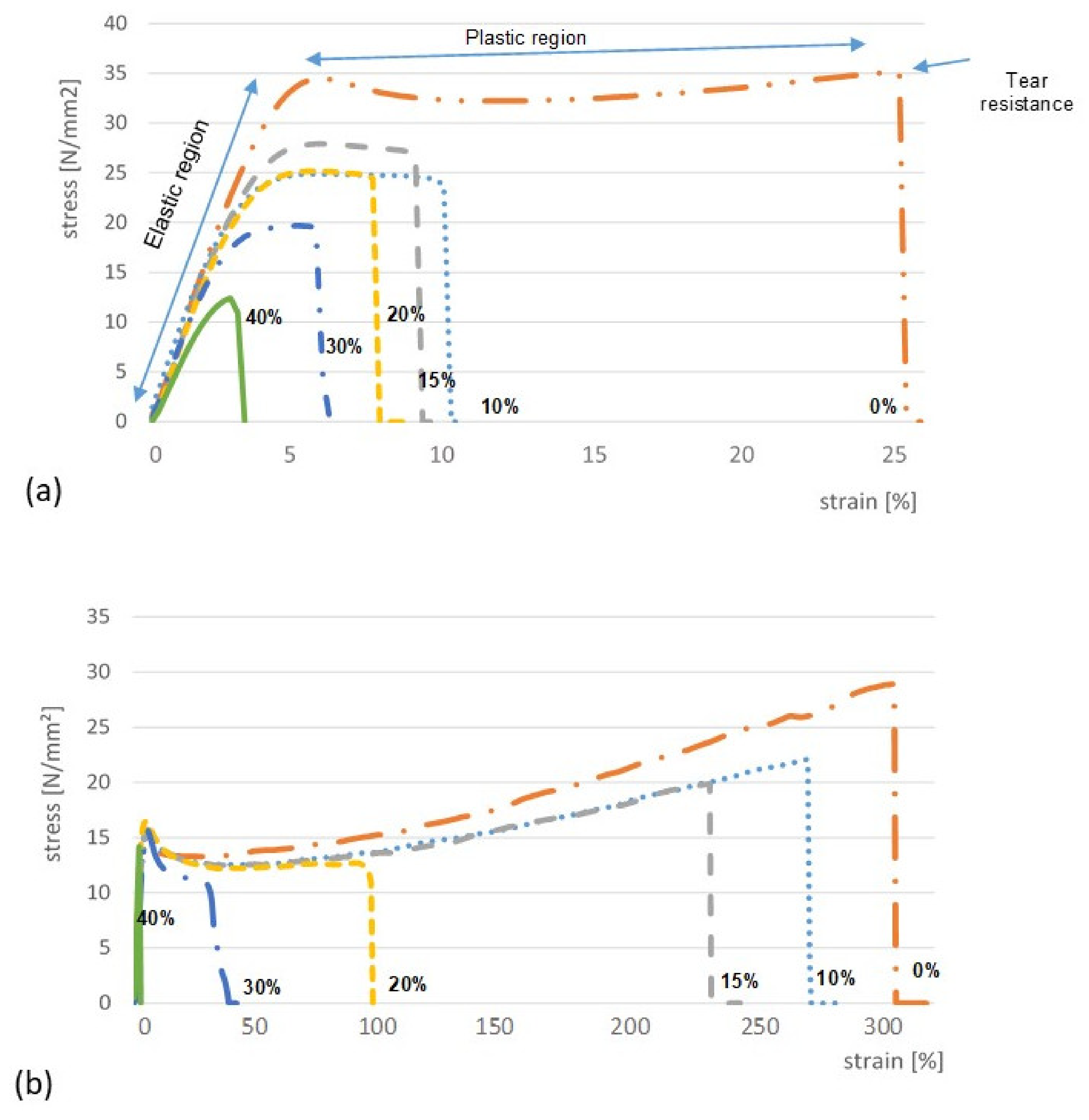
3.2. Mechanical Properties of ODFs
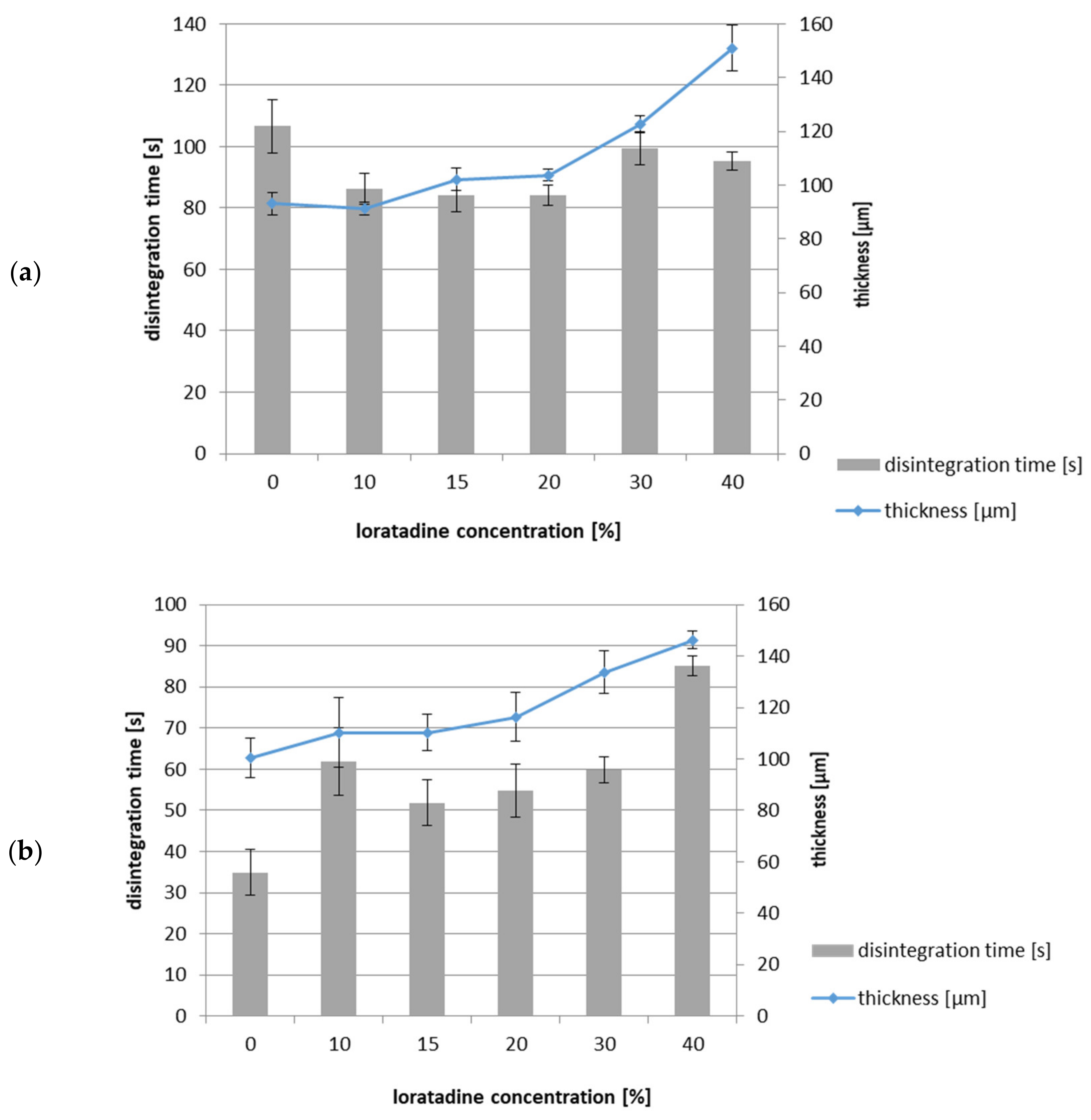
3.3. Disintegration Time
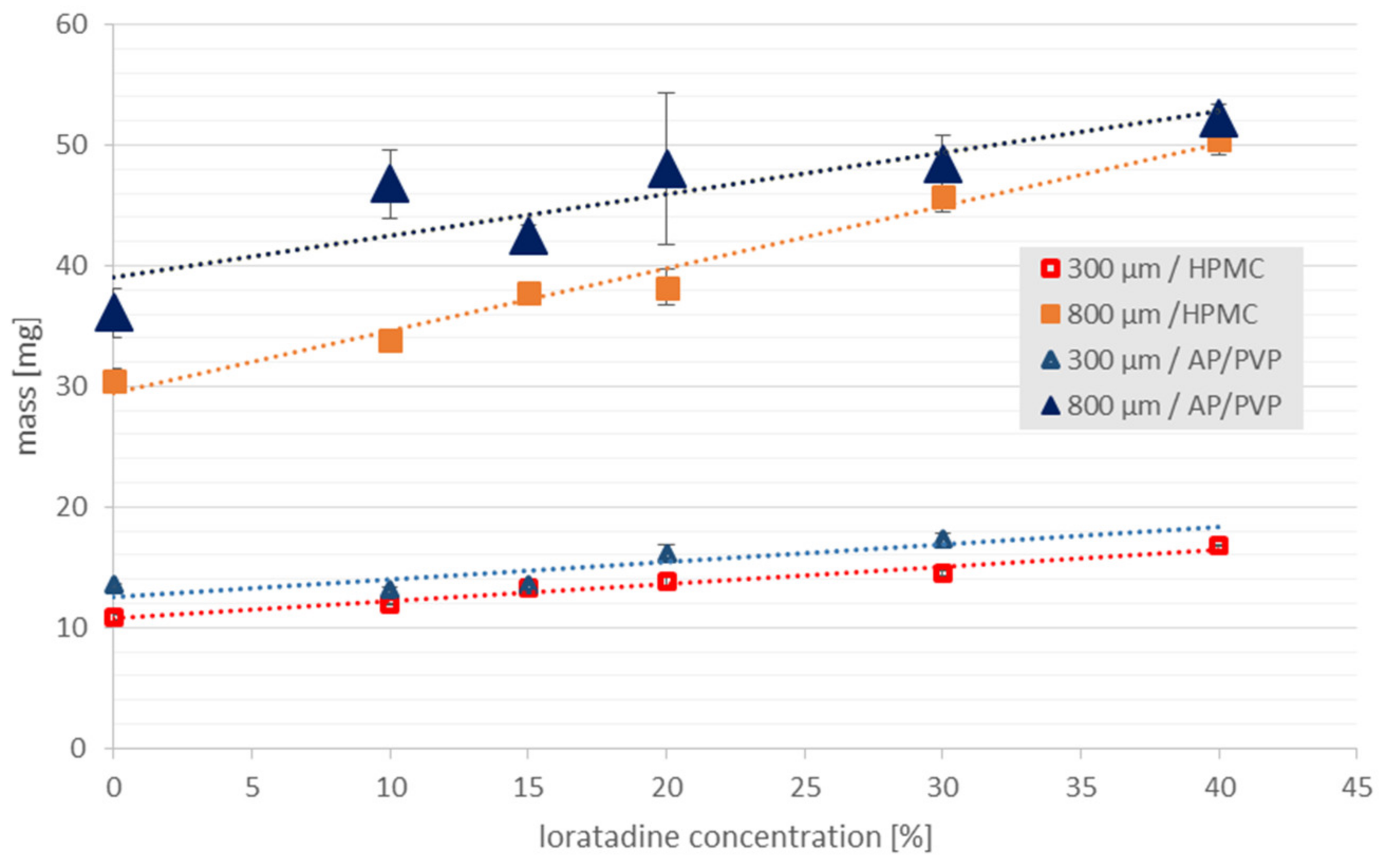
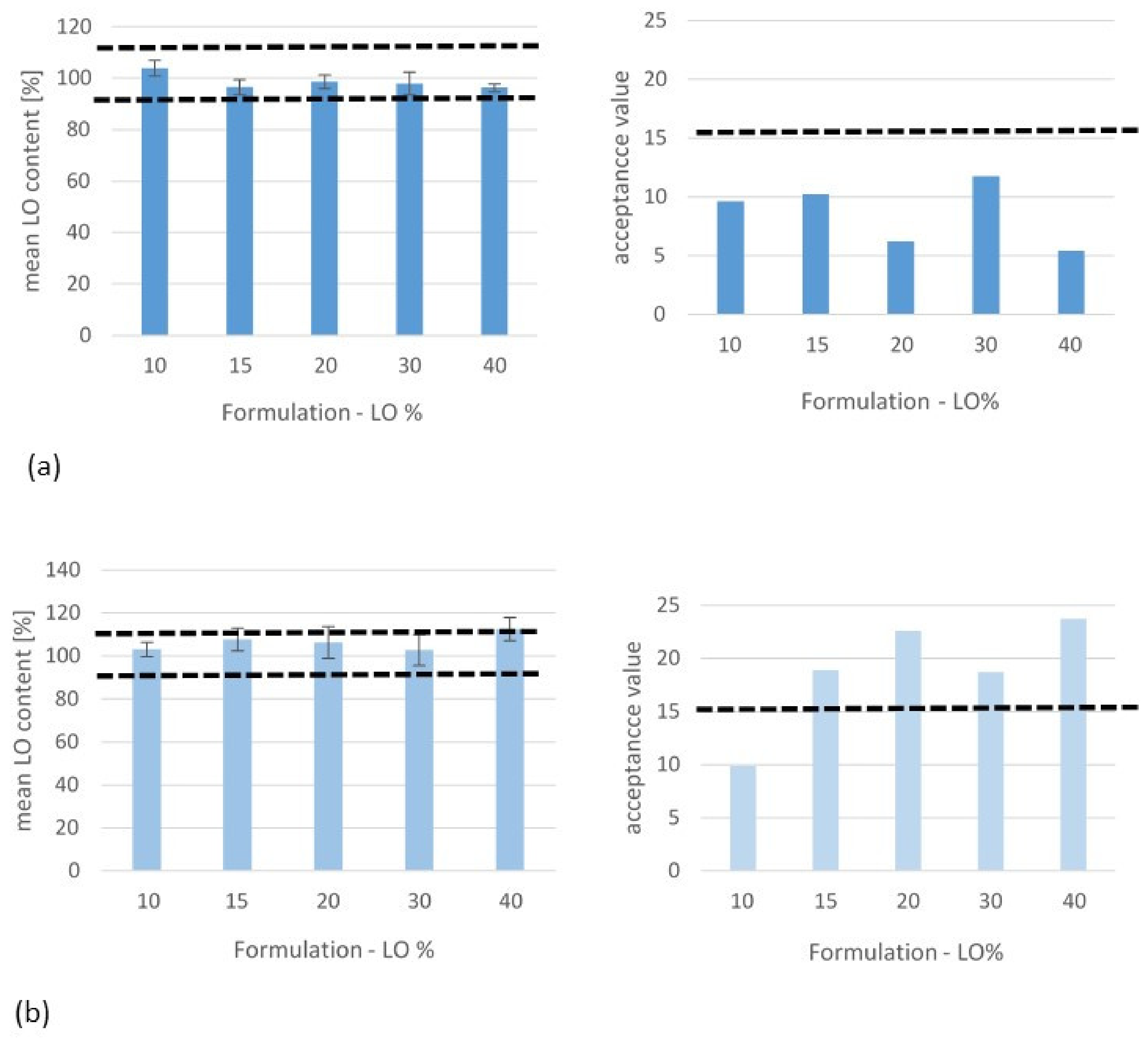
3.4. Uniformity of Drug Content
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- EDQM. European Pharmacopoeia, 10th ed; EDQM: Strasbourg, France, 2019; Volume 1. [Google Scholar]
- Satyanarayana, D.A.; Keshavarao, K.P. Fast disintegrating films containing anastrozole as a dosage form for dysphagia patients. Arch. Pharm. Res. 2012, 35, 2171–2182. [Google Scholar] [CrossRef] [PubMed]
- Slavkova, M.; Breitkreutz, J. Orodispersible drug formulations for children and elderly. Eur. J. Pharm. Sci. 2015, 75, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Visser, J.C.; Woerdenbag, H.J.; Hanff, L.M.; Frijlink, H.W. Personalized Medicine in Pediatrics: The Clinical Potential of Orodispersible Films. AAPS PharmSciTech 2017, 18, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, M.; Matsuura, K.; Tsukioka, T.; Yamashita, H.; Inagaki, N.; Sugiyama, T.; Itoh, Y. In vitro and in vivo characteristics of prochlorperazine oral disintegrating film. Int. J. Pharm. 2009, 368, 98–102. [Google Scholar] [CrossRef]
- Orlu, M.; Ranmal, S.R.; Sheng, Y.; Tuleu, C.; Seddon, P. Acceptability of orodispersible films for delivery of medicines to infants and preschool children. Drug Deliv. 2017, 24, 1243–1248. [Google Scholar] [CrossRef]
- Krampe, R.; Sieber, D.; Pein-Hackelbusch, M.; Breitkreutz, J. A new biorelevant dissolution method for orodispersible films. Eur. J. Pharm. Biopharm. 2016, 98, 20–25. [Google Scholar] [CrossRef]
