Supramolecular and Macromolecular Matrix Nanocarriers for Drug Delivery in Inflammation-Associated Skin Diseases
Abstract
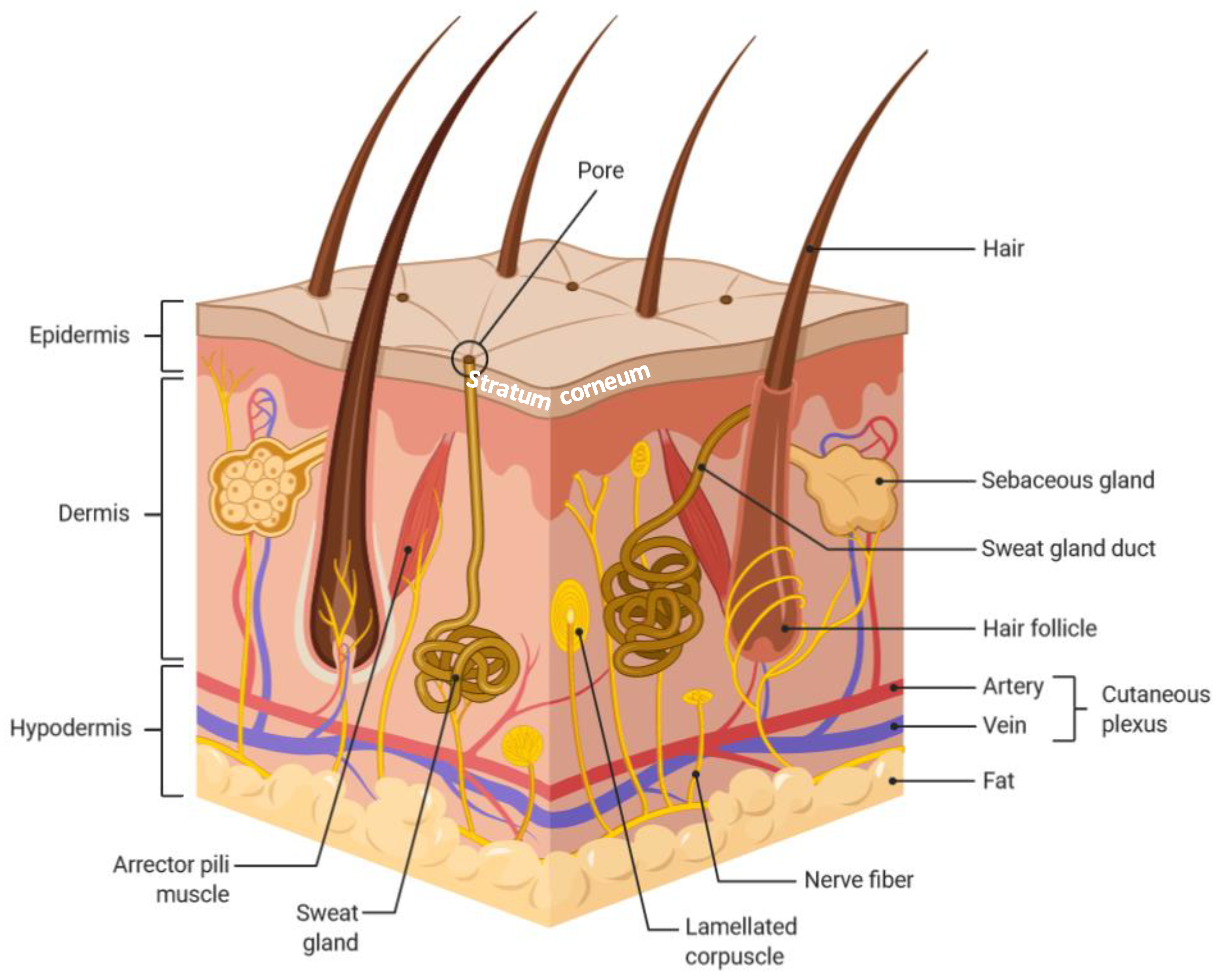
:1. Introduction
2. Supramolecular Matrix Nanocarriers
2.1. Microemulsions and Nanoemulsions
2.2. Nanospheres
2.3. Solid Lipid Nanoparticles (SLNs)
2.4. Nanostructured Lipid Carriers (NLCs)
3. Macromolecular Matrix Nanocarriers: Dendrimers
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Proksch, E.; Brandner, J.M.; Jensen, J.M. The skin: An indispensable barrier. Exp. Dermatol. 2008, 17, 1063–1072. [Google Scholar] [CrossRef]
- Benson, H.A.E. Skin structure, function, and permeation. In Topical and Transdermal Drug Delivery: Principles and Practice, 1st ed.; Benson, H.A.E., Watkinson, A.C., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2012; pp. 1–22. [Google Scholar]
- Walters, K.A.; Roberts, M.S. The structure and function of skin. In Dermatological and Transdermal Formulations, 1st ed.; Walters, K.A., Ed.; Taylor & Francis Group: Boca Raton, FL, USA, 2002; pp. 1–40. [Google Scholar]
- Langan, S.M.; Irvine, A.D.; Weidinger, S. Atopic dermatitis. Lancet 2020, 396, 345–360. [Google Scholar] [CrossRef]
- Boehncke, W.H.; Schön, M.P. Psoriasis. Lancet 2015, 326, 983–994. [Google Scholar] [CrossRef]
- Raychaudhuri, S.P.; Farber, E.M. The prevalence of psoriasis in the world. J. Eur. Acad. Dermatol. Venereol. 2001, 15, 16–17. [Google Scholar] [CrossRef] [PubMed]
- Nestle, F.O.; Di Meglio, P.; Qin, J.Z.; Nickoloff, B.J. Skin immune sentinels in health and disease. Nat. Rev. Immunol. 2009, 9, 679–691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsakovska, I.; Pajeva, I.; Al Sharif, M.; Alov, P.; Fioravanzo, E.; Kovarich, S.; Worth, A.P.; Richarz, A.N.; Yang, C.; Mostrag-Szlichtyng, A.; et al. Quantitative structure-skin permeability relationships. Toxicology 2017, 387, 27–42. [Google Scholar] [CrossRef]
- Supe, S.; Takudage, P. Methods for evaluating penetration of drug in the skin: A review. Skin Res. Technol. 2020. [Google Scholar] [CrossRef]
- Choy, Y.B.; Prausnitz, M.R. The rule of five for non-oral routes of delivery: Ophthalmic, inhalation and transdermal. Pharm. Res. 2011, 28, 943–948. [Google Scholar] [CrossRef] [Green Version]
- Hashida, M. Role of pharmacokinetic consideration for the development of drug delivery systems: A historical overview. Adv. Drug Deliv. Rev. 2020. [Google Scholar] [CrossRef]
- Poupot, R.; Bergozza, D.; Fruchon, S. Nanoparticle-based strategies to treat neuro-inflammation. Materials 2018, 11, 270. [Google Scholar] [CrossRef] [Green Version]
