Metal Organic Frameworks as Drug Targeting Delivery Vehicles in the Treatment of Cancer
Abstract
1. Introduction
2. Synthesis, Functionalization, and Biomedical Applications of MOFs
2.1. MOFs Synthesis and Functionalization
2.2. Properties of Metal Organic Frameworks Regarding Drug Delivery Applications
2.2.1. The Effect of Pore Size of MOFs on the Drug Loading Capacity
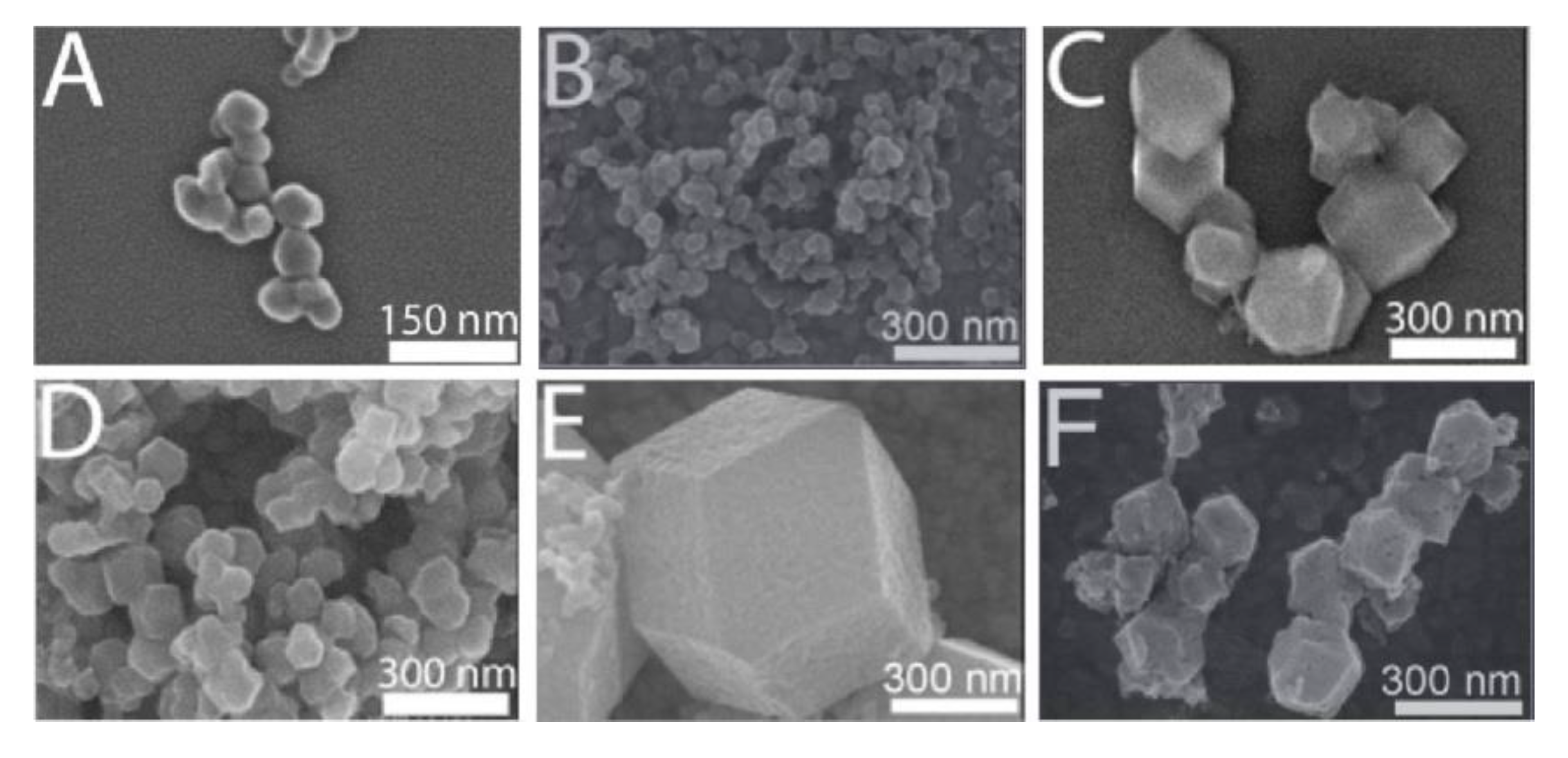
2.2.2. Particle Size Control to Achieve Functional Transfer of MOFs and Improve Biocompatibility
2.2.3. Stability of MOFs: Another Property to Consider
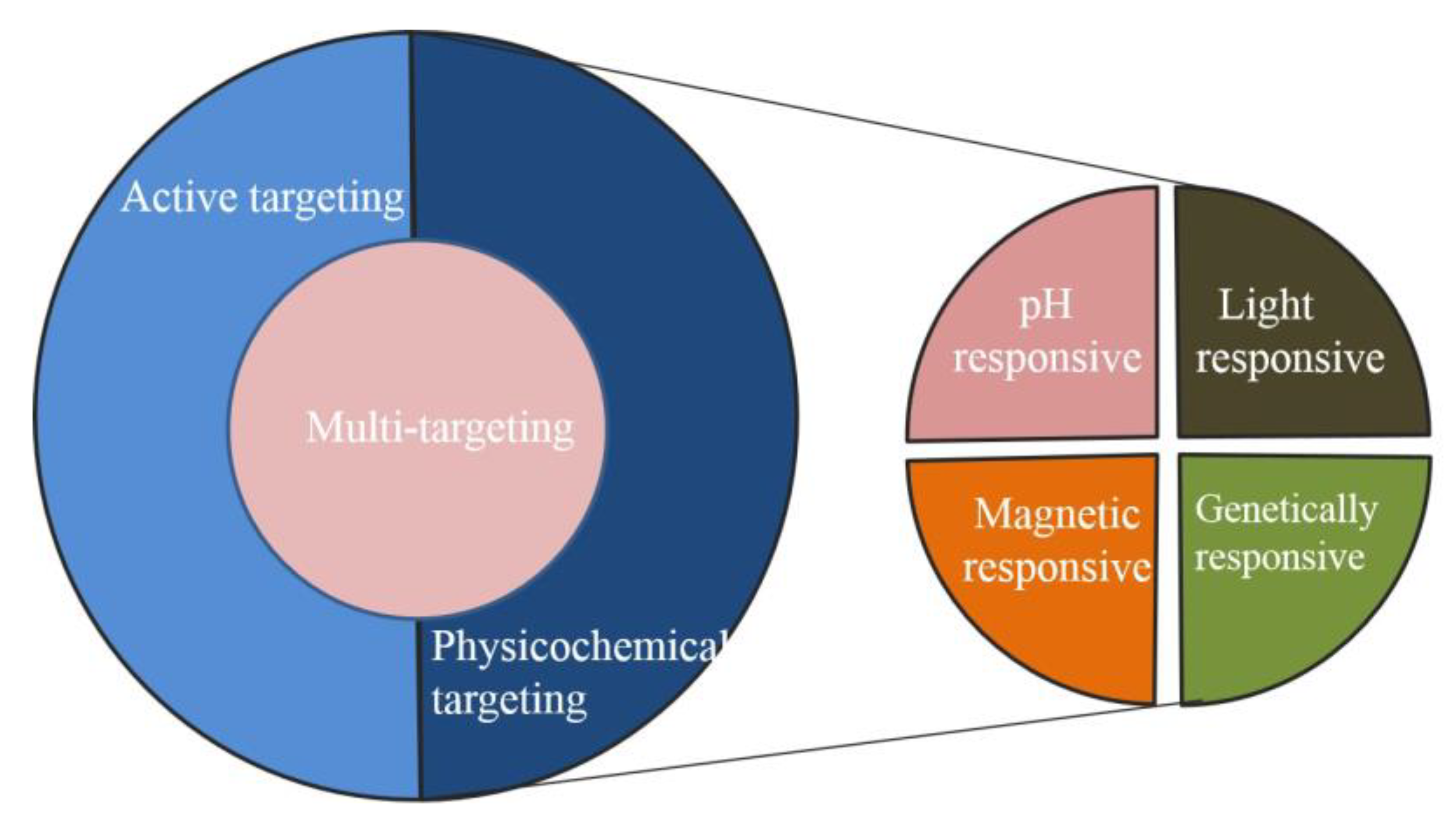
3. Applications of Metal Organic Frameworks in Targeting Cancer
3.1. Passive Targeted Therapy Used Metal Organic Frameworks
3.2. Active Targeted Therapy Based on Metal Organic Frameworks
3.3. Physicochemical Targeting Depended on Metal Organic Frameworks
3.3.1. Metal Organic Frameworks for pH-Responsive Targeted Treatment
3.3.2. Light-Responsive Targeting of Cancer with MOF-Based Nano-Therapeutics
3.3.3. Magnetic-Field-Responsive Metal Organic Frameworks-Based Targeted Anticancer Treatment
3.3.4. Targeted Drug Delivery Strategy Based on Thermosensitive MOFs
3.3.5. Targeted Anti-Tumor Therapy Strategy Based on Ion-Responsive MOFs
3.3.6. Redox Phase-Responsive-Metal Organic Frameworks-Based Targeted Anticancer Treatment
3.4. MOF-Based Nanotherapeutics for Gene Delivery
3.5. MOFs-Based Bionic Immune Escape Strategy
3.6. MOFs-Based Core-Shell Nanomedicine Carriers
3.7. Multi-Targeted Response of MOF Nanomaterial for Anticancer Treatment
4. Possible Challenges of MOFs Application in Cancer Therapy
4.1. Quality Control: from Small-Scale Production in Laboratories to Large-Scale Industrial Production
4.2. Toxicity and Biocompatibility
4.3. Avoid Drug Release or Immune Clearance Before Reaching the Target Site
5. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- DeSantis, C.E.; Miller, K.D.; Sauer, A.G.; Jemal, A.; Siegel, R.L. Cancer statistics for African Americans, 2019. Ca-A Cancer J. Clin. 2019, 69, 211–233. [Google Scholar] [CrossRef]
- Meng, Y.M.; Sun, J.; Qv, N.; Zhang, G.R.; Yu, T.; Piao, H.Z. Application of molecular imaging technology in tumor immunotherapy. Cell. Immunol. 2020, 348, 104039. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Xie, J.J.; Chen, H.J.; Gu, S.E.; Zhao, R.L.; Shao, J.W.; Jia, L. Nanotechnology-based intelligent drug design for cancer metastasis treatment. Biotechnol. Adv. 2014, 32, 761–777. [Google Scholar] [CrossRef] [PubMed]
- Gul, R.; Ahmed, N.; Shah, K.U.; Khan, G.M.; Rehman, A.U. Functionalised nanostructures for transdermal delivery of drug cargos. J. Drug Target. 2018, 26, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Castillo, R.R.; Lozano, D.; Gonzalez, B.; Manzano, M.; Izquierdo-Barba, I.; Vallet-Regi, M. Advances in mesoporous silica nanoparticles for targeted stimuli-responsive drug delivery: An update. Expert Opin. Drug Deliv. 2019, 16, 415–439. [Google Scholar] [CrossRef]
- Della Rocca, J.; Liu, D.M.; Lin, W.B. Nanoscale Metal-Organic Frameworks for Biomedical Imaging and Drug Delivery. Acc. Chem. Res. 2011, 44, 957–968. [Google Scholar] [CrossRef]
- Farrusseng, D.; Aguado, S.; Pinel, C. Metal-Organic Frameworks: Opportunities for Catalysis. Angew. Chem. Int. Ed. 2009, 48, 7502–7513. [Google Scholar] [CrossRef]
- Lee, J.; Farha, O.K.; Roberts, J.; Scheidt, K.A.; Nguyen, S.T.; Hupp, J.T. Metal-organic framework materials as catalysts. Chem. Soc. Rev. 2009, 38, 1450–1459. [Google Scholar] [CrossRef]
- Yang, B.C.; Shen, M.; Liu, J.Q.; Ren, F. Post-Synthetic Modification Nanoscale Metal-Organic Frameworks for Targeted Drug Delivery in Cancer Cells. Pharm. Res. 2017, 34, 2440–2450. [Google Scholar] [CrossRef]
- Chowdhuri, A.R.; Bhattacharya, D.; Sahu, S.K. Magnetic nanoscale metal organic frameworks for potential targeted anticancer drug delivery, imaging and as an MRI contrast agent. Dalton Trans. 2016, 45, 2963–2973. [Google Scholar] [CrossRef]
- Nian, F.Y.; Huang, Y.F.; Song, M.R.; Chen, J.J.; Xue, J.P. A novel fabricated material with divergent chemical handles based on UiO-66 and used for targeted photodynamic therapy. J. Mater. Chem. B 2017, 5, 6227–6232. [Google Scholar] [CrossRef]
