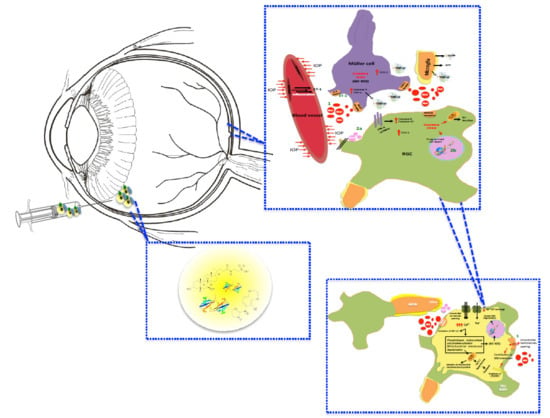
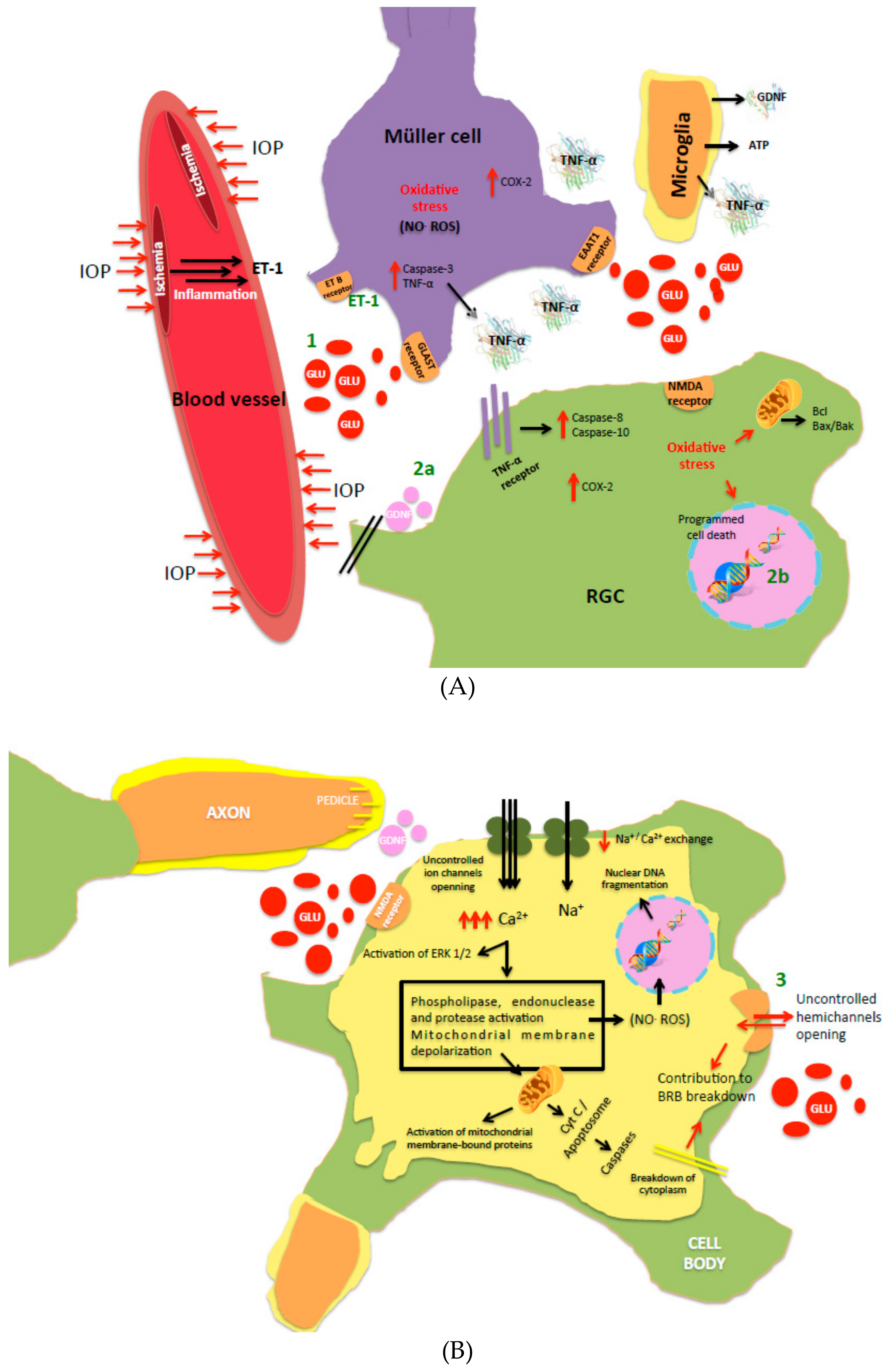
Retinal Cell Protection in Ocular Excitotoxicity Diseases. Possible Alternatives Offered by Microparticulate Drug Delivery Systems and Future Prospects
Abstract
1. Introduction
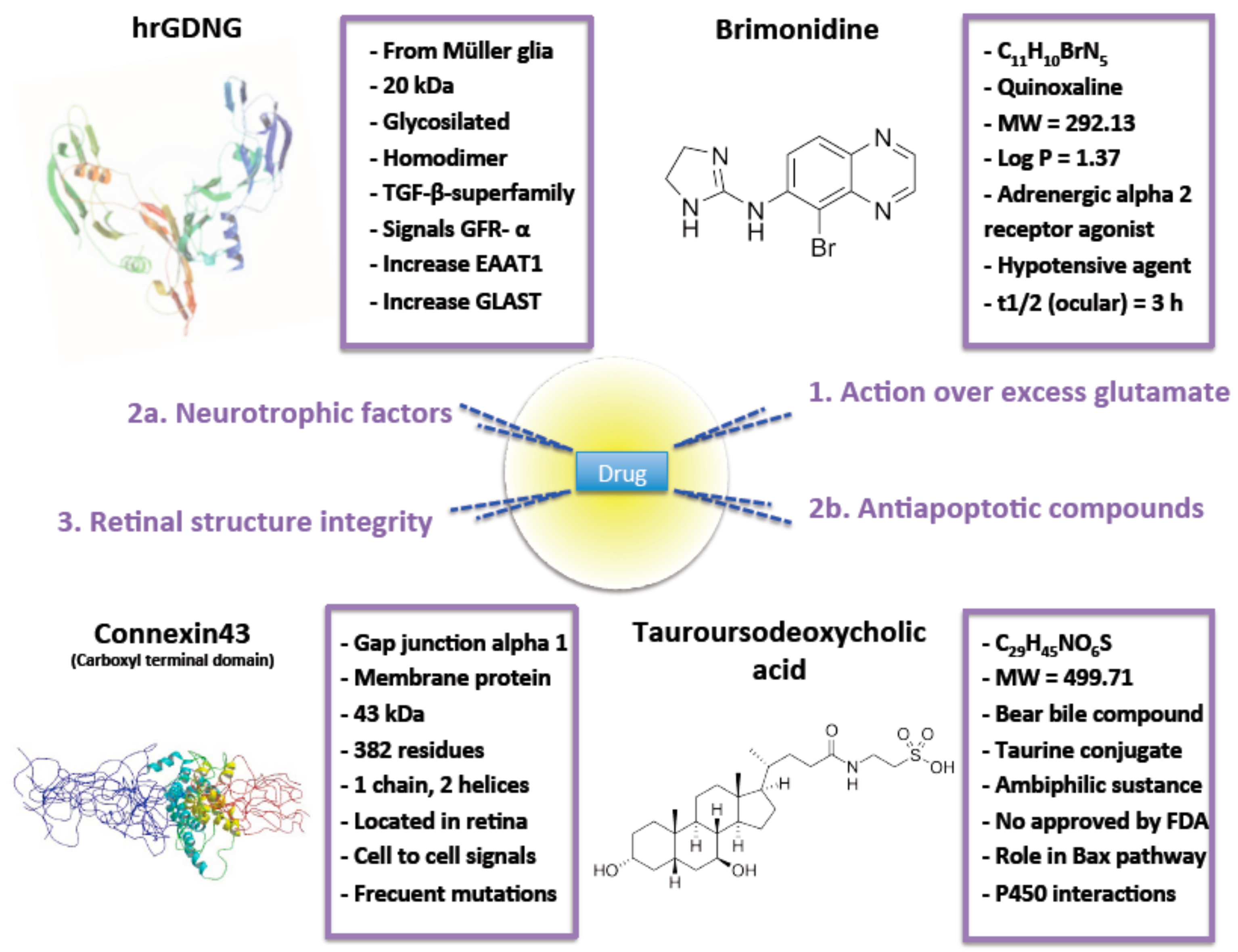
2. Intervention over Excess Glutamate
3. Neuroprotection
3.1. Neurotrophic Factors
3.2. Antiapoptotic Compounds
4. Retinal Structure Integrity
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Platt, S.R. The role of glutamate in central nervous system health and disease—A review. Vet. J. 2007, 173, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Russo, R.; Cavaliere, F.; Varano, G.P.; Milanese, M.; Adornetto, A.; Nucci, C.; Bonanno, G.; Morrone, L.A.; Corasaniti, M.T.; Bagetta, G. Impairment of Neuronal Glutamate Uptake and Modulation of the Glutamate Transporter GLT-1 Induced by Retinal Ischemia. PLoS ONE 2013, 8, e69250. [Google Scholar] [CrossRef] [PubMed]
- Baxter, P.S.; Bell, K.F.; Hasel, P.; Kaindl, A.M.; Fricker, M.; Thomson, D.; Cregan, S.P.; Gillingwater, T.H.; Hardingham, G.E. Synaptic NMDA receptor activity is coupled to the transcriptional control of the glutathione system. Nat. Commun. 2015, 6, 6761. [Google Scholar] [CrossRef] [PubMed]
- Meunier, C.; Wang, N.; Yi, C.; Dallerac, G.; Ezan, P.; Koulakoff, A.; Leybaert, L.; Giaume, C. Contribution of Astroglial Cx43 Hemichannels to the Modulation of Glutamatergic Currents by D-Serine in the Mouse Prefrontal Cortex. J. Neurosci. Off. J. Soc. Neurosci. 2017, 37, 9064–9075. [Google Scholar] [CrossRef]
- Cuenca, N.; Fernandez-Sanchez, L.; Campello, L.; Maneu, V.; De la Villa, P.; Lax, P.; Pinilla, I. Cellular responses following retinal injuries and therapeutic approaches for neurodegenerative diseases. Prog. Retin. Eye Res. 2014, 43, 17–75. [Google Scholar] [CrossRef]
- Opere, C.A.; Heruye, S.; Njie-Mbye, Y.F.; Ohia, S.E.; Sharif, N.A. Regulation of Excitatory Amino Acid Transmission in the Retina: Studies on Neuroprotection. J. Ocul. Pharmacol. Ther. 2018, 34, 107–118. [Google Scholar] [CrossRef]
- Luo, X.; Yu, Y.; Xiang, Z.; Wu, H.; Ramakrishna, S.; Wang, Y.; So, K.F.; Zhang, Z.; Xu, Y. Tetramethylpyrazine nitrone protects retinal ganglion cells against N-methyl-d-aspartate-induced excitotoxicity. J. Neurochem. 2017, 141, 373–386. [Google Scholar] [CrossRef]
- Gomez-Vicente, V.; Lax, P.; Fernandez-Sanchez, L.; Rondon, N.; Esquiva, G.; Germain, F.; de la Villa, P.; Cuenca, N. Neuroprotective Effect of Tauroursodeoxycholic Acid on N-Methyl-D-Aspartate-Induced Retinal Ganglion Cell Degeneration. PLoS ONE 2015, 10, e0137826. [Google Scholar] [CrossRef]
- Vyklicky, V.; Korinek, M.; Smejkalova, T.; Balik, A.; Krausova, B.; Kaniakova, M.; Lichnerova, K.; Cerny, J.; Krusek, J.; Dittert, I.; et al. Structure, function, and pharmacology of NMDA receptor channels. Physiol. Res. 2014, 63, S191–S203. [Google Scholar]
- Daruich, A.; Parcq, J.; Delaunay, K.; Naud, M.C.; Le Rouzic, Q.; Picard, E.; Crisanti, P.; Vivien, D.; Berdugo, M.; Behar-Cohen, F. Retinal safety of intravitreal rtPA in healthy rats and under excitotoxic conditions. Mol. Vis. 2016, 22, 1332–1341. [Google Scholar]
- Lam, T.T.; Abler, A.S.; Kwong, J.M.; Tso, M.O. N-methyl-D-aspartate (NMDA)--induced apoptosis in rat retina. Investig. Ophthalmol. Vis. Sci. 1999, 40, 2391–2397. [Google Scholar]
