Theoretical and Experimental Gas Volume Quantification of Micro- and Nanobubble Ultrasound Contrast Agents
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Bubble Formulation
2.3. Size Characterization of Bubbles
2.4. Quantification of Bubble Perfluoropropane Gas (C3F8) Volume via GC/MS
2.5. Quantification of Bubble Population Acoustic Response
2.6. Theoretical Calculation of Bubble Gas Volume
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- de Leon, A.; Perera, R.; Nittayacharn, P.; Cooley, M.; Jung, O.; Exner, A.A. Ultrasound Contrast Agents and Delivery Systems in Cancer Detection and Therapy. Adv. Cancer Res. 2018, 139, 57–84. [Google Scholar] [PubMed]
- American Society of Echocardiography (ASE). The Basics. Available online: https://www.asecho.org/contrast-zone/the-basics/ (accessed on 25 August 2019).
- Klibanov, A.L. Targeted delivery of gas-filled microspheres, contrast agents for ultrasound imaging. Adv. Drug Deliv. Rev. 1999, 37, 139–157. [Google Scholar] [CrossRef]
- Perera, R.H.; Hernandez, C.; Zhou, H.; Kota, P.; Burke, A.; Exner, A.A. Ultrasound imaging beyond the vasculature with new generation contrast agents. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015, 7, 593–608. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Abenojar, E.C.; Perera, R.; De Leon, A.C.; An, T.; Exner, A.A. Time-intensity-curve Analysis and Tumor Extravasation of Nanobubble Ultrasound Contrast Agents. Ultrasound Med. Biol. 2019, 45, 2502–2514. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, Y.; Deng, C.; Zhang, L.; Sun, Z.; Wang, J.; Yang, Y.; Lv, Q.; Han, W.; Xie, M. The optimized fabrication of a novel nanobubble for tumor imaging. Front. Pharmacol. 2019, 10. [Google Scholar] [CrossRef]
- Cai, W.B.; Yang, H.L.; Zhang, J.; Yin, J.K.; Yang, Y.L.; Yuan, L.J.; Zhang, L.; Duan, Y.Y. The Optimized Fabrication of Nanobubbles as Ultrasound Contrast Agents for Tumor Imaging. Sci. Rep. 2015, 5, 1–11. [Google Scholar] [CrossRef]