- Jones, C.G.; Stavila, V.; Conroy, M.A.; Feng, P.; Slaughter, B.V.; Ashley, C.E.; Allendorf, M.D. Versatile Synthesis and Fluorescent Labeling of ZIF-90 Nanoparticles for Biomedical Applications. ACS Appl. Mater. Interfaces 2016, 8, 7623–7630. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.Q.; Chen, X.R.; Zhang, L.; Ding, S.P.; Wang, X.; Lei, Q.F.; Fang, W.J. FA-PEG decorated MOF nanoparticles as a targeted drug delivery system for controlled release of an autophagy inhibitor. Biomater. Sci. 2018, 6, 2582–2590. [Google Scholar] [CrossRef]
- Song, M.R.; Li, D.Y.; Nian, F.Y.; Xue, J.P.; Chen, J.J. Zeolitic imidazolate metal organic framework-8 as an efficient pH-controlled delivery vehicle for zinc phthalocyanine in photodynamic therapy. J. Mater. Sci. 2018, 53, 2351–2361. [Google Scholar] [CrossRef]
- Wang, X.G.; Dong, Z.Y.; Cheng, H.; Wan, S.S.; Chen, W.H.; Zou, M.Z.; Huo, J.W.; Deng, H.X.; Zhang, X.Z. A multifunctional metal-organic framework based tumor targeting drug delivery system for cancer therapy. Nanoscale 2015, 7, 16061–16070. [Google Scholar] [CrossRef]
- Shi, P.F.; Zhang, Y.C.; Yu, Z.P.; Zhang, S.S. Label-free Electrochemical Detection of ATP Based on Amino-functionalized Metal-organic Framework. Sci. Rep. 2017, 7, 1–7. [Google Scholar] [CrossRef]
- Su, F.F.; Jia, Q.J.; Li, Z.Z.; Wang, M.H.; He, L.H.; Peng, D.L.; Song, Y.P.; Zhang, Z.H.; Fang, S.M. Aptamer-templated silver nanoclusters embedded in zirconium metal-organic framework for targeted antitumor drug delivery. Microporous Mesoporous Mater. 2019, 275, 152–162. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Li, Q.; Liu, R.L.; Zhang, X.K.; Li, Z.H.; Luan, Y.X. A Versatile Prodrug Strategy to In Situ Encapsulate Drugs in MOF Nanocarriers: A Case of Cytarabine-IR820 Prodrug Encapsulated ZIF-8 toward Chemo-Photothermal Therapy. Adv. Funct. Mater. 2018, 28, 1802830. [Google Scholar] [CrossRef]
- Zhang, F.M.; Dong, H.; Zhang, X.; Sun, X.J.; Liu, M.; Yang, D.D.; Liu, X.; Wei, J.Z. Postsynthetic Modification of ZIF-90 for Potential Targeted Codelivery of Two Anticancer Drugs. ACS Appl. Mater. Interfaces 2017, 9, 27332–27337. [Google Scholar] [CrossRef]
- Jia, J.; Zhang, Y.; Zheng, M.; Shan, C.; Yan, H.; Wu, W.; Gao, X.; Cheng, B.; Liu, W.; Tang, Y. Functionalized Eu(III)-Based Nanoscale Metal-Organic Framework To Achieve Near-IR-Triggered and -Targeted Two-Photon Absorption Photodynamic Therapy. Inorg. Chem. 2018, 57, 300–310. [Google Scholar] [CrossRef]
- Cai, X.C.; Deng, X.R.; Xie, Z.X.; Shi, Y.S.; Pang, M.L.; Lin, J. Controllable synthesis of highly monodispersed nanoscale Fe-soc-MOF and the construction of Fe-soc-MOF@polypyrrole core-shell nanohybrids for cancer therapy. Chem. Eng. J. 2019, 358, 369–378. [Google Scholar] [CrossRef]
- Yu, H.Z.; Qiu, X.Y.; Neelakanda, P.; Deng, L.; Khashab, N.M.; Nunes, S.P.; Peinemann, K.V. Hollow ZIF-8 Nanoworms from Block Copolymer Templates. Sci. Rep. 2015, 5, 15275. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Dai, L.; Wang, L.Y.; Liu, J.; Lei, J.D. A surfactant template-assisted strategy for synthesis of ZIF-8 hollow nanospheres. Mater. Lett. 2015, 161, 682–685. [Google Scholar] [CrossRef]
- Wang, D.D.; Wu, H.H.; Zhou, J.J.; Xu, P.P.; Wang, C.L.; Shi, R.H.; Wang, H.B.; Wang, H.; Guo, Z.; Chen, Q.W. In Situ One-Pot Synthesis of MOF-Polydopamine Hybrid Nanogels with Enhanced Photothermal Effect for Targeted Cancer Therapy. Adv. Sci. 2018, 5, 1800287. [Google Scholar] [CrossRef]
- Bhattacharjee, A.; Gumma, S.; Purkait, M.K. Fe3O4 promoted metal organic framework MIL-100(Fe) for the controlled release of doxorubicin hydrochloride. Microporous Mesoporous Mater. 2018, 259, 203–210. [Google Scholar] [CrossRef]
- Zheng, H.Q.; Zhang, Y.N.; Liu, L.F.; Wan, W.; Guo, P.; Nystrom, A.M.; Zou, X.D. One-pot Synthesis of Metal Organic Frameworks with Encapsulated Target Molecules and Their Applications for Controlled Drug Delivery. J. Am. Chem. Soc. 2016, 138, 962–968. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Jiang, W.; Liu, R.L.; Zhang, J.; Zhang, D.; Li, Z.H.; Luan, Y.X. Rational Design of Metal Organic Framework Nanocarrier-Based Codelivery System of Doxorubicin Hydrochloride/Verapamil Hydrochloride for Overcoming Multidrug Resistance with Efficient Targeted Cancer Therapy. ACS Appl. Mater. Interfaces 2017, 9, 19687–19697. [Google Scholar] [CrossRef]
- Sharma, S.; Sethi, K.; Roy, I. Magnetic nanoscale metal-organic frameworks for magnetically aided drug delivery and photodynamic therapy. New J. Chem. 2017, 41, 11860–11866. [Google Scholar] [CrossRef]