- Steiner, D.; Finke, J.H.; Kwade, A. Model-based description of disintegration time and dissolution rate of nanoparticle-loaded orodispersible films. Eur. J. Pharm. Sci. 2019, 132, 18–26. [Google Scholar] [CrossRef]
- Krull, S.M.; Moreno, J.; Li, M.; Bilgili, E.; Davé, R.N. Critical material attributes (CMAs) of strip films loaded with poorly water-soluble drug nanoparticles: III. Impact of drug nanoparticle loading. Int. J. Pharm. 2017, 523, 33–41. [Google Scholar] [CrossRef]
- Vuddanda, P.R.; Montenegro-Nicolini, M.; Morales, J.O.; Velaga, S. Effect of surfactants and drug load on physico-mechanical and dissolution properties of nanocrystalline tadalafil-loaded oral films. Eur. J. Pharm. Sci. 2017, 109, 372–380. [Google Scholar] [CrossRef]
- Borges, A.F.; Silva, C.; Coelho, J.F.J.; Simões, S. Oral films: Current status and future perspectives: I-Galenical development and quality attributes. J. Control. Release 2015, 206, 1–19. [Google Scholar] [CrossRef]
- Dixit, R.P.; Puthli, S.P. Oral strip technology: Overview and future potential. J. Control. Release 2009, 139, 94–107. [Google Scholar] [CrossRef] [PubMed]
- Irfan, M.; Rabel, S.; Bukhtar, Q.; Qadir, M.I.; Jabeen, F.; Khan, A. Orally disintegrating films: A modern expansion in drug delivery system. Saudi Pharm. J. 2016, 24, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, E.M.; Breitenbach, A.; Breitkreutz, J. Advances in orodispersible films for drug delivery. Expert Opin. Drug Deliv. 2011, 8, 299–316. [Google Scholar] [CrossRef] [PubMed]