- Maeda, H.; Wu, J.; Sawa, T.; Matsumura, Y.; Hori, K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J. Control. Release 2000, 65, 271–284. [Google Scholar] [CrossRef]
- Maeda, H. Toward a full understanding of the EPR effect in primary and metastatic tumors as well as issues related to its heterogeneity. Adv. Drug Deliv. Rev. 2015, 91, 3–6. [Google Scholar] [CrossRef]
- de Leon, A.; Perera, R.; Hernandez, C.; Cooley, M.; Jung, O.; Jeganathan, S.; Abenojar, E.; Fishbein, G.; Sojahrood, A.J.; Emerson, C.C.; et al. Contrast enhanced ultrasound imaging by nature-inspired ultrastable echogenic nanobubbles. Nanoscale 2019, 11, 15647–15658. [Google Scholar] [CrossRef]
- Garg, S.; Thomas, A.A.; Borden, M.A. The effect of lipid monolayer in-plane rigidity on invivo microbubble circulation persistence. Biomaterials 2013, 34, 6862–6870. [Google Scholar] [CrossRef]
- Ferrara, K.W.; Borden, M.A.; Zhang, H. Lipid-Shelled Vehicles: Engineering for Ultrasound Molecular Imaging and Drug Delivery. Acc. Chem. Res. 2009, 42. [Google Scholar] [CrossRef] [PubMed]
- Kwan, J.J.; Borden, M.A. Lipid monolayer collapse and microbubble stability. Adv. Colloid Interface Sci. 2012, 183–184, 82–99. [Google Scholar] [CrossRef] [PubMed]
- Kanbar, E.; Fouan, D.; Sennoga, C.A.; Doinikov, A.A.; Bouakaz, A. Impact of Filling Gas on Subharmonic Emissions of Phospholipid Ultrasound Contrast Agents. Ultrasound Med. Biol. 2017, 43, 1004–1015. [Google Scholar] [CrossRef] [PubMed]
- Omata, D.; Maruyama, T.; Unga, J.; Hagiwara, F.; Munakata, L.; Kageyama, S.; Shima, T.; Suzuki, Y.; Maruyama, K.; Suzuki, R. Effects of encapsulated gas on stability of lipid-based microbubbles and ultrasound-triggered drug delivery. J. Control. Release 2019, 311–312, 65–73. [Google Scholar] [CrossRef]