- Li, S.Y.; Cheng, H.; Xie, B.R.; Qiu, W.X.; Zeng, J.Y.; Li, C.X.; Wan, S.S.; Zhang, L.; Liu, W.L.; Zhang, X.Z. Cancer Cell Membrane Camouflaged Cascade Bioreactor for Cancer Targeted Starvation and Photodynamic Therapy. ACS Nano 2017, 11, 7006–7018. [Google Scholar] [CrossRef]
- Huxford, R.C.; deKrafft, K.E.; Boyle, W.S.; Liu, D.M.; Lin, W.B. Lipid-coated nanoscale coordination polymers for targeted delivery of antifolates to cancer cells. Chem. Sci. 2012, 3, 198–204. [Google Scholar] [CrossRef]
- Gao, X.; Zhai, M.; Guan, W.; Liu, J.; Liu, Z.; Damirin, A.J.A.A.M.I. Controllable Synthesis of a Smart Multifunctional Nanoscale Metal–Organic Framework for Magnetic Resonance/Optical Imaging and Targeted Drug Delivery. ACS Appl. Mater. Interfaces 2017, 9, 3455–3462. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.C.; Hai, X.; Baigude, H.; Guan, W.H.; Liu, Z.L. Fabrication of functional hollow microspheres constructed from MOF shells: Promising drug delivery systems with high loading capacity and targeted transport. Sci. Rep. 2016, 6, 37705. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Yang, G.X.; Zhang, X.; Meng, X.B.; Sheng, J.L.; Sun, X.J.; Feng, Y.J.; Zhang, F.M. Folic Acid Functionalized Zirconium-Based Metal-Organic Frameworks as Drug Carriers for Active Tumor-Targeted Drug Delivery. Chem. A Eur. J. 2018, 24, 17148–17154. [Google Scholar] [CrossRef]
- Ebrahimi, A.K.; Barani, M.; Sheikhshoaie, I. Fabrication of a new superparamagnetic metal-organic framework with core-shell nanocomposite structures: Characterization, biocompatibility, and drug release study. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 92, 349–355. [Google Scholar] [CrossRef]
- Hu, D.; Xu, H.X.; Xiao, B.; Li, D.D.; Zhou, Z.X.; Liu, X.R.; Tang, J.B.; Shen, Y.Q. Albumin-Stabilized Metal-Organic Nanoparticles for Effective Delivery of Metal Complex Anticancer Drugs. Acs Appl. Mater. Interfaces 2018, 10, 34974–34982. [Google Scholar] [CrossRef]
- Jiang, K.; Zhang, L.; Hu, Q.; Zhang, Q.; Lin, W.X.; Cui, Y.J.; Yang, Y.; Qian, G.D. Thermal Stimuli-Triggered Drug Release from a Biocompatible Porous Metal-Organic Framework. Chem. A Eur. J. 2017, 23, 10215–10221. [Google Scholar] [CrossRef]
- Duan, D.; Liu, H.; Xu, M.; Chen, M.; Han, Y.; Shi, Y.; Liu, Z. Size-Controlled Synthesis of Drug-Loaded Zeolitic Imidazolate Framework in Aqueous Solution and Size Effect on Their Cancer Theranostics in Vivo. Acs Appl. Mater. Interfaces 2018, 10, 42165–42174. [Google Scholar] [CrossRef]
- Gao, X.C.; Cui, R.X.; Ji, G.F.; Liu, Z.L. Size and surface controllable metal-organic frameworks (MOFs) for fluorescence imaging and cancer therapy. Nanoscale 2018, 10, 6205–6211. [Google Scholar] [CrossRef]
- Lim, E.K.; Kim, T.; Paik, S.; Haam, S.; Huh, Y.M.; Lee, K. Nanomaterials for Theranostics: Recent Advances and Future Challenges. Chem. Rev. 2015, 115, 327–394. [Google Scholar] [CrossRef]
- Baeza, A.; Colilla, M.; Vallet-Regi, M. Advances in mesoporous silica nanoparticles for targeted stimuli-responsive drug delivery. Expert Opin. Drug Deliv. 2015, 12, 319–337. [Google Scholar] [CrossRef]
- Chen, D.Q.; Yang, D.Z.; Dougherty, C.A.; Lu, W.F.; Wu, H.W.; He, X.R.; Cai, T.; Van Dort, M.E.; Ross, B.D.; Hong, H. In Vivo Targeting and Positron Emission Tomography Imaging of Tumor with Intrinsically Radioactive Metal-Organic Frameworks Nanomaterials. ACS Nano 2017, 11, 4315–4327. [Google Scholar] [CrossRef]
- Wei, Z.; Yao, L.; Zhe, Y.; Li, Z.; Xiao, L.; Pei, L.; Jing, W.; Yi, C.; Xu, Z.; Ren, J. Albumin/sulfonamide stabilized iron porphyrin metal organic framework nanocomposites: Targeting tumor hypoxia by carbonic anhydrase IX inhibition and: T 1-T 2 dual mode MRI guided photodynamic/photothermal therapy. J. Mater. Chem. B 2017, 6, 265–276. [Google Scholar]
- Zhang, L.; Lei, J.P.; Ma, F.J.; Ling, P.H.; Liu, J.T.; Ju, H.X. A porphyrin photosensitized metal-organic framework for cancer cell apoptosis and caspase responsive theranostics. Chem. Commun. 2015, 51, 10831–10834. [Google Scholar] [CrossRef]
- Wu, Y.F.; Han, J.Y.; Xue, P.; Xu, R.; Kang, Y.J. Nano metal-organic framework (NMOF)-based strategies for multiplexed microRNA detection in solution and living cancer cells. Nanoscale 2015, 7, 1753–1759. [Google Scholar] [CrossRef]
