RETRACTED: Drug Flux across RPE Cell Models: The Hunt for an Appropriate Outer Blood–Retinal Barrier Model for Use in Early Drug Discovery
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Permeation Studies
2.3. Drug Concentration Measurements
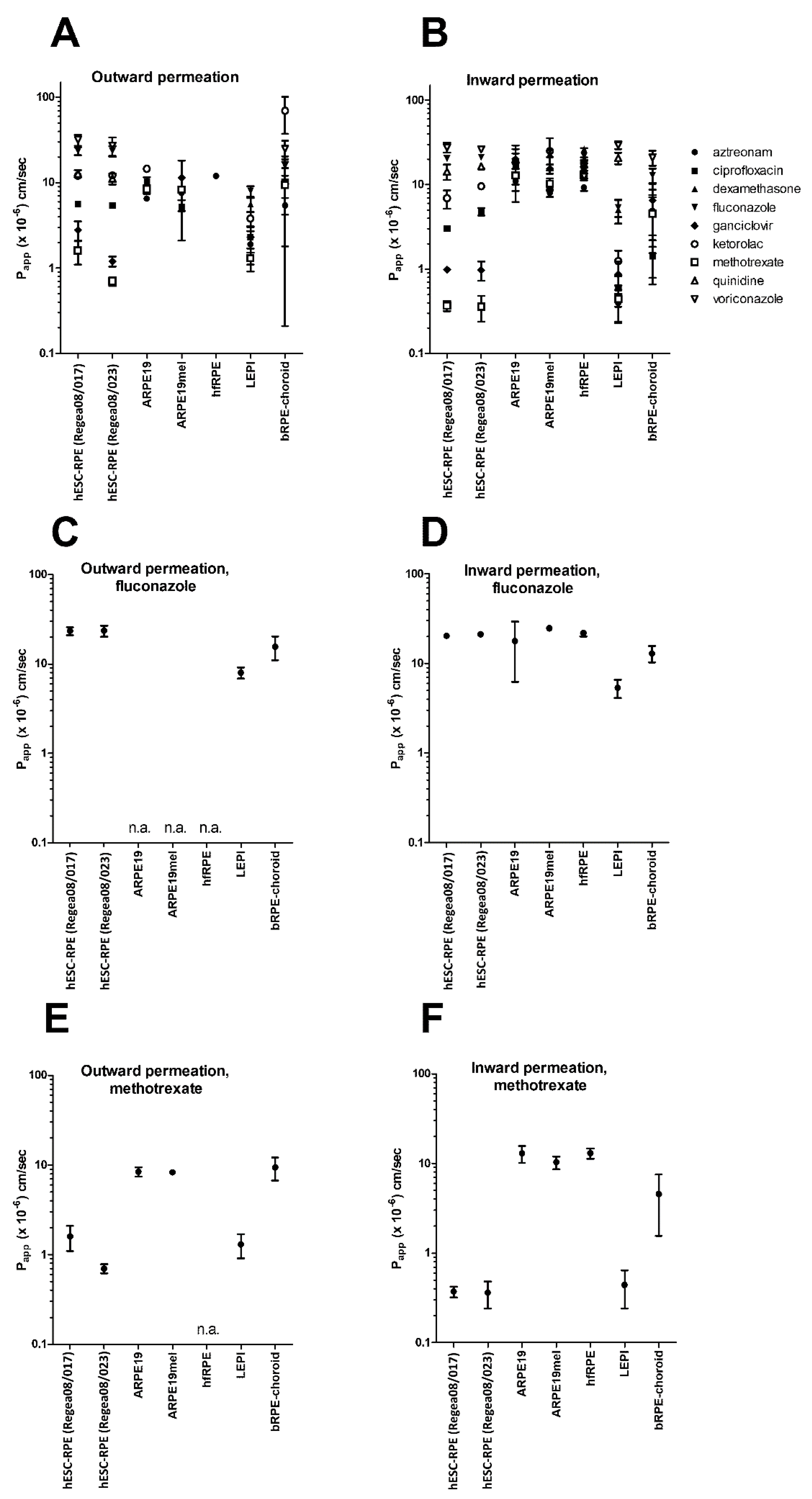
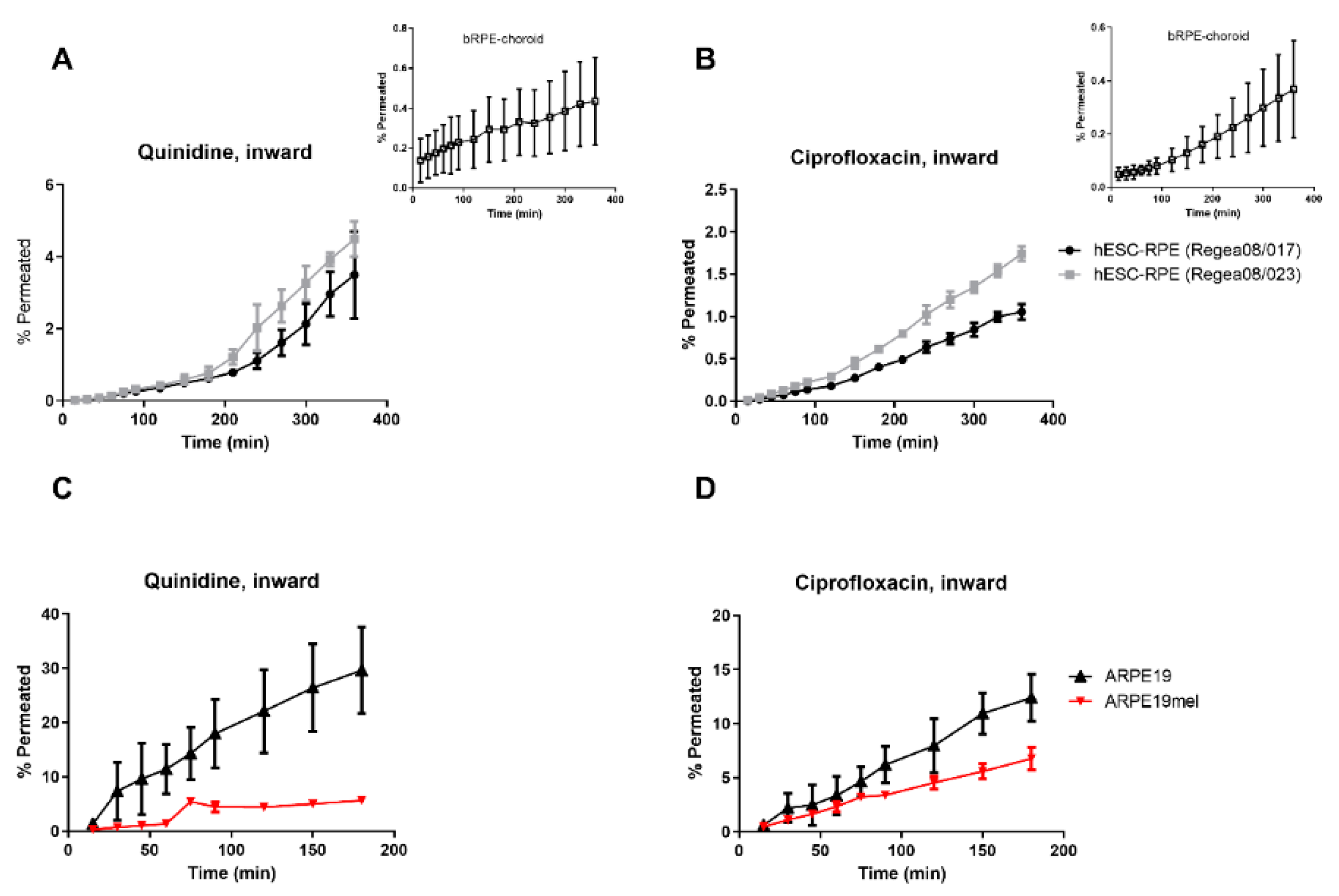
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Strauss, O. The Retinal Pigment Epithelium in Visual Function. Physiol. Rev. 2005, 85, 845–881. [Google Scholar] [CrossRef]
- Ramsay, E.; Hagstrom, M.; Vellonen, K.S.; Boman, S.; Toropainen, E.; Del Amo, E.M.; Kidron, H.; Urtti, A.; Ruponen, M. Role of Retinal Pigment Epithelium Permeability in Drug Transfer between Posterior Eye Segment and Systemic Blood Circulation. Eur. J. Pharm. Biopharm. 2019, 143, 18–23. [Google Scholar] [CrossRef]
- Del Amo, E.M.; Rimpela, A.K.; Heikkinen, E.; Kari, O.K.; Ramsay, E.; Lajunen, T.; Schmitt, M.; Pelkonen, L.; Bhattacharya, M.; Richardson, D.; et al. Pharmacokinetic Aspects of Retinal Drug Delivery. Prog. Retin. Eye Res. 2016, 57, 134–185. [Google Scholar] [CrossRef]
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.; Klein, R.; Cheng, C.Y.; Wong, T.Y. Global Prevalence of Age-Related Macular Degeneration and Disease Burden Projection for 2020 and 2040: A Systematic Review and Meta-Analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef]
- Del Amo, E.M.; Vellonen, K.S.; Kidron, H.; Urtti, A. Intravitreal Clearance and Volume of Distribution of Compounds in Rabbits: In Silico Prediction and Pharmacokinetic Simulations for Drug Development. Eur. J. Pharm. Biopharm. 2015, 95, 215–226. [Google Scholar] [CrossRef]
- Del Amo, E.M.; Urtti, A. Rabbit as an Animal Model for Intravitreal Pharmacokinetics: Clinical Predictability and Quality of the Published Data. Exp. Eye Res. 2015, 137, 111–124. [Google Scholar] [CrossRef]
- Vellonen, K.S.; Malinen, M.; Mannermaa, E.; Subrizi, A.; Toropainen, E.; Lou, Y.R.; Kidron, H.; Yliperttula, M.; Urtti, A. A Critical Assessment of in Vitro Tissue Models for ADME and Drug Delivery. J. Control. Release 2014, 190, 94–114. [Google Scholar] [CrossRef]