- Chomas, J.E.; Dayton, P.; Allen, J.; Morgan, K.; Ferrara, K.W. Mechanisms of contrast agent destruction. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2001, 48, 232–248. [Google Scholar] [CrossRef]
- Cheng, K.T. Perflutren Lipid Microspheres; National Center for Biotechnology Information: Bethesda, MD, USA, 2004. [Google Scholar]
- Schutt, E.G.; Klein, D.H.; Mattrey, R.M.; Riess, J.G. Injectable Microbubbles as Contrast Agents for Diagnostic Ultrasound Imaging: The Key Role of Perfluorochemicals. Angew. Chemie Int. Ed. 2003, 42, 3218–3235. [Google Scholar] [CrossRef]
- Klibanov, A.L. Ultrasound Contrast Agents: Development of the Field and Current Status. In Topics in Current Chemistry, Vol. 222; Krause, W., Ed.; Springer: Berlin/Heidelberg, Germany, 2002; pp. 73–106. [Google Scholar]
- Sheeran, P.S.; Dayton, P.A. Phase-Change Contrast Agents for Imaging and Therapy. Curr. Pharm. Des. 2012, 18, 2152–2165. [Google Scholar] [CrossRef]
- Hernandez, C.; Nieves, L.; de Leon, A.C.; Advincula, R.; Exner, A.A. Role of Surface Tension in Gas Nanobubble Stability Under Ultrasound. ACS Appl. Mater. Interfaces 2018. [Google Scholar] [CrossRef]
- Nguyen, P.N.; Trinh Dang, T.T.; Waton, G.; Vandamme, T.; Krafft, M.P. A nonpolar, nonamphiphilic molecule can accelerate adsorption of phospholipids and lower their surface tension at the air/water interface. ChemPhysChem 2011, 12, 2646–2652. [Google Scholar] [CrossRef]
- Gorce, J.-M.; Arditi, M.; Schneider, M. Influence of Bubble Size Distribution on the Echogenicity of Ultrasound Contrast Agents. Invest. Radiol. 2000, 35, 661–671. [Google Scholar] [CrossRef]
- de Jong, N.; Cornet, R.; Lancée, C.T. Higher harmonics of vibrating gas-filled microspheres. Part one: Simulations. Ultrasonics 1994, 32, 447–453. [Google Scholar] [CrossRef]