- Rowe, M.D.; Thamm, D.H.; Kraft, S.L.; Boyes, S.G. Polymer-Modified Gadolinium Metal-Organic Framework Nanoparticles Used as Multifunctional Nanomedicines for the Targeted Imaging and Treatment of Cancer. Biomacromolecules 2009, 10, 983–993. [Google Scholar] [CrossRef]
- Liu, J.T.; Zhang, L.; Lei, J.P.; Shen, H.; Ju, H.X. Multifunctional Metal-Organic Framework Nanoprobe for Cathepsin B-Activated Cancer Cell Imaging and Chemo-Photodynamic Therapy. ACS Appl. Mater. Interfaces 2017, 9, 2150–2158. [Google Scholar] [CrossRef]
- He, L.C.; Brasino, M.; Mao, C.C.; Cho, S.; Park, W.; Goodwin, A.P.; Cha, J.N. DNA-Assembled Core-Satellite Upconverting-Metal-Organic Framework Nanoparticle Superstructures for Efficient Photodynamic Therapy. Small 2017, 13, 1700504. [Google Scholar] [CrossRef]
- Deng, K.R.; Hou, Z.Y.; Li, X.J.; Li, C.X.; Zhang, Y.X.; Deng, X.R.; Cheng, Z.Y.; Lin, J. Aptamer-Mediated Up-conversion Core/MOF Shell Nanocomposites for Targeted Drug Delivery and Cell Imaging. Sci. Rep. 2015, 5, 7851. [Google Scholar] [CrossRef]
- Chowdhuri, A.R.; Laha, D.; Pal, S.; Karmakar, P.; Sahu, S.K. One-pot synthesis of folic acid encapsulated upconversion nanoscale metal organic frameworks for targeting, imaging and pH responsive drug release. Dalton Trans. 2016, 45, 18120–18132. [Google Scholar] [CrossRef]
- Cai, W.; Gao, H.Y.; Chu, C.C.; Wang, X.Y.; Wang, J.Q.; Zhang, P.F.; Lin, G.; Li, W.G.; Liu, G.; Chen, X.Y. Engineering Phototheranostic Nanoscale Metal-Organic Frameworks for Multimodal Imaging-Guided Cancer Therapy. ACS Appl. Mater. Interfaces 2017, 9, 2040–2051. [Google Scholar] [CrossRef]
- Alizadeh, N.; Salimi, A.; Hallaj, R.; Fathi, F.; Soleimani, F. Ni-hemin metal-organic framework with highly efficient peroxidase catalytic activity: Toward colorimetric cancer cell detection and targeted therapeutics. J. Nanobiotechnol. 2018, 16, 1–14. [Google Scholar] [CrossRef]
- Chen, X.R.; Shi, Z.Q.; Tong, R.L.; Ding, S.P.; Wang, X.; Wu, J.; Lei, Q.F.; Fang, W.J. Derivative of Epigallocatechin-3-gallatea Encapsulated in ZIF-8 with Polyethylene Glycol-Folic Acid Modification for Target and pH-Responsive Drug Release in Anticancer Research. ACS Biomater. Sci. Eng. 2018, 4, 4183–4192. [Google Scholar] [CrossRef]
- Zhang, X.L.; Zeng, Y.Y.; Zheng, A.X.; Cai, Z.X.; Huang, A.M.; Zeng, J.H.; Liu, X.L.; Liu, J.F. A fluorescence based immunoassay for galectin-4 using gold nanoclusters and a composite consisting of glucose oxidase and a metal-organic framework. Microchim. Acta 2017, 184, 1933–1940. [Google Scholar] [CrossRef]
- Dong, K.; Zhang, Y.; Zhang, L.; Wang, Z.Z.; Ren, J.S.; Qu, X.G. Facile preparation of metal organic frameworks-based hydrophobic anticancer drug delivery nanoplatform for targeted and enhanced cancer treatment. Talanta 2019, 194, 703–708. [Google Scholar] [CrossRef]
- Zhang, H.; Shang, Y.; Li, Y.H.; Sun, S.K.; Yin, X.B. Smart Metal-Organic Framework-Based Nanoplatforms for Imaging-Guided Precise Chemotherapy. ACS Appl. Mater. Interfaces 2019, 11, 1886–1895. [Google Scholar] [CrossRef]
- Park, J.; Jiang, Q.; Feng, D.W.; Mao, L.Q.; Zhou, H.C. Size-Controlled Synthesis of Porphyrinic Metal-Organic Framework and Functionalization for Targeted Photodynamic Therapy. J. Am. Chem. Soc. 2016, 138, 3518–3525. [Google Scholar] [CrossRef]
- Li, Y.A.; Zhao, X.D.; Yin, H.P.; Chen, G.J.; Yang, S.; Dong, Y.B. A drug-loaded nanoscale metal-organic framework with a tumor targeting agent for highly effective hepatoma therapy. Chem. Commun. 2016, 52, 14113–14116. [Google Scholar] [CrossRef]
- Laha, D.; Pal, K.; Chowdhuri, A.R.; Parida, P.K.; Sahu, S.K.; Jana, K.; Karmakar, P. Fabrication of curcumin-loaded folic acid-tagged metal organic framework for triple negative breast cancer therapy in in vitro and in vivo systems. New J. Chem. 2019, 43, 217–229. [Google Scholar] [CrossRef]
- Chen, W.H.; Sung, S.Y.; Fadeev, M.; Cecconello, A.; Nechushtai, R.; Willner, I. Targeted VEGF-triggered release of an anti-cancer drug from aptamer-functionalized metal-organic framework nanoparticles. Nanoscale 2018, 10, 4650–4657. [Google Scholar] [CrossRef]
- Qi, X.Y.; Chang, Z.Y.; Zhang, D.; Binder, K.J.; Shen, S.S.; Huang, Y.Y.S.; Bai, Y.; Wheatley, A.E.H.; Liu, H.W. Harnessing Surface-Functionalized Metal-Organic Frameworks for Selective Tumor Cell Capture. Chem. Mater. 2017, 29, 8052–8056. [Google Scholar] [CrossRef]
- Li, S.Y.; Xie, B.R.; Cheng, H.; Li, C.X.; Zhang, M.K.; Qiu, W.X.; Liu, W.L.; Wang, X.S.; Zhang, X.Z. A biomimetic theranostic O-2-meter for cancer targeted photodynamic therapy and phosphorescence imaging. Biomaterials 2018, 151, 1–12. [Google Scholar] [CrossRef]