- Geeraerts, E.; Dekeyster, E.; Gaublomme, D.; Salinas-Navarro, M.; De Groef, L.; Moons, L. A freely available semi-automated method for quantifying retinal ganglion cells in entire retinal flatmounts. Exp. Eye Res. 2016, 147, 105–113. [Google Scholar] [CrossRef]
- Gao, L.; Chen, X.; Tang, Y.; Zhao, J.; Li, Q.; Fan, X.; Xu, H.; Yin, Z.Q. Neuroprotective effect of memantine on the retinal ganglion cells of APPswe/PS1DeltaE9 mice and its immunomodulatory mechanisms. Exp. Eye Res. 2015, 135, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Sanchez, L.; Bravo-Osuna, I.; Lax, P.; Arranz-Romera, A.; Maneu, V.; Esteban-Perez, S.; Pinilla, I.; Puebla-Gonzalez, M.D.M.; Herrero-Vanrell, R.; Cuenca, N. Controlled delivery of tauroursodeoxycholic acid from biodegradable microspheres slows retinal degeneration and vision loss in P23H rats. PLoS ONE 2017, 12, e0177998. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Rizzolo, L.J. Effects of diabetic retinopathy on the barrier functions of the retinal pigment epithelium. Vis. Res. 2017. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.C.; Sood, S.; Narang, S.; Ichhpujani, P. Role of brimonidine in the treatment of clinically significant macular edema with ischemic changes in diabetic maculopathy. Int. Ophthalmol. 2014, 34, 787–792. [Google Scholar] [CrossRef]
- Rodriguez Villanueva, J.; Bravo-Osuna, I.; Herrero-Vanrell, R.; Molina Martinez, I.T.; Guzman Navarro, M. Optimising the controlled release of dexamethasone from a new generation of PLGA-based microspheres intended for intravitreal administration. Eur. J. Pharm. Sci. 2016, 92, 287–297. [Google Scholar] [CrossRef]
- Bringmann, A.; Grosche, A.; Pannicke, T.; Reichenbach, A. GABA and Glutamate Uptake and Metabolism in Retinal Glial (Müller) Cells. Front Endocrinol (Lausanne) 2013, 4, 48. [Google Scholar] [CrossRef]
- Blanco, R.; Martinez-Navarrete, G.; Valiente-Soriano, F.J.; Aviles-Trigueros, M.; Perez-Rico, C.; Serrano-Puebla, A.; Boya, P.; Fernandez, E.; Vidal-Sanz, M.; de la Villa, P. The S1P1 receptor-selective agonist CYM-5442 protects retinal ganglion cells in endothelin-1 induced retinal ganglion cell loss. Exp. Eye Res. 2017, 164, 37–45. [Google Scholar] [CrossRef]
- Jung, K.I.; Kim, J.H.; Park, C.K. alpha2-Adrenergic modulation of the glutamate receptor and transporter function in a chronic ocular hypertension model. Eur. J. Pharmacol. 2015, 765, 274–283. [Google Scholar] [CrossRef]
- Almasieh, M.; Levin, L.A. Neuroprotection in Glaucoma: Animal Models and Clinical Trials. Annu. Rev. Vis. Sci. 2017, 3, 91–120. [Google Scholar] [CrossRef]
- Omodaka, K.; Nishiguchi, K.M.; Yasuda, M.; Tanaka, Y.; Sato, K.; Nakamura, O.; Maruyama, K.; Nakazawa, T. Neuroprotective effect against axonal damage-induced retinal ganglion cell death in apolipoprotein E-deficient mice through the suppression of kainate receptor signaling. Brain Res. 2014, 1586, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Vohra, R.; Tsai, J.C.; Kolko, M. The role of inflammation in the pathogenesis of glaucoma. Surv. Ophthalmol. 2013, 58, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Colligris, B.; Crooke, A.; Gasull, X.; Escribano, J.; Herrero-Vanrell, R.; Benitez-del-Castillo, J.M.; Garcia-Feijoo, J.; Pintor, J. Recent patents and developments in glaucoma biomarkers. Recent Pat. Endocr. Metab. Immune Drug Discov. 2012, 6, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Lopez, E.; Egea, M.A.; Davis, B.M.; Guo, L.; Espina, M.; Silva, A.M.; Calpena, A.C.; Souto, E.M.B.; Ravindran, N.; Ettcheto, M.; et al. Memantine-Loaded PEGylated Biodegradable Nanoparticles for the Treatment of Glaucoma. Small (Weinh. Der Bergstr. Ger.) 2018, 14. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Moore, M.J.; Zhang, X.; Klassen, H.; Langer, R.; Young, M. Intravitreal injections of GDNF-loaded biodegradable microspheres are neuroprotective in a rat model of glaucoma. Mol. Vis. 2007, 13, 1783–1792. [Google Scholar]