- Abenojar, E.C.; Nittayacharn, P.; de Leon, A.C.; Perera, R.; Wang, Y.; Bederman, I.; Exner, A.A. Effect of Bubble Concentration on the in Vitro and in Vivo Performance of Highly Stable Lipid Shell-Stabilized Micro- and Nanoscale Ultrasound Contrast Agents. Langmuir 2019, 35, 10192–10202. [Google Scholar] [CrossRef] [PubMed]
- Sirsi, S.; Feshitan, J.; Kwan, J.; Homma, S.; Borden, M. Effect of microbubble size on fundamental mode high frequency ultrasound imaging in mice. Ultrasound Med. Biol. 2010, 36, 935–948. [Google Scholar] [CrossRef]
- Song, K.-H.; Fan, A.C.; Hinkle, J.J.; Newman, J.; Borden, M.A.; Harvey, B.K. Microbubble gas volume: A unifying dose parameter in blood-brain barrier opening by focused ultrasound. Theranostics 2017, 7, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Bing, C.; Hong, Y.; Hernandez, C.; Rich, M.; Cheng, B.; Munaweera, I.; Szcz, D.; Xi, Y.; Bolding, M.; Exner, A.; et al. Characterization of different bubble formulations for blood-brain barrier opening using a focused ultrasound system with acoustic feedback control. Sci. Rep. 2018, 8, 7986. [Google Scholar] [CrossRef]
- Shim, J.S.; Geng, J.; Ahn, C.H.; Guo, P. Formation of lipid bilayers inside microfluidic channel array for monitoring membrane-embedded nanopores of phi29 DNA packaging nanomotor. Biomed. Microdevices 2012, 14, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Østensen, J.; Sontum, P.C.; Hoff, L.; Bendiksen, R. Microbubble volume concentration: A better efficacy parameter for US contrast agents than the number concentration. Acad. Radiol. 2002, 9, S38–S40. [Google Scholar]
- Kobayashi, H.; Maeda, S.; Kashiwa, M.; Fujita, T. Measurements of ultrafine bubbles using different types of particle size measuring instruments. In Proceedings of SPIE; Aya, N., Iki, N., Shimura, T., Shirai, T., Eds.; International Society for Optics and Photonics: Tokyo, Japan, 2014; Volume 9232, p. 92320U. [Google Scholar]