- Chen, X.; Tong, R.; Shi, Z.; Yang, B.; Liu, H.; Ding, S.; Wang, X.; Lei, Q.; Wu, J.; Fang, W. MOF Nanoparticles with Encapsulated Autophagy Inhibitor in Controlled Drug Delivery System for Antitumor. ACS Appl. Mater. Interfaces 2018, 10, 2328–2337. [Google Scholar] [CrossRef]
- Liang, Z.Z.; Yang, Z.Y.; Yuan, H.T.; Wang, C.; Qi, J.; Liu, K.Q.; Cao, R.; Zheng, H.Q. A protein@metal-organic framework nanocomposite for pH-triggered anticancer drug delivery. Dalton Trans. 2018, 47, 10223–10228. [Google Scholar] [CrossRef]
- Tang, L.; Shi, J.; Wang, X.; Zhang, S.; Wu, H.; Sun, H.; Jiang, Z. Coordination polymer nanocapsules prepared using metal-organic framework templates for pH-responsive drug delivery. Nanotechnology 2017, 28, 275601. [Google Scholar] [CrossRef]
- Wang, D.; Zhou, J.; Chen, R.; Shi, R.; Wang, C.; Lu, J.; Zhao, G.; Xia, G.; Zhou, S.; Liu, Z.; et al. Core–Shell Metal-Organic Frameworks as Fe2+ Suppliers for Fe2+-Mediated Cancer Therapy under Multimodality Imaging. Chem. Mater. 2017, 29, 3477–3489. [Google Scholar] [CrossRef]
- Gupta, V.; Tyagi, S.; Paul, A.K. Development of Biocompatible Iron-Carboxylate Metal Organic Frameworks for pH-Responsive Drug Delivery Application. J. Nanosci. Nanotechnol. 2019, 19, 646–654. [Google Scholar] [CrossRef]
- Chen, L.; Yu, H.; Li, Y.; Zhang, X.; Du, Y. Fabrication of a microporous Dy(III)-organic framework with polar channels for 5-Fu (fluorouracil) delivery and inhibiting human brain tumor cells. Struct. Chem. 2018, 29, 1885–1891. [Google Scholar] [CrossRef]
- Park, J.H.; Saravanakumar, G.; Kim, K.; Kwon, I.C. Targeted delivery of low molecular drugs using chitosan and its derivatives. Adv. Drug Deliv. Rev. 2010, 62, 28–41. [Google Scholar] [CrossRef]
- Abazari, R.; Mahjoub, A.R.; Ataei, F.; Morsali, A.; Carpenter-Warren, C.L.; Mehdizadeh, K.; Slawin, A.M.Z. Chitosan Immobilization on Bio-MOF Nanostructures: A Biocompatible pH-Responsive Nanocarrier for Doxorubicin Release on MCF-7 Cell Lines of Human Breast Cancer. Inorg. Chem. 2018, 57, 13364–13379. [Google Scholar] [CrossRef]
- Cárdenas-Jirón, G.; Borges-Martínez, M.; Mera-Adasme, R.; Pino-Rios, R. Quantum chemical studies of porphyrin- and expanded porphyrin-based systems and their potential applications in nanoscience. Latin America research review. Int. J. Quantum Chem. 2018, 119, e25821. [Google Scholar]
- Yang, Y.; Liu, J.; Liang, C.; Feng, L.; Fu, T.; Dong, Z.; Chao, Y.; Li, Y.; Lu, G.; Chen, M.; et al. Nanoscale Metal-Organic Particles with Rapid Clearance for Magnetic Resonance Imaging-Guided Photothermal Therapy. ACS Nano 2016, 10, 2774–2781. [Google Scholar] [CrossRef]
- Jin, Q.; Zhu, W.; Jiang, D.; Zhang, R.; Kutyreff, C.J.; Engle, J.W.; Huang, P.; Cai, W.; Liu, Z.; Cheng, L. Ultra-small iron-gallic acid coordination polymer nanoparticles for chelator-free labeling of (64)Cu and multimodal imaging-guided photothermal therapy. Nanoscale 2017, 9, 12609–12617. [Google Scholar] [CrossRef]
- Fang, L.; Hu, Q.; Jiang, K.; Zhang, X.; Li, B.; Cui, Y.; Yang, Y.; Qian, G. An inner light integrated metal-organic framework photodynamic therapy system for effective elimination of deep-seated tumor cells. J. Solid State Chem. 2019, 276, 205–209. [Google Scholar] [CrossRef]
- Ke, F.; Yuan, Y.-P.; Qiu, L.-G.; Shen, Y.-H.; Xie, A.-J.; Zhu, J.-F.; Tian, X.-Y.; Zhang, L.-D. Facile fabrication of magnetic metal–organic framework nanocomposites for potential targeted drug delivery. J. Mater. Chem. 2011, 21, 3843–3848. [Google Scholar] [CrossRef]
- Yang, D.; Yang, G.; Gai, S.; He, F.; An, G.; Dai, Y.; Lv, R.; Yang, P. Au25 cluster functionalized metal-organic nanostructures for magnetically targeted photodynamic/photothermal therapy triggered by single wavelength 808 nm near-infrared light. Nanoscale 2015, 7, 19568–19578. [Google Scholar] [CrossRef]
- Xue, Z.; Zhu, M.; Dong, Y.; Feng, T.; Chen, Z.; Feng, Y.; Shan, Z.; Xu, J.; Meng, S. An integrated targeting drug delivery system based on the hybridization of graphdiyne and MOFs for visualized cancer therapy. Nanoscale 2019, 11, 11709–11718. [Google Scholar] [CrossRef]
- Taylor, K.M.L.; Rieter, W.J.; Lin, W.B. Manganese-Based Nanoscale Metal-Organic Frameworks for Magnetic Resonance Imaging. J. Am. Chem. Soc. 2008, 130, 14358. [Google Scholar] [CrossRef]