- Steuer, H.; Jaworski, A.; Elger, B.; Kaussmann, M.; Keldenich, J.; Schneider, H.; Stoll, D.; Schlosshauer, B. Functional Characterization and Comparison of the Outer Blood-Retina Barrier and the Blood-Brain Barrier. Investig. Ophthalmol. Vis. Sci. 2005, 46, 1047–1053. [Google Scholar] [CrossRef]
- Pitkanen, L.; Ranta, V.P.; Moilanen, H.; Urtti, A. Permeability of Retinal Pigment Epithelium: Effects of Permeant Molecular Weight and Lipophilicity. Investig. Ophthalmol. Vis. Sci. 2005, 46, 641–646. [Google Scholar] [CrossRef]
- Dunn, K.C.; Aotaki-Keen, A.E.; Putkey, F.R.; Hjelmeland, L.M. ARPE-19, a Human Retinal Pigment Epithelial Cell Line with Differentiated Properties. Exp. Eye Res. 1996, 62, 155–169. [Google Scholar] [CrossRef]
- Mannermaa, E.; Reinisalo, M.; Ranta, V.P.; Vellonen, K.S.; Kokki, H.; Saarikko, A.; Kaarniranta, K.; Urtti, A. Filter-Cultured ARPE-19 Cells as Outer Blood-Retinal Barrier Model. Eur. J. Pharm. Sci. 2010, 40, 289–296. [Google Scholar] [CrossRef]
- Rimpela, A.K.; Reinisalo, M.; Hellinen, L.; Grazhdankin, E.; Kidron, H.; Urtti, A.; Del Amo, E.M. Implications of Melanin Binding in Ocular Drug Delivery. Adv. Drug Deliv. Rev. 2018, 126, 23–43. [Google Scholar] [CrossRef]
- Rimpela, A.K.; Hagstrom, M.; Kidron, H.; Urtti, A. Melanin Targeting for Intracellular Drug Delivery: Quantification of Bound and Free Drug in Retinal Pigment Epithelial Cells. J. Control. Release 2018, 283, 261–268. [Google Scholar] [CrossRef]
- Jakubiak, P.; Reutlinger, M.; Mattei, P.; Schuler, F.; Urtti, A.; Alvarez-Sanchez, R. Understanding Molecular Drivers of Melanin Binding to Support Rational Design of Small Molecule Ophthalmic Drugs. J. Med. Chem. 2018, 61, 10106–10115. [Google Scholar] [CrossRef]
- Robbie, S.J.; Lundh von Leithner, P.; Ju, M.; Lange, C.A.; King, A.G.; Adamson, P.; Lee, D.; Sychterz, C.; Coffey, P.; Ng, Y.S.; et al. Assessing a Novel Depot Delivery Strategy for Noninvasive Administration of VEGF/PDGF RTK Inhibitors for Ocular Neovascular Disease. Investig. Ophthalmol. Vis. Sci. 2013, 54, 1490–1500. [Google Scholar] [CrossRef]