- Sennoga, C.A.; Yeh, J.S.M.; Alter, J.; Stride, E.; Nihoyannopoulos, P.; Seddon, J.M.; Haskard, D.O.; Hajnal, J.V.; Tang, M.X.; Eckersley, R.J. Evaluation of Methods for Sizing and Counting of Ultrasound Contrast Agents. Ultrasound Med. Biol. 2012, 38, 834–845. [Google Scholar] [CrossRef]
- Alheshibri, M.; Craig, V.S.J. Differentiating between Nanoparticles and Nanobubbles by Evaluation of the Compressibility and Density of Nanoparticles. J. Phys. Chem. C 2018, 122, 21998–22007. [Google Scholar] [CrossRef]
- Kobayashi, H.; Maeda, S.; Kashiwa, M.; Fujita, T. Measurement and identification of ultrafine bubbles by resonant mass measurement method. In Proeedings of SPIE; Aya, N., Iki, N., Shimura, T., Shirai, T., Eds.; International Society for Optics and Photonics: Tokyo, Japan, 2014; Volume 9232, p. 92320S. [Google Scholar]
- Krueger, A.B.; Hadley, J.; Cheney, P.P.; Markova, N.; Carpenter, J.F.; Fradkin, A.H. Pharmaceutical Biotechnology Application of a Best Practice Approach Using Resonant Mass Measurement for Biotherapeutic Product Characterization. J. Pharm. Sci. 2019, 108, 1675–1685. [Google Scholar] [CrossRef]
- Alheshibri, M.; Craig, V.S.J. Armoured nanobubbles; ultrasound contrast agents under pressure. J. Colloid Interface Sci. 2019, 537, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, C.; Abenojar, E.C.; Hadley, J.; de Leon, A.C.; Coyne, R.; Perera, R.; Gopalakrishnan, R.; Basilion, J.P.; Kolios, M.C.; Exner, A.A. Sink or float? Characterization of shell-stabilized bulk nanobubbles using a resonant mass measurement technique. Nanoscale 2019, 11, 851–855. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, Y.; Li, X.; Pan, D.; Li, L.; Peng, J.; Hou, L.; Chen, Z. Rapid determination of a fluorinated gas in a lipid microbubble contrast agent by ultrasound-mediated microbubble destruction and GC-MS. Anal. Methods 2016, 8, 3353–3358. [Google Scholar] [CrossRef]