- Krampe, R.; Visser, J.C.; Frijlink, H.W.; Breitkreutz, J.; Woerdenbag, H.J.; Preis, M. Oromucosal film preparations: Points to consider for patient centricity and manufacturing processes. Expert Opin. Drug Deliv. 2016, 13, 493–506. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Y.; Abed, M.; Davé, R.N. Incorporation of surface-modified dry micronized poorly water-soluble drug powders into polymer strip films. Int. J. Pharm. 2018, 535, 462–472. [Google Scholar] [CrossRef]
- Woertz, C.; Kleinebudde, P. Development of orodispersible polymer films containing poorly water soluble active pharmaceutical ingredients with focus on different drug loadings and storage stability. Int. J. Pharm. 2015, 493, 134–145. [Google Scholar] [CrossRef]
- Khan, S.; Boateng, J.S.; Mitchell, J.; Trivedi, V. Formulation, Characterisation and Stabilisation of Buccal Films for Paediatric Drug Delivery of Omeprazole. AAPS PharmSciTech 2015, 16, 800–810. [Google Scholar] [CrossRef]
- Beck, C.; Sievens-Figueroa, L.; Gärtner, K.; Jerez-Rozo, J.I.; Romañach, R.J.; Bilgili, E.; Davé, R.N. Effects of stabilizers on particle redispersion and dissolution from polymer strip films containing liquid antisolvent precipitated griseofulvin particles. Powder Technol. 2013, 236, 37–51. [Google Scholar] [CrossRef]
- Steiner, D.; Finke, J.H.; Kwade, A. Efficient production of nanoparticle-loaded orodispersible films by process integration in a stirred media mill. Int. J. Pharm. 2016, 511, 804–813. [Google Scholar] [CrossRef]