- Tian, Y.; He, Y.; Song, W.; Zhang, E.; Xia, X. Neuroprotective effect of deferoxamine on N-methyl-d-aspartate-induced excitotoxicity in RGC-5 cells. Acta Biochim. Et Biophys. Sin. 2017, 49, 827–834. [Google Scholar] [CrossRef]
- Zhao, L.; Chen, G.; Li, J.; Fu, Y.; Mavlyutov, T.A.; Yao, A.; Nickells, R.W.; Gong, S.; Guo, L.W. An intraocular drug delivery system using targeted nanocarriers attenuates retinal ganglion cell degeneration. J. Control. Release 2017, 247, 153–166. [Google Scholar] [CrossRef]
- Sandalon, S.; Konnecke, B.; Levkovitch-Verbin, H.; Simons, M.; Hein, K.; Sattler, M.B.; Bahr, M.; Ofri, R. Functional and structural evaluation of lamotrigine treatment in rat models of acute and chronic ocular hypertension. Exp. Eye Res. 2013, 115, 47–56. [Google Scholar] [CrossRef]
- Andres-Guerrero, V.; Perucho-Gonzalez, L.; Garcia-Feijoo, J.; Morales-Fernandez, L.; Saenz-Frances, F.; Herrero-Vanrell, R.; Julvez, L.P.; Llorens, V.P.; Martinez-de-la-Casa, J.M.; Konstas, A.G. Current Perspectives on the Use of Anti-VEGF Drugs as Adjuvant Therapy in Glaucoma. Adv. Ther. 2017, 34, 378–395. [Google Scholar] [CrossRef]
- Calvo, M.; Sanz-Blasco, S.; Caballero, E.; Villalobos, C.; Nunez, L. Susceptibility to excitotoxicity in aged hippocampal cultures and neuroprotection by non-steroidal anti-inflammatory drugs: Role of mitochondrial calcium. J. Neurochem. 2015, 132, 403–417. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Caballero, C.; Prieto-Calvo, E.; Checa-Casalengua, P.; Garcia-Martin, E.; Polo-Llorens, V.; Garcia-Feijoo, J.; Molina-Martinez, I.T.; Bravo-Osuna, I.; Herrero-Vanrell, R. Six month delivery of GDNF from PLGA/vitamin E biodegradable microspheres after intravitreal injection in rabbits. Eur. J. Pharm. Sci. 2017, 103, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Bravo-Osuna, I.; Andres-Guerrero, V.; Pastoriza Abal, P.; Molina-Martinez, I.T.; Herrero-Vanrell, R. Pharmaceutical microscale and nanoscale approaches for efficient treatment of ocular diseases. Drug Deliv. Transl. Res. 2016, 6, 686–707. [Google Scholar] [CrossRef] [PubMed]
- Barcia, E.; Herrero-Vanrell, R.; Diez, A.; Alvarez-Santiago, C.; Lopez, I.; Calonge, M. Downregulation of endotoxin-induced uveitis by intravitreal injection of polylactic-glycolic acid (PLGA) microspheres loaded with dexamethasone. Exp. Eye Res. 2009, 89, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez Villanueva, J.; Rodriguez Villanueva, L. Turning the screw even further to increase microparticle retention and ocular bioavailability of associated drugs: The bioadhesion goal. Int. J. Pharm. 2017, 531, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Bansal, P.; Garg, S.; Sharma, Y.; Venkatesh, P. Posterior Segment Drug Delivery Devices: Current and Novel Therapies in Development. J. Ocul. Pharmacol. Ther. 2016, 32, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.S.; Green, C.R.; Wang, K.; Danesh-Meyer, H.V.; Rupenthal, I.D. Sustained intravitreal delivery of connexin43 mimetic peptide by poly(D,L-lactide-co-glycolide) acid micro- and nanoparticles--Closing the gap in retinal ischaemia. Eur. J. Pharm. Biopharm. 2015, 95, 378–386. [Google Scholar] [CrossRef]
