Intranasal Nanoemulsions for Direct Nose-to-Brain Delivery of Actives for CNS Disorders
Abstract
1. Introduction
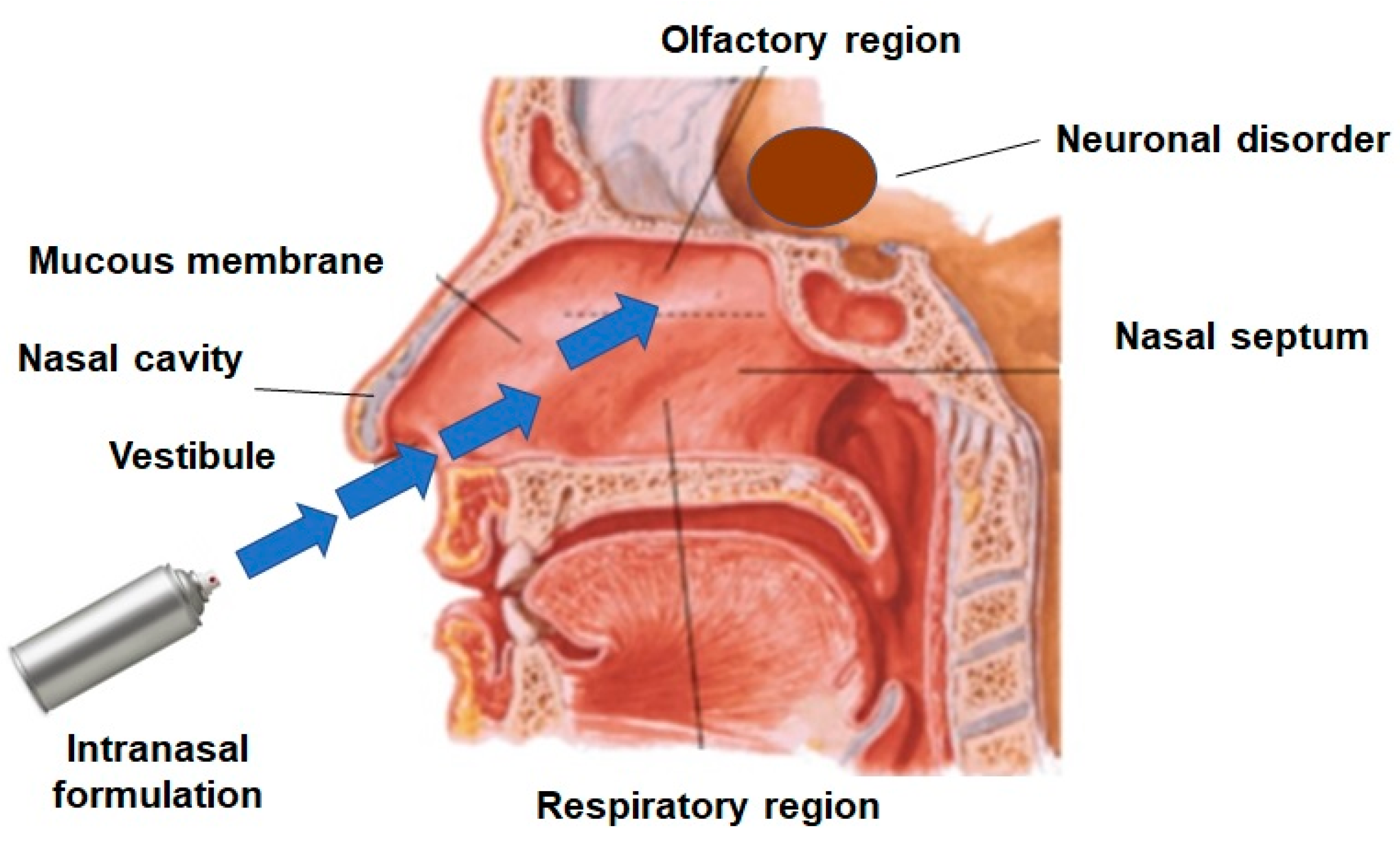
2. Pathways for Brain Delivery through the Intranasal Route
2.1. Olfactory Pathway
2.2. Trigeminal Pathway
2.3. Lymphatic Pathway
2.4. Systemic Pathway
3. Nanoemulsions
3.1. Overview of Nanoemulsion Components
3.1.1. Oils
3.1.2. Surfactants
3.1.3. Cosurfactants
3.2. Significant Factors of Nanoemulsions for Nose-to-Brain Delivery
3.2.1. Globule Size
3.2.2. Zeta Potential
4. Intranasal NEs for Brain Disorders
4.1. NEs for Alzheimer’s Disease
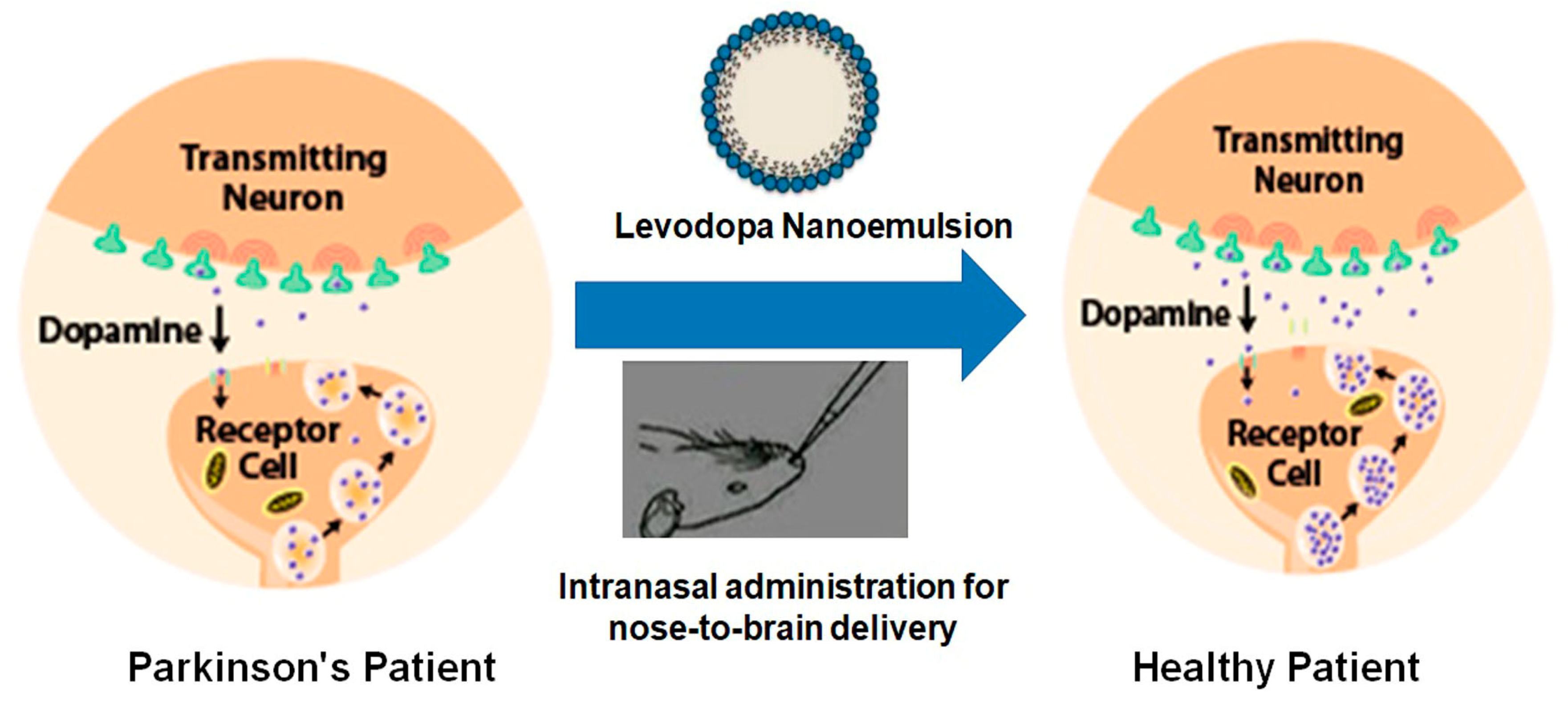
4.2. NE for Parkinson’s Disease
4.3. NE for Migraines
4.4. NE for Epilepsy
4.5. NE for Psychosis
4.6. NE for CNS Infection
4.7. NE for Senile Dementia and Cerebrovascular Spasms
4.8. NE for Depression
4.9. NE for Brain Tumors
4.10. NE for Neuroprotection
4.11. NE for Multiple Sclerosis and Amyotrophic Lateral Sclerosis
4.12. NE for Cerebral Ischemia
5. Current Challenges and Future Prospects
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Martins, P.P.; Smyth, H.D.; Cui, Z. Strategies to facilitate or block nose-to-brain drug delivery. Int. J. Pharm. 2019, 570, 118635. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. The blood-brain barrier: Bottleneck in brain drug development. NeuroRX 2005, 2, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Gadhave, D.; Choudhury, H.; Kokare, C.R. Neutropenia and leukopenia protective intranasal olanzapine-loaded lipid-based nanocarriers engineered for brain delivery. Appl. Nanosci. 2019, 9, 151–168. [Google Scholar] [CrossRef]
- Pardeshi, C.V.; Belgamwar, V.S. Direct nose to brain drug deliveryviaintegrated nerve pathways bypassing the blood–brain barrier: An excellent platform for brain targeting. Expert Opin. Drug Deliv. 2013, 10, 957–972. [Google Scholar] [CrossRef]
- Hada, N.; Netzer, W.J.; Belhassan, F.; Wennogle, L.P.; Gizurarson, S. Nose-to-brain transport of imatinib mesylate: A pharmacokinetic evaluation. Eur. J. Pharm. Sci. 2017, 102, 46–54. [Google Scholar] [CrossRef]
- Bonaccorso, A.; Gigliobianco, M.R.; Pellitteri, R.; Santonocito, D.; Carbone, C.; Di Martino, P.; Puglisi, G.; Musumeci, T. Optimization of Curcumin Nanocrystals as Promising Strategy for Nose-to-Brain Delivery Application. Pharmaceutics 2020, 12, 476. [Google Scholar] [CrossRef]
- Fahmy, U.A.; Badr-Eldin, S.M.; Ahmed, O.A.A.; Aldawsari, H.M.; Tima, S.; Asfour, H.Z.; Al-Rabia, M.W.; Negm, A.A.; Sultan, M.H.; Madkhali, O.A.A.; et al. Intranasal Niosomal In Situ Gel as a Promising Approach for Enhancing Flibanserin Bioavailability and Brain Delivery: In Vitro Optimization and Ex Vivo/In Vivo Evaluation. Pharmaceutics 2020, 12, 485. [Google Scholar] [CrossRef]
- Bourganis, V.; Kammona, O.; Alexopoulos, A.; Kiparissides, C. Recent advances in carrier mediated nose-to-brain delivery of pharmaceutics. Eur. J. Pharm. Biopharm. 2018, 128, 337–362. [Google Scholar] [CrossRef]
- Shingaki, T.; Hidalgo, I.J.; Furubayashi, T.; Sakane, T.; Katsumi, H.; Yamamoto, A.; Yamashita, S. Nasal delivery of P-gp substrates to the brain through the nose-brain pathway. Drug Metab. Pharmacokinet. 2011, 26, 248–255. [Google Scholar] [CrossRef]
- Choudhury, H.; Gorain, B.; Chatterjee, B.; Mandal, U.K.; Sengupta, P.; Tekade, R.K. Pharmacokinetic and Pharmacodynamic Features of Nanoemulsion Following Oral, Intravenous, Topical and Nasal Route. Curr. Pharm. Des. 2017, 23, 2504–2531. [Google Scholar] [CrossRef]
- Illum, L. Transport of drugs from the nasal cavity to the central nervous system. Eur. J. Pharm. Sci. 2000, 11, 1–18. [Google Scholar] [CrossRef]
- Illum, L. Is nose-to-brain transport of drugs in man a reality? J. Pharm. Pharmacol. 2004, 56, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Bonferoni, M.C.; Rossi, S.; Sandri, G.; Ferrari, F.; Gavini, E.; Rassu, G.; Giunchedi, P. Nanoemulsions for “Nose-to-Brain” Drug Delivery. Pharmaceutics 2019, 11, 84. [Google Scholar] [CrossRef] [PubMed]
- Rassu, G.; Soddu, E.; Cossu, M.; Brundu, A.; Cerri, G.; Marchetti, N.; Ferraro, L.; Regan, R.F.; Giunchedi, P.; Gavini, E.; et al. Solid microparticles based on chitosan or methyl-β-cyclodextrin: A first formulative approach to increase the nose-to-brain transport of deferoxamine mesylate. J. Control. Release 2015, 201, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Mistry, A.; Stolnik, S.; Illum, L. Nose-to-Brain Delivery: Investigation of the Transport of Nanoparticles with Different Surface Characteristics and Sizes in Excised Porcine Olfactory Epithelium. Mol. Pharm. 2015, 12, 2755–2766. [Google Scholar] [CrossRef] [PubMed]