- Göke, K.; Lorenz, T.; Repanas, A.; Schneider, F.; Steiner, D.; Baumann, K.; Kwade, A. Novel strategies for the formulation and processing of poorly water-soluble drugs. Eur. J. Pharm. Biopharm. 2018, 126, 40–56. [Google Scholar] [CrossRef]
- Speer, I.; Lenhart, V.; Preis, M.; Breitkreutz, J. Prolonged release from orodispersible films by incorporation of diclofenac-loaded micropellets. Int. J. Pharm. 2019, 554, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Speer, I.; Preis, M.; Breitkreutz, J. Prolonged drug release properties for orodispersible films by combining hot-melt extrusion and solvent casting methods. Eur. J. Pharm. Biopharm. 2018, 129, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, Y.; Kawamoto, M.; Tahara, K.; Takeuchi, H. Design of a new disintegration test system for the evaluation of orally disintegrating films. Int. J. Pharm. 2018, 553, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Frizon, F.; Eloy, J.O.; Donaduzzi, C.M.; Mitsui, M.L.; Marchetti, J.M. Dissolution rate enhancement of loratadine in polyvinylpyrrolidone K-30 solid dispersions by solvent methods. Powder Technol. 2013, 235, 532–539. [Google Scholar] [CrossRef]
- Motola, D.; Donati, M.; Biagi, C.; Calamelli, E.; Cipriani, F.; Melis, M.; Ricci, G. Safety profile of H1-antihistamines in pediatrics: An analysis based on data from VigiBase. Pharmacoepidemiol. Drug Saf. 2017, 26, 1164–1171. [Google Scholar] [CrossRef]
- Niese, S.; Breitkreutz, J.; Quodbach, J. Development of a dosing device for individualized dosing of orodispersible warfarin films. Int. J. Pharm. 2019, 561, 314–323. [Google Scholar] [CrossRef]
