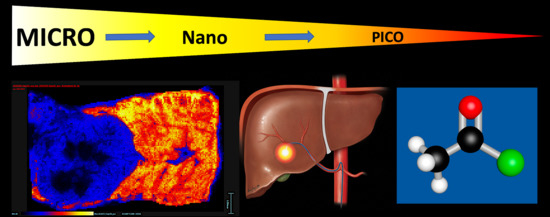
Combining Chemistry and Engineering for Hepatocellular Carcinoma: Nano-Scale and Smaller Therapies
Abstract
1. Introduction
2. Patient Selection and Technical Aspects in Large or Multifocal HCC
3. Measuring Success in TACE for HCC: Evidence from Imaging and Pathology Correlations
4. Adjunct Strategies: Transcatheter Hyperthermia and Nanotechnologies
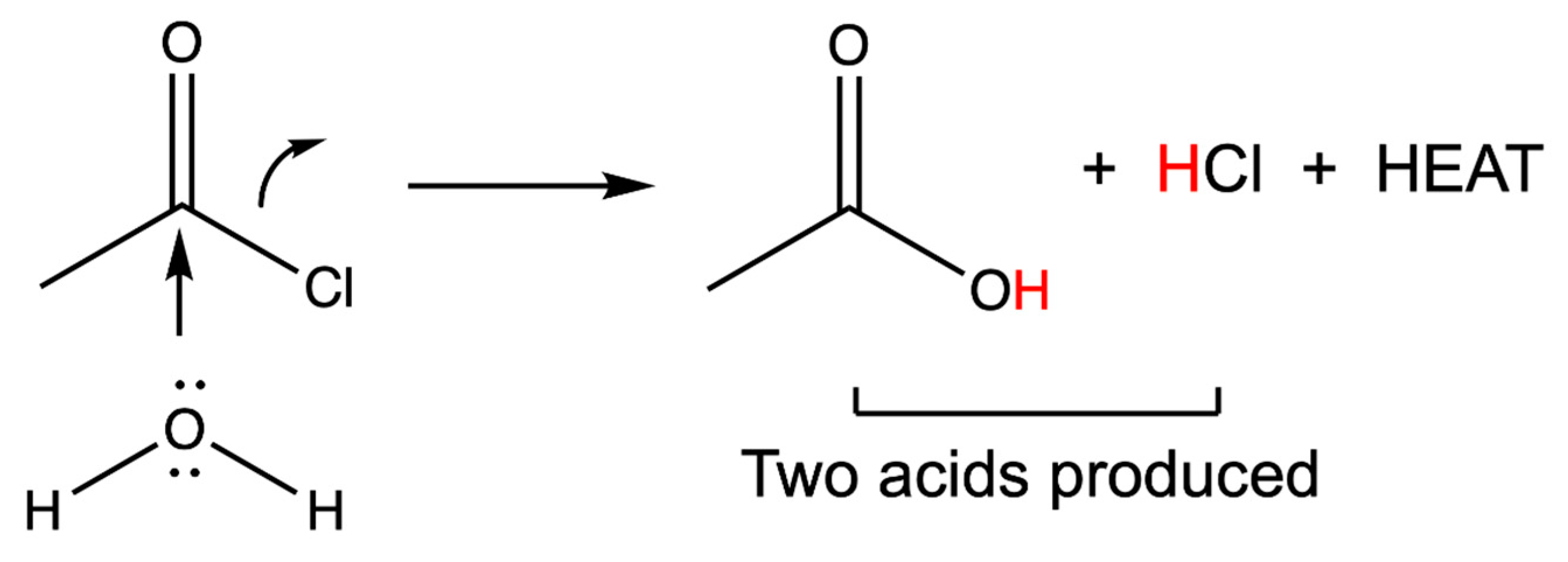
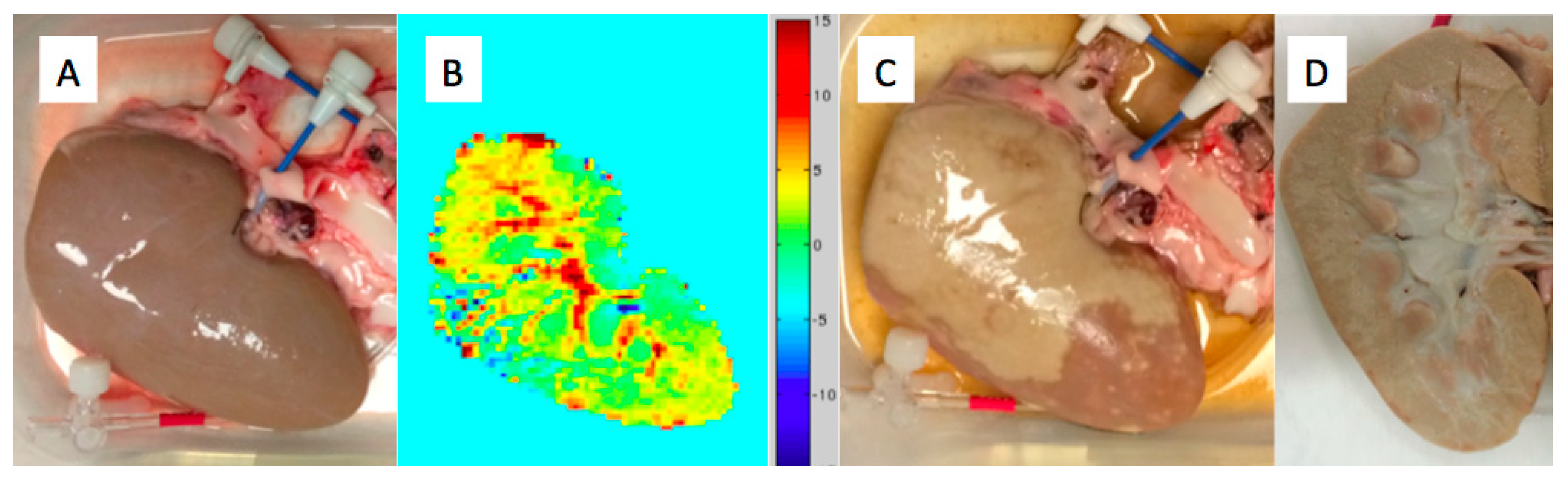
5. Pico-Scale Technology Using Chemical Energy: The Thermoembolic Strategy
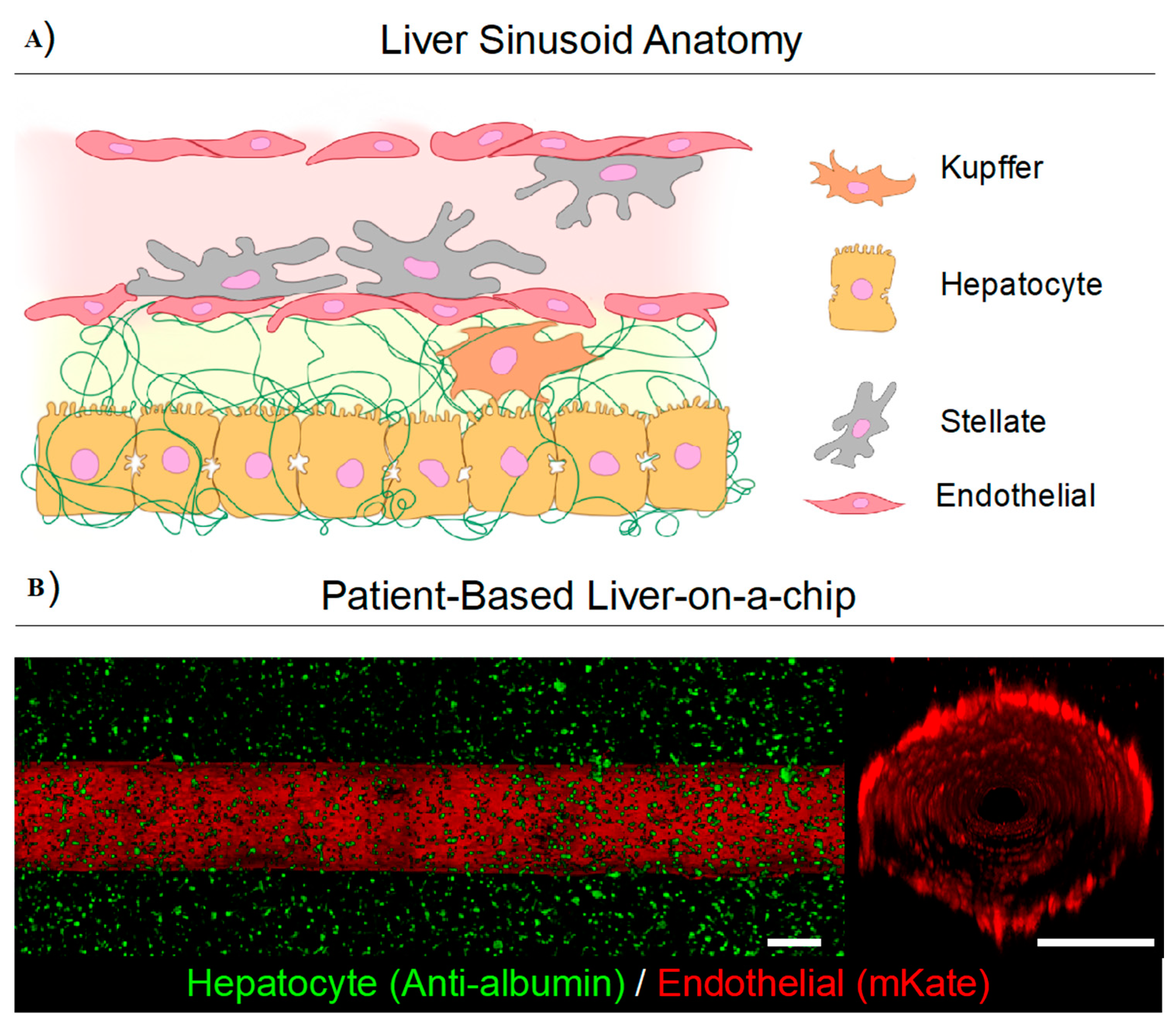
6. Engineering New Micro-Scale Model Systems for the Nano and Pico Scales: Collagen Hydrogels