- Quintana, D.S.; Guastella, A.J.; Westlye, L.T.; Andreassen, O. The promise and pitfalls of intranasally administering psychopharmacological agents for the treatment of psychiatric disorders. Mol. Psychiatry 2015, 21, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Hu, K.; Jiang, X. From nose to brain: Understanding transport capacity and transport rate of drugs. Expert Opin. Drug Deliv. 2008, 5, 1159–1168. [Google Scholar] [CrossRef]
- Agrawal, M.; Saraf, S.; Saraf, S.; Antimisiaris, S.G.; Chougule, M.B.; Shoyele, S.A.; Alexander, A. Nose-to-brain drug delivery: An update on clinical challenges and progress towards approval of anti-Alzheimer drugs. J. Control. Release 2018, 281, 139–177. [Google Scholar] [CrossRef]
- Bors, L.A.; Bajza, Á.; Mándoki, M.; Tasi, B.J.; Cserey, G.; Imre, T.; Szabó, P.; Erdő, F. Modulation of nose-to-brain delivery of a P-glycoprotein (MDR1) substrate model drug (quinidine) in rats. Brain Res. Bull. 2020, 160, 65–73. [Google Scholar] [CrossRef]
- Mittal, D.; Ali, A.; Md, S.; Baboota, S.; Sahni, J.K.; Ali, J. Insights into direct nose to brain delivery: Current status and future perspective. Drug Deliv. 2013, 21, 75–86. [Google Scholar] [CrossRef]
- Casettari, L.; Illum, L. Chitosan in nasal delivery systems for therapeutic drugs. J. Control. Release 2014, 190, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Kesarla, R.; Omri, A. Approaches for CNS delivery of drugs—Nose to brain targeting of antiretroviral agents as a potential attempt for complete elimination of major reservoir site of HIV to aid AIDS treatment. Expert Opin. Drug Deliv. 2019, 16, 287–300. [Google Scholar] [CrossRef] [PubMed]
- Ruigrok, M.J.; De Lange, E.C. Emerging Insights for Translational Pharmacokinetic and Pharmacokinetic-Pharmacodynamic Studies: Towards Prediction of Nose-to-Brain Transport in Humans. AAPS J. 2015, 17, 493–505. [Google Scholar] [CrossRef] [PubMed]
- Crowe, T.P.; Greenlee, M.H.W.; Kanthasamy, A.G.; Hsu, W.H. Mechanism of intranasal drug delivery directly to the brain. Life Sci. 2018, 195, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Samaridou, E.; Alonso, M.J. Nose-to-brain peptide delivery – The potential of nanotechnology. Bioorg. Med. Chem. 2018, 26, 2888–2905. [Google Scholar] [CrossRef]
- Lochhead, J.J.; Thorne, R.G. Intranasal delivery of biologics to the central nervous system. Adv. Drug Deliv. Rev. 2012, 64, 614–628. [Google Scholar] [CrossRef]
- Mistry, A.; Stolnik, S.; Illum, L. Nanoparticles for direct nose-to-brain delivery of drugs. Int. J. Pharm. 2009, 379, 146–157. [Google Scholar] [CrossRef]
- Feng, Y.; He, H.; Li, F.; Lu, Y.; Qi, J.; Wu, W. An update on the role of nanovehicles in nose-to-brain drug delivery. Drug Discov. Today 2018, 23, 1079–1088. [Google Scholar] [CrossRef]
- Kozlovskaya, L.; Abou-Kaoud, M.; Stepensky, D. Quantitative analysis of drug delivery to the brain via nasal route. J. Control. Release 2014, 189, 133–140. [Google Scholar] [CrossRef]
- Sessa, M.; Balestrieri, M.L.; Ferrari, G.; Servillo, L.; Castaldo, D.; D’Onofrio, N.; Donsì, F.; Tsao, R. Bioavailability of encapsulated resveratrol into nanoemulsion-based delivery systems. Food Chem. 2014, 147, 42–50. [Google Scholar] [CrossRef]
- Savale, S.; Mahajan, H. Nose to brain: A versatile mode of drug delivery system. Asian J. Biomater. Res. 2017, 3, 16–38. [Google Scholar]
- Pandey, M.; Choudhury, H.; Yeun, O.C.; Yin, H.M.; Lynn, T.W.; Tine, C.L.; Wi, N.S.; Yen, K.C.; Phing, C.S.; Kesharwani, P.; et al. Perspectives of Nanoemulsion Strategies in The Improvement of Oral, Parenteral and Transdermal Chemotherapy. Curr. Pharm. Biotechnol. 2018, 19, 276–292. [Google Scholar] [CrossRef] [PubMed]
- Sood, S.; Jain, K.; Kuppusamy, G. Optimization of curcumin nanoemulsion for intranasal delivery using design of experiment and its toxicity assessment. Colloids Surf. B Biointerfaces 2014, 113, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, H.; Gorain, B.; Karmakar, S.; Biswas, E.; Dey, G.; Barik, R.; Mandal, M.; Pal, T.K. Improvement of cellular uptake, in vitro antitumor activity and sustained release profile with increased bioavailability from a nanoemulsion platform. Int. J. Pharm. 2014, 460, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, C.B.; Soni, U.; Patravale, V. Nano-interventions for neurodegenerative disorders. Pharmacol. Res. 2010, 62, 166–178. [Google Scholar] [CrossRef] [PubMed]
- Edmond, J. Essential Polyunsaturated Fatty Acids and the Barrier to the Brain: The Components of a Model for Transport. J. Mol. Neurosci. 2001, 16, 181–194. [Google Scholar] [CrossRef]
- Khunt, D.; Shah, B.; Misra, M. Role of butter oil in brain targeted delivery of Quetiapine fumarate microemulsion via intranasal route. J. Drug Deliv. Sci. Technol. 2017, 40, 11–20. [Google Scholar] [CrossRef]
- Hosny, K.M.; Banjar, Z.M. The formulation of a nasal nanoemulsion zaleplonin situgel for the treatment of insomnia. Expert Opin. Drug Deliv. 2013, 10, 1033–1041. [Google Scholar] [CrossRef]