- Ambrus, R.; Alshweiat, A.; Csóka, I.; Ovari, G.; Esmail, A.; Radacsi, N. 3D-printed electrospinning setup for the preparation of loratadine nanofibers with enhanced physicochemical properties. Int. J. Pharm. 2019, 567, 118455. [Google Scholar] [CrossRef]
- Zeng, A.; Yao, X.; Gui, Y.; Li, Y.; Jones, K.J.; Yu, L. Inhibiting Surface Crystallization and Improving Dissolution of Amorphous Loratadine by Dextran Sulfate Nanocoating. J. Pharm. Sci. 2019, 108, 2391–2396. [Google Scholar] [CrossRef]
- Salmun, L.M.; Herron, J.M.; Banfield, C.; Padhi, D.; Lorber, R.; Affrime, M.B. The pharmacokinetics, electrocardiographic effects, and tolerability of loratadine syrup in children aged 2 to 5 years. Clin. Ther. 2000, 22, 613–621. [Google Scholar] [CrossRef]
- Visser, J.C.; Woerdenbag, H.J.; Crediet, S.; Gerrits, E.; Lesschen, M.A.; Hinrichs, W.L.J.; Breitkreutz, J.; Frijlink, H.W. A Orodispersible films in individualized pharmacotherapy: The development of a formulation for pharmacy preparations. Int. J. Pharm. 2015, 478, 155–163. [Google Scholar] [CrossRef]
- Liew, K.B.; Tan, Y.T.F.; Peh, K.K. Characterization of Oral Disintegrating Film Containing Donepezil for Alzheimer Disease. AAPS PharmSciTech 2012, 13, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Park, G.T.; Park, M.H.; Shin, Y.G.; Cho, C.W. Preparation and evaluation of oral dissolving film containing local anesthetic agent, lidocaine. J. Pharm. Investig. 2017, 47, 575–581. [Google Scholar] [CrossRef]