- Hellinen, L.; Hagstrom, M.; Knuutila, H.; Ruponen, M.; Urtti, A.; Reinisalo, M. Characterization of Artificially Re-Pigmented ARPE-19 Retinal Pigment Epithelial Cell Model. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Hellinen, L.; Pirskanen, L.; Tengvall-Unadike, U.; Urtti, A.; Reinisalo, M. Retinal Pigment Epithelial Cell Line with Fast Differentiation and Improved Barrier Properties. Pharmaceutics 2019, 11. [Google Scholar] [CrossRef]
- Pelkonen, L.; Reinisalo, M.; Morin-Picardat, E.; Kidron, H.; Urtti, A. Isolation of Intact and Functional Melanosomes from the Retinal Pigment Epithelium. PLoS ONE 2016, 11, e0160352. [Google Scholar] [CrossRef]
- Da Cruz, L.; Fynes, K.; Georgiadis, O.; Kerby, J.; Luo, Y.H.; Ahmado, A.; Vernon, A.; Daniels, J.T.; Nommiste, B.; Hasan, S.M.; et al. Phase 1 Clinical Study of an Embryonic Stem Cell-Derived Retinal Pigment Epithelium Patch in Age-Related Macular Degeneration. Nat. Biotechnol. 2018, 36, 328–337. [Google Scholar] [CrossRef]
- Carr, A.J.; Vugler, A.; Lawrence, J.; Chen, L.L.; Ahmado, A.; Chen, F.K.; Semo, M.; Gias, C.; da Cruz, L.; Moore, H.D.; et al. Molecular Characterization and Functional Analysis of Phagocytosis by Human Embryonic Stem Cell-Derived RPE Cells using a Novel Human Retinal Assay. Mol. Vis. 2009, 15, 283–295. [Google Scholar]
- Vaajasaari, H.; Ilmarinen, T.; Juuti-Uusitalo, K.; Rajala, K.; Onnela, N.; Narkilahti, S.; Suuronen, R.; Hyttinen, J.; Uusitalo, H.; Skottman, H. Toward the Defined and Xeno-Free Differentiation of Functional Human Pluripotent Stem Cell-Derived Retinal Pigment Epithelial Cells. Mol. Vis. 2011, 17, 558–575. [Google Scholar] [PubMed]
- Bennis, A.; Jacobs, J.G.; Catsburg, L.A.E.; Ten Brink, J.B.; Koster, C.; Schlingemann, R.O.; van Meurs, J.; Gorgels, T.G.M.F.; Moerland, P.D.; Heine, V.M.; et al. Stem Cell Derived Retinal Pigment Epithelium: The Role of Pigmentation as Maturation Marker and Gene Expression Profile Comparison with Human Endogenous Retinal Pigment Epithelium. Stem Cell. Rev. Rep. 2017, 13, 659–669. [Google Scholar] [CrossRef]
- Hongisto, H.; Ilmarinen, T.; Vattulainen, M.; Mikhailova, A.; Skottman, H. Xeno- and Feeder-Free Differentiation of Human Pluripotent Stem Cells to Two Distinct Ocular Epithelial Cell Types using Simple Modifications of One Method. Stem Cell. Res. Ther. 2017, 8, 291. [Google Scholar] [CrossRef] [PubMed]