- Zheng, L.; Yapa, P.D. Buoyant Velocity of Spherical and Nonspherical Bubbles/Droplets. J. Hydraul. Eng. 2000, 126, 852–854. [Google Scholar] [CrossRef]
- Feshitan, J.A.; Chen, C.C.; Kwan, J.J.; Borden, M.A. Microbubble size isolation by differential centrifugation. J. Colloid Interface Sci. 2009, 329, 316–324. [Google Scholar] [CrossRef]
- Thery, C.; Boussac, M.; Veron, P.; Ricciardi-Castagnoli, P.; Raposo, G.; Garin, J.; Amigorena, S. Proteomic Analysis of Dendritic Cell-Derived Exosomes: A Secreted Subcellular Compartment Distinct from Apoptotic Vesicles. J. Immunol. 2001, 166, 7309–7318. [Google Scholar] [CrossRef]
- Théry, C.; Ostrowski, M.; Segura, E. Membrane vesicles as conveyors of immune responses. Nat. Rev. Immunol. 2009, 9, 581–593. [Google Scholar] [CrossRef]
- Nittayacharn, P.; Dai, K.; Leon, A.D.; Therdrattanawong, C.; Exner, A.A. The Effect of Freeze/Thawing on the Physical Properties and Acoustic Performance of Perfluoropropane Nanobubble Suspensions. In Proceedings of the 2019 IEEE International Ultrasonics Symposium, IUS, Glasgow, UK, 6–9 October 2019; pp. 2279–2282. [Google Scholar]
- Hernandez, C.; Gulati, S.; Fioravanti, G.; Stewart, P.L.; Exner, A.A. Cryo-EM Visualization of Lipid and Polymer-Stabilized Perfluorocarbon Gas Nanobubbles - A Step Towards Nanobubble Mediated Drug Delivery. Sci. Rep. 2017, 7, 13517. [Google Scholar] [CrossRef]
- Krafft, M.P. Fluorocarbons and fluorinated amphiphiles in drug delivery and biomedical research. Adv. Drug Deliv. Rev. 2001, 47, 209–228. [Google Scholar] [CrossRef]
- Riess, J.G. Oxygen Carriers (“Blood Substitutes”)Raison d’Etre, Chemistry, and Some Physiology Blut ist ein ganz besondrer Saft 1. Chem. Rev. 2001, 101, 2797–2920. [Google Scholar] [CrossRef]