- Roh, J.; Han, M.; Kim, K.N.; Kim, K.M. The in vivo and in vitro effects of a fast-dissolving mucoadhesive bi-layered strip as topical anesthetics. Dent. Mater. J. 2016, 35, 601–605. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Thabet, Y.; Lunter, D.; Breitkreutz, J. Continuous manufacturing and analytical characterization of fixed-dose, multilayer orodispersible films. Eur. J. Pharm. Sci. 2018, 117, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.P.; Phani, A.R.; Prasad, R.G.S.V.; Sanganal, J.S.; Manali, N.; Gupta, R.; Raju, D.B. Polyvinylpyrrolidone oral films of enrofloxacin: Film characterization and drug release. Int. J. Pharm. 2014, 471, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Guo, X.; Sun, Y.; Yang, M. Anti-solvent Precipitation Method Coupled Electrospinning Process to Produce Poorly Water-Soluble Drug-Loaded Orodispersible Films. AAPS Pharm. Sci. Tech. 2019, 20, 273. [Google Scholar] [CrossRef]
- Alipour, S.; Akbari, S.; Ahmadi, F. Development and in vitro evaluation of fast-dissolving oral films of ondansetron hydrochloride. Trends Pharm. Sci. 2015, 1, 25–30. [Google Scholar]
- Cole, G.; Hogan, J.; Aulton, M. Pharmaceutical Coating Technology; Taylor and Francis: London, UK, 2002; pp. 288–362. [Google Scholar]
- Cilurzo, F.; Cupone, I.E.; Minghetti, P.; Selmin, F.; Montanari, L. Fast dissolving films made of maltodextrins. Eur. J. Pharm. Biopharm. 2008, 70, 895–900. [Google Scholar] [CrossRef]
- Visser, J.C.; Weggemans, O.A.F.; Boosman, R.J.; Loos, K.U.; Frijlink, H.W.; Woerdenbag, H.J. Increased drug load and polymer compatibility of bilayered orodispersible films. Eur. J. Pharm. Sci. 2017, 107, 183–190. [Google Scholar] [CrossRef]
