Brain and Nasal Cavity Anatomy of the Cynomolgus Monkey: Species Differences from the Viewpoint of Direct Delivery from the Nose to the Brain
Abstract
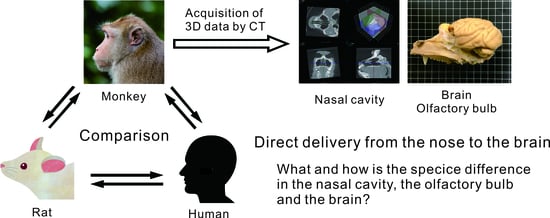
1. Introduction
2. Materials and Methods
| Voxel size: | 0.04 mm × 0.04 mm |
| Image number: | 2000 |
| Accelerating voltage: | 140 kV |
| Filament current: | 120 mA |
3. Results
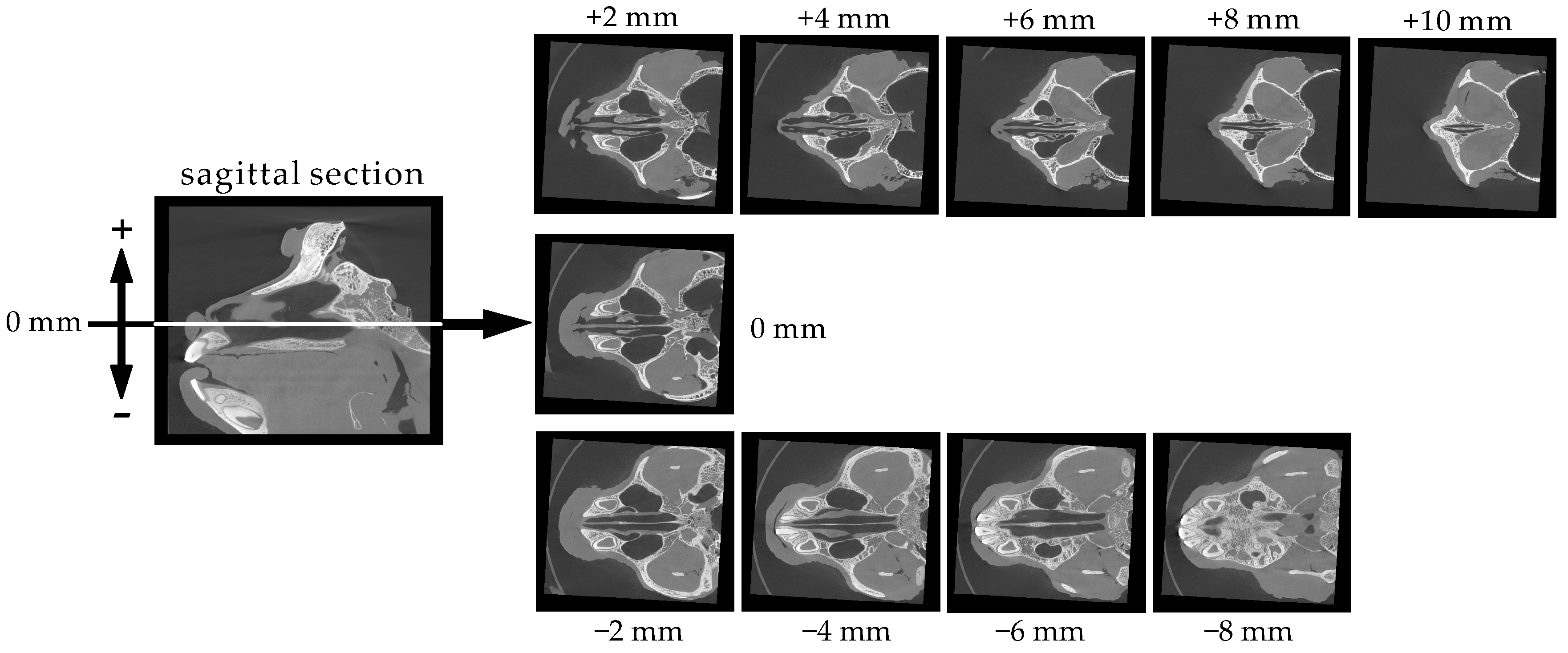
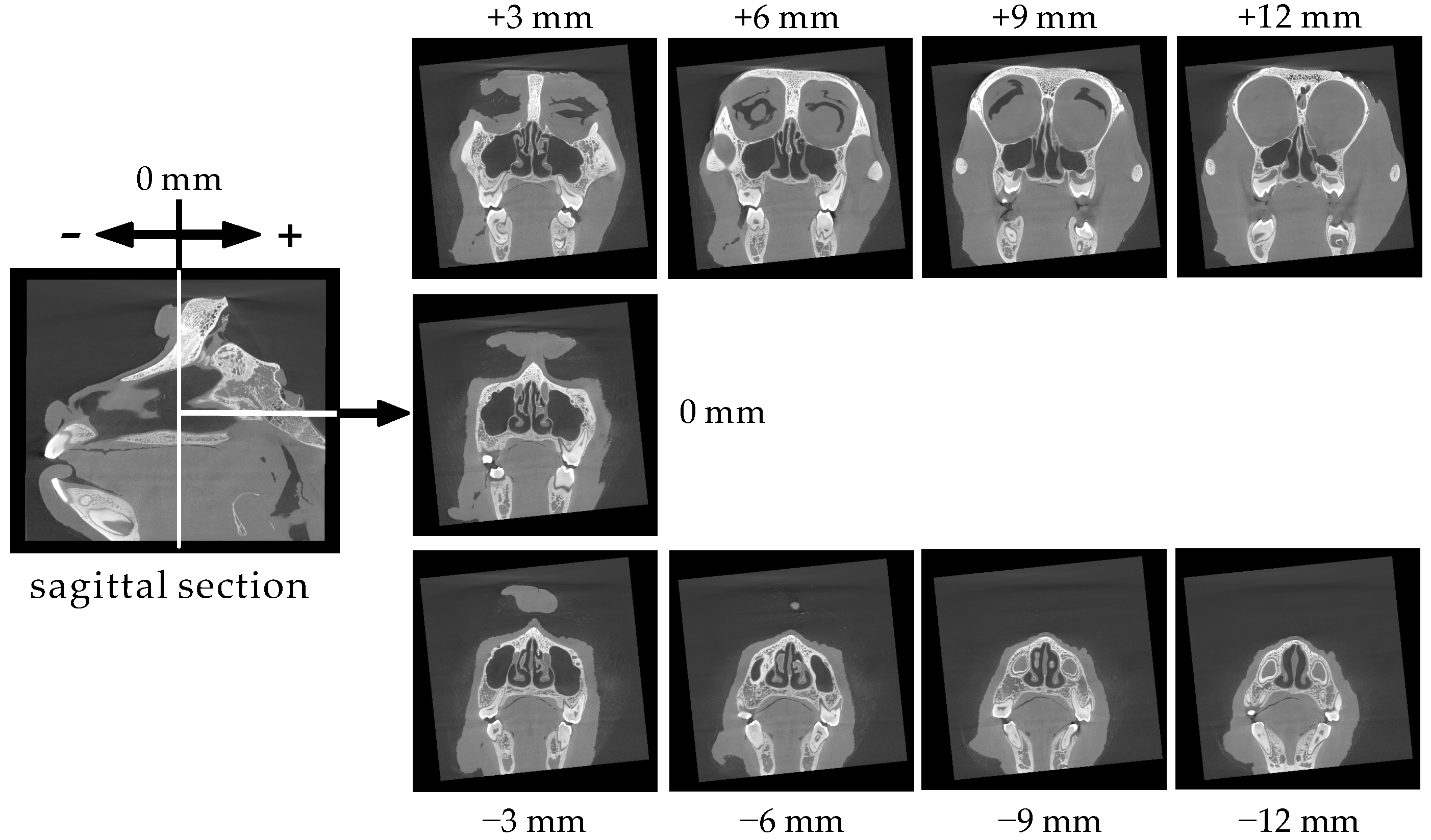
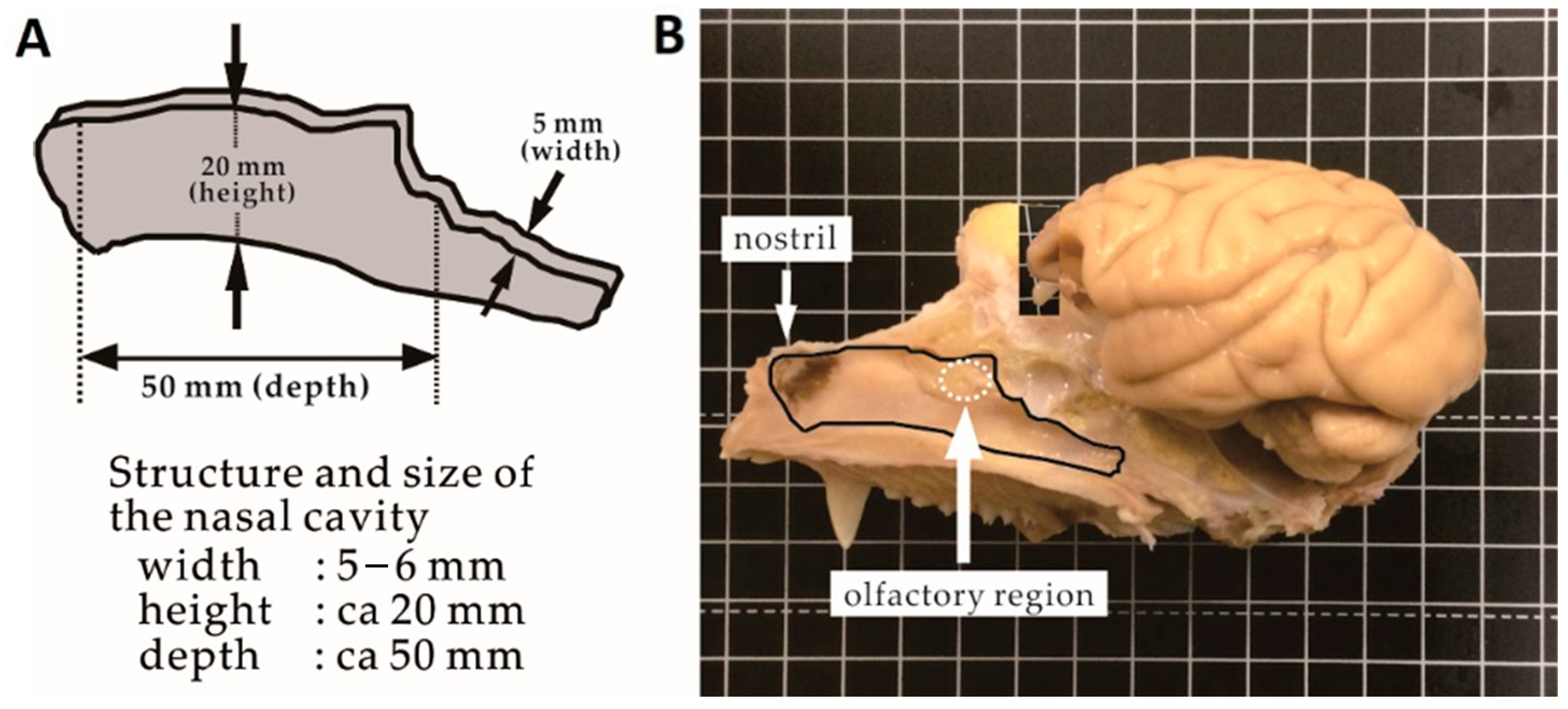
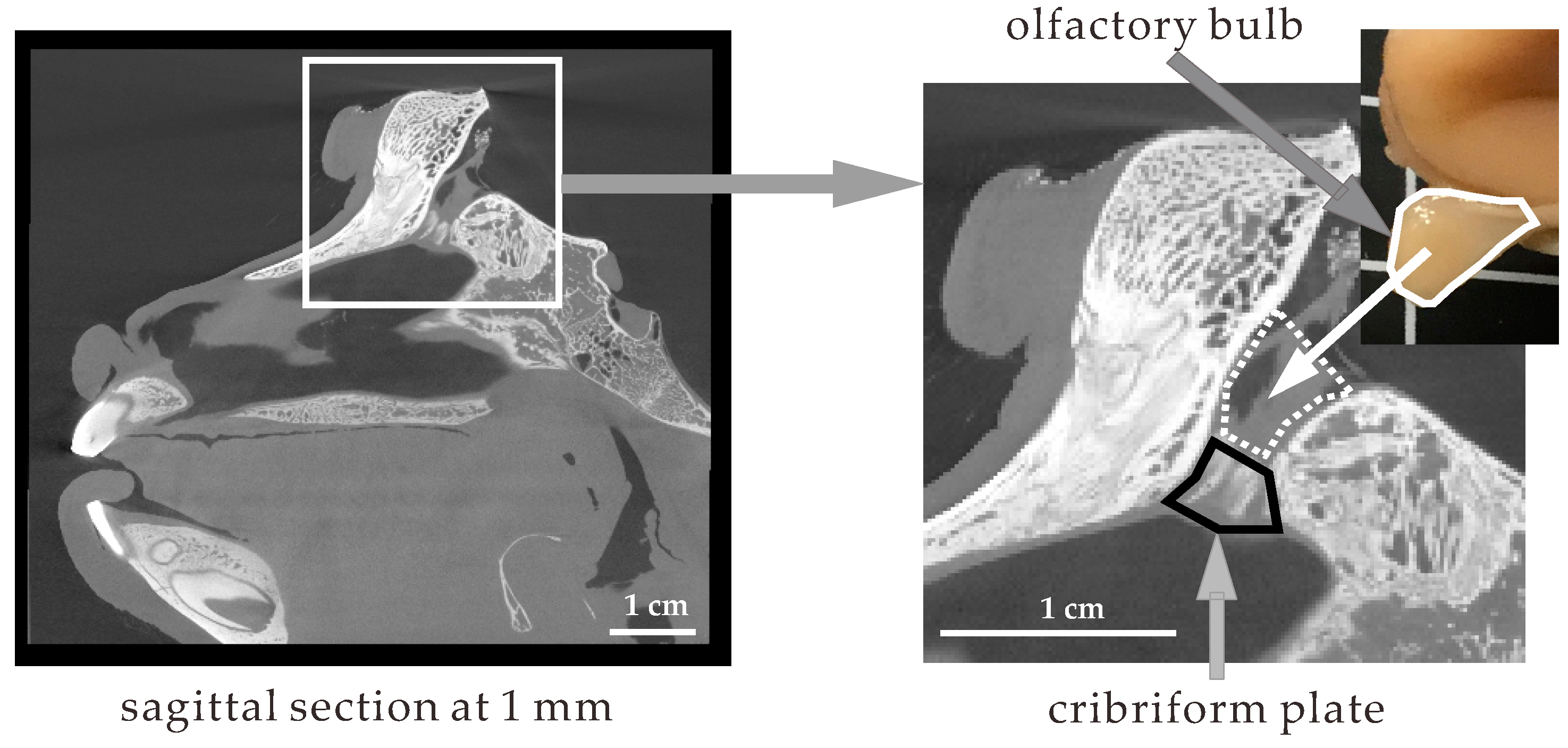
3.1. Structure and Size of the Cynomolgus Monkey Nasal Cavity
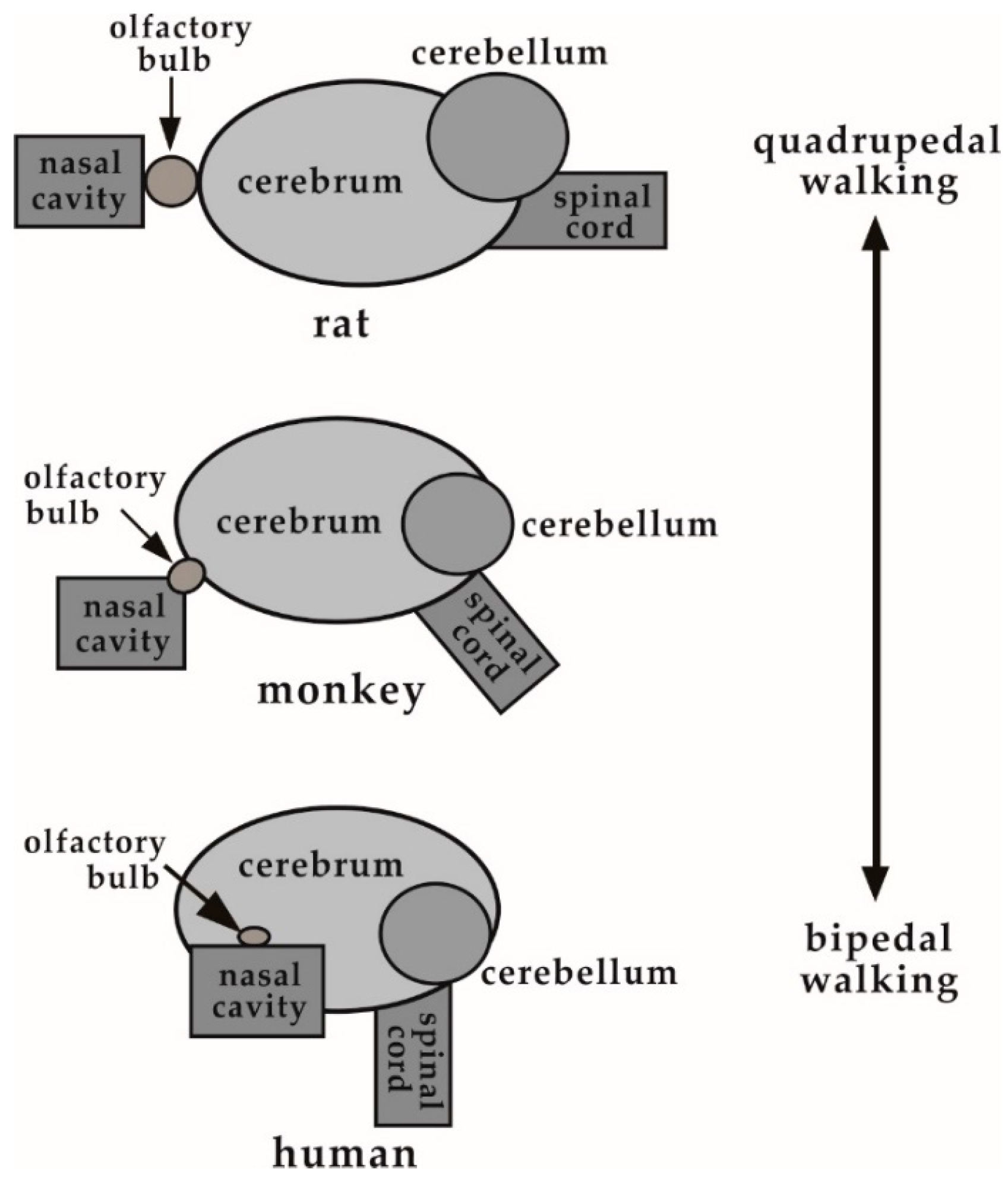
3.2. Size of the Nasal Cavity and Relative Position with the Brain
3.3. Size of the Brain
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Daneman, R.; Prat, A. The Blood–Brain Barrier. Cold Spring Harb. Perspect. Biol. 2015, 7, a020412. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.M.; Patel, B.M. Crossing the Blood-Brain Barrier: Recent Advances in Drug Delivery to the Brain. CNS Drugs 2017, 31, 109–133. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Furubayashi, T.; Arai, M.; Inoue, D.; Kimura, S.; Kiriyama, A.; Kusamori, K.; Katsumi, H.; Yutani, R.; Sakane, T.; et al. Delivery of Oxytocin to the Brain for the Treatment of Autism Spectrum Disorder by Nasal Application. Mol. Pharm. 2018, 15, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Takayama, K.; Furubayashi, T.; Kenji Mori, K.; Takemura, Y.; Amano, A.; Maeda, C.; Inoue, D.; Kimura, S.; Kiriyama, A.; et al. Transnasal Delivery of the Peptide Agonist Specific to Neuromedin-U Receptor 2 to the Brain for the Treatment of Obesity. Mol. Pharm. 2020, 17, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Han, I.-K.; Kim, M.I.; Byun, H.-M.; Hwang, T.S.; Kim, J.M.; Hwang, K.W.; Park, T.G.; Jung, W.-W.; Chun, T.; Jeong, G.-J.; et al. Enhanced brain targeting efficiency of intranasally administered plasmid DNA: An alternative route for brain gene therapy. J. Mol. Med. 2007, 85, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Danielyan, L.; Schäfer, R.; Ameln-Mayerhofer, A.; Buadze, M.; Geisler, J.; Klopfer, T.; Burkhardt, U.; Proksch, B.; Verleysdonk, S.; Ayturan, M.; et al. Intranasal delivery of cells to the brain. Eur. J. Cell Biol. 2009, 88, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Yu-Taeger, L.; Stricker-Shaver, J.; Arnold, K.; Bambynek-Dziuk, P.; Novati, A.; Singer, E.; Lourhmati, A.; Fabian, C.; Magg, J.; Riess, O.; et al. Intranasal administration of mesenchymal stem cells ameliorates the abnormal dopamine transmission system and inflammatory reaction in the R6/2 mouse model of Huntington disease. Cells 2019, 8, 595. [Google Scholar] [CrossRef] [PubMed]
- Galeano, C.; Qiu, Z.; Mishra, A.; Farnsworth, S.L.; Hemmi, J.J.; Moreira, A.; Edenhoer, P.; Hornsby, P.J. The route by which intranasally delivered stem cells enter the central nervous system. Cell Transpl. 2018, 27, 501–514. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 7th ed.; Academic Press: New York, NY, USA, 2013. [Google Scholar]
- Greene, C.G. Anatomy of the Rat; Hafner Publishing: New York, NY, USA, 1963. [Google Scholar]