- Xing, K.; Fan, R.; Wang, F.; Nie, H.; Du, X.; Gai, S.; Wang, P.; Yang, Y. Dual-Stimulus-Triggered Programmable Drug Release and Luminescent Ratiometric pH Sensing from Chemically Stable Biocompatible Zinc Metal-Organic Framework. ACS Appl. Mater. Interfaces 2018, 10, 22746–22756. [Google Scholar] [CrossRef]
- Lin, W.; Hu, Q.; Yu, J.; Jiang, K.; Yang, Y.; Xiang, S.; Cui, Y.; Yang, Y.; Wang, Z.; Qian, G. Low Cytotoxic Metal-Organic Frameworks as Temperature-Responsive Drug Carriers. ChemPlusChem 2016, 81, 804–810. [Google Scholar] [CrossRef]
- Yang, Y.; Hu, Q.; Zhang, Q.; Jiang, K.; Lin, W.; Yang, Y.; Cui, Y.; Qian, G. A Large Capacity Cationic Metal-Organic Framework Nanocarrier for Physiological pH Responsive Drug Delivery. Mol. Pharm. 2016, 13, 2782–2786. [Google Scholar] [CrossRef]
- Wu, M.X.; Gao, J.; Wang, F.; Yang, J.; Song, N.; Jin, X.; Mi, P.; Tian, J.; Luo, J.; Liang, F.; et al. Multistimuli Responsive Core-Shell Nanoplatform Constructed from Fe3 O4 @MOF Equipped with Pillar[6]arene Nanovalves. Small 2018, 14, e1704440. [Google Scholar] [CrossRef]
- Yi, J.T.; Chen, T.T.; Huo, J.; Chu, X. Nanoscale Zeolitic Imidazolate Framework-8 for Ratiometric Fluorescence Imaging of MicroRNA in Living Cells. Anal. Chem. 2017, 89, 12351–12359. [Google Scholar] [CrossRef]
- Qiu, G.H.; Lu, W.Z.; Hu, P.P.; Jiang, Z.H.; Bai, L.P.; Wang, T.R.; Li, M.M.; Chen, J.X. A metal-organic framework based PCR-free biosensor for the detection of gastric cancer associated microRNAs. J. Inorg. Biochem. 2017, 177, 138–142. [Google Scholar] [CrossRef]
- Chen, W.-H.; Yu, X.; Liao, W.-C.; Sohn, Y.S.; Cecconello, A.; Kozell, A.; Nechushtai, R.; Willner, I. ATP-Responsive Aptamer-Based Metal-Organic Framework Nanoparticles (NMOFs) for the Controlled Release of Loads and Drugs. Adv. Funct. Mater. 2017, 27, 1702102. [Google Scholar] [CrossRef]
- Cheng, H.; Zhu, J.-Y.; Li, S.-Y.; Zeng, J.-Y.; Lei, Q.; Chen, K.-W.; Zhang, C.; Zhang, X.-Z. An O2Self-Sufficient Biomimetic Nanoplatform for Highly Specific and Efficient Photodynamic Therapy. Adv. Funct. Mater. 2016, 26, 7847–7860. [Google Scholar] [CrossRef]
- Li, S.Y.; Cheng, H.; Qiu, W.X.; Zhang, L.; Wan, S.S.; Zeng, J.Y.; Zhang, X.Z. Cancer cell membrane-coated biomimetic platform for tumor targeted photodynamic therapy and hypoxia-amplified bioreductive therapy. Biomaterials 2017, 142, 149–161. [Google Scholar] [CrossRef]
- Wan, S.S.; Zeng, J.Y.; Cheng, H.; Zhang, X.Z. ROS-induced NO generation for gas therapy and sensitizing photodynamic therapy of tumor. Biomaterials 2018, 185, 51–62. [Google Scholar] [CrossRef]
- Chowdhuri, A.R.; Laha, D.; Chandra, S.; Karmakar, P.; Sahu, S.K. Synthesis of multifunctional upconversion NMOFs for targeted antitumor drug delivery and imaging in triple negative breast cancer cells. Chem. Eng. J. 2017, 319, 200–211. [Google Scholar] [CrossRef]
- Au, K.M.; Satterlee, A.; Min, Y.; Tian, X.; Kim, Y.S.; Caster, J.M.; Zhang, L.; Zhang, T.; Huang, L.; Wang, A.Z. Folate-targeted pH-responsive calcium zoledronate nanoscale metal-organic frameworks: Turning a bone antiresorptive agent into an anticancer therapeutic. Biomaterials 2016, 82, 178–193. [Google Scholar] [CrossRef]
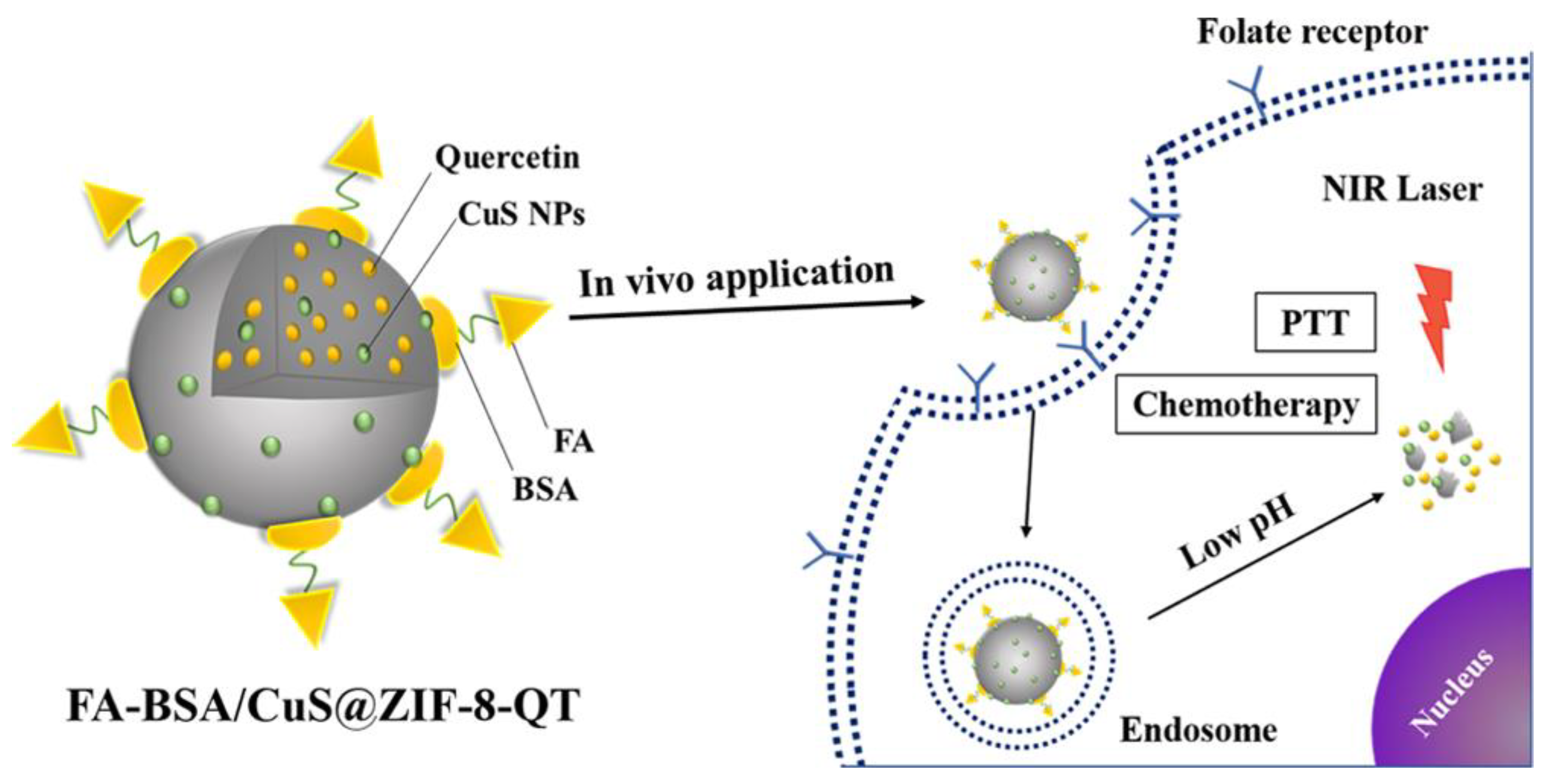
- Jiang, W.; Zhang, H.; Wu, J.; Zhai, G.; Li, Z.; Luan, Y.; Garg, S. CuS@MOF-Based Well-Designed Quercetin Delivery System for Chemo-Photothermal Therapy. ACS Appl. Mater. Interfaces 2018, 10, 34513–34523. [Google Scholar] [CrossRef]
- Wu, M.X.; Yan, H.J.; Gao, J.; Cheng, Y.; Yang, J.; Wu, J.R.; Gong, B.J.; Zhang, H.Y.; Yang, Y.W. Multifunctional Supramolecular Materials Constructed from Polypyrrole@UiO-66 Nanohybrids and Pillararene Nanovalves for Targeted Chemophotothermal Therapy. ACS Appl. Mater. Interfaces 2018, 10, 34655–34663. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhu, H.; Shi, Y.; Ge, Y.; Feng, X.; Liu, R.; Li, Y.; Ma, Y.; Wang, L. Novel catalytic micromotor of porous zeolitic imidazolate framework-67 for precise drug delivery. Nanoscale 2018, 10, 11384–11391. [Google Scholar] [CrossRef] [PubMed]