- Hongisto, H.; Jylha, A.; Nattinen, J.; Rieck, J.; Ilmarinen, T.; Vereb, Z.; Aapola, U.; Beuerman, R.; Petrovski, G.; Uusitalo, H.; et al. Comparative Proteomic Analysis of Human Embryonic Stem Cell-Derived and Primary Human Retinal Pigment Epithelium. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef]
- Subrizi, A.; Hiidenmaa, H.; Ilmarinen, T.; Nymark, S.; Dubruel, P.; Uusitalo, H.; Yliperttula, M.; Urtti, A.; Skottman, H. Generation of hESC-Derived Retinal Pigment Epithelium on Biopolymer Coated Polyimide Membranes. Biomaterials 2012, 33, 8047–8054. [Google Scholar] [CrossRef]
- Skottman, H.; Muranen, J.; Lahdekorpi, H.; Pajula, E.; Makela, K.; Koivusalo, L.; Koistinen, A.; Uusitalo, H.; Kaarniranta, K.; Juuti-Uusitalo, K. Contacting Co-Culture of Human Retinal Microvascular Endothelial Cells Alters Barrier Function of Human Embryonic Stem Cell Derived Retinal Pigment Epithelial Cells. Exp. Cell Res. 2017, 359, 101–111. [Google Scholar] [CrossRef]
- Pelkonen, L.; Tengvall-Unadike, U.; Ruponen, M.; Kidron, H.; Del Amo, E.M.; Reinisalo, M.; Urtti, A. Melanin Binding Study of Clinical Drugs with Cassette Dosing and Rapid Equilibrium Dialysis Inserts. Eur. J. Pharm. Sci. 2017, 109, 162–168. [Google Scholar] [CrossRef]
- Pelkonen, L.; Sato, K.; Reinisalo, M.; Kidron, H.; Tachikawa, M.; Watanabe, M.; Uchida, Y.; Urtti, A.; Terasaki, T. LC-MS/MS Based Quantitation of ABC and SLC Transporter Proteins in Plasma Membranes of Cultured Primary Human Retinal Pigment Epithelium Cells and Immortalized ARPE19 Cell Line. Mol. Pharm. 2017, 14, 605–613. [Google Scholar] [CrossRef]
- Skottman, H. Derivation and Characterization of Three New Human Embryonic Stem Cell Lines in Finland. In Vitro Cell. Dev. Biol. Anim. 2010, 46, 206–209. [Google Scholar] [CrossRef]
- Ramsay, E.; Ruponen, M.; Picardat, T.; Tengvall, U.; Tuomainen, M.; Auriola, S.; Toropainen, E.; Urtti, A.; Del Amo, E.M. Impact of Chemical Structure on Conjunctival Drug Permeability: Adopting Porcine Conjunctiva and Cassette Dosing for Construction of in Silico Model. J. Pharm. Sci. 2017, 106, 2463–2471. [Google Scholar] [CrossRef]