- Herrero-Vanrell, R.; Bravo-Osuna, I.; Andres-Guerrero, V.; Vicario-de-la-Torre, M.; Molina-Martinez, I.T. The potential of using biodegradable microspheres in retinal diseases and other intraocular pathologies. Prog. Retin. Eye Res. 2014, 42, 27–43. [Google Scholar] [CrossRef]
- Garbayo, E.; Montero-Menei, C.N.; Ansorena, E.; Lanciego, J.L.; Aymerich, M.S.; Blanco-Prieto, M.J. Effective GDNF brain delivery using microspheres--a promising strategy for Parkinson’s disease. J. Control. Release 2009, 135, 119–126. [Google Scholar] [CrossRef]
- Chen, L.; Feng, W.; Zhou, X.; Yin, Z.; He, C. Thermo-and pH dual-responsive mesoporous silica nanoparticles for controlled drug release. J. Control. Release 2015, 213, e69–e70. [Google Scholar] [CrossRef]
- Pannicke, T.; Fischer, W.; Biedermann, B.; Schadlich, H.; Grosche, J.; Faude, F.; Wiedemann, P.; Allgaier, C.; Illes, P.; Burnstock, G.; et al. P2X7 receptors in Muller glial cells from the human retina. J. Neurosci. Off. J. Soc. Neurosci. 2000, 20, 5965–5972. [Google Scholar] [CrossRef]
- Zhou, X.; Li, G.; Zhang, S.; Wu, J. 5-HT1A Receptor Agonist Promotes Retinal Ganglion Cell Function by Inhibiting OFF-Type Presynaptic Glutamatergic Activity in a Chronic Glaucoma Model. Front Cell Neurosci 2019, 13, 167. [Google Scholar] [CrossRef] [PubMed]
- Chiang, B.; Venugopal, N.; Edelhauser, H.F.; Prausnitz, M.R. Distribution of particles, small molecules and polymeric formulation excipients in the suprachoroidal space after microneedle injection. Exp. Eye Res. 2016, 153, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, L.A.; Woldemussie, E. Alpha-2 adrenergic receptor agonists are neuroprotective in experimental models of glaucoma. Eur. J. Ophthalmol. 2001, 11 (Suppl. 2), S30–S35. [Google Scholar] [CrossRef]
- Lee, D.; Kim, K.Y.; Noh, Y.H.; Chai, S.; Lindsey, J.D.; Ellisman, M.H.; Weinreb, R.N.; Ju, W.K. Brimonidine blocks glutamate excitotoxicity-induced oxidative stress and preserves mitochondrial transcription factor a in ischemic retinal injury. PLoS ONE 2012, 7, e47098. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.J.; Guo, Y.; Agey, P.; Wheeler, L.; Hare, W.A. Alpha2 adrenergic modulation of NMDA receptor function as a major mechanism of RGC protection in experimental glaucoma and retinal excitotoxicity. Investig. Ophthalmol. Vis. Sci. 2008, 49, 4515–4522. [Google Scholar] [CrossRef]
- Zanoni, D.S.; Da Silva, G.A.; Ezra-Elia, R.; Carvalho, M.; Quitzan, J.G.; Ofri, R.; Laus, J.L.; Laufer-Amorim, R. Histological, morphometric, protein and gene expression analyses of rat retinas with ischaemia-reperfusion injury model treated with sildenafil citrate. Int. J. Exp. Pathol. 2017, 98, 147–157. [Google Scholar] [CrossRef]
- Kwong, J.M.; Quan, A.; Kyung, H.; Piri, N.; Caprioli, J. Quantitative analysis of retinal ganglion cell survival with Rbpms immunolabeling in animal models of optic neuropathies. Investig. Ophthalmol. Vis. Sci. 2011, 52, 9694–9702. [Google Scholar] [CrossRef]
- Koeberle, P.D.; Bahr, M. The upregulation of GLAST-1 is an indirect antiapoptotic mechanism of GDNF and neurturin in the adult CNS. Cell Death Differ. 2008, 15, 471–483. [Google Scholar] [CrossRef]
- Nakazawa, M. Effects of calcium ion, calpains, and calcium channel blockers on retinitis pigmentosa. J. Ophthalmol. 2011, 2011, 292040. [Google Scholar] [CrossRef]