- Kumar, L.; Verma, S.; Singh, M.; Chalotra, T.; Utreja, P. Advanced drug delivery systems for transdermal delivery of non-steroidal anti-inflammatory drugs: A review. Curr. Drug Deliv. 2018, 15, 1087–1099. [Google Scholar] [CrossRef]
- Neubert, R.H.H. Potentials of new nanocarriers for dermal and transdermal drug delivery. Eur. J. Pharm. Biopharm. 2011, 77, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Soussan, E.; Cassel, S.; Blanzat, M.; Rico-Lattes, I. Drug delivery by soft matter: Matrix and vesicular carriers. Angew. Chem. Int. Ed. 2009, 48, 274–288. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.W.; Gao, P. Emulsions and microemulsions for topical and transdermal drug delivery. In Handbook of Non-Invasive Drug Delivery Systems, 1st ed.; Kulkarny, V.S., Ed.; Elsevier Inc.: Amsterdam, The Netherland, 2010; pp. 59–94. [Google Scholar]
- Danielsson, I.; Lindman, B. The definition of microemulsion. Colloids Surf. 1980, 3, 391–392. [Google Scholar] [CrossRef]
- McClements, D.J. Nanoemulsions versus microemulsions: Terminology, differences, and similarities. Soft Matter 2012, 8, 1719–1729. [Google Scholar] [CrossRef]
- Shukla, T.; Upmanyu, N.; Agrawal, M.; Saraf, S.; Alexander, A. Biomedical applications of microemulsion through dermal and transdermal route. Biomed. Pharmacother. 2018, 108, 1477–1494. [Google Scholar] [CrossRef]
- Abd, E.; Benson, H.; Roberts, M.; Grice, J. Minoxidil skin delivery from nanoemulsion formulations containing eucalyptol or oleic acid: Enhanced diffusivity and follicular targeting. Pharmaceutics 2018, 10, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salim, N.; Ahmad, N.; Musa, S.H.; Hashim, R.; Tadros, T.F.; Basri, M. Nanoemulsion as a topical delivery system of antipsoriatic drugs. RSC Adv. 2016, 6, 6234–6250. [Google Scholar] [CrossRef]
- Tung, N.T.; Vu, V.D.; Nguyen, P.L. DoE-based development, physicochemical characterization, and pharmacological evaluation of a topical hydrogel containing betamethasone dipropionate microemulsion. Colloids Surf. B Biointerfaces 2019, 181, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Praça, F.G.; Viegas, J.S.R.; Peh, H.Y.; Garbin, T.N.; Medina, W.S.G.; Bentley, M.V.L.B. Microemulsion co-delivering vitamin A and vitamin E as a new platform for topical treatment of acute skin inflammation. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 110, 110639. [Google Scholar] [CrossRef]
- Latter, G.; Grice, J.E.; Mohammed, Y.; Roberts, M.S.; Benson, H.A.E. Targeted topical delivery of retinoids in the management of acne vulgaris: Current formulations and novel delivery systems. Pharmaceutics 2019, 11, 490. [Google Scholar] [CrossRef] [Green Version]