- Lin, H.; Gebhardt, M.; Bian, S.; Kwon, K.A.; Shim, C.-K.; Chung, S.-J.; Kim, D.-D. Enhancing effect of surfactants on fexofenadine·HCl transport across the human nasal epithelial cell monolayer. Int. J. Pharm. 2007, 330, 23–31. [Google Scholar] [CrossRef]
- Chatterjee, B.; Gorain, B.; Mohananaidu, K.; Sengupta, P.; Mandal, U.K.; Choudhury, H. Targeted drug delivery to the brain via intranasal nanoemulsion: Available proof of concept and existing challenges. Int. J. Pharm. 2019, 565, 258–268. [Google Scholar] [CrossRef]
- Sood, S.; Jain, K.; Gowthamarajan, K. Intranasal therapeutic strategies for management of Alzheimer’s disease. J. Drug Target. 2014, 22, 279–294. [Google Scholar] [CrossRef] [PubMed]
- Azeem, A.; Rizwan, M.; Ahmad, F.J.; Iqbal, Z.; Khar, R.K.; Aqil, M.; Talegaonkar, S. Nanoemulsion Components Screening and Selection: A Technical Note. AAPS PharmSciTech 2009, 10, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Morrison, E.E.; Costanzo, R.M. Morphology of olfactory epithelium in humans and other vertebrates. Microsc. Res. Tech. 1992, 23, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Ahmad, R.; Naqvi, A.A.; Alam, A.; Ashafaq, M.; Rub, R.A.; Ahmad, F.J. Intranasal delivery of quercetin-loaded mucoadhesive nanoemulsion for treatment of cerebral ischaemia. Artif. Cells Nanomed. Biotechnol. 2017, 46, 717–729. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, E.; Feng, Y.; Qi, J.; Fan, W.; Ma, Y.; He, H.; Xia, F.; Dong, X.; Zhao, W.; Lu, Y.; et al. Evidence of nose-to-brain delivery of nanoemulsions: Cargoes but not vehicles. Nanoscale 2017, 9, 1174–1183. [Google Scholar] [CrossRef] [PubMed]
- Win, T.; Rajagopal, J.; Manda, U.K.; Sengupta, P.; Chatterjee, B. Incorporation of carbopol to palm olein based analgesic cream: Effect on formulation characteristics. Lat. Am. J. Pharm. 2017, 36, 2144–2152. [Google Scholar]
- Espinoza, L.C.; Silva-Abreu, M.; Clares, B.; Rodríguez-Lagunas, M.J.; Halbaut-Bellowa, L.; Cañas, M.-A.; Calpena, A.C. Formulation Strategies to Improve Nose-to-Brain Delivery of Donepezil. Pharmaceutics 2019, 11, 64. [Google Scholar] [CrossRef]
- Haider, F.; Khan, S.; Gaba, B.; Alam, T.; Baboota, S.; Ali, J.; Ali, A. Optimization of rivastigmine nanoemulsion for enhanced brain delivery: In-vivo and toxicity evaluation. J. Mol. Liq. 2018, 255, 384–396. [Google Scholar] [CrossRef]
- Nasr, M. Development of an optimized hyaluronic acid-based lipidic nanoemulsion co-encapsulating two polyphenols for nose to brain delivery. Drug Deliv. 2016, 23, 1444–1452. [Google Scholar] [CrossRef]
- Kumar, S.; Ali, J.; Baboota, S. Design Expert® supported optimization and predictive analysis of selegiline nanoemulsion via the olfactory region with enhanced behavioural performance in Parkinson’s disease. Nanotechnology 2016, 27, 435101. [Google Scholar] [CrossRef]
- Iqbal, R.; Ahmed, S.; Jain, G.K.; Vohora, D. Design and development of letrozole nanoemulsion: A comparative evaluation of brain targeted nanoemulsion with free letrozole against status epilepticus and neurodegeneration in mice. Int. J. Pharm. 2019, 565, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Ahmad, R.; Alam, A.; Ahmad, F.J.; Amir, M. Impact of ultrasonication techniques on the preparation of novel Amiloride-nanoemulsion used for intranasal delivery in the treatment of epilepsy. Artif. Cells Nanomed. Biotechnol. 2018, 46, S192–S207. [Google Scholar] [CrossRef] [PubMed]
- Abdou, E.M.; Kandil, S.M.; El Miniawy, H.M. Brain targeting efficiency of antimigrain drug loaded mucoadhesive intranasal nanoemulsion. Int. J. Pharm. 2017, 529, 667–677. [Google Scholar] [CrossRef] [PubMed]
- Bhanushali, R.S.; Gatne, M.M.; Gaikwad, R.V.; Bajaj, A.N.; Morde, M.A. Nanoemulsion based Intranasal Delivery of Antimigraine Drugs for Nose to Brain Targeting. Indian J. Pharm. Sci. 2009, 71, 707–709. [Google Scholar]
- Yadav, S.; Gattacceca, F.; Panicucci, R.; Amiji, M. Comparative Biodistribution and Pharmacokinetic Analysis of Cyclosporine-A in the Brain upon Intranasal or Intravenous Administration in an Oil-in-Water Nanoemulsion Formulation. Mol. Pharm. 2015, 12, 1523–1533. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Melchiades, G.D.L.; Figueiró, F.; Battastini, A.M.O.; Teixeira, H.F.; Koester, L.S. Validation of an HPLC-UV method for analysis of Kaempferol-loaded nanoemulsion and its application to in vitro and in vivo tests. J. Pharm. Biomed. Anal. 2017, 145, 831–837. [Google Scholar] [CrossRef] [PubMed]
- Bahadur, S.; Pathak, K. Buffered nanoemulsion for nose to brain delivery of ziprasidone hydrochloride: Preformulation and pharmacodynamic evaluation. Curr. Drug Deliv. 2012, 9, 596–607. [Google Scholar] [CrossRef]
- Boche, M.; Pokharkar, V.B. Quetiapine Nanoemulsion for Intranasal Drug Delivery: Evaluation of Brain-Targeting Efficiency. AAPS PharmSciTech 2016, 18, 686–696. [Google Scholar] [CrossRef]
- Mehta, M.; Adem, A.; Sabbagh, M.N. New Acetylcholinesterase Inhibitors for Alzheimer’s Disease. Int. J. Alzheimer’s Dis. 2012, 2012, 728983. [Google Scholar] [CrossRef]
- Arias, J.L. Nanotechnology and Drug Delivery: Volume 1: Nanoplatforms in Drug Delivery; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Surve, D.H.; Jindal, A.B. Recent advances in long-acting nanoformulations for delivery of antiretroviral drugs. J. Control. Release 2020, 324, 379–404. [Google Scholar] [CrossRef]
- Eqian, Z.M.; Ke, Y. Huperzine A: Is it an Effective Disease-Modifying Drug for Alzheimer’s Disease? Front. Aging Neurosci. 2014, 6, 216. [Google Scholar] [CrossRef]
- Zhang, H.-Y. New insights into huperzine A for the treatment of Alzheimer’s disease. Acta Pharmacol. Sin. 2012, 33, 1170–1175. [Google Scholar] [CrossRef] [PubMed]
- Rascol, O.; Brooks, D.J.; Korczyn, A.D.; De Deyn, P.P.; Clarke, C.E.; Lang, A.E. A Five-Year Study of the Incidence of Dyskinesia in Patients with Early Parkinson’s Disease Who Were Treated with Ropinirole or Levodopa. N. Engl. J. Med. 2000, 342, 1484–1491. [Google Scholar] [CrossRef] [PubMed]
- Smith, Y.; Wichmann, T.; Factor, S.; Delong, M.R. Parkinson’s Disease Therapeutics: New Developments and Challenges Since the Introduction of Levodopa. Neuropsychopharmacol. 2012, 37, 213–246. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, C.; Zhai, W.; Zhuang, N.; Han, T.; Ding, Z. The Optimization Design of Lactoferrin Loaded HupA Nanoemulsion for Targeted Drug Transport Via Intranasal Route. Int. J. Nanomed. 2019, 14, 9217–9234. [Google Scholar] [CrossRef]
- Singh, D.; Kapahi, H.; Rashid, M.; Prakash, A.; Majeed, A.B.A.; Mishra, N. Recent prospective of surface engineered Nanoparticles in the management of Neurodegenerative disorders. Artif. Cells Nanomed. Biotechnol. 2015, 44, 1–12. [Google Scholar] [CrossRef]
- Abbott, A. Levodopa: The story so far. Nat. Cell Biol. 2010, 466, S6–S7. [Google Scholar] [CrossRef]
- Zainol, S.; Bin Basri, H.; Bin Basri, H.; Shamsuddin, A.F.; Abdul-Gani, S.S.; Karjiban, R.A.; Abdul-Malek, E. Formulation Optimization of a Palm-Based Nanoemulsion System Containing Levodopa. Int. J. Mol. Sci. 2012, 13, 13049–13064. [Google Scholar] [CrossRef]
- Pangeni, R.; Sharma, S.; Mustafa, G.; Ali, J.; Baboota, S. Vitamin E loaded resveratrol nanoemulsion for brain targeting for the treatment of Parkinson’s disease by reducing oxidative stress. Nanotechnology 2014, 25, 485102. [Google Scholar] [CrossRef]
- Gaba, B.; Khan, T.; Haider, F.; Alam, T.; Baboota, S.; Parvez, S.; Ali, J. Vitamin E Loaded Naringenin Nanoemulsion via Intranasal Delivery for the Management of Oxidative Stress in a 6-OHDA Parkinson’s Disease Model. BioMed Res. Int. 2019, 2019, 2382563. [Google Scholar] [CrossRef]
- Mustafa, G.; Baboota, S.; Ahuja, A.; Ali, J. Formulation Development of Chitosan Coated Intra Nasal Ropinirole Nanoemulsion for Better Management Option of Parkinson: An In Vitro Ex Vivo Evaluation. Curr. Nanosci. 2012, 8, 348–360. [Google Scholar] [CrossRef]
- Sahni, J.K.; Doggui, S.; Ali, J.; Baboota, S.; Dao, L.; Ramassamy, C. Neurotherapeutic applications of nanoparticles in Alzheimer’s disease. J. Control. Release 2011, 152, 208–231. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Bishnoi, R.S.; Shukla, A.K.; Jain, C.P. Techniques for Formulation of Nanoemulsion Drug Delivery System: A Review. Prev. Nutr. Food Sci. 2019, 24, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.-Y.; Hung, C.-F.; Chi, C.-H.; Chen, C.-C. Transdermal permeation of selegiline from hydrogel-membrane drug delivery systems. Int. J. Pharm. 2009, 380, 33–39. [Google Scholar] [CrossRef]
- Mustafa, G.; Ahuja, A.; Al Rohaimi, A.H.; Muslim, S.; Hassan, A.; Baboota, S.; Ali, J. Nano-ropinirole for the management of Parkinsonism: Blood–brain pharmacokinetics and carrier localization. Expert Rev. Neurother. 2015, 15, 695–710. [Google Scholar] [CrossRef]
- Lipton, R.B.; Bigal, M.; Diamond, M.; Freitag, F.; Reed, M.L.; Stewart, W.F.; on behalf of the AMPP Advisory Group. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology 2007, 68, 343–349. [Google Scholar] [CrossRef]
- Newman, L. Expert commentary. Headache J. Head Face Pain 2013, 53, 1502–1503. [Google Scholar] [CrossRef]
- Tepper, S.J.; Cady, R.; Silberstein, S.D.; Messina, J.; Mahmoud, R.A.; Djupesland, P.G.; Shin, P.; Siffert, J. AVP-825 Breath-Powered Intranasal Delivery System Containing 22 mg Sumatriptan Powder vs 100 mg Oral Sumatriptan in the Acute Treatment of Migraines (The COMPASS Study): A Comparative Randomized Clinical Trial Across Multiple Attacks. Headache J. Head Face Pain 2015, 55, 621–635. [Google Scholar] [CrossRef]
- Brodie, M.J.; Kwan, P. Current position of phenobarbital in epilepsy and its future. Epilepsia 2012, 53, 40–46. [Google Scholar] [CrossRef]
- Kokel, D.; Peterson, R.T. Chemobehavioural phenomics and behaviour-based psychiatric drug discovery in the zebrafish. Brief. Funct. Genom. Proteom. 2008, 7, 483–490. [Google Scholar] [CrossRef]
- Brodie, M.J. Do we need any more new antiepileptic drugs? Epilepsy Res. 2001, 45, 3–6. [Google Scholar] [CrossRef]
- Rivers, F.; O’Brien, T.J.; Callaghan, R. Exploring the possible interaction between anti-epilepsy drugs and multidrug efflux pumps; in vitro observations. Eur. J. Pharmacol. 2008, 598, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Patsalos, P.N. Properties of Antiepileptic Drugs in the Treatment of Idiopathic Generalized Epilepsies. Epilepsia 2005, 46, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Löscher, W.; Potschka, H. Drug resistance in brain diseases and the role of drug efflux transporters. Nat. Rev. Neurosci. 2005, 6, 591–602. [Google Scholar] [CrossRef]
- Barcia, J.A.; Gallego, J.M. Intraventricular and intracerebral delivery of anti-epileptic drugs in the kindling model. Neurotherapeutics 2009, 6, 337–343. [Google Scholar] [CrossRef]
- Abdel-Bar, H.M.; Abdel-Reheem, A.Y.; Awad, G.A.S.; Mortada, N. Evaluation of Brain Targeting and Mucosal Integrity of Nasally Administrated Nanostructured Carriers of a CNS Active Drug, Clonazepam. J. Pharm. Pharm. Sci. 2013, 16, 456–469. [Google Scholar] [CrossRef]
- Wu, X.-Y.; Liu, J.-S.; Shen, T.; Wang, J.-H.; Zhou, J.; Tang, X.-H.; Xu, L.; Hong, Z. Enhanced brain delivery of lamotrigine with Pluronic® P123-based nanocarrier. Int. J. Nanomed. 2014, 9, 3923–3935. [Google Scholar] [CrossRef]
- Wilson, B.; Lavanya, Y.; Priyadarshini, S.; Ramasamy, M.; Jenita, J.L. Albumin nanoparticles for the delivery of gabapentin: Preparation, characterization and pharmacodynamic studies. Int. J. Pharm. 2014, 473, 73–79. [Google Scholar] [CrossRef]
- Jain, N.; Akhter, S.; Jain, G.; Khan, Z.; Khar, R.; Ahmad, F. Antiepileptic Intranasal Amiloride Loaded Mucoadhesive Nanoemulsion: Development and Safety Assessment. J. Biomed. Nanotechnol. 2011, 7, 142–143. [Google Scholar] [CrossRef]
- Kumar, M.; Pathak, K.; Misra, A. Formulation and Characterization of Nanoemulsion-Based Drug Delivery System of Risperidone. Drug Dev. Ind. Pharm. 2009, 35, 387–395. [Google Scholar] [CrossRef]
- Kumar, M.; Misra, A.; Babbar, A.; Mishra, A.; Mishra, P.; Pathak, K. Intranasal nanoemulsion based brain targeting drug delivery system of risperidone. Int. J. Pharm. 2008, 358, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Misra, A.; Pathak, K. Formulation and characterization of nanoemulsion of olanzapine for intranasal delivery. PDA J. Pharm. Sci. Technol. 2010, 63, 501–511. [Google Scholar]
- Kumar, M.; Misra, A.; Mishra, A.K.; Mishra, P.; Pathak, K. Mucoadhesive nanoemulsion-based intranasal drug delivery system of olanzapine for brain targeting. J. Drug Target. 2008, 16, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Parikh, V.; Tucci, V.; Galwankar, S. Infections of the nervous system. Int. J. Crit. Illn. Inj. Sci. 2012, 2, 82–97. [Google Scholar] [CrossRef]
- DeMarino, C.; Schwab, A.; Pleet, M.; Mathiesen, A.; Friedman, J.; El-Hage, N.; Kashanchi, F. Biodegradable Nanoparticles for Delivery of Therapeutics in CNS Infection. J. Neuroimmune Pharmacol. 2016, 12, 31–50. [Google Scholar] [CrossRef]
- Mahajan, H.; Mahajan, M.S.; Nerkar, P.P.; Agrawal, A. Nanoemulsion-based intranasal drug delivery system of saquinavir mesylate for brain targeting. Drug Deliv. 2014, 21, 148–154. [Google Scholar] [CrossRef]
- Prabhakar, K.; Afzal, S.M.; Surender, G.; Kishan, V. Tween 80 containing lipid nanoemulsions for delivery of indinavir to brain. Acta Pharm. Sin. B 2013, 3, 345–353. [Google Scholar] [CrossRef]
- Musa, S.H.; Basri, M.; Masoumi, H.R.F.; Karjiban, R.A.; Malek, E.A.; Bin Basri, H.; Shamsuddin, A.F. Formulation optimization of palm kernel oil esters nanoemulsion-loaded with chloramphenicol suitable for meningitis treatment. Colloids Surf. B Biointerfaces 2013, 112, 113–119. [Google Scholar] [CrossRef]
- Scott, K.R.; Barrett, A.M. Dementia syndromes: Evaluation and treatment. Expert Rev. Neurother. 2007, 7, 407–422. [Google Scholar] [CrossRef]
- Birks, J.; Arrieta, J.M.L.; López-Arrieta, J.M. Nimodipine for primary degenerative, mixed and vascular dementia. Cochrane Database Syst. Rev. 2002, 2002, CD000147. [Google Scholar] [CrossRef]
- Pathak, R.; Dash, R.P.; Misra, M.; Nivsarkar, M. Role of mucoadhesive polymers in enhancing delivery of nimodipine microemulsion to brain via intranasal route. Acta Pharm. Sin. B 2014, 4, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Treatments in depression. Dialog-Clin. Neurosci. 2006, 8, 191–206. [CrossRef]
- Bahr, R.; Lopez, A.; Rey, J. Intranasal Esketamine (SpravatoTM) for Use in Treatment-Resistant Depression in Conjunction with an Oral Antidepressant. Pharm. Ther. 2019, 44, 340–375. [Google Scholar]
- Pandey, Y.R.; Kumar, S.; Gupta, B.K.; Ali, J.; Baboota, S. Intranasal delivery of paroxetine nanoemulsion via the olfactory region for the management of depression: Formulation, behavioural and biochemical estimation. Nanotechnology 2015, 27, 25102. [Google Scholar] [CrossRef]
- Mishra, D.K.; Kumar, A.; Raj, R.; Chaturvedi, A. Capmul MCM based nanoemulsion for intranasal delivery of an antidepressant. Bull. Pharm. Res. 2013, 3, 34–39. [Google Scholar]
- Groothuis, D.R. The blood-brain and blood-tumor barriers: A review of strategies for increasing drug delivery. Neuro Oncol. 2000, 2, 45–59. [Google Scholar] [CrossRef]
- Dobrovoljac, M.; Hengartner, H.; Boltshauser, E.; Grotzer, M. Delay in the diagnosis of paediatric brain tumours. Eur. J. Nucl. Med. Mol. Imaging 2002, 161, 663–667. [Google Scholar] [CrossRef]
- Basics of Brain Tumors. Available online: https://www.hopkinsmedicine.org/health/conditions-and-diseases/basics-of-brain-tumors (accessed on 17 December 2020).
- Harter, D.H.; Wilson, T.A.; Karajannis, M.A. Glioblastoma multiforme: State of the art and future therapeutics. Surg. Neurol. Int. 2014, 5, 64. [Google Scholar] [CrossRef]
- Azambuja, J.H.; Schuh, R.S.; Michels, L.R.; Gelsleichter, N.E.; Beckenkamp, L.R.; Iser, I.C.; Lenz, G.S.; De Oliveira, F.H.; Venturin, G.; Greggio, S.; et al. Nasal Administration of Cationic Nanoemulsions as CD73-siRNA Delivery System for Glioblastoma Treatment: A New Therapeutical Approach. Mol. Neurobiol. 2019, 57, 635–649. [Google Scholar] [CrossRef]
- Colombo, M.; Figueiró, F.; Dias, A.D.F.; Teixeira, H.F.; Battastini, A.M.O.; Koester, L.S. Kaempferol-loaded mucoadhesive nanoemulsion for intranasal administration reduces glioma growth in vitro. Int. J. Pharm. 2018, 543, 214–223. [Google Scholar] [CrossRef]
- Khan, A.; Aqil, M.; Imam, S.S.; Ahad, A.; Sultana, Y.; Ali, A.; Khan, K. Temozolomide loaded nano lipid based chitosan hydrogel for nose to brain delivery: Characterization, nasal absorption, histopathology and cell line study. Int. J. Biol. Macromol. 2018, 116, 1260–1267. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.; Vyas, T.; Amiji, M. Cytotoxicity and Apoptosis Enhancement in Brain Tumor Cells upon Coadministration of Paclitaxel and Ceramide in Nanoemulsion Formulations. J. Pharm. Sci. 2008, 97, 2745–2756. [Google Scholar] [CrossRef]
- Nachiappan, K. Targeted drug delivery system of 5-Fluorouracil with recombinant epidermal growth factor for brain tumor. J. Pharm. Care Health Syst. 2017, 4, 27. [Google Scholar]
- Choi, K.Y.; Liu, G.; Lee, S.; Chen, X. Theranostic nanoplatforms for simultaneous cancer imaging and therapy: Current approaches and future perspectives. Nanoscale 2011, 4, 330–342. [Google Scholar] [CrossRef]
- Primo, F.L.; Rodrigues, M.M.A.; Simioni, A.R.; Lacava, Z.G.M.; Morais, P.C.; Tedesco, A.C. Photosensitizer-Loaded Magnetic Nanoemulsion for Use in Synergic Photodynamic and Magnetohyperthermia Therapies of Neoplastic Cells. J. Nanosci. Nanotechnol. 2008, 8, 5873–5877. [Google Scholar] [CrossRef] [PubMed]
- De Paula, L.B.; Primo, F.L.; Pinto, M.R.; Morais, P.C.; Tedesco, A.C. Evaluation of a chloroaluminium phthalocyanine-loaded magnetic nanoemulsion as a drug delivery device to treat glioblastoma using hyperthermia and photodynamic therapy. RSC Adv. 2017, 7, 9115–9122. [Google Scholar] [CrossRef]
- Najlah, M.; Kadam, A.; Wan, K.-W.; Ahmed, W.; Taylor, K.M.; Elhissi, A. Novel paclitaxel formulations solubilized by parenteral nutrition nanoemulsions for application against glioma cell lines. Int. J. Pharm. 2016, 506, 102–109. [Google Scholar] [CrossRef]
- Pang, Z.; Feng, L.; Hua, R.; Chen, J.; Gao, H.; Pan, S.; Jiang, X.; Zhang, P. Lactoferrin-Conjugated Biodegradable Polymersome Holding Doxorubicin and Tetrandrine for Chemotherapy of Glioma Rats. Mol. Pharm. 2010, 7, 1995–2005. [Google Scholar] [CrossRef]
- Zhan, C.; Gu, B.; Xie, C.; Li, J.; Liu, Y.; Lu, W. Cyclic RGD conjugated poly(ethylene glycol)-co-poly(lactic acid) micelle enhances paclitaxel anti-glioblastoma effect. J. Control. Release 2010, 143, 136–142. [Google Scholar] [CrossRef]
- Gao, H.; Pang, Z.; Pan, S.; Cao, S.; Yang, Z.; Chen, C.; Jiang, X. Anti-glioma effect and safety of docetaxel-loaded nanoemulsion. Arch. Pharmacal Res. 2012, 35, 333–341. [Google Scholar] [CrossRef]