- Du, T.; Zhao, C.; ur Rehman, F.; Lai, L.; Li, X.; Sun, Y.; Luo, S.; Jiang, H.; Gu, N.; Selke, M.; et al. In Situ Multimodality Imaging of Cancerous Cells Based on a Selective Performance of Fe2+-Adsorbed Zeolitic Imidazolate Framework-8. Adv. Funct. Mater. 2017, 27, 1603926. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, C.; Gao, Y.; Li, Z.; Xing, J.; Ren, W.; Zhang, L.; Li, A.; Lu, G.; Wu, A.; et al. ZD2-Engineered Gold Nanostar@Metal-Organic Framework Nanoprobes for T1 -Weighted Magnetic Resonance Imaging and Photothermal Therapy Specifically Toward Triple-Negative Breast Cancer. Adv. Healthc. Mater. 2018, 7, 1801144. [Google Scholar] [CrossRef]
- Chen, W.H.; Yu, X.; Cecconello, A.; Sohn, Y.S.; Nechushtai, R.; Willner, I. Stimuli-responsive nucleic acid-functionalized metal-organic framework nanoparticles using pH-and metal-ion-dependent DNAzymes as locks. Chem. Sci. 2017, 8, 5769–5780. [Google Scholar] [CrossRef]
- Zhao, Q.-G.; Wang, J.; Zhang, Y.-P.; Zhang, J.; Tang, A.-N.; Kong, D.-M. A ZnO-gated porphyrinic metal–organic framework-based drug delivery system for targeted bimodal cancer therapy. J. Mater. Chem. B 2018, 6, 7898–7907. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Y.H.; Chen, Y.; Wang, M.M.; Wang, X.S.; Yin, X.B. Fluorescence and magnetic resonance dual-modality imaging-guided photothermal and photodynamic dual-therapy with magnetic porphyrin-metal organic framework nanocomposites. Scientific Reports, 2017, 7: 44153. Sci. Rep. 2017, 7, 44153. [Google Scholar] [CrossRef]
- Baati, T.; Njim, L.; Neffati, F.; Kerkeni, A.; Bouttemi, M.; Gref, R.; Najjar, M.F.; Zakhama, A.; Couvreur, P.; Serre, C.; et al. In depth analysis of the in vivo toxicity of nanoparticles of porous iron(III) metal-organic frameworks. Chem. Sci. 2013, 4, 1597–1607. [Google Scholar] [CrossRef]
- Chen, Z.X.; Liu, M.D.; Zhang, M.K.; Wang, S.B.; Xu, L.; Li, C.X.; Gao, F.; Xie, B.R.; Zhong, Z.L.; Zhang, X.Z. Interfering with Lactate-Fueled Respiration for Enhanced Photodynamic Tumor Therapy by a Porphyrinic MOF Nanoplatform. Adv. Funct. Mater. 2018, 28, 1803498. [Google Scholar] [CrossRef]
- Ma, T.C.; Liu, Y.D.; Wu, Q.; Luo, L.F.; Cui, Y.L.; Wang, X.H.; Chen, X.W.; Tan, L.F.; Meng, X.W. Quercetin-Modified Metal-Organic Frameworks for Dual Sensitization of Radiotherapy in Tumor Tissues by Inhibiting the Carbonic Anhydrase IX. ACS Nano 2019, 13, 4209–4219. [Google Scholar] [CrossRef]
- Wang, H.; Yu, D.Q.; Fang, J.; Cao, C.C.; Liu, Z.; Ren, J.S.; Qu, X.G. Renal-Clearable Porphyrinic Metal-Organic Framework Nanodots for Enhanced Photodynamic Therapy. ACS Nano 2019, 13, 9206–9217. [Google Scholar] [CrossRef] [PubMed]
| Drug Delivery System | Synthetic Method | Loading Capacity | Release Rate | Achievement | Ref. |
|---|---|---|---|---|---|
| DOX @ZIF-8 | One-pot synthesis | 20% | 95% (pH 5–6, 37 °C, 7–9 days) | pH-responsive | [26] |
| PEG-FA/(DOX+VER)@ZIF-8 | One-pot synthesis | 8.9% (DOX) 32% (VER) | 27.37% (DOX) 76.48% (VER) (pH 5, 37 °C, 24 h) | pH-responsive, Overcoming multidrug resistance | [27] |
| 5-FU+DOX @ZIF-90 | Ultrasonic stirring | 36.35% (5-FU) 13.5% (DOX) | 95% (5-FU, 15 h) 91% (DOX, 25 h) (pH 5, 37 °C) | pH-responsive, Combination therapy | [19] |
| DOX @ZIF-8 NTs | Template-directed synthesis | 350% (drug/mo-fs) | 72% (DOX) (pH 5, 37 °C, 50 h) | pH-responsive, Ultra-high drug loading and long-acting cycle | [22] |
| FA/5-FU @IRMOF-3 | Solvothermal method | 20.4% | 68% (37 °C, 96 h) | pH-responsive, Active targeting | [9] |
| DOX @TTMOF | One-pot synthesis | 14.3% | 78% (10 mM DTT, pH 7.4, 37 °C, 140 h) | pH-responsive, Redox responsive | [15] |
| PD/M-NMOF | AOT microemulsion method | 4.3% (MB) 0.69% (dox) | 72% (MB) 95% (Dox) | Magnetic-responsive Light-responsive | [28] |
| PTX/Fe3O4@IRMOF-3 | mixed solvent solvothermal method | 12.32% | 65% (pH 7.4, 37 °C, 100 h) | Magnetic-responsive | [10] |
| mCGP | solvothermal method | 13.5% (glucose oxidase and catalase) | / | Starvation and Photodynamic Therapy | [29] |
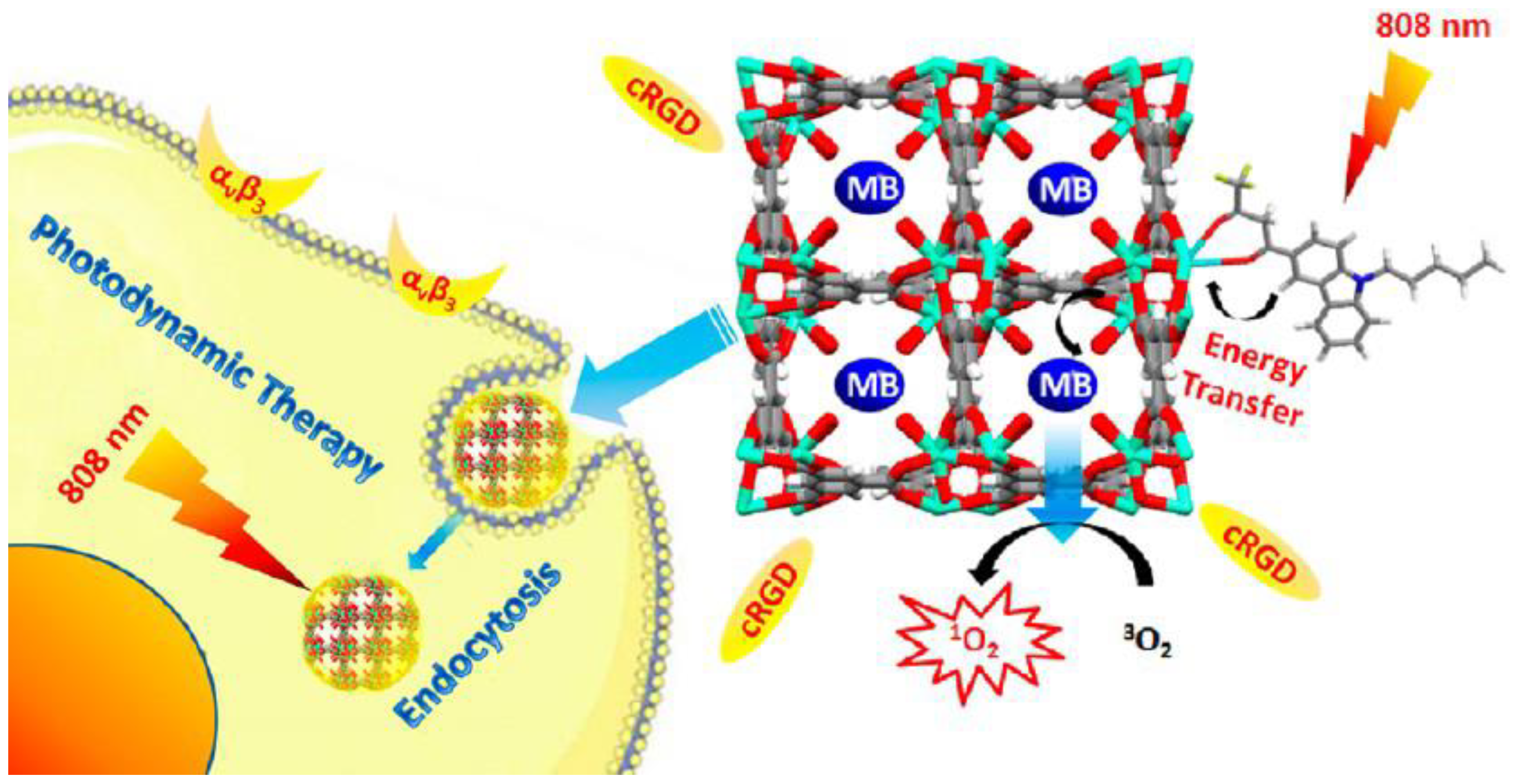
| MB @THA-NMOF-76 @cRGD | rapid microwave-assisted method | 3 ug/mg (MB) | / | Light-responsive, Active targeting | [20] |
| Gd-MTX NCP | microwave heating | 79.1% (MTX) | 100% (pH 7.4, 37 °C 192 h) | Active targeting | [30] |
| Fe-MIL-53- NH2-FA-5- FAM/5-FU | a reflux method at low temperature | 23% | the gentle release for 25 h in pH 7.4 for 20 h in pH 5 | Light-responsive, Magnetic-responsive Active targeting | [31] |
| ZIF-8/5-FU @FA-CHI- 5-FAM | solvothermal method | 51% | complete release (pH 7.4, 37 °C, 45 h pH 5, 37 °C, 21 h) | Light-responsive, pH-responsive, Active targeting | [32] |