- Mai, J.; Majtanik, M.; Paxinos, G. Atlas of the Human Brain, 4th ed.; Academic Press: New York, NY, USA, 2015. [Google Scholar]
- DeArmond, S.J.; Fusco, M.M.; Dewey, M.M. Structure of the Human Brain: A Photographic Atlas, 3rd ed.; Oxford University Press: Oxford, UK, 1989. [Google Scholar]
- Kumar, T.C.A.; David, G.F.; Sankaranarayanan, A.; Puri, V.; Sundram, K.R. Pharmacokinetics of progesterone after its administration to ovariectomized rhesus monkeys by injection, infusion, or nasal spraying. Proc. Natl. Acad. Sci. USA 1982, 79, 4185–4189. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Hasegawa, M.; Kametani, S.; Ito, S. Nose-to-brain delivery of TS-002, prostaglandin D2 analogue. J. Drug Target. 2007, 15, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Thorne, R.G.; Hanson, L.R.; Ross, T.M.; Tung, D.; Frey, W.H., 2nd. Delivery of interferon-beta to the monkey nervous system following intranasal administration. Neuroscience 2008, 152, 785–797. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, S.; Yamamoto, S.; Sano, S.; Tohyama, K.; Kosugi, Y.; Furuta, A.; Hamada, T.; Igari, T.; Fujioka, Y.; Hirabayashi, H.; et al. Direct drug delivery of low-permeable compounds to the central nervous system via intranasal administration in rats and monkeys. Pharm. Res. 2019, 36, 76–89. [Google Scholar] [CrossRef] [PubMed]
- Born, J.; Lange, T.; Kern, W.; McGregor, G.P.; Bickel, U.; Fehm, H.L. Sniffing neuropeptides: A transnasal approach to the human brain. Nat. Neurosci. 2002, 5, 514–516. [Google Scholar] [CrossRef] [PubMed]
- Djupesland, P.G.; Messina, J.C.; Mahmoud, R.A. The nasal approach to delivering treatment for brain diseases: An anatomic, physiologic, and delivery technology overview. Therap. Deliv. 2014, 5, 709–733. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakane, T.; Okabayashi, S.; Kimura, S.; Inoue, D.; Tanaka, A.; Furubayashi, T. Brain and Nasal Cavity Anatomy of the Cynomolgus Monkey: Species Differences from the Viewpoint of Direct Delivery from the Nose to the Brain. Pharmaceutics 2020, 12, 1227. https://doi.org/10.3390/pharmaceutics12121227
Sakane T, Okabayashi S, Kimura S, Inoue D, Tanaka A, Furubayashi T. Brain and Nasal Cavity Anatomy of the Cynomolgus Monkey: Species Differences from the Viewpoint of Direct Delivery from the Nose to the Brain. Pharmaceutics. 2020; 12(12):1227. https://doi.org/10.3390/pharmaceutics12121227
Chicago/Turabian StyleSakane, Toshiyasu, Sachi Okabayashi, Shunsuke Kimura, Daisuke Inoue, Akiko Tanaka, and Tomoyuki Furubayashi. 2020. "Brain and Nasal Cavity Anatomy of the Cynomolgus Monkey: Species Differences from the Viewpoint of Direct Delivery from the Nose to the Brain" Pharmaceutics 12, no. 12: 1227. https://doi.org/10.3390/pharmaceutics12121227
APA StyleSakane, T., Okabayashi, S., Kimura, S., Inoue, D., Tanaka, A., & Furubayashi, T. (2020). Brain and Nasal Cavity Anatomy of the Cynomolgus Monkey: Species Differences from the Viewpoint of Direct Delivery from the Nose to the Brain. Pharmaceutics, 12(12), 1227. https://doi.org/10.3390/pharmaceutics12121227