- Azeem, A.; Khan, Z.I.; Aqil, M.; Ahmad, F.J.; Khar, R.K.; Talegaonkar, S. Microemulsions as a surrogate carrier for dermal drug delivery. Drug Dev. Ind. Pharm. 2009, 35, 525–547. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, R.H.; Pratavale, V.B.; Joshi, M.D. Polymeric nanoparticles for targeted treatment in oncology: Current insights. Int. J. Nanomed. 2015, 1001–1018. [Google Scholar] [CrossRef] [Green Version]
- Goyal, R.; Macri, L.K.; Kaplan, H.M.; Kohn, J. Nanoparticles and nanofibers for topical drug delivery. J. Control. Release 2016, 240, 77–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, X.; Price, G.J.; Guy, R.H. Disposition of nanoparticles and an associated lipophilic permeant following topical application to the skin. Mol. Pharm. 2009, 6, 1441–1448. [Google Scholar] [CrossRef] [Green Version]
- Campbell, C.S.J.; Contreras-Rojas, L.R.; Delgado-Charro, M.B.; Guy, R.H. Objective assessment of nanoparticle disposition in mammalian skin after topical exposure. J. Control. Release 2012, 162, 201–207. [Google Scholar] [CrossRef] [Green Version]
- Kilfoyle, B.E.; Sheihet, L.; Zhang, Z.; Laohoo, M.; Kohn, J.; Michniak-Kohn, B.B. Development of paclitaxel-TyroSpheres for topical skin treatment. J. Control. Release 2012, 163, 18–24. [Google Scholar] [CrossRef] [Green Version]
- Ramezanli, T.; Kilfoyle, B.E.; Zhang, Z.; Michniak-Kohn, B.B. Polymeric nanospheres for topical delivery of vitamin D3. Int. J. Pharm. 2017, 516, 196–203. [Google Scholar] [CrossRef] [Green Version]
- Goyal, R.; Macri, L.; Kohn, J. Formulation strategy for the delivery of cyclosporine A: Comparison of two polymeric nanospheres. Sci. Rep. 2015, 5, 13065. [Google Scholar] [CrossRef] [Green Version]
- Ramezanli, T.; Zhang, Z.; Michniak-Kohn, B.B. Development and characterization of polymeric nanoparticle-based formulation of adapalene for topical acne therapy. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 143–152. [Google Scholar] [CrossRef]
- Smith, A.; Hunneyball, I.M. Evaluation of poly(lactic acid) as a biodegradable drug delivery system for parenteral administration. Int. J. Pharm. 1986, 30, 215–220. [Google Scholar] [CrossRef]
- Muller, R.H.; Mäder, K.; Gohla, S. Solid lipid nanoparticles (SLN) for controlled drug delivery—A review of the state of the art. Eur. J. Pharm. Biopharm. 2000, 50, 161–177. [Google Scholar] [CrossRef]
- Duan, Y.; Dhar, A.; Patel, C.; Khimani, M.; Neogi, S.; Sharma, P.; Kumar, N.S.; Vekariya, R.L. A brief review on solid lipid nanoparticles: Part and parcel of contemporary drug delivery systems. RCS Adv. 2020, 10, 26777–26791. [Google Scholar] [CrossRef]