- Preis, M.; Knop, K.; Breitkreutz, J. Mechanical strength test for orodispersible and buccal films. Int. J. Pharm. 2014, 461, 22–29. [Google Scholar] [CrossRef]
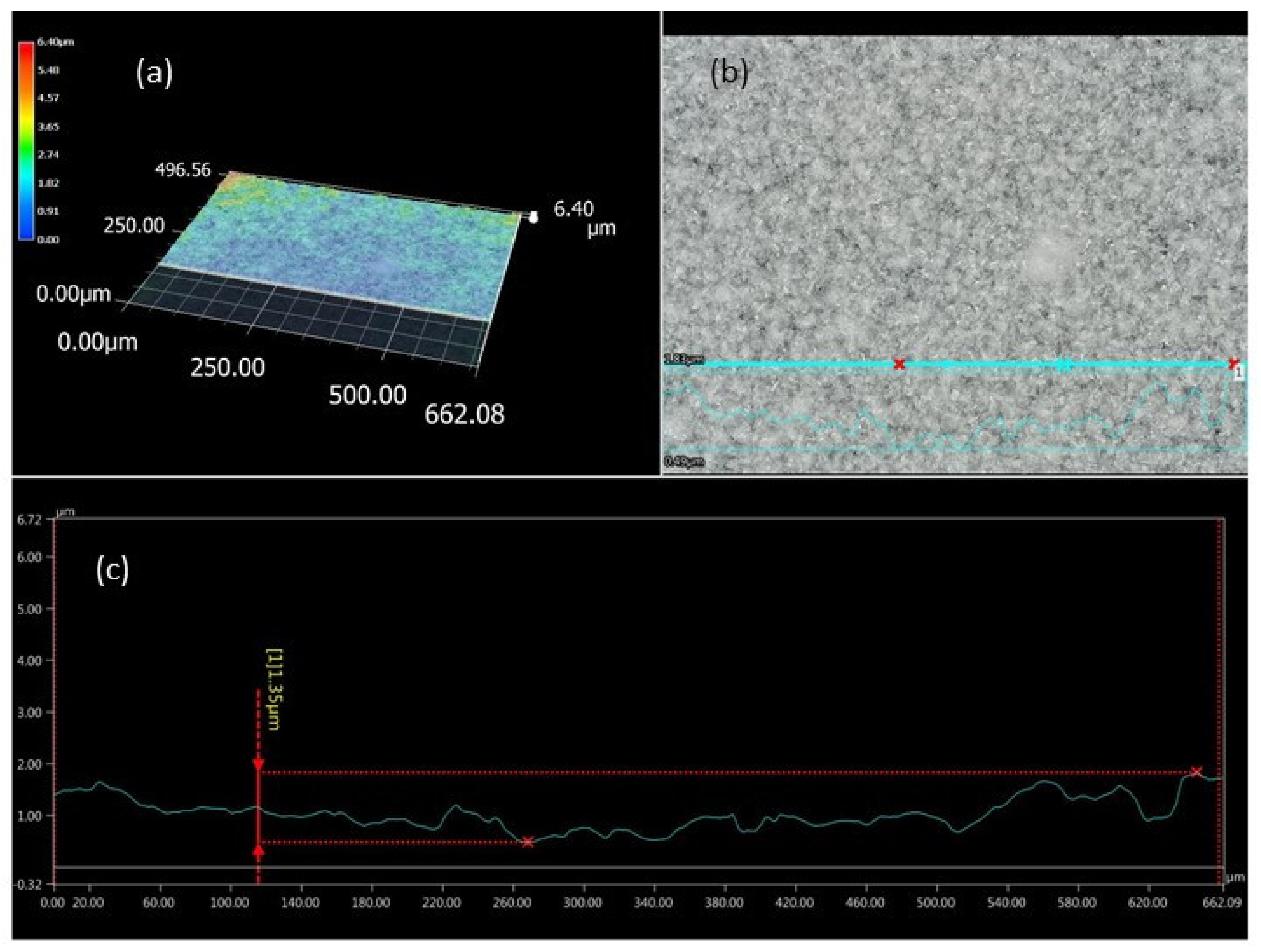
| Method | Casting Dispersion | Film Morphology | ||
|---|---|---|---|---|
| HPMC | AP/PVP | HPMC | AP/PVP | |
| TM | homogenous, degassed | homogenous, degassed | homogenous, peelable, elastic | homogenous, peelable, very elastic |
| UT | non-homogenous (LO aggregates up to 660 µm), highly aerated | non-homogenous (LO aggregates up to 220 µm), aerated | non-homogenous, rough upper surface, aerated, peelable, elastic | non-homogenous, rough upper surface, aerated, peelable, very elastic |
| US | local non-homogenous gelation, degassed, LO homogenously dispersed | local non-homogenous gelation, degassed, LO homogenously dispersed | unformable | unformable |
| Polymer | LO (%) | Thickness * (µm) | Young’s Modulus E (N/mm2) | Tear Resistance TR (N) | Elongation at Break %E (%) | Folding Endurance FE |
|---|---|---|---|---|---|---|
| HPMC | 0 | 93.0 ± 4.1 | 771.3 ± 26.5 | 33.4 ± 1.5 | 26.2 ± 0.85 | > 200 |
| 10 | 91.2 ± 2.5 | 526.3 ± 30.3 | 22.3 ± 0.3 | 6.3 ± 0.3 | > 200 | |
| 15 | 102.1 ± 4.2 | 557.0 ± 16.1 | 27.2 ± 1.1 | 6.0 ± 0.4 | 39 | |
| 20 | 103.7 ± 2.2 | 522.4 ± 33.4 | 26.2 ± 1.2 | 5.7 ± 0.3 | 12 | |
| 30 | 122.7 ± 3.0 | 396.3 ± 25.1 | 23.1 ± 0.5 | 5.6 ± 0.4 | 6 | |
| 40 | 150.9 ± 8.8 | 449.6 ± 55.6 | 17.6 ± 0.3 | 3.1 ± 0.1 | 1 | |