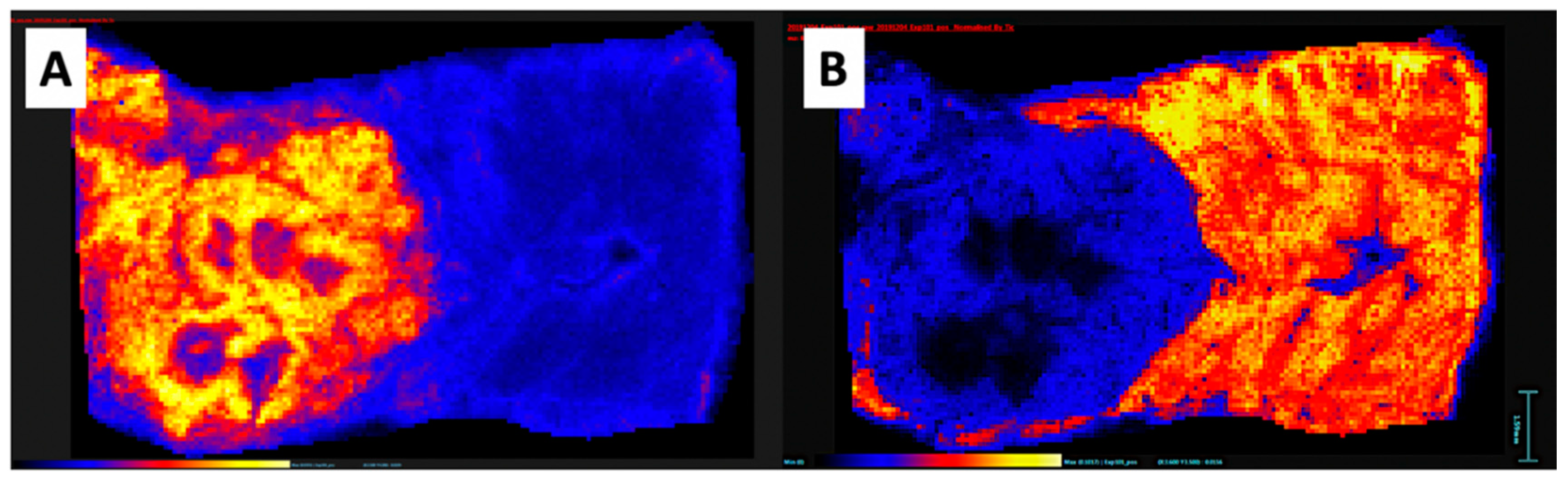
7. Pico-Scale Technology with Mass Spectrometry Imaging: A New Versatile Analytical Method
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Yang, J.D.; Hainaut, P.; Gores, G.J.; Amadou, A.; Plymoth, A.; Roberts, L.R. A global view of hepatocellular carcinoma: Trends, risk, prevention and management. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 589–604. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. Ca Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- NCI. Liver (Hepatocellular) Cancer Screening (PDQ®)–Health Professional Version. Available online: https://www.cancer.gov/types/liver/hp/liver-screening-pdq (accessed on 25 October 2020).