- Sarkar, S.; Raymick, J.; Imam, S.Z. Neuroprotective and Therapeutic Strategies against Parkinson’s Disease: Recent Perspectives. Int. J. Mol. Sci. 2016, 17, 904. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Gandham, S.K.; Panicucci, R.; Amiji, M. Intranasal brain delivery of cationic nanoemulsion-encapsulated TNFα siRNA in prevention of experimental neuroinflammation. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 987–1002. [Google Scholar] [CrossRef] [PubMed]
- Jadidi-Niaragh, F.; Mirshafiey, A. Histamine and histamine receptors in pathogenesis and treatment of multiple sclerosis. Neuropharmacology 2010, 59, 180–189. [Google Scholar] [CrossRef] [PubMed]
- English, C.; Aloi, J.J. New FDA-Approved Disease-Modifying Therapies for Multiple Sclerosis. Clin. Ther. 2015, 37, 691–715. [Google Scholar] [CrossRef]
- Sarker, D.K. Engineering of Nanoemulsions for Drug Delivery. Curr. Drug Deliv. 2005, 2, 297–310. [Google Scholar] [CrossRef]
- Parikh, R.H.; Patel, R.J. Nanoemulsions for Intranasal Delivery of Riluzole to Improve Brain Bioavailability: Formulation Development and Pharmacokinetic Studies. Curr. Drug Deliv. 2015, 13, 1130–1143. [Google Scholar] [CrossRef]
- Ashafaq, M.; Khan, M.M.; Raza, S.S.; Ahmad, A.; Khuwaja, G.; Javed, H.; Khan, A.; Islam, F.; Siddiqui, M.S.; Safhi, M.M.; et al. S-allyl cysteine mitigates oxidative damage and improves neurologic deficit in a rat model of focal cerebral ischemia. Nutr. Res. 2012, 32, 133–143. [Google Scholar] [CrossRef]
- Al-Majed, A.A.; Al-Omar, F.A.; Nagi, M.N. Neuroprotective effects of thymoquinone against transient forebrain ischemia in the rat hippocampus. Eur. J. Pharmacol. 2006, 543, 40–47. [Google Scholar] [CrossRef]
- Ahmad, N.; Ahmad, R.; Alam, A.; Samim, M.; Iqbal, Z.; Ahmad, F.J. Quantification and evaluation of thymoquinone loaded mucoadhesive nanoemulsion for treatment of cerebral ischemia. Int. J. Biol. Macromol. 2016, 88, 320–332. [Google Scholar] [CrossRef]
- Yero, T.; Rey, J.A. Tetrabenazine (Xenazine), an FDA-Approved Treatment Option for Huntington’s Disease–Related Chorea. Pharm. Ther. 2008, 33, 690–694. [Google Scholar]
- Wang, Z.; Xiong, G.; Tsang, W.C.; Schätzlein, A.G.; Uchegbu, I.F. Nose-to-Brain Delivery. J. Pharmacol. Exp. Ther. 2019, 370, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Matsuyama, T.; Morita, T.; Horikiri, Y.; Yamahara, H.; Yoshino, H. Enhancement of nasal absorption of large molecular weight compounds by combination of mucolytic agent and nonionic surfactant. J. Control. Release 2006, 110, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Inthavong, K.; Tu, J.; Wang, S. Numerical simulations for detailed airflow dynamics in a human nasal cavity. Respir. Physiol. Neurobiol. 2008, 161, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Matida, E.A.; Gu, J.; Johnson, M.R. Numerical simulation of aerosol deposition in a 3-D human nasal cavity using RANS, RANS/EIM, and LES. J. Aerosol Sci. 2007, 38, 683–700. [Google Scholar] [CrossRef]
- Cu, Y.; Saltzman, W.M. Mathematical modeling of molecular diffusion through mucus. Adv. Drug Deliv. Rev. 2009, 61, 101–114. [Google Scholar] [CrossRef]

| Drug | Therapy for | Characterization Parameters | Study Model (s) | Relevant Therapeutic Outcomes | Ref. |
|---|---|---|---|---|---|
| Donepezil | Alzheimer’s disease | GS = 127.13 ± 4.14 nm PDI = 0.182 ± 0.011 | In vitro drug diffusion study. Ex vivo drug permeation study. Tolerability study through in vitro and in vivo models. | The permeation of donepezil was found to be significant through intranasal NE. The polymers can be used as an effective strategy to improve the bioadhesion and drug penetration through nasal mucosa, which enhances the bioavailability of donepezil. | [47] |
| Rivastigmine | Alzheimer’s disease | GS = 35.75 ± 0.21 nm PDI = 0.247 ± 0.04 ZP = −24.4 ± 0.67 mV | In vitro drug release study. Ex vivo diffusion study. In vivo pharmacokinetic and biodistribution study in rat. Nasal ciliotoxicity studies in goat nasal mucosa. | Rivastigmine-loaded NE showed significantly higher drug concentration in brain than the solution. The optimized formulation was devoid of nasal ciliotoxicity. | [48] |
| Resveratrol | Parkinson’s disease | GS = 176.3 ± 3.5 nm PDI = 0.17 ± 0.03 ZP = 18.5 ± 1.77 mV | In vitro drug release study. Ex vivo diffusion study. In vivo drug biodistribution study in Wistar rat’s brain. | Diffusion controlled release of resveratrol was for 6 h with flux of 2.86 mg/cm2 h through sheep nasal mucosa. The drug level in the brain from intranasal resveratrol mucoadhesive NE was higher than the resveratrol solution. Bioavailability was seven times higher through this approach. | [49] |
| Selegiline | Parkinson’s disease | GS = 61.43 ± 4.10 nm PDI = 0.203 ± 0.005 ZP = −34.00 ± 0.17 mV | In vitro drug release study. Ex vivo diffusion study. Behavioral activities of Parkinson’s disease in Wistar rats. | Selegiline NE showed 3.7-fold more penetration than the drug solution. Haloperidol-induced Parkinson’s disease in animals with selegiline intranasal NE showed significant improvement in behavioral activities in comparison to conventional drug delivery. | [50] |
| Letrozole | Epilepsy | GS = 95.59 ± 2.34nm PDI = 0.162 ± 0.012 ZP = −7.12 ± 0.12 mV | In vitro and ex vivo drug release study. A behavioral seizure; biochemical and histopathological studies were performed. | Intranasal administration of NE showed the prolonged drug release profile as compared to suspension. High concentration of drug was found in brain. | [51] |
| Amiloride | Antiepileptic | GS = 89.36 ± 11.18 nm PDI = 0.231 ± 0.018 ZP = −9.83 ± 0.12 mV | In vitro drug release study. Ex vivo diffusion study. In vivo pharmacodynamic and pharmacokinetic study in Wistar rats. | Bioavailability and brain-targeting efficiency with efficacy of developed amiloride NE was enhanced though nasal administration. | [52] |
| Zolmitriptan | Migraine | GS = 54.63 ± 3.24 nm ZP = −0.086 ± 0.014 mV PDI = 0.17 ± 0.01 | In vitro mucoadhesion study. Ex vivo drug permeation studies. In vivo pharmacokinetic and biodistribution studies. | Zolmitriptan mucoadhesive NE showed higher permeability coefficients than the solution through the nasal mucosa. In vivo study of zolmitriptan mucoadhesive NE showed higher AUC0–8 and shorter Tmax in the brain in comparison to intravenous and nasal solutions. | [53] |
| Rizatriptan | Migraine | GS = 20–120 nm | In vitro drug diffusion study. Nasal irritation study on sheep nasal mucosa. In vivo brain-targeting potential. | Ex vivo drug diffusion-defined controlled release with 86% in 4 h. Brain targeting through intranasal NE (AUC = 302.52 μg min/g) was more than intranasal gel (AUC = 115 μg min/g) and intravenous route (AUC = 109.63 μg min/g). | [54] |
| Cyclosporine-A | Neuroprotective | GS = 158.47 ± 3.02 nm ZP = −30 mV | In vitro drug diffusion study. In vivo brain uptake study. | The brain/blood ratios of cyclosporine-A by intranasal and intravenous was found to be 4.49 and 0.01, respectively. Cyclosporine-A NE can be used for direct nose-to-brain delivery, bypassing the BBB. | [55] |
| Kaempferol | Neuroprotective and anti-tumor | GS = 170.4 ± 4.1 nm PDI = 0.155 ± 0.015 ZP = −18.71 ± 1.72 | Ex vivo diffusion study. In vivo drug biodistribution study in Wistar rats. | The drug concentration through intranasal NE was found to be 4 to 5-fold higher than the solution. Ex vivo permeation and in vivo biodistribution studies showed higher drug concentrations in the brain with chitosan NE through intranasal administration in compared to NE and the kaempferol solution. | [56] |
| Ziprasidone hydrochloride | Antipsychotic | GS = 145.24 ± 4.75 nm PDI = 0.186 ± 0.40 ZP = −30.2 ± 3.21 mV DC = 0.3418 ± 0.03 CM2/min | Ex vivo diffusion study. In vivo pharmacodynamic study in Wistar rats. Nasal ciliotoxicity studies in goat nasal mucosa. | Higher drug diffusion of ziprasidone NE than the solution was found. Pharmacodynamic study revealed the superiority of mucoadhesive NE than NE in the locomotor activity and paw test. Formulation was devoid of acute nasal ciliotoxicity. | [57] |
| Quetiapine | Antipsychotic | GS = 144 ± 0.5 nm | In vitro dissolution study. In vivo drug distribution study in Wistar rats. | Higher drug transport efficiency (DTE%) via intranasal NE. | [58] |
| Drug | Method of Preparation | GS and ZP | In Vivo Model | Ref. |
|---|---|---|---|---|
| Ecto-50-nucleotidase (CD73) | Microfluidization | 262.7 ± 12.8 nm +3.5 ± 3.0 | C6 rat glioma | [111] |
| Kaempferol | High-pressure homogenization | 180.53 ± 4.90 nm (coated) +26.09 ± 2.67 (coated) 145.07 ± 4.91 nm (uncoated) −18.10 ± 2.55 (uncoated) | N/A | [112] |
| Temozolomide | High-pressure homogenization | 134 nm −13.11 | N/A | [113] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bahadur, S.; Pardhi, D.M.; Rautio, J.; Rosenholm, J.M.; Pathak, K. Intranasal Nanoemulsions for Direct Nose-to-Brain Delivery of Actives for CNS Disorders. Pharmaceutics 2020, 12, 1230. https://doi.org/10.3390/pharmaceutics12121230
Bahadur S, Pardhi DM, Rautio J, Rosenholm JM, Pathak K. Intranasal Nanoemulsions for Direct Nose-to-Brain Delivery of Actives for CNS Disorders. Pharmaceutics. 2020; 12(12):1230. https://doi.org/10.3390/pharmaceutics12121230
Chicago/Turabian StyleBahadur, Shiv, Dinesh M. Pardhi, Jarkko Rautio, Jessica M. Rosenholm, and Kamla Pathak. 2020. "Intranasal Nanoemulsions for Direct Nose-to-Brain Delivery of Actives for CNS Disorders" Pharmaceutics 12, no. 12: 1230. https://doi.org/10.3390/pharmaceutics12121230
APA StyleBahadur, S., Pardhi, D. M., Rautio, J., Rosenholm, J. M., & Pathak, K. (2020). Intranasal Nanoemulsions for Direct Nose-to-Brain Delivery of Actives for CNS Disorders. Pharmaceutics, 12(12), 1230. https://doi.org/10.3390/pharmaceutics12121230