- Yamada, H.; Chen, Y.N.; Aihara, M.; Araie, M. Neuroprotective effect of calcium channel blocker against retinal ganglion cell damage under hypoxia. Brain Res. 2006, 1071, 75–80. [Google Scholar] [CrossRef]
- Zamponi, G.W. Targeting voltage-gated calcium channels in neurological and psychiatric diseases. Nat. Reviews. Drug Discov. 2016, 15, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Isiegas, C.; Marinich-Madzarevich, J.A.; Marchena, M.; Ruiz, J.M.; Cano, M.J.; de la Villa, P.; Hernandez-Sanchez, C.; de la Rosa, E.J.; de Pablo, F. Intravitreal Injection of Proinsulin-Loaded Microspheres Delays Photoreceptor Cell Death and Vision Loss in the rd10 Mouse Model of Retinitis Pigmentosa. Investig. Ophthalmol. Vis. Sci. 2016, 57, 3610–3618. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P. Glutamate and Neurotrophic Factors in Neuronal Plasticity and Disease. Ann. New York Acad. Sci. 2008, 1144, 97–112. [Google Scholar] [CrossRef]
- Martinez-Moreno, C.G.; Fleming, T.; Carranza, M.; Avila-Mendoza, J.; Luna, M.; Harvey, S.; Aramburo, C. Growth hormone protects against kainate excitotoxicity and induces BDNF and NT3 expression in chicken neuroretinal cells. Exp. Eye Res. 2018, 166, 1–12. [Google Scholar] [CrossRef]
- Klocker, N.; Braunling, F.; Isenmann, S.; Bahr, M. In vivo neurotrophic effects of GDNF on axotomized retinal ganglion cells. Neuroreport 1997, 8, 3439–3442. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.Y.; Cao, L.; Lu, C.L.; He, C.; Bao, X. [Protective effect of exogenous glial cell line derived neurotrophic factor on neurons after sciatic nerve injury in rats]. Sheng Li Xue Bao 2000, 52, 295–300. [Google Scholar]
- Naskar, R.; Vorwerk, C.K.; Dreyer, E.B. Concurrent downregulation of a glutamate transporter and receptor in glaucoma. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1940–1944. [Google Scholar]
- Kyhn, M.V.; Warfvinge, K.; Scherfig, E.; Kiilgaard, J.F.; Prause, J.U.; Klassen, H.; Young, M.; la Cour, M. Acute retinal ischemia caused by controlled low ocular perfusion pressure in a porcine model. Electrophysiological and histological characterisation. Exp. Eye Res. 2009, 88, 1100–1106. [Google Scholar] [CrossRef]
- Lin, L.F.; Doherty, D.H.; Lile, J.D.; Bektesh, S.; Collins, F. GDNF: A glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science 1993, 260, 1130–1132. [Google Scholar] [CrossRef]
- Ward, M.S.; Khoobehi, A.; Lavik, E.B.; Langer, R.; Young, M.J. Neuroprotection of retinal ganglion cells in DBA/2J mice with GDNF-loaded biodegradable microspheres. J. Pharm. Sci. 2007, 96, 558–568. [Google Scholar] [CrossRef] [PubMed]
- Checa-Casalengua, P.; Jiang, C.; Bravo-Osuna, I.; Tucker, B.A.; Molina-Martinez, I.T.; Young, M.J.; Herrero-Vanrell, R. Retinal ganglion cells survival in a glaucoma model by GDNF/Vit E PLGA microspheres prepared according to a novel microencapsulation procedure. J. Control. Release 2011, 156, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Checa-Casalengua, P.; Jiang, C.; Bravo-Osuna, I.; Tucker, B.A.; Molina-Martinez, I.T.; Young, M.J.; Herrero-Vanrell, R. Preservation of biological activity of glial cell line-derived neurotrophic factor (GDNF) after microencapsulation and sterilization by gamma irradiation. Int. J. Pharm. 2012, 436, 545–554. [Google Scholar] [CrossRef]
- Valenciano, A.I.; Corrochano, S.; de Pablo, F.; de la Villa, P.; de la Rosa, E.J. Proinsulin/insulin is synthesized locally and prevents caspase- and cathepsin-mediated cell death in the embryonic mouse retina. J. Neurochem. 2006, 99, 524–536. [Google Scholar] [CrossRef]