- Forner, A.; Reig, M.; Bruix, J. Hepatocellular carcinoma. Lancet 2018, 391, 1301–1314. [Google Scholar] [CrossRef]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.F.; de Oliveira, A.C.; Santoro, A.; Raoul, J.L.; Forner, A.; et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef]
- Kudo, M.; Finn, R.S.; Qin, S.; Han, K.H.; Ikeda, K.; Piscaglia, F.; Baron, A.; Park, J.W.; Han, G.; Jassem, J.; et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet 2018, 391, 1163–1173. [Google Scholar] [CrossRef]
- Pinato, D.J.; Guerra, N.; Fessas, P.; Murphy, R.; Mineo, T.; Mauri, F.A.; Mukherjee, S.K.; Thursz, M.; Wong, C.N.; Sharma, R.; et al. Immune-based therapies for hepatocellular carcinoma. Oncogene 2020, 39, 3620–3637. [Google Scholar] [CrossRef]
- Yau, T.; Hsu, C.; Kim, T.Y.; Choo, S.P.; Kang, Y.K.; Hou, M.M.; Numata, K.; Yeo, W.; Chopra, A.; Ikeda, M.; et al. Nivolumab in advanced hepatocellular carcinoma: Sorafenib-experienced Asian cohort analysis. J. Hepatol. 2019, 71, 543–552. [Google Scholar] [CrossRef]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef]
- Lee, M.S.; Ryoo, B.Y.; Hsu, C.H.; Numata, K.; Stein, S.; Verret, W.; Hack, S.P.; Spahn, J.; Liu, B.; Abdullah, H.; et al. Atezolizumab with or without bevacizumab in unresectable hepatocellular carcinoma (GO30140): An open-label, multicentre, phase 1b study. Lancet Oncol. 2020, 21, 808–820. [Google Scholar] [CrossRef]
- Marrero, J.A.; Kulik, L.M.; Sirlin, C.B.; Zhu, A.X.; Finn, R.S.; Abecassis, M.M.; Roberts, L.R.; Heimbach, J.K. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018, 68, 723–750. [Google Scholar] [CrossRef]
- Heimbach, J.K.; Kulik, L.M.; Finn, R.S.; Sirlin, C.B.; Abecassis, M.M.; Roberts, L.R.; Zhu, A.X.; Murad, M.H.; Marrero, J.A. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018, 67, 358–380. [Google Scholar] [CrossRef] [PubMed]
- Op den Winkel, M.; Nagel, D.; Op den Winkel, P.; Paprottka, P.M.; Schmidt, L.; Bourhis, H.; Trojan, J.; Goeller, M.; Reiter, F.P.; Stecher, S.S.; et al. The Munich-Transarterial Chemoembolisation Score Holds Superior Prognostic Capacities Compared to TACE-Tailored Modifications of 9 Established Staging Systems for Hepatocellular Carcinoma. Digestion 2019, 100, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Yamada, R.; Sato, M.; Kawabata, M.; Nakatsuka, H.; Nakamura, K.; Takashima, S. Hepatic artery embolization in 120 patients with unresectable hepatoma. Radiology 1983, 148, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Kanematsu, T.; Furuta, T.; Takenaka, K.; Matsumata, T.; Yoshida, Y.; Nishizaki, T.; Hasuo, K.; Sugimachi, K. A 5-year experience of lipiodolization: Selective regional chemotherapy for 200 patients with hepatocellular carcinoma. Hepatology 1989, 10, 98–102. [Google Scholar] [CrossRef]
- Llovet, J.M.; Real, M.I.; Montaña, X.; Planas, R.; Coll, S.; Aponte, J.; Ayuso, C.; Sala, M.; Muchart, J.; Solà, R.; et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: A randomised controlled trial. Lancet 2002, 359, 1734–1739. [Google Scholar] [CrossRef]
- Lo, C.M.; Ngan, H.; Tso, W.K.; Liu, C.L.; Lam, C.M.; Poon, R.T.; Fan, S.T.; Wong, J. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology 2002, 35, 1164–1171. [Google Scholar] [CrossRef]
- Ahmed, O.; Funaki, B. Lack of Technique Standardization Limits Evaluation of Toxicity following Transarterial Chemoembolization. J. Vasc. Interv. Radiol. 2020, 31, 1300–1301. [Google Scholar] [CrossRef]
- Gaba, R.C. Chemoembolization practice patterns and technical methods among interventional radiologists: Results of an online survey. Ajr Am. J. Roentgenol. 2012, 198, 692–699. [Google Scholar] [CrossRef]
- de Baère, T.; Denys, A.; Briquet, R.; Chevallier, P.; Dufaux, J.; Roche, A. Modification of Arterial and Portal Hemodynamics after Injection of Iodized Oils and Different Emulsions of Iodized Oils in the Hepatic Artery: An Experimental Study. J. Vasc. Interv. Radiol. 1998, 9, 305–310. [Google Scholar] [CrossRef]