- Sala, M.; Diab, R.; Elaissari, A.; Fessi, H. Lipid nanocarriers as skin drug delivery systems: Properties, mechanisms of skin interactions and medical applications. Int. J. Pharm. 2018, 535, 1–17. [Google Scholar] [CrossRef]
- Desai, P.; Patlolla, R.R.; Singh, M. Interaction of nanoparticles and cell-penetrating peptides with skin for transdermal drug delivery. Mol. Membr. Biol. 2010, 27, 247–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jensen, L.B.; Petersson, K.; Nielsen, H.M. In vitro penetration properties of solid lipid nanoparticles in intact and barrier-impaired skin. Eur. J. Pharm. Biopharm. 2011, 79, 68–75. [Google Scholar] [CrossRef]
- Madan, J.R.; Khude, P.A.; Dua, K. Development and evaluation of solid lipid nanoparticles of mometasone furoate for topical delivery. Int. J. Pharm. Investig. 2014, 4, 60–64. [Google Scholar] [CrossRef] [Green Version]
- Natarajan, J.; Karri, V.; Anindita, D. Nanostructured lipid carrier (NLC): A promising drug delivery system. Glob. J. Nano 2017, 1, 555575. [Google Scholar] [CrossRef]
- Zhai, Y.; Zhai, G. Advances in lipid-based colloid systems as drug carrier for topic delivery. J. Control. Release 2014, 193, 90–99. [Google Scholar] [CrossRef]
- Kaur, N.; Sharma, K.; Bedi, N. Topical nanostructured lipid carrier based hydrogel of mometasone furoate for the treatment of psoriasis. Pharm. Nanotechnol. 2018, 6, 133–143. [Google Scholar] [CrossRef]
- Viegas, J.S.R.; Praça, F.G.; Caron, A.L.; Suzuki, I.; Silvestrini, A.V.P.; Medina, W.S.G.; Del Campio, J.O.; Kravicz, M.; Bentley, M.V.L.B. Nanostructured lipid carrier co-delivering tacrolimus and TNF-α siRNA as an innovate approach to psoriasis. Drug Deliv. Transl. Res. 2020, 10, 646–660. [Google Scholar] [CrossRef]
- Venuganti, V.V.K.; Perumal, O.P. Poly(amidoamine) dendrimers as skin penetration enhancers: Influence of charge, generation, and concentration. J. Pharm. Sci. 2009, 98, 2345–2356. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, A.S.; Sridevi, S.; Chalasani, K.B.; Jain, A.K.; Jain, S.K.; Jain, N.K.; Diwan, P.V. Dendrimer-mediated transdermal delivery: Enhanced bioavailability of indomethacin. J. Control. Release 2003, 90, 335–343. [Google Scholar] [CrossRef]
- Guo, C.; Zhang, Y.; Sun, M.; Wang, Z.; Fan, A.; Zhao, Y. Modulating topical drug delivery via skin pre-treatment with low-generation poly(amidoamine) dendrimers. J. Drug Del. Sci. Technol. 2014, 24, 555–557. [Google Scholar] [CrossRef]
- Agrawal, U.; Mehra, N.K.; Gupta, U.; Jain, N.K. Hyperbranched dendritic nano-carrier for topical delivery of dithