Ent-Peniciherqueinone Suppresses Acetaldehyde-Induced Cytotoxicity and Oxidative Stress by Inducing ALDH and Suppressing MAPK Signaling
Abstract
1. Introduction
2. Materials and Methods
2.1. Purification and Identification of Ent-Peniciherqueinone (EPQ)
2.2. Cell Culture
2.3. Antibodies and Reagents
2.4. Cell Viability Assay and Anti-Acetaldehyde Activity Assay
2.5. Nuclear Staining with Hoechst 33342 and Propidium Iodide (PI)
2.6. Annexin V/PI Staining
2.7. DPPH Assay
2.8. In Vitro GSH-Glo Assay
2.9. Cellular H2O2 Generation Assay
2.10. Measurement of Intracellular Oxidative Stress
2.11. Detection of Mitochondrial Membrane Potential (ΔΨm)
2.12. Western Blot Analysis
2.13. Statistics
3. Results
3.1. EPQ Effectively Suppresses Acetaldehyde-Induced Cytotoxicity in PC12 Cells
3.2. Protective Effects of EPQ against Acetaldehyde-Induced Oxidative Stress
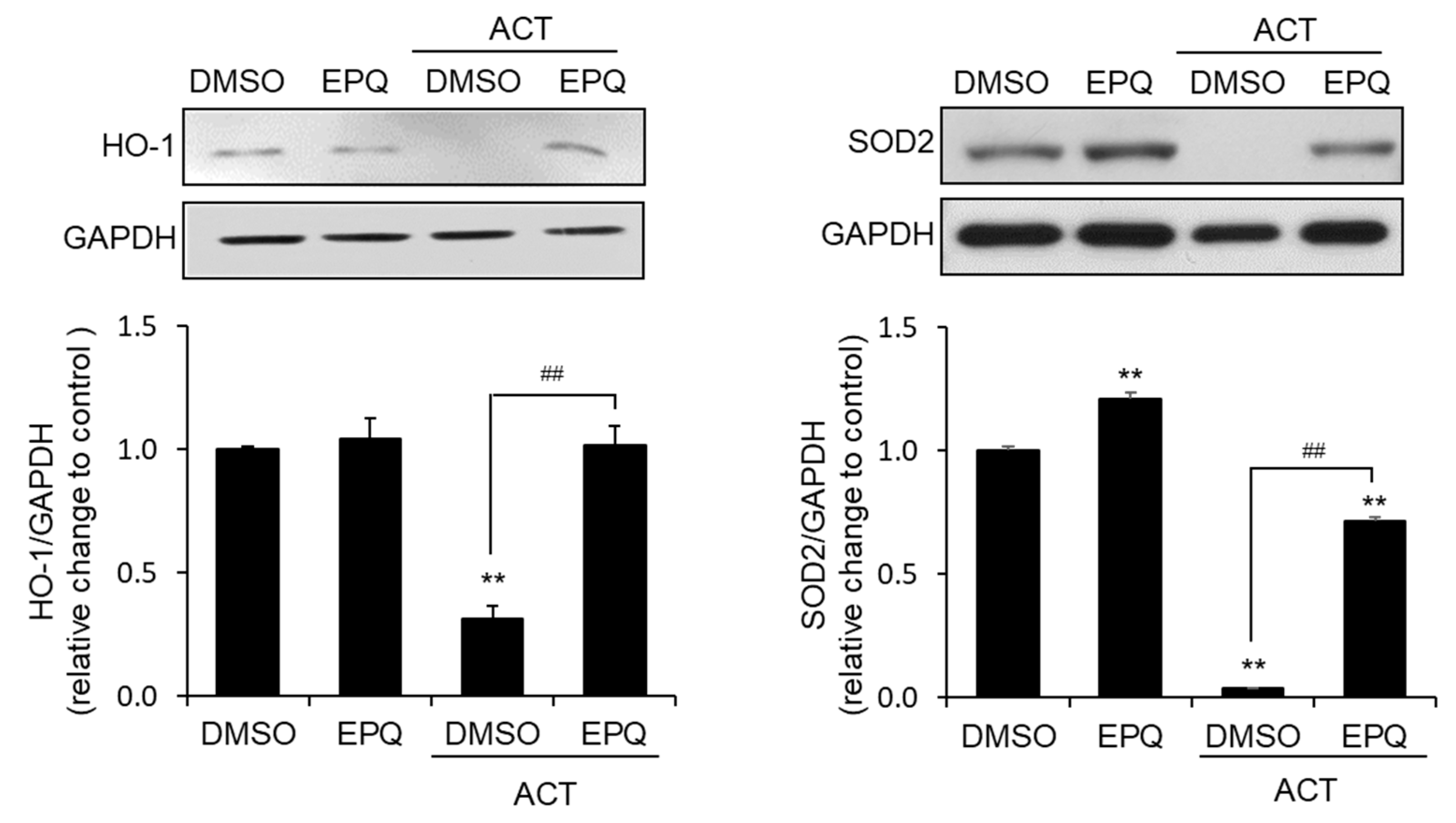
3.3. EPQ Enhances Antioxidant Enzyme Expression in Acetaldehyde-Treated PC12 Cells
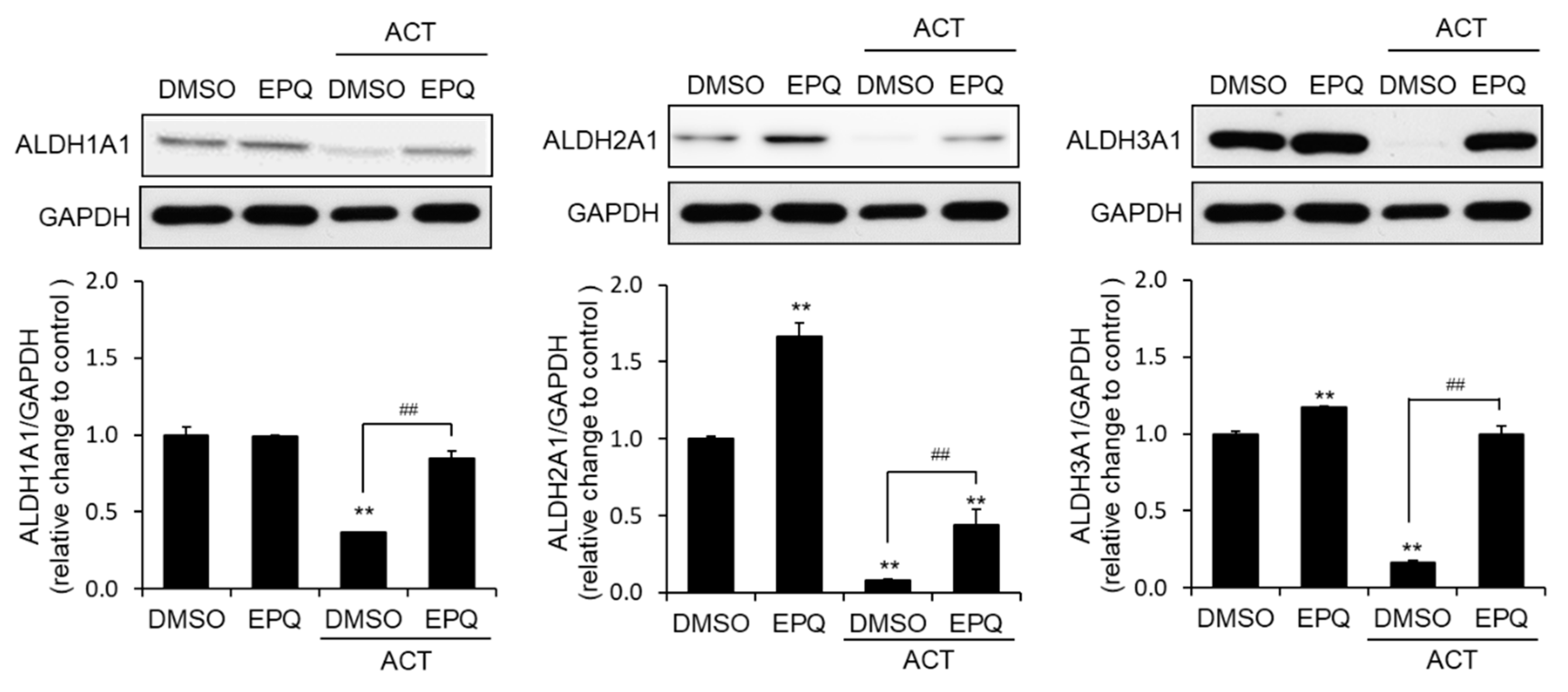
3.4. EPQ Enhances ALDH Expression in Acetaldehyde-Treated PC12 Cells
3.5. EPQ Treatment Reduces Acetaldehyde-Induced MAPK Phosphorylation in PC12 Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kitakaze, T.; Yuan, S.; Inoue, M.; Yoshioka, Y.; Yamashita, Y.; Ashida, H. 6-(methylsulfinyl) hexyl isothiocyanate protects acetaldehyde-caused cytotoxicity through the induction of aldehyde dehydrogenase in hepatocytes. Arch. Biochem. Biophys. 2020, 686, 108329. [Google Scholar] [CrossRef] [PubMed]
- Koch, O.R.; Pani, G.; Borrello, S.; Colavitti, R.; Cravero, A.; Farrè, S.; Galeotti, T. Oxidative stress and antioxidant defenses in ethanol-induced cell injury. Mol. Asp. Med. 2004, 25, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Cederbaum, A.I. Alcohol metabolism. Clin. Liver Dis. 2012, 16, 667–685. [Google Scholar] [CrossRef] [PubMed]
- Weathermon, R.; Crabb, D.W. Alcohol and medication interactions. Alcohol Res. Health 1999, 23, 40–54. [Google Scholar]
- Edenberg, H.J.; McClintick, J.N. Alcohol dehydrogenases, aldehyde dehydrogenases, and alcohol use disorders: A critical review. Alcohol. Clin. Exp. Res. 2018, 42, 2281–2297. [Google Scholar] [CrossRef]
- Zakhari, S. Overview: How is alcohol metabolized by the body? Alcohol Res. Health 2006, 29, 245–254. [Google Scholar]
- Harper, C. The neuropathology of alcohol-related brain damage. Alcohol Alcohol. 2009, 44, 136–140. [Google Scholar] [CrossRef]
- Massey, V.L.; Arteel, G.E. Acute alcohol-induced liver injury. Front. Physiol. 2012, 3, 193. [Google Scholar] [CrossRef]
- Jung, T.W.; Lee, J.Y.; Shim, W.S.; Kang, E.S.; Kim, S.K.; Ahn, C.W.; Lee, H.C.; Cha, B.S. Rosiglitazone protects human neuroblastoma sh-sy5y cells against acetaldehyde-induced cytotoxicity. Biochem. Biophys. Res. Commun. 2006, 340, 221–227. [Google Scholar] [CrossRef]
- Setshedi, M.; Wands, J.R.; de la Monte, S.M. Acetaldehyde adducts in alcoholic liver disease. Oxid. Med. Cell. Longev. 2010, 3, 178–185. [Google Scholar] [CrossRef]
- Labonne, B.E.F.; Gutiérrez, M.; Gómez-Quiroz, L.E.; Fainstein, M.K.; Bucio, L.; Souza, V.; Flores, O.; Ortíz, V.; Hernández, E.; Kershenobich, D.; et al. Acetaldehyde-induced mitochondrial dysfunction sensitizes hepatocytes to oxidative damage. Cell Biol. Toxicol. 2009, 25, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Leal, J.F.M.; Barbancho, M. Acetaldehyde detoxification mechanisms in drosophila melanogaster adults involving aldehyde dehydrogenase (aldh) and alcohol dehydrogenase (adh) enzymes. Insect Biochem. Mol. Biol. 1992, 22, 885–892. [Google Scholar] [CrossRef]
- Ku, B.M.; Lee, Y.K.; Jeong, J.Y.; Mun, J.; Han, J.Y.; Roh, G.S.; Kim, H.J.; Cho, G.J.; Choi, W.S.; Yi, G.-S.; et al. Ethanol-induced oxidative stress is mediated by p38 mapk pathway in mouse hippocampal cells. Neurosci. Lett. 2007, 419, 64–67. [Google Scholar] [CrossRef] [PubMed]
- San Miguel, S.M.; Opperman, L.A.; Allen, E.P.; Zielinski, J.; Svoboda, K.K. Bioactive polyphenol antioxidants protect oral fibroblasts from ros-inducing agents. Arch. Oral Biol. 2012, 57, 1657–1667. [Google Scholar] [CrossRef] [PubMed]
- McCubrey, J.A.; Lahair, M.M.; Franklin, R.A. Reactive oxygen species-induced activation of the map kinase signaling pathways. Antioxid. Redox Signal. 2006, 8, 1775–1789. [Google Scholar] [CrossRef] [PubMed]
- Cowan, K.J.; Storey, K.B. Mitogen-activated protein kinases: New signaling pathways functioning in cellular responses to environmental stress. J. Exp. Biol. 2003, 206, 1107–1115. [Google Scholar] [CrossRef]
- Munshi, A.; Ramesh, R. Mitogen-activated protein kinases and their role in radiation response. Genes Cancer 2013, 4, 401–408. [Google Scholar] [CrossRef]
- Roux, P.P.; Blenis, J. Erk and p38 mapk-activated protein kinases: A family of protein kinases with diverse biological functions. Microbiol. Mol. Biol. Rev. 2004, 68, 320–344. [Google Scholar] [CrossRef]
- Coulthard, L.R.; White, D.E.; Jones, D.L.; McDermott, M.F.; Burchill, S.A. P38(mapk): Stress responses from molecular mechanisms to therapeutics. Trends Mol. Med. 2009, 15, 369–379. [Google Scholar] [CrossRef]
- Wang, F.; Li, Y.; Zhang, Y.-J.; Zhou, Y.; Li, S.; Li, H.-B. Natural products for the prevention and treatment of hangover and alcohol use disorder. Molecules 2016, 21, 64. [Google Scholar] [CrossRef]
- Aroor, A.R.; Shukla, S.D. Map kinase signaling in diverse effects of ethanol. Life Sci. 2004, 74, 2339–2364. [Google Scholar] [CrossRef] [PubMed]
- So, H.M.; Eom, H.J.; Lee, D.; Kim, S.; Kang, K.S.; Lee, I.K.; Baek, K.H.; Park, J.Y.; Kim, K.H. Bioactivity evaluations of betulin identified from the bark of betula platyphylla var. Jpn. Cancer Ther. Arch. Pharmacal Res. 2018, 41, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.S.; Roh, H.S.; Baek, K.H.; Lee, S.; Kim, S.; So, H.M.; Moon, E.; Pang, C.; Jang, T.S.; Kim, K.H. Bioactivity-guided isolation of ginsenosides from korean red ginseng with cytotoxic activity against human lung adenocarcinoma cells. J. Ginseng Res. 2018, 42, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.C.; Choi, E.; Eom, H.J.; Jo, M.S.; Kim, S.; So, H.M.; Kim, S.H.; Kang, K.S.; Kim, K.H. LC/MS-based analysis of bioactive compounds from the bark of Betula platyphylla var. japonica and their effects on regulation of adipocyte and osteoblast differentiation. Nat. Prod. Sci. 2018, 24, 235–240. [Google Scholar] [CrossRef]
- Lee, S.R.; Yi, S.A.; Nam, K.H.; Ryoo, R.; Lee, J.; Kim, K.H. Pantheric acids a–c from a poisonous mushroom, amanita pantherina, promote lipid accumulation in adipocytes. J. Nat. Prod. 2019, 82, 3489–3493. [Google Scholar] [CrossRef]
- Trinh, T.A.; Park, E.-J.; Lee, D.; Song, J.H.; Lee, H.L.; Kim, K.H.; Kim, Y.; Jung, K.; Kang, K.S.; Yoo, J.-E. Estrogenic activity of sanguiin h-6 through activation of estrogen receptor α coactivator-binding site. Nat. Prod. Sci. 2019, 25, 28–33. [Google Scholar] [CrossRef][Green Version]
- Elsebai, M.F.; Saleem, M.; Tejesvi, M.V.; Kajula, M.; Mattila, S.; Mehiri, M.; Turpeinen, A.; Pirttilä, A.M. Fungal phenalenones: Chemistry, biology, biosynthesis and phylogeny. Nat. Prod. Rep. 2014, 31, 628–645. [Google Scholar] [CrossRef]
- Park, S.C.; Julianti, E.; Ahn, S.; Kim, D.; Lee, S.K.; Noh, M.; Oh, D.-C.; Oh, K.-B.; Shin, J. Phenalenones from a marine-derived fungus penicillium sp. Mar. Drugs 2019, 17, 176. [Google Scholar] [CrossRef]
- Yu, J.S.; Li, C.; Kwon, M.; Oh, T.; Lee, T.H.; Kim, D.H.; Ahn, J.S.; Ko, S.-K.; Kim, C.S.; Cao, S.; et al. Herqueilenone a, a unique rearranged benzoquinone-chromanone from the hawaiian volcanic soil-associated fungal strain penicillium herquei ft729. Bioorganic Chem. 2020, 105, 104397. [Google Scholar] [CrossRef]
- Lee, S.R.; Kreuzenbeck, N.B.; Jang, M.; Oh, T.; Ko, S.-K.; Ahn, J.S.; Beemelmanns, C.; Kim, K.H. Xyloneside a: A new glycosylated incisterol derivative from xylaria sp. Fb. Chembiochem Eur. J. Chem. Biol. 2020, 21, 2253–2258. [Google Scholar] [CrossRef]
- Aquilano, K.; Baldelli, S.; Ciriolo, M.R. Glutathione: New roles in redox signaling for an old antioxidant. Front. Pharmacol. 2014, 5, 196. [Google Scholar] [CrossRef] [PubMed]
- Nita, M.; Grzybowski, A. The role of the reactive oxygen species and oxidative stress in the pathomechanism of the age-related ocular diseases and other pathologies of the anterior and posterior eye segments in adults. Oxid. Med. Cell. Longev. 2016, 2016, 3164734. [Google Scholar] [CrossRef] [PubMed]
- Aranda, A.; Sequedo, L.; Tolosa, L.; Quintas, G.; Burello, E.; Castell, J.V.; Gombau, L. Dichloro-dihydro-fluorescein diacetate (dcfh-da) assay: A quantitative method for oxidative stress assessment of nanoparticle-treated cells. Toxicol. In Vitro 2013, 27, 954–963. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.K.; Kim, J.; Na, D.C.; Park, S.; Park, S.H.; Hyun, J.Y.; Baek, K.H.; Kim, N.D.; Kim, N.K.; Park, Y.N.; et al. A small molecule inhibitor of atpase activity of hsp70 induces apoptosis and has antitumor activities. Chem. Biol. 2015, 22, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Antico Arciuch, V.G.; Elguero, M.E.; Poderoso, J.J.; Carreras, M.C. Mitochondrial regulation of cell cycle and proliferation. Antioxid. Redox Signal. 2012, 16, 1150–1180. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, T.; Kwon, M.; Yu, J.S.; Jang, M.; Kim, G.-H.; Kim, K.H.; Ko, S.-K.; Ahn, J.S. Ent-Peniciherqueinone Suppresses Acetaldehyde-Induced Cytotoxicity and Oxidative Stress by Inducing ALDH and Suppressing MAPK Signaling. Pharmaceutics 2020, 12, 1229. https://doi.org/10.3390/pharmaceutics12121229
Oh T, Kwon M, Yu JS, Jang M, Kim G-H, Kim KH, Ko S-K, Ahn JS. Ent-Peniciherqueinone Suppresses Acetaldehyde-Induced Cytotoxicity and Oxidative Stress by Inducing ALDH and Suppressing MAPK Signaling. Pharmaceutics. 2020; 12(12):1229. https://doi.org/10.3390/pharmaceutics12121229
Chicago/Turabian StyleOh, Taehoon, Mincheol Kwon, Jae Sik Yu, Mina Jang, Gun-Hee Kim, Ki Hyun Kim, Sung-Kyun Ko, and Jong Seog Ahn. 2020. "Ent-Peniciherqueinone Suppresses Acetaldehyde-Induced Cytotoxicity and Oxidative Stress by Inducing ALDH and Suppressing MAPK Signaling" Pharmaceutics 12, no. 12: 1229. https://doi.org/10.3390/pharmaceutics12121229
APA StyleOh, T., Kwon, M., Yu, J. S., Jang, M., Kim, G.-H., Kim, K. H., Ko, S.-K., & Ahn, J. S. (2020). Ent-Peniciherqueinone Suppresses Acetaldehyde-Induced Cytotoxicity and Oxidative Stress by Inducing ALDH and Suppressing MAPK Signaling. Pharmaceutics, 12(12), 1229. https://doi.org/10.3390/pharmaceutics12121229