- de Baere, T.; Arai, Y.; Lencioni, R.; Geschwind, J.F.; Rilling, W.; Salem, R.; Matsui, O.; Soulen, M.C. Treatment of Liver Tumors with Lipiodol TACE: Technical Recommendations from Experts Opinion. Cardiovasc. Intervent. Radiol. 2016, 39, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.T.; Do, R.K.; Gonen, M.; Covey, A.M.; Getrajdman, G.I.; Sofocleous, C.T.; Jarnagin, W.R.; D'Angelica, M.I.; Allen, P.J.; Erinjeri, J.P.; et al. Randomized Trial of Hepatic Artery Embolization for Hepatocellular Carcinoma Using Doxorubicin-Eluting Microspheres Compared With Embolization With Microspheres Alone. J. Clin. Oncol. 2016, 34, 2046–2053. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.J.; Lee, B.C.; Kim, J.K.; Yim, N.Y.; Kim, H.O.; Cho, S.B.; Jeong, Y.Y. Conventional Versus Small Doxorubicin-eluting Bead Transcatheter Arterial Chemoembolization for Treating Barcelona Clinic Liver Cancer Stage 0/A Hepatocellular Carcinoma. Cardiovasc. Intervent. Radiol. 2020, 43, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Marelli, L.; Stigliano, R.; Triantos, C.; Senzolo, M.; Cholongitas, E.; Davies, N.; Tibballs, J.; Meyer, T.; Patch, D.W.; Burroughs, A.K. Transarterial therapy for hepatocellular carcinoma: Which technique is more effective? A systematic review of cohort and randomized studies. Cardiovasc. Intervent. Radiol. 2007, 30, 6–25. [Google Scholar] [CrossRef] [PubMed]
- Gade, T.P.F.; Tucker, E.; Nakazawa, M.S.; Hunt, S.J.; Wong, W.; Krock, B.; Weber, C.N.; Nadolski, G.J.; Clark, T.W.I.; Soulen, M.C.; et al. Ischemia Induces Quiescence and Autophagy Dependence in Hepatocellular Carcinoma. Radiology 2017, 283, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Lencioni, R. mRECIST for HCC: Performance and novel refinements. J. Hepatol. 2020, 72, 288–306. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Pellerin, O.; Bhagat, N.; Rao, P.P.; Loffroy, R.; Ardon, R.; Mory, B.; Reyes, D.K.; Geschwind, J.F. Quantitative and volumetric European Association for the Study of the Liver and Response Evaluation Criteria in Solid Tumors measurements: Feasibility of a semiautomated software method to assess tumor response after transcatheter arterial chemoembolization. J. Vasc. Interv. Radiol. 2012, 23, 1629–1637. [Google Scholar] [CrossRef]
- Zhao, Y.; Duran, R.; Bai, W.; Sahu, S.; Wang, W.; Kabus, S.; Lin, M.; Han, G.; Geschwind, J.F. Which Criteria Applied in Multi-Phasic CT Can Predict Early Tumor Response in Patients with Hepatocellular Carcinoma Treated Using Conventional TACE: RECIST, mRECIST, EASL or qEASL? Cardiovasc. Intervent. Radiol. 2018, 41, 433–442. [Google Scholar] [CrossRef]
- Najmi Varzaneh, F.; Pandey, A.; Aliyari Ghasabeh, M.; Shao, N.; Khoshpouri, P.; Pandey, P.; Zarghampour, M.; Fouladi, D.; Liddell, R.; Anders, R.A.; et al. Prediction of post-TACE necrosis of hepatocellular carcinoma usingvolumetric enhancement on MRI and volumetric oil deposition on CT, with pathological correlation. Eur. Radiol. 2018, 28, 3032–3040. [Google Scholar] [CrossRef]
- Dioguardi Burgio, M.; Ronot, M.; Bruno, O.; Francoz, C.; Paradis, V.; Castera, L.; Durand, F.; Soubrane, O.; Vilgrain, V. Correlation of tumor response on computed tomography with pathological necrosis in hepatocellular carcinoma treated by chemoembolization before liver transplantation. Liver Transpl. 2016, 22, 1491–1500. [Google Scholar] [CrossRef]
- Bargellini, I.; Bozzi, E.; Campani, D.; Carrai, P.; De Simone, P.; Pollina, L.; Cioni, R.; Filipponi, F.; Bartolozzi, C. Modified RECIST to assess tumor response after transarterial chemoembolization of hepatocellular carcinoma: CT-pathologic correlation in 178 liver explants. Eur. J. Radiol. 2013, 82, e212–e218. [Google Scholar] [CrossRef] [PubMed]
- Bittermann, T.; Hoteit, M.A.; Abt, P.L.; Forde, K.A.; Goldberg, D. Waiting time and explant pathology in transplant recipients with hepatocellular carcinoma: A novel study using national data. Am. J. Transplant. 2014, 14, 1657–1663. [Google Scholar] [CrossRef] [PubMed]
- El-Gazzaz, G.; Sourianarayanane, A.; Menon, K.V.N.; Sanabria, J.; Hashimoto, K.; Quintini, C.; Kelly, D.; Eghtesad, B.; Miller, C.; Fung, J.; et al. Radiologic-histological correlation of hepatocellular carcinoma treated via pre-liver transplant locoregional therapies. Hepatobiliary Pancreat. Dis. Int. 2013, 12, 34–41. [Google Scholar] [CrossRef]
- DiNorcia, J.; Florman, S.S.; Haydel, B.; Tabrizian, P.; Ruiz, R.M.; Klintmalm, G.B.; Senguttuvan, S.; Lee, D.D.; Taner, C.B.; Verna, E.C.; et al. Pathologic Response to Pretransplant Locoregional Therapy is Predictive of Patient Outcome After Liver Transplantation for Hepatocellular Carcinoma: Analysis From the US Multicenter HCC Transplant Consortium. Ann. Surg. 2020, 271, 616–624. [Google Scholar] [CrossRef]
- Marin, H.L.; Furth, E.E.; Olthoff, K.; Shaked, A.; Soulen, M.C. Histopathologic outcome of neoadjuvant image-guided therapy of hepatocellular carcinoma. J. Gastrointestin. Liver Dis. 2009, 18, 169–176. [Google Scholar]
- Higuchi, T.; Kikuchi, M.; Okazaki, M. Hepatocellular carcinoma after transcatheter hepatic arterial embolization. A histopathologic study of 84 resected cases. Cancer 1994, 73, 2259–2267. [Google Scholar] [CrossRef]
- Xiao, E.H.; Li, J.Q.; Huang, J.F. Effect of preoperative transcatheter arterial chemoembolization on proliferation of hepatocellular carcinoma cells. World J. Gastroenterol. 2007, 13, 4509–4513. [Google Scholar] [CrossRef]
- Liu, L.; Ren, Z.G.; Shen, Y.; Zhu, X.D.; Zhang, W.; Xiong, W.; Qin, Y.; Tang, Z.Y. Influence of hepatic artery occlusion on tumor growth and metastatic potential in a human orthotopic hepatoma nude mouse model: Relevance of epithelial-mesenchymal transition. Cancer Sci. 2010, 101, 120–128. [Google Scholar] [CrossRef]
- Kok, H.P.; Cressman, E.N.K.; Ceelen, W.; Brace, C.L.; Ivkov, R.; Grull, H.; Ter Haar, G.; Wust, P.; Crezee, J. Heating technology for malignant tumors: A review. Int. J. Hyperth. 2020, 37, 711–741. [Google Scholar] [CrossRef]
- Hildebrandt, B.; Wust, P.; Ahlers, O.; Dieing, A.; Sreenivasa, G.; Kerner, T.; Felix, R.; Riess, H. The cellular and molecular basis of hyperthermia. Crit. Rev. Oncol. Hematol. 2002, 43, 33–56. [Google Scholar] [CrossRef]
- Issels, R.D. Hyperthermia adds to chemotherapy. Eur. J. Cancer 2008, 44, 2546–2554. [Google Scholar] [CrossRef] [PubMed]
- Harada, M.; Yamashita, Y.; Hirai, T.; Yamamoto, H.; Miyazaki, T.; Takahashi, M. Intravascular hyperthermia: Experimental study of transcatheter treatment. Acad. Radiol. 1995, 2, 475–483. [Google Scholar] [CrossRef]
- Cao, W.; Li, J.H.; Feng, D.Y.; Wan, Y.; Liu, Y.F.; Yang, Q.F.; Cheng, J.W.; Zhao, S.Y.; Zhang, H.X. Effect of transarterial pulsed perfusion with heated saline on tumor vascular permeability in a rabbit VX2 liver tumor model. Eur. J. Radiol. 2012, 81, e209–e216. [Google Scholar] [CrossRef]
- Cao, W.; Lu, Q.; Li, J.H.; Zhou, C.X.; Zhu, J.; Wan, Y.; Liu, Y.F. Transcatheter arterial infusion with heated saline changes the vascular permeability of rabbit hepatic tumors. Acad. Radiol. 2011, 18, 1569–1576. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Wan, Y.; Liang, Z.H.; Duan, Y.Y.; Liu, X.; Wang, Z.M.; Liu, Y.Y.; Zhu, J.; Liu, X.T.; Zhang, H.X. Heated lipiodol as an embolization agent for transhepatic arterial embolization in VX2 rabbit liver cancer model. Eur. J. Radiol. 2010, 73, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Xu, X.; Zhang, J.; Duan, Y. Tumor angiogenesis after heated lipiodol infusion via the hepatic artery in a rabbit model of VX2 liver cancer. PLoS ONE 2013, 8, e61583. [Google Scholar] [CrossRef] [PubMed]
- Kozissnik, B.; Bohorquez, A.C.; Dobson, J.; Rinaldi, C. Magnetic fluid hyperthermia: Advances, challenges, and opportunity. Int. J. Hyperth. 2013, 29, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Hensley, D.; Tay, Z.W.; Dhavalikar, R.; Zheng, B.; Goodwill, P.; Rinaldi, C.; Conolly, S. Combining magnetic particle imaging and magnetic fluid hyperthermia in a theranostic platform. Phys. Med. Biol. 2017, 62, 3483–3500. [Google Scholar] [CrossRef]
- Chen, K.H.; Chen, B.C.; Ho, C.Y. Hyperthermia Temperature of Magnetic Fluid with Superparamagnetic Nanoparticles Subjected to an Alternating Magnetic Field. J. Nanosci. Nanotechnol. 2018, 18, 3018–3023. [Google Scholar] [CrossRef]
- Das, P.; Colombo, M.; Prosperi, D. Recent advances in magnetic fluid hyperthermia for cancer therapy. Colloids Surf. B. Biointerfaces 2019, 174, 42–55. [Google Scholar] [CrossRef]
- Kandala, S.K.; Liapi, E.; Whitcomb, L.L.; Attaluri, A.; Ivkov, R. Temperature-controlled power modulation compensates for heterogeneous nanoparticle distributions: A computational optimization analysis for magnetic hyperthermia. Int. J. Hyperth. 2019, 36, 115–129. [Google Scholar] [CrossRef] [PubMed]
- Attaluri, A.; Seshadri, M.; Mirpour, S.; Wabler, M.; Marinho, T.; Furqan, M.; Zhou, H.; De Paoli, S.; Gruettner, C.; Gilson, W.; et al. Image-guided thermal therapy with a dual-contrast magnetic nanoparticle formulation: A feasibility study. Int. J. Hyperth. 2016, 32, 543–557. [Google Scholar] [CrossRef] [PubMed]
- Cressman, E.N.K.; Guo, C.; Karbasian, N. Image-guided chemistry altering biology: An in vivo study of thermoembolization. PLoS ONE 2018, 13, e0200471. [Google Scholar] [CrossRef] [PubMed]
- Devore, J.A.; O'Neal, H.E. Heats of formation of the acetyl halides and of the acetyl radical. J. Phys. Chem. 1969, 73, 2644–2648. [Google Scholar] [CrossRef]
- Carson, A.S.; Skinner, H.A. Carbon-Halogen Bond Energies in the Acetyl Halides. J. Chem. Soc. 1949. [Google Scholar] [CrossRef]
- Liang, H.L.; Yang, C.F.; Pan, H.B.; Lai, K.H.; Cheng, J.S.; Lo, G.H.; Chen, C.K.; Lai, P.H. Small hepatocellular carcinoma: Safety and efficacy of single high-dose percutaneous acetic acid injection for treatment. Radiology 2000, 214, 769–774. [Google Scholar] [CrossRef]
- Ishihara, Y.; Calderon, A.; Watanabe, H.; Okamoto, K.; Suzuki, Y.; Kuroda, K.; Suzuki, Y. A precise and fast temperature mapping using water proton chemical shift. Magn. Reson. Med. 1995, 34, 814–823. [Google Scholar] [CrossRef]
- Fahrenholtz, S.J.; Guo, C.; Maclellan, C.J.; Yung, J.P.; Hwang, K.-P.; Stafford, R.J.; Layman, R.R.; Cressman, E.N.K. Temperature mapping of exothermic in situ chemistry: Imaging of thermoembolization via MR. Int. J. Hyperth. 2019, 36, 730–738. [Google Scholar] [CrossRef]
- Cressman, E.N.K.; Guo, C.X. Feasibility study using tissue as reagent for cancer therapy: Endovascular ablation via thermochemistry. Converg. Sci. Phys. Oncol. 2018, 4, 025003. [Google Scholar] [CrossRef]
- Cressman, E.N.K.; Guo, C. First In Vivo Test of Thermoembolization: Turning Tissue Against Itself Using Transcatheter Chemistry in a Porcine Model. Cardiovasc. Intervent. Radiol. 2018, 41, 1611–1617. [Google Scholar] [CrossRef]
- Anson, M.L.; Mirsky, A.E. The Effect of Denaturation on the Viscosity of Protein Systems. J. Gen. Physiol. 1932, 15, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; De, S. Thermal injury of skin and subcutaneous tissues: A review of experimental approaches and numerical models. Burns 2017, 43, 909–932. [Google Scholar] [CrossRef]
- Chick, H. On the heat coagulation of proteins. J. Physiol. 1910, 40, 404–430. [Google Scholar] [CrossRef] [PubMed]
- Wolf, E.P. Experimental Studies on Inflammation: Ii. Experimental Chemical Inflammation in Vivo. J. Exp. Med. 1923, 37, 511–524. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lewis, P.S. The Kinetics of Protein Denaturation: Part III. The Influence of Neutral Salts on the Velocity of the Heat Denaturation of Oxyhaemoglobin. Biochem. J. 1926, 20, 984–992. [Google Scholar] [CrossRef]
- Nunes, P.; Ernandez, T.; Roth, I.; Qiao, X.; Strebel, D.; Bouley, R.; Charollais, A.; Ramadori, P.; Foti, M.; Meda, P.; et al. Hypertonic stress promotes autophagy and microtubule-dependent autophagosomal clusters. Autophagy 2013, 9, 550–567. [Google Scholar] [CrossRef]
- Aravalli, R.N.; Golzarian, J.; Cressman, E.N. Animal models of cancer in interventional radiology. Eur. Radiol. 2009, 19, 1049–1053. [Google Scholar] [CrossRef]
- Kan, Z.; Ivancev, K.; Lunderquist, A.; McCuskey, P.A.; Wright, K.C.; Wallace, S.; McCuskey, R.S. In vivo microscopy of hepatic tumors in animal models: A dynamic investigation of blood supply to hepatic metastases. Radiology 1993, 187, 621–626. [Google Scholar] [CrossRef]
- Kumagai, K.; Horikawa, M.; Yamada, K.; Uchida, B.T.; Farsad, K. Transtail Artery Access in Rats: A New Technique for Repeatable Selective Angiography. J. Vasc. Interv. Radiol. 2020, 31, 678–681 e674. [Google Scholar] [CrossRef]
- Ju, S.; McLennan, G.; Bennett, S.L.; Liang, Y.; Bonnac, L.; Pankiewicz, K.W.; Jayaram, H.N. Technical aspects of imaging and transfemoral arterial treatment of N1-S1 tumors in rats: An appropriate model to test the biology and therapeutic response to transarterial treatments of liver cancers. J. Vasc. Interv. Radiol. 2009, 20, 410–414. [Google Scholar] [CrossRef]
- Nishiofuku, H.; Cortes, A.C.; Ensor, J.E.; Minhaj, A.A.; Polak, U.; Lopez, M.S.; Kiefer, R.; Hunt, S.J.; Kichikawa, K.; Hicks, M.E.; et al. Factors impacting technical success rate of image-guided intra-arterial therapy in rat orthotopic liver tumor model. Am. J. Transl. Res. 2019, 11, 3761–3770. [Google Scholar] [PubMed]
- Wilkins, L.R.; Stone, J.R.; Mata, J.; Hawrylack, A.; Kubicka, E.; Brautigan, D.L. The Use of the Woodchuck as an Animal Model for Evaluation of Transarterial Embolization. J. Vasc. Interv. Radiol. 2017, 28, 1467–1471. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, W.F.; Woods, D.L.; Esparza-Trujillo, J.A.; Starost, M.F.; Mauda-Havakuk, M.; Mikhail, A.S.; Bakhutashvili, I.; Leonard, S.; Jones, E.C.; Krishnasamy, V.; et al. Transarterial Chemoembolization in a Woodchuck Model of Hepatocellular Carcinoma. J. Vasc. Interv. Radiol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Schachtschneider, K.M.; Schwind, R.M.; Newson, J.; Kinachtchouk, N.; Rizko, M.; Mendoza-Elias, N.; Grippo, P.; Principe, D.R.; Park, A.; Overgaard, N.H.; et al. The Oncopig Cancer Model: An Innovative Large Animal Translational Oncology Platform. Front. Oncol. 2017, 7, 190. [Google Scholar] [CrossRef]
- Bowyer, C.; Lewis, A.L.; Lloyd, A.W.; Phillips, G.J.; Macfarlane, W.M. Hypoxia as a target for drug combination therapy of liver cancer. Anticancer Drugs 2017, 28, 771–780. [Google Scholar] [CrossRef]
- Coriat, R.; Nicco, C.; Chereau, C.; Mir, O.; Alexandre, J.; Ropert, S.; Weill, B.; Chaussade, S.; Goldwasser, F.; Batteux, F. Sorafenib-induced hepatocellular carcinoma cell death depends on reactive oxygen species production in vitro and in vivo. Mol. Cancer Ther. 2012, 11, 2284–2293. [Google Scholar] [CrossRef]
- Ebrahimkhani, M.R.; Neiman, J.A.; Raredon, M.S.; Hughes, D.J.; Griffith, L.G. Bioreactor technologies to support liver function in vitro. Adv. Drug. Deliv. Rev. 2014, 69–70, 132–157. [Google Scholar] [CrossRef]
- Deng, J.; Wei, W.; Chen, Z.; Lin, B.; Zhao, W.; Luo, Y.; Zhang, X. Engineered Liver-on-a-Chip Platform to Mimic Liver Functions and Its Biomedical Applications: A Review. Micromachines 2019, 10, 676. [Google Scholar] [CrossRef]
- Baudoin, R.; Alberto, G.; Paullier, P.; Legallais, C.; Leclerc, E. Parallelized microfluidic biochips in multi well plate applied to liver tissue engineering. Sens. Actuators B Chem. 2012, 173, 919–926. [Google Scholar] [CrossRef]
- Bhatia, S.N.; Ingber, D.E. Microfluidic organs-on-chips. Nat. Biotechnol. 2014, 32, 760–772. [Google Scholar] [CrossRef]
- Jang, K.J.; Otieno, M.A.; Ronxhi, J.; Lim, H.K.; Ewart, L.; Kodella, K.R.; Petropolis, D.B.; Kulkarni, G.; Rubins, J.E.; Conegliano, D.; et al. Reproducing human and cross-species drug toxicities using a Liver-Chip. Sci. Transl. Med. 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Materne, E.M.; Tonevitsky, A.G.; Marx, U. Chip-based liver equivalents for toxicity testing--organotypicalness versus cost-efficient high throughput. Lab A Chip 2013, 13, 3481–3495. [Google Scholar] [CrossRef] [PubMed]
- Ware, B.R.; Khetani, S.R. Engineered Liver Platforms for Different Phases of Drug Development. Trends Biotechnol. 2017, 35, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Ozkan, A.; Ghousifam, N.; Hoopes, P.J.; Yankeelov, T.E.; Rylander, M.N. In vitro vascularized liver and tumor tissue microenvironments on a chip for dynamic determination of nanoparticle transport and toxicity. Biotechnol. Bioeng. 2019, 116, 1201–1219. [Google Scholar] [CrossRef]
- Michna, R.; Gadde, M.; Ozkan, A.; DeWitt, M.; Rylander, M. Vascularized microfluidic platforms to mimic the tumor microenvironment. Biotechnol. Bioeng. 2018, 115, 2793–2806. [Google Scholar] [CrossRef]
- Antoine, E.E.; Vlachos, P.P.; Rylander, M.N. Tunable collagen I hydrogels for engineered physiological tissue micro-environments. PLoS ONE [Electron. Resour.] 2015, 10, e0122500. [Google Scholar] [CrossRef]
- Gadde, M.; Phillips, C.; Ghousifam, N.; Sorace, A.G.; Wong, E.; Krishnamurthy, S.; Syed, A.; Rahal, O.; Yankeelov, T.E.; Woodward, W.A.; et al. In Vitro Vascularized Tumor Platform for Modeling Tumor-Vasculature Interactions of Inflammatory Breast Cancer. Biotechnol. Bioeng. 2020. [Google Scholar] [CrossRef]
- Ozkan, A.; Stolley, D.; Cressman, E.N.K.; McMillin, M.; DeMorrow, S.; Yankeelov, T.E.; Rylander, M.N. The Influence of Chronic Liver Diseases on Hepatic Vasculature: A Liver-on-a-chip Review. Micromachines 2020, 11, 487. [Google Scholar] [CrossRef]
- Herland, A.; Maoz, B.M.; Das, D.; Somayaji, M.R.; Prantil-Baun, R.; Novak, R.; Cronce, M.; Huffstater, T.; Jeanty, S.S.F.; Ingram, M.; et al. Quantitative prediction of human pharmacokinetic responses to drugs via fluidically coupled vascularized organ chips. Nat. Biomed. Eng. 2020, 4, 421–436. [Google Scholar] [CrossRef]
- Chevallay, B.; Herbage, D. Collagen-based biomaterials as 3D scaffold for cell cultures: Applications for tissue engineering and gene therapy. Med. Biol. Eng. Comput. 2000, 38, 211–218. [Google Scholar] [CrossRef]
- Whitney, J.; DeWitt, M.; Whited, B.M.; Carswell, W.; Simon, A.; Rylander, C.G.; Rylander, M.N. 3D viability imaging of tumor phantoms treated with single-walled carbon nanohorns and photothermal therapy. Nanotechnology 2013, 24, 275102. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Whitney, J.R.; Rodgers, A.; Harvie, E.; Carswell, W.F.; Torti, S.; Puretzky, A.A.; Rouleau, C.M.; Geohegan, D.B.; Rylander, C.G.; Rylander, M.N. Spatial and temporal measurements of temperature and cell viability in response to nanoparticle-mediated photothermal therapy. Nanomedicine 2012, 7, 1729–1742. [Google Scholar] [CrossRef] [PubMed]
- Caprioli, R.M.; Farmer, T.B.; Gile, J. Molecular imaging of biological samples: Localization of peptides and proteins using MALDI-TOF MS. Anal. Chem. 1997, 69, 4751–4760. [Google Scholar] [CrossRef] [PubMed]
- Buchberger, A.R.; DeLaney, K.; Johnson, J.; Li, L. Mass Spectrometry Imaging: A Review of Emerging Advancements and Future Insights. Anal. Chem. 2018, 90, 240–265. [Google Scholar] [CrossRef] [PubMed]
- Porta Siegel, T.; Hamm, G.; Bunch, J.; Cappell, J.; Fletcher, J.S.; Schwamborn, K. Mass Spectrometry Imaging and Integration with Other Imaging Modalities for Greater Molecular Understanding of Biological Tissues. Mol. Imaging Biol. 2018. [Google Scholar] [CrossRef]
- Scupakova, K.; Soons, Z.; Ertaylan, G.; Pierzchalski, K.A.; Eijkel, G.B.; Ellis, S.R.; Greve, J.W.; Driessen, A.; Verheij, J.; De Kok, T.M.; et al. Spatial Systems Lipidomics Reveals Nonalcoholic Fatty Liver Disease Heterogeneity. Anal. Chem. 2018, 90, 5130–5138. [Google Scholar] [CrossRef]
- Wattacheril, J.; Seeley, E.H.; Angel, P.; Chen, H.; Bowen, B.P.; Lanciault, C.; Caprioli, R.M.; Abumrad, N.; Flynn, C.R. Differential intrahepatic phospholipid zonation in simple steatosis and nonalcoholic steatohepatitis. PLoS ONE 2013, 8, e57165. [Google Scholar] [CrossRef]
- West, C.A.; Wang, M.; Herrera, H.; Liang, H.; Black, A.; Angel, P.M.; Drake, R.R.; Mehta, A.S. N-Linked Glycan Branching and Fucosylation Are Increased Directly in Hcc Tissue As Determined through in Situ Glycan Imaging. J. Proteome Res. 2018, 17, 3454–3462. [Google Scholar] [CrossRef]
- Abu Sammour, D.; Marsching, C.; Geisel, A.; Erich, K.; Schulz, S.; Ramallo Guevara, C.; Rabe, J.H.; Marx, A.; Findeisen, P.; Hohenberger, P.; et al. Quantitative Mass Spectrometry Imaging Reveals Mutation Status-independent Lack of Imatinib in Liver Metastases of Gastrointestinal Stromal Tumors. Sci. Rep. 2019, 9, 10698. [Google Scholar] [CrossRef]
- Fuchs, K.; Kiss, A.; Bize, P.E.; Duran, R.; Denys, A.; Hopfgartner, G.; Borchard, G.; Jordan, O. Mapping of drug distribution in the rabbit liver tumor model by complementary fluorescence and mass spectrometry imaging. J. Control. Release 2018, 269, 128–135. [Google Scholar] [CrossRef]
- Guo, C.; Baluya, D.L.; Thompson, E.A.; Whitley, E.M.; Cressman, E.N.K. Correlation of molecular and morphologic effects of thermoembolization in a swine model using mass spectrometry imaging. J. Mass Spectrom. 2020, 55, e4477. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stolley, D.L.; Crouch, A.C.; Özkan, A.; Seeley, E.H.; Whitley, E.M.; Rylander, M.N.; Cressman, E.N.K. Combining Chemistry and Engineering for Hepatocellular Carcinoma: Nano-Scale and Smaller Therapies. Pharmaceutics 2020, 12, 1243. https://doi.org/10.3390/pharmaceutics12121243
Stolley DL, Crouch AC, Özkan A, Seeley EH, Whitley EM, Rylander MN, Cressman ENK. Combining Chemistry and Engineering for Hepatocellular Carcinoma: Nano-Scale and Smaller Therapies. Pharmaceutics. 2020; 12(12):1243. https://doi.org/10.3390/pharmaceutics12121243
Chicago/Turabian StyleStolley, Danielle L., Anna Colleen Crouch, Aliçan Özkan, Erin H. Seeley, Elizabeth M. Whitley, Marissa Nichole Rylander, and Erik N. K. Cressman. 2020. "Combining Chemistry and Engineering for Hepatocellular Carcinoma: Nano-Scale and Smaller Therapies" Pharmaceutics 12, no. 12: 1243. https://doi.org/10.3390/pharmaceutics12121243
APA StyleStolley, D. L., Crouch, A. C., Özkan, A., Seeley, E. H., Whitley, E. M., Rylander, M. N., & Cressman, E. N. K. (2020). Combining Chemistry and Engineering for Hepatocellular Carcinoma: Nano-Scale and Smaller Therapies. Pharmaceutics, 12(12), 1243. https://doi.org/10.3390/pharmaceutics12121243