- Shadforth, A.M.A.; Suzuki, S.; Theodoropoulos, C.; Richardson, N.A.; Chirila, T.V.; Harkin, D.G. A Bruch’s Membrane Substitute Fabricated from Silk Fibroin Supports the Function of Retinal Pigment Epithelial Cells in Vitro. J. Tissue Eng. Regen. Med. 2017, 11, 1915–1924. [Google Scholar] [CrossRef]
- Samuel, W.; Jaworski, C.; Postnikova, O.A.; Kutty, R.K.; Duncan, T.; Tan, L.X.; Poliakov, E.; Lakkaraju, A.; Redmond, T.M. Appropriately Differentiated ARPE-19 Cells Regain Phenotype and Gene Expression Profiles Similar to those of Native RPE Cells. Mol. Vis. 2017, 23, 60–89. [Google Scholar]
- Philp, N.J.; Wang, D.; Yoon, H.; Hjelmeland, L.M. Polarized Expression of Monocarboxylate Transporters in Human Retinal Pigment Epithelium and ARPE-19 Cells. Investig. Ophthalmol. Vis. Sci. 2003, 44, 1716–1721. [Google Scholar] [CrossRef]
- Hellinen, L.; Sato, K.; Reinisalo, M.; Kidron, H.; Rilla, K.; Tachikawa, M.; Uchida, Y.; Terasaki, T.; Urtti, A. Quantitative Protein Expression in the Human Retinal Pigment Epithelium: Comparison between Apical and Basolateral Plasma Membranes with Emphasis on Transporters. Investig. Ophthalmol. Vis. Sci. 2019, 60, 5022–5034. [Google Scholar] [CrossRef]
- Sorkio, A.; Hongisto, H.; Kaarniranta, K.; Uusitalo, H.; Juuti-Uusitalo, K.; Skottman, H. Structure and Barrier Properties of Human Embryonic Stem Cell-Derived Retinal Pigment Epithelial Cells are Affected by Extracellular Matrix Protein Coating. Tissue Eng. Part A 2014, 20, 622–634. [Google Scholar] [CrossRef]
- European Medicines Agency. Guideline on the Investigation of Drug Interactions. 2012, PMP/EWP/560/95/Rev. 1 Corr. 2 **; Committee for Human Medicinal Products (CHMP): London, UK, 2012. [Google Scholar]
- Juuti-Uusitalo, K.; Vaajasaari, H.; Ryhanen, T.; Narkilahti, S.; Suuronen, R.; Mannermaa, E.; Kaarniranta, K.; Skottman, H. Efflux Protein Expression in Human Stem Cell-Derived Retinal Pigment Epithelial Cells. PLoS ONE 2012, 7, e30089. [Google Scholar] [CrossRef]
- Vellonen, K.S.; Soini, E.M.; Del Amo, E.M.; Urtti, A. Prediction of Ocular Drug Distribution from Systemic Blood Circulation. Mol. Pharm. 2016, 13, 2906–2911. [Google Scholar] [CrossRef]