| AP/PVP | 0 | 100.40 ± 7.8 | 300.0 ± 26.1 | 27.6 ± 2.6 | 338.5 ± 10.3 | > 200 |
| 10 | 110.30 ± 13.5 | 325.2 ± 18.9 | 28.2 ± 2.1 | 284.9 ± 20.5 | > 200 | |
| 15 | 110.30 ± 6.9 | 339.6 ± 25.4 | 20.3 ± 0.8 | 257.5 ± 6.9 | > 200 | |
| 20 | 116.30 ± 9.6 | 321.3 ± 11.7 | 20.1 ± 0.6 | 5.3 ± 0.1 | > 200 | |
| 30 | 133.70 ± 8.3 | 343.1 ± 57.5 | 21.8 ± 1.4 | 5.0 ± 0.5 | > 200 | |
| 40 | 146.30 ± 3.3 | 946.0 ± 77.6 | 21.5 ± 1.9 | ± 0.1 | 1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Centkowska, K.; Ławrecka, E.; Sznitowska, M. Technology of Orodispersible Polymer Films with Micronized Loratadine—Influence of Different Drug Loadings on Film Properties. Pharmaceutics 2020, 12, 250. https://doi.org/10.3390/pharmaceutics12030250
Centkowska K, Ławrecka E, Sznitowska M. Technology of Orodispersible Polymer Films with Micronized Loratadine—Influence of Different Drug Loadings on Film Properties. Pharmaceutics. 2020; 12(3):250. https://doi.org/10.3390/pharmaceutics12030250
Chicago/Turabian StyleCentkowska, Katarzyna, Elżbieta Ławrecka, and Malgorzata Sznitowska. 2020. "Technology of Orodispersible Polymer Films with Micronized Loratadine—Influence of Different Drug Loadings on Film Properties" Pharmaceutics 12, no. 3: 250. https://doi.org/10.3390/pharmaceutics12030250
APA StyleCentkowska, K., Ławrecka, E., & Sznitowska, M. (2020). Technology of Orodispersible Polymer Films with Micronized Loratadine—Influence of Different Drug Loadings on Film Properties. Pharmaceutics, 12(3), 250. https://doi.org/10.3390/pharmaceutics12030250