- Vergara, M.N.; de la Rosa, E.J.; Canto-Soler, M.V. Focus on molecules: Proinsulin in the eye: Precursor or pioneer? Exp. Eye Res. 2012, 101, 109–110. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Boatright, J.H.; Nickerson, J.M.; Moring, A.G.; Pardue, M.T. Bile acids in treatment of ocular disease. J. Ocul. Biol. Dis. Inform. 2009, 2, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Bovolenta, P.; Cisneros, E. Retinitis pigmentosa: Cone photoreceptors starving to death. Nat. Neurosci. 2009, 12, 5–6. [Google Scholar] [CrossRef]
- Ishimaru, Y.; Sumino, A.; Kajioka, D.; Shibagaki, F.; Yamamuro, A.; Yoshioka, Y.; Maeda, S. Apelin protects against NMDA-induced retinal neuronal death via an APJ receptor by activating Akt and ERK1/2, and suppressing TNF-alpha expression in mice. J. Pharmacol. Sci. 2017, 133, 34–41. [Google Scholar] [CrossRef]
- Kokona, D.; Thermos, K. Synthetic and endogenous cannabinoids protect retinal neurons from AMPA excitotoxicity in vivo, via activation of CB1 receptors: Involvement of PI3K/Akt and MEK/ERK signaling pathways. Exp. Eye Res. 2015, 136, 45–58. [Google Scholar] [CrossRef]
- Sakamoto, K.; Kuroki, T.; Sagawa, T.; Ito, H.; Mori, A.; Nakahara, T.; Ishii, K. Opioid receptor activation is involved in neuroprotection induced by TRPV1 channel activation against excitotoxicity in the rat retina. Eur. J. Pharmacol. 2017, 812, 57–63. [Google Scholar] [CrossRef]
- Lakk, M.; Denes, V.; Gabriel, R. Pituitary Adenylate Cyclase-Activating Polypeptide Receptors Signal via Phospholipase C Pathway to Block Apoptosis in Newborn Rat Retina. Neurochem. Res. 2015, 40, 1402–1409. [Google Scholar] [CrossRef] [PubMed]
- Galvao, J.; Elvas, F.; Martins, T.; Cordeiro, M.F.; Ambrosio, A.F.; Santiago, A.R. Adenosine A3 receptor activation is neuroprotective against retinal neurodegeneration. Exp. Eye Res. 2015, 140, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Shinmei, Y.; Dong, Y.; Inafuku, S.; Fukuhara, J.; Ando, R.; Kitaichi, N.; Kanda, A.; Tanaka, K.; Noda, K.; et al. Effect of geranylgeranylacetone on the protection of retinal ganglion cells in a mouse model of normal tension glaucoma. Heliyon 2016, 2, e00191. [Google Scholar] [CrossRef] [PubMed]
- Tsutsumi, T.; Iwao, K.; Hayashi, H.; Kirihara, T.; Kawaji, T.; Inoue, T.; Hino, S.; Nakao, M.; Tanihara, H. Potential Neuroprotective Effects of an LSD1 Inhibitor in Retinal Ganglion Cells via p38 MAPK Activity. Investig. Ophthalmol. Vis. Sci. 2016, 57, 6461–6473. [Google Scholar] [CrossRef] [PubMed]
- Del Valle Bessone, C.; Fajreldines, H.D.; de Barboza, G.E.D.; Tolosa de Talamoni, N.G.; Allemandi, D.A.; Carpentieri, A.R.; Quinteros, D.A. Protective role of melatonin on retinal ganglionar cell: In vitro an in vivo evidences. Life Sci. 2019, 218, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhang, R.; Zhang, S.; Wu, J.; Sun, X. Activation of 5-HT1A Receptors Promotes Retinal Ganglion Cell Function by Inhibiting the cAMP-PKA Pathway to Modulate Presynaptic GABA Release in Chronic Glaucoma. J. Neurosci. 2019, 39, 1484–1504. [Google Scholar] [CrossRef]
- Eriksen, A.Z.; Eliasen, R.; Oswald, J.; Kempen, P.J.; Melander, F.; Andresen, T.L.; Young, M.; Baranov, P.; Urquhart, A.J. Multifarious Biologic Loaded Liposomes that Stimulate the Mammalian Target of Rapamycin Signaling Pathway Show Retina Neuroprotection after Retina Damage. ACS Nano 2018, 12, 7497–7508. [Google Scholar] [CrossRef]