- Lampaskis, M.; Averkiou, M. Investigation of the Relationship of Nonlinear Backscattered Ultrasound Intensity with Microbubble Concentration at Low MI. Ultrasound Med. Biol. 2010, 36, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Haghi, H.; Sojahrood, A.J.; de Leon, A.C.; Exner, A.A.; Kolios, M.C. Experimental and numerical investigation of backscattered signal strength from different concentrations of nanobubble and microbubble clusters. J. Acoust. Soc. Am. 2018, 144, 1888. [Google Scholar] [CrossRef]
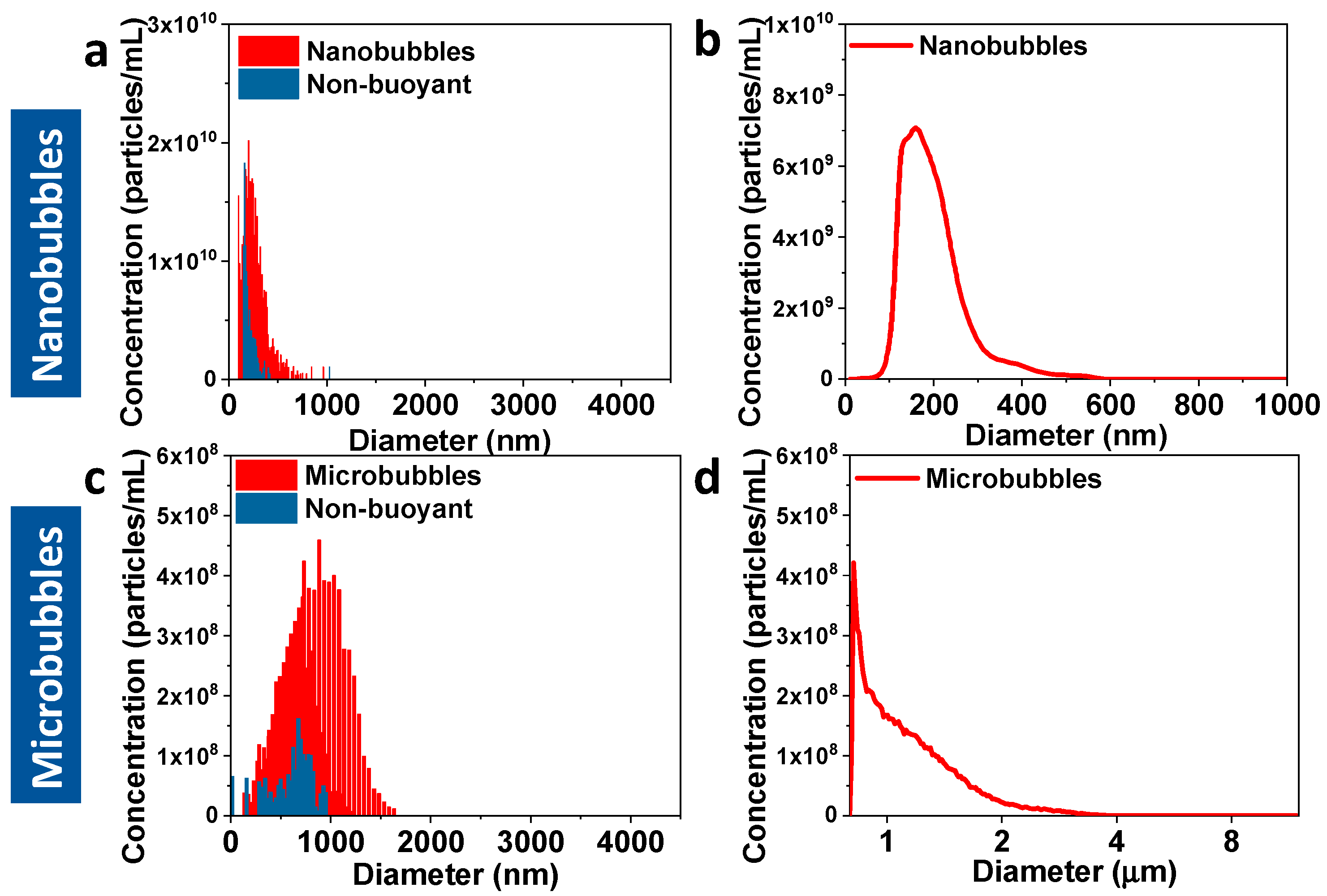
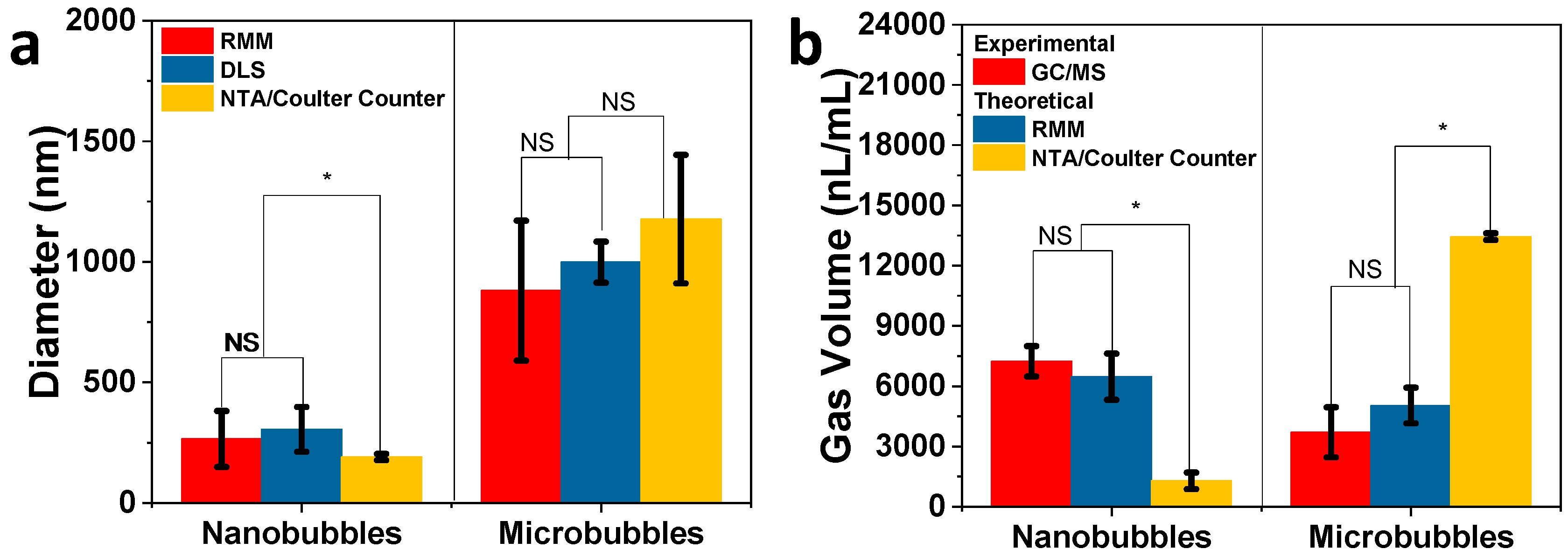
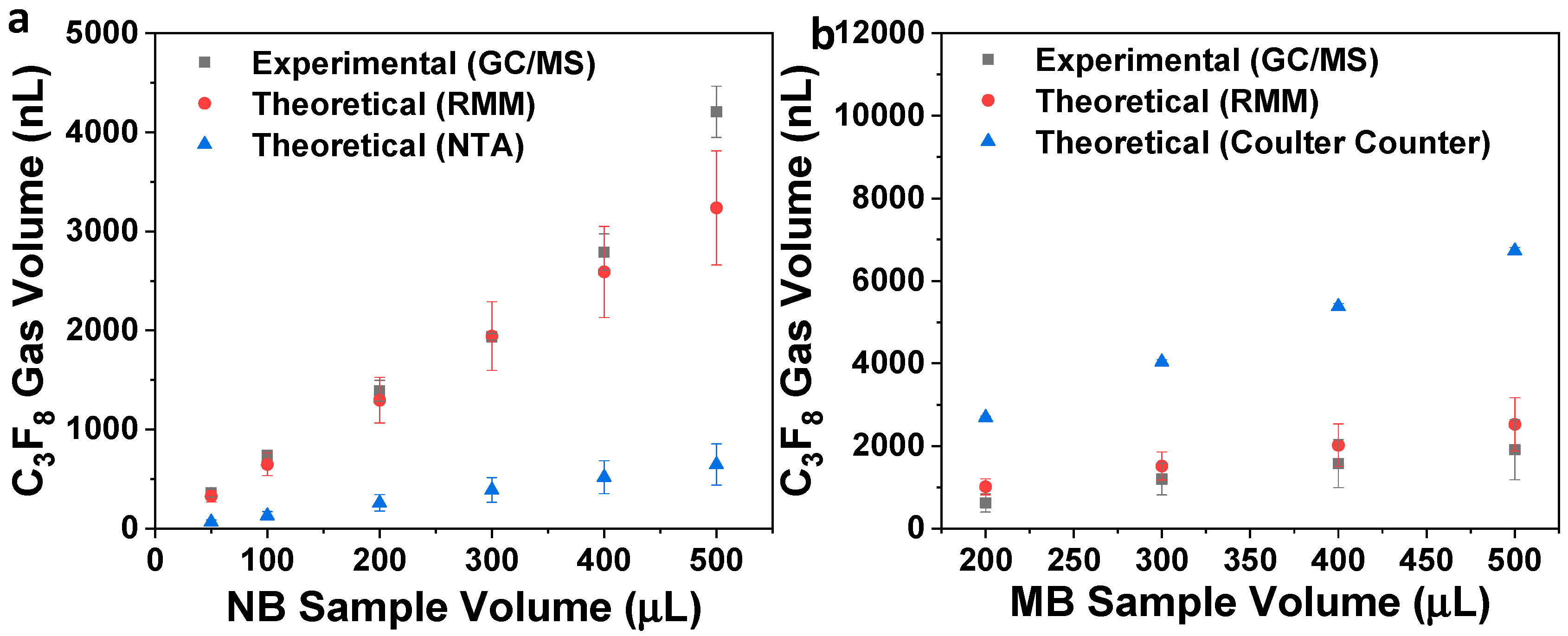
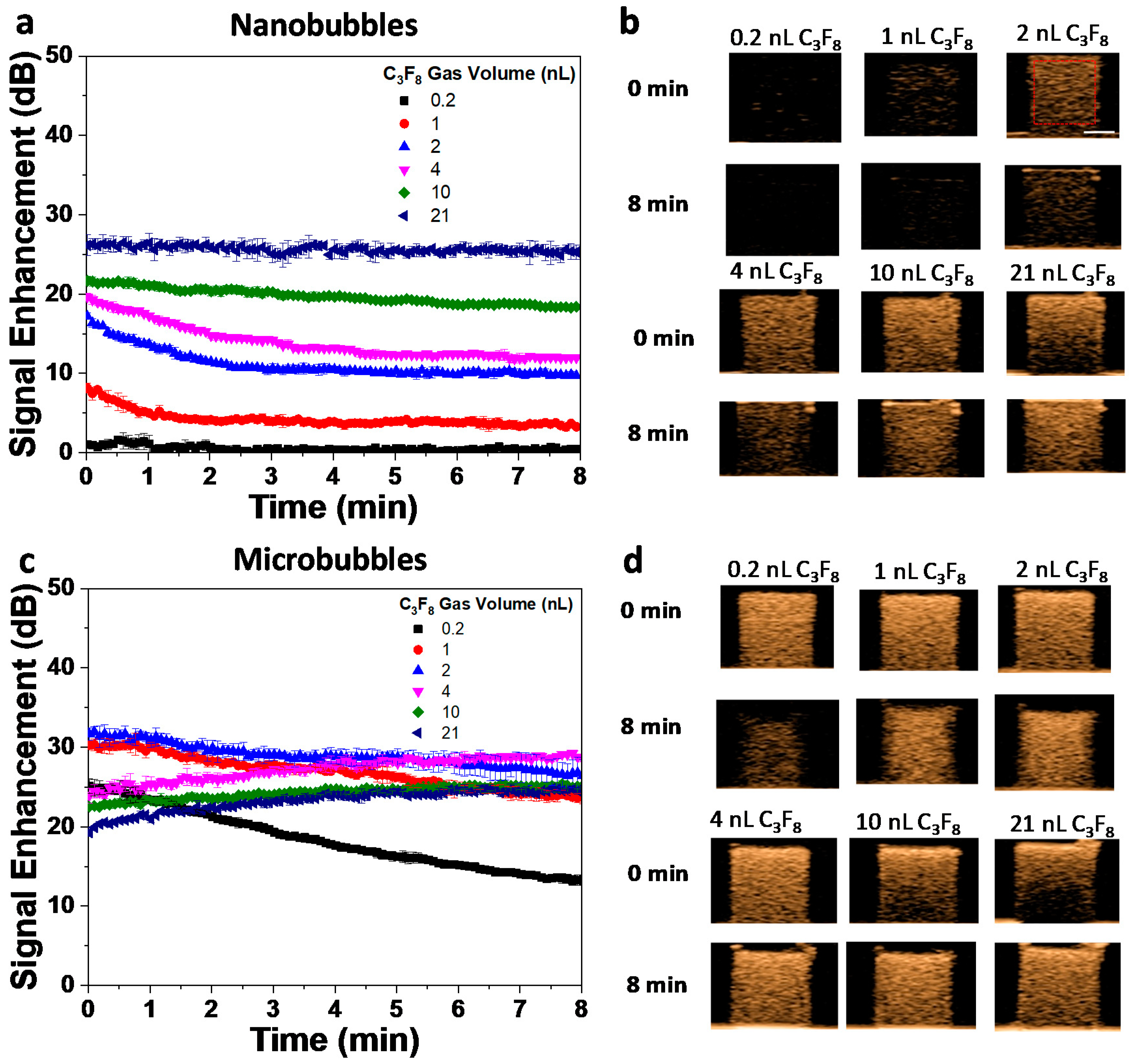
| Sample | RMM (Bubbles/mL) | NTA/Coulter Counter (Particles/mL) |
|---|---|---|
| Nanobubbles | 4.07 ± 0.11 × 1011 | 4.16 ± 0.28 × 1011 |
| Microbubbles | 1.08 ± 0.23 × 1010 | 1.14 ± 0.05 × 1010 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abenojar, E.C.; Bederman, I.; de Leon, A.C.; Zhu, J.; Hadley, J.; Kolios, M.C.; Exner, A.A. Theoretical and Experimental Gas Volume Quantification of Micro- and Nanobubble Ultrasound Contrast Agents. Pharmaceutics 2020, 12, 208. https://doi.org/10.3390/pharmaceutics12030208
Abenojar EC, Bederman I, de Leon AC, Zhu J, Hadley J, Kolios MC, Exner AA. Theoretical and Experimental Gas Volume Quantification of Micro- and Nanobubble Ultrasound Contrast Agents. Pharmaceutics. 2020; 12(3):208. https://doi.org/10.3390/pharmaceutics12030208
Chicago/Turabian StyleAbenojar, Eric C., Ilya Bederman, Al C. de Leon, Jinle Zhu, Judith Hadley, Michael C. Kolios, and Agata A. Exner. 2020. "Theoretical and Experimental Gas Volume Quantification of Micro- and Nanobubble Ultrasound Contrast Agents" Pharmaceutics 12, no. 3: 208. https://doi.org/10.3390/pharmaceutics12030208
APA StyleAbenojar, E. C., Bederman, I., de Leon, A. C., Zhu, J., Hadley, J., Kolios, M. C., & Exner, A. A. (2020). Theoretical and Experimental Gas Volume Quantification of Micro- and Nanobubble Ultrasound Contrast Agents. Pharmaceutics, 12(3), 208. https://doi.org/10.3390/pharmaceutics12030208