- Vellonen, K.S.; Hellinen, L.; Mannermaa, E.; Ruponen, M.; Urtti, A.; Kidron, H. Expression, Activity and Pharmacokinetic Impact of Ocular Transporters. Adv. Drug Deliv. Rev. 2018, 126, 3–22. [Google Scholar] [CrossRef]
- Rimpela, A.I.; Schmitt, M.; Latonen, S.; Hagstrom, M.; Antopolsky, M.; Manzanares, J.A.; Kidron, H.; Urtti, A.O. Drug Distribution to Retinal Pigment Epithelium: Studies on Melanin Binding, Cellular Kinetics, and SPECT/CT Imaging. Mol. Pharm. 2016, 57, 107. [Google Scholar]
- Westerhout, J.; van de Steeg, E.; Grossouw, D.; Zeijdner, E.E.; Krul, C.A.; Verwei, M.; Wortelboer, H.M. A New Approach to Predict Human Intestinal Absorption using Porcine Intestinal Tissue and Biorelevant Matrices. Eur. J. Pharm. Sci. 2014, 63, 167–177. [Google Scholar] [CrossRef]
- Kiamehr, M.; Klettner, A.; Richert, E.; Koskela, A.; Koistinen, A.; Skottman, H.; Kaarniranta, K.; Aalto-Setala, K.; Juuti-Uusitalo, K. Compromised Barrier Function in Human Induced Pluripotent Stem-Cell-Derived Retinal Pigment Epithelial Cells from Type 2 Diabetic Patients. Int. J. Mol. Sci. 2019, 20. [Google Scholar] [CrossRef] [PubMed]
| Compound | 1 LogD7.4 (Predicted, ACDLabs) | Molecular Weight (g/mol) | 2 Manufacturer | Exposure Concentration (µg/mL) |
|---|---|---|---|---|
| Aztreonam | −4.32 | 435.4 | Fluka | 10 |
| Ciprofloxacin | −0.29 | 331.3 | BioChemica | 1 |
| Dexamethasone | 1.92 | 392.5 | Sigma-Aldrich | 10 |
| Fluconazole | 0.45 | 306.3 | Sigma-Aldrich | 1 |
| Ganciclovir | −1.61 | 255.2 | Sigma-Aldrich | 1 |
| Ketorolac | −0.34 | 255.3 | Sigma-Aldrich | 1 |
| Methotrexate | -5.1 | 454.4 | Fluka | 1 |
| Quinidine | 1.17 | 324.4 | Sigma-Aldrich | 10 |
| Voriconazole | 1.21 | 349.3 | Fluka | 10 |
| Compound | LEPI | hESC-RPE (Regea08/017) | hESC-RPE (Regea08/023) | Bovine RPE-Choroid 1 |
|---|---|---|---|---|
| Aztreonam | 4.8 | n.a. | n.a. | 1.2 |
| Ciprofloxacin | 3.9 | 1.9 | 1.1 | 6.7 |
| Dexamethasone | 1.1 | n.a. | n.a. | n.d. |
| Fluconazole | 1.5 | 1.1 | 1.1 | 1.2 |
| Ganciclovir | 2.7 | 2.9 | 1.3 | 1.5 |
| Ketorolac | 3.1 | 1.8 | 1.3 | 14.5 |
| Methotrexate | 3.0 | 4.4 | 1.8 | 2.1 |
| Quinidine | n.a. | 0.9 | 0.7 | n.a. |
| Voriconazole | n.a. | 1.1 | 1.0 | 1.2 |