- Mallozzi, C.; Parravano, M.; Gaddini, L.; Villa, M.; Pricci, F.; Malchiodi-Albedi, F.; Matteucci, A. Curcumin Modulates the NMDA Receptor Subunit Composition Through a Mechanism Involving CaMKII and Ser/Thr Protein Phosphatases. Cell. Mol. Neurobiol. 2018, 38, 1315–1320. [Google Scholar] [CrossRef]
- Lambuk, L.; Jafri, A.J.; Arfuzir, N.N.; Iezhitsa, I.; Agarwal, R.; Rozali, K.N.; Agarwal, P.; Bakar, N.S.; Kutty, M.K.; Yusof, A.P.; et al. Neuroprotective Effect of Magnesium Acetyltaurate Against NMDA-Induced Excitotoxicity in Rat Retina. Neurotox. Res. 2017, 31, 31–45. [Google Scholar] [CrossRef]
- Arranz-Romera, A.; Davis, B.M.; Bravo-Osuna, I.; Esteban-Perez, S.; Molina-Martinez, I.T.; Shamsher, E.; Ravindran, N.; Guo, L.; Cordeiro, M.F.; Herrero-Vanrell, R. Simultaneous co-delivery of neuroprotective drugs from multi-loaded PLGA microspheres for the treatment of glaucoma. J. Control. Release 2019, 297, 26–38. [Google Scholar] [CrossRef]
- Shin, J.A.; Kim, H.S.; Vargas, A.; Yu, W.Q.; Eom, Y.S.; Craft, C.M.; Lee, E.J. Inhibition of Matrix Metalloproteinase 9 Enhances Rod Survival in the S334ter-line3 Retinitis Pigmentosa Model. PLoS ONE 2016, 11, e0167102. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Cheng, M.; Chintala, S.K. Kainic acid-mediated upregulation of matrix metalloproteinase-9 promotes retinal degeneration. Investig. Ophthalmol. Vis. Sci. 2004, 45, 2374–2383. [Google Scholar] [CrossRef] [PubMed]
- Sreekumar, P.G.; Li, Z.; Wang, W.; Spee, C.; Hinton, D.R.; Kannan, R.; MacKay, J.A. Intra-vitreal alphaB crystallin fused to elastin-like polypeptide provides neuroprotection in a mouse model of age-related macular degeneration. J. Control. Release 2018, 283, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Trudeau, K.; Muto, T.; Roy, S. Downregulation of mitochondrial connexin 43 by high glucose triggers mitochondrial shape change and cytochrome C release in retinal endothelial cells. Investig. Ophthalmol. Vis. Sci. 2012, 53, 6675–6681. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Jiang, J.X.; Li, A.F.; Kim, D. Connexin channel and its role in diabetic retinopathy. Prog. Retin. Eye Res. 2017. [Google Scholar] [CrossRef] [PubMed]
| Compounds | Pathway/Mechanism Involved | Bibliography |
|---|---|---|
| Apelin-36 and apelin- 17 | Akt and ERK1/2 signaling pathways. | [68] |
| Cannabinoids | CB1 receptors, PI3K/Akt and MEK/ERK1/2 signaling pathways. | [69] |
| Capsaicin | Opioid, calcitonin gene-related peptide and tachykinin NK1 receptor. | [70] |
| Pituitary adenylate cyclase-activating polypeptide | Phosphatidylcholine-specific PLC pathway and cAMP production. | [71] |
| Adenosine A3 receptor agonists | Attenuates the rise in calcium in RGC. | [72] |
| Geranylgeranylacetone | Reduction in the activities of caspase-9 and caspase-3. | [73] |
| CYM-5442 | Sphingosine 1-phosphate receptor agonism. | [19] |
| Adamantane derivatives | Blockage of NMDARs excessive overactivation | [25] |
| Tetramethylpyrazines | Blockage of L-type voltaje-gated Ca2+ channels. | [7] |
| Tranylcypromine | P38 MAPK and KEGG pathway genes expression. | [74] |
| Dual compounds (e.g., Mg acetyltaurate) | NMDAR inhibition + antioxidant effect. | [79] |
| Melatonin | Direct and indirect free radical scavenger. | [75] |
| 5-HT1A agonists | Inhibition of cAMP-PKA signaling pathway. | [76] |
| Ciliary neurotrophic factors, lipopeptide N-fragment osteopontin mimic, lipopeptide phosphatase tension homologue inhibitors | mTOR pathway stimulation. | [77] |