| FA/5-FU @MOF-808 | stirring-reflux method | 38.42% | 60–70% (pH 5, 37 °C, 24 h) | pH-responsive, Active targeting | [33] |
| FA/5-FU@ NH2-UiO-66 | stirring-reflux method | 30.26% | 60–70% (pH 5, 37 °C, 24 h) | pH-responsive, Active targeting | [33] |
| CoFe2O4@Mn-MOF | layer to layer method | 75 ± 1.22% (Encapsu-lation efficienc-y) | 55% (pH 7.4, 37 °C, 20 h) | Magnetic-responsive | [34] |
| BSA/Cu/NQ NP | protein-nanoreactorv method | 13.6% | / | Active targeting | [35] |
| Fe-soc-MOF@PPy | The liquid-solid- solution (LSS) method | 15% | / | Light-responsive | [21] |
| Classifications | Abbreviations | Examples | The Molecular Formula | Ref |
|---|---|---|---|---|
| Isoreticular Metal Organic Frameworks | IRMOF-n | IRMOF-3 | C24H5N3O13Zn4 | [9] |
| Materials of Institute Lavoisier | MIL-n | NH2-MIL-53 (Fe) | C8H6NO5Fe | [31] |
| Zeolitic Imidazolate Frameworks | ZIF-n | ZIF-8 | C8H10N4Zn | [27] |
| ZIF-90 | C4H4N2OZn | [12] | ||
| University of Oslo | UiO-n | NH2-UiO-66 | C48H30NO32Zr6 | [11] |
| Zhejiang University | ZJU-n | ZJU-801 | C12H4O32Zr6 | [36] |
| Targeting Cancer Cell | ||||
|---|---|---|---|---|
| Drug | Target | Target Cell Line | Targeting Type | Ref. |
| BSA/SAs@MOF | CA IX | 4T1 cancer cells | Light-responsive | [42] |
| Caspase-FA/TMPyP@MOF | FRs | HeLa cells | Light-responsive | [43] |
| FA/5-FU @IRMOF-3 | FRs | HeLa cells, lung adenocarcinoma A549 cells, KB cells | Active targeting | [9] |
| PNA@UiO-66 | miRNAs | MDA-MB-231, MCF-7 | Gene-responsive | [44] |
| Polymer-Modified Gd MOF | αvβ3-integrins | FITZ-HSA tumor cells | Magnetic-responsive Light-responsive | [45] |
| CPC@MOF | CaB | HeLa cells | Light-responsive | [46] |
| mCGP | (4T1) cancer cell membrane | 4T1 cancer cell, B16F10 cells, HepG2 cells, COS7 cells | Starvation and Photodynamic Therapy | [29] |
| MB @THA-NMOF-76 @cRGD | αvβ3-integrins | HeLa cells, A549 cells | Light-responsive, Active targeting | [20] |
| Gd-MTX NCP | sigma receptors | Jurkat ALL cells | Active targeting | [30] |
| Zr(IV)-based porphyrinic MOF–UCNP | epidermal growth factor receptor | The MDA-MB-468 cells | Gene-responsive, Light-responsive | [47] |
| Fe-MIL-53- NH2-FA-5- FAM/5-FU | FRs | MGC-803 and HASMC cells | Light-responsive, Magnetic-responsive Active targeting | [31] |
| ZIF-8/5-FU @FA-CHI- 5-FAM | FRs | MGC-803 cells | Light-responsive, pH-responsive, Active targeting | [32] |
| UCNPs@MOF- DOX-AS1411 | nucleolin | MCF-7 cells | Light-responsive, pH-responsive, Active targeting | [48] |
| UCNPs@ZIF-8/FA/5-FU | FRs | HeLa cells, mouse fibroblast (L929) cells | Light-responsive, pH-responsive, Active targeting | [49] |
| MOF@HA@ICG | CD44 | MCF-7 cancer cells | Light-responsive, Active targeting | [50] |
| Fe-soc-MOF@PPy | / | 4T1 cancer cells | Light-responsive | [21] |
| FA@Ni-hemin metal organic framework | FRs | MCF-7 cancer cells | Active targeting, Redox responsive | [51] |
| PEG-FA/PEGCG@ZIF-8 NPs | FRs | HeLa cells | pH-responsive, Active targeting | [52] |
| Streptavidin/GOx@ZIF-8-AuNCs | biotinylated antibody against galectin-4 | colorectal cancer, breast hepatocellular carcinoma, gastric cancer, etc. | Active targeting, Light-responsive | [53] |
| RGD@CPT@ZIF-8 | αvβ3 receptor | HeLa cells | Active targeting, pH-responsive | [54] |
| DOX@MOFs-Glu | glucose-transported protein (GLUT1) | HeLa cells | Active targeting, pH-responsive, the magnetic resonance (MR) imaging | [55] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, M.; Chen, G.; Qin, L.; Qu, C.; Dong, X.; Ni, J.; Yin, X. Metal Organic Frameworks as Drug Targeting Delivery Vehicles in the Treatment of Cancer. Pharmaceutics 2020, 12, 232. https://doi.org/10.3390/pharmaceutics12030232
Cai M, Chen G, Qin L, Qu C, Dong X, Ni J, Yin X. Metal Organic Frameworks as Drug Targeting Delivery Vehicles in the Treatment of Cancer. Pharmaceutics. 2020; 12(3):232. https://doi.org/10.3390/pharmaceutics12030232
Chicago/Turabian StyleCai, Mengru, Gongsen Chen, Liuying Qin, Changhai Qu, Xiaoxv Dong, Jian Ni, and Xingbin Yin. 2020. "Metal Organic Frameworks as Drug Targeting Delivery Vehicles in the Treatment of Cancer" Pharmaceutics 12, no. 3: 232. https://doi.org/10.3390/pharmaceutics12030232
APA StyleCai, M., Chen, G., Qin, L., Qu, C., Dong, X., Ni, J., & Yin, X. (2020). Metal Organic Frameworks as Drug Targeting Delivery Vehicles in the Treatment of Cancer. Pharmaceutics, 12(3), 232. https://doi.org/10.3390/pharmaceutics12030232