| Cell Model | Culture Conditions | Tight Junction Protein Expression | Pigmentation | Barrier Properties: Conclusions of this Study | Assays in Which the Cell Model can be Utilized in Early Drug Discovery |
|---|---|---|---|---|---|
| Cell lines | |||||
| ARPE19 | simple to demanding; variation between laboratories | yes | no | leaky | Drug uptake, active transport |
| ARPE19mel | simple | yes | can be controlled; from low to heavy | leaky | Drug uptake: quantitative effects of pigmentation |
| LEPI | simple | yes | no | tight | Drug uptake and permeation |
| Primary RPE cells | |||||
| hfRPE | simple | yes | low/modest | leaky | Drug uptake, active transport |
| Stem-cell based RPE cells | |||||
| hESC-RPE | demanding; long differentiation time, requires specialized conditions and expensive supplements | yes | heavy | tight | Drug uptake and permeation |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hellinen, L.; Hongisto, H.; Ramsay, E.; Kaarniranta, K.; Vellonen, K.-S.; Skottman, H.; Ruponen, M. RETRACTED: Drug Flux across RPE Cell Models: The Hunt for an Appropriate Outer Blood–Retinal Barrier Model for Use in Early Drug Discovery. Pharmaceutics 2020, 12, 176. https://doi.org/10.3390/pharmaceutics12020176
Hellinen L, Hongisto H, Ramsay E, Kaarniranta K, Vellonen K-S, Skottman H, Ruponen M. RETRACTED: Drug Flux across RPE Cell Models: The Hunt for an Appropriate Outer Blood–Retinal Barrier Model for Use in Early Drug Discovery. Pharmaceutics. 2020; 12(2):176. https://doi.org/10.3390/pharmaceutics12020176
Chicago/Turabian StyleHellinen, Laura, Heidi Hongisto, Eva Ramsay, Kai Kaarniranta, Kati-Sisko Vellonen, Heli Skottman, and Marika Ruponen. 2020. "RETRACTED: Drug Flux across RPE Cell Models: The Hunt for an Appropriate Outer Blood–Retinal Barrier Model for Use in Early Drug Discovery" Pharmaceutics 12, no. 2: 176. https://doi.org/10.3390/pharmaceutics12020176
APA StyleHellinen, L., Hongisto, H., Ramsay, E., Kaarniranta, K., Vellonen, K.-S., Skottman, H., & Ruponen, M. (2020). RETRACTED: Drug Flux across RPE Cell Models: The Hunt for an Appropriate Outer Blood–Retinal Barrier Model for Use in Early Drug Discovery. Pharmaceutics, 12(2), 176. https://doi.org/10.3390/pharmaceutics12020176