| Curcumin | Modulation of NMDA receptor subunits composition. | [78] |
| Neuroprotection based on | Compound | Properties | Encapsulation | Observations | Bibliography |
|---|---|---|---|---|---|
| Intervention over excess glutamate | Brimonidine | See Figure 2 | Poly-lactic acid (RESOMER® 202H) MS | Particle size between 20 to 45 µm. Reduced burst effect. After a month, only 75% of the drug was released. Reduction of IOP after a month. No serious adverse effects noticed and eyes did not look inflamed and the animals did not show signs of pain, irritation or distress. RGC protective activity was evaluated. | [43] |
| Neuroprotective therapies | |||||
| Neurotrophic factors | GDNF | See Figure 2 | PLGA (50:50) MS | Particle size ≈ 20 µm. Drug loading ≈ 25 ng/mg. EE ≈ 28%. RGC survival in-vitro > 70%. 50% higher preservation of RGC in vivo compared to the same dose of GDNF administered in bolus. No side effects observed on retina. | [32,62,63] |
| Antiapoptotic compounds | TUDCA | See Figure 2 | PLGA (50:50) MS | Particle size ≈ 20 µm. Spherical MS. High production yield. Low burst effect. Significant photoreceptor’s survival. Well-preserved contact between photoreceptor cells and second order neurons. | [14] |
| Dexamethasone (DX) Melatonin (Mel) Coenzyme Q10 (CoQ10) | Antiapoptotic Antioxidant Anti-inflammatory | PLGA (50:50) MS | Particle size ≈ 24 µm. Spherical MS. Production yield ≈ 72% EE ≈ 78% DX; 62% Mel; 96% CoQ10 Low burst effect and triphasic release. Neuroprotection—high RGC density—in the Morrison’s model of ocular hypertension; whole retina density measures demonstrated that MS administration preserved RGC to a comparable extent as naïve retinas. Multidrug MS demonstrated less side effects than the same amount of drug administered in single-drug loaded MS. | [80] | |
| Retinal structure intregrity | Connexin43 mimetic peptide | See Figure 2 | PLGA (50:50) MP | Particle size ≈ 9 µm and narrow distribution. Spherical morphology. Smooth surface. Neutral zeta potential. MP release drug in sustained release more than 3 months. No enough drug released after a day to exert effective protection maybe due to rapid RGC death after ischemia lesion. | [37] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez Villanueva, J.; Martín Esteban, J.; Rodríguez Villanueva, L.J. Retinal Cell Protection in Ocular Excitotoxicity Diseases. Possible Alternatives Offered by Microparticulate Drug Delivery Systems and Future Prospects. Pharmaceutics 2020, 12, 94. https://doi.org/10.3390/pharmaceutics12020094
Rodríguez Villanueva J, Martín Esteban J, Rodríguez Villanueva LJ. Retinal Cell Protection in Ocular Excitotoxicity Diseases. Possible Alternatives Offered by Microparticulate Drug Delivery Systems and Future Prospects. Pharmaceutics. 2020; 12(2):94. https://doi.org/10.3390/pharmaceutics12020094
Chicago/Turabian StyleRodríguez Villanueva, Javier, Jorge Martín Esteban, and Laura J. Rodríguez Villanueva. 2020. "Retinal Cell Protection in Ocular Excitotoxicity Diseases. Possible Alternatives Offered by Microparticulate Drug Delivery Systems and Future Prospects" Pharmaceutics 12, no. 2: 94. https://doi.org/10.3390/pharmaceutics12020094
APA StyleRodríguez Villanueva, J., Martín Esteban, J., & Rodríguez Villanueva, L. J. (2020). Retinal Cell Protection in Ocular Excitotoxicity Diseases. Possible Alternatives Offered by Microparticulate Drug Delivery Systems and Future Prospects. Pharmaceutics, 12(2), 94. https://doi.org/10.3390/pharmaceutics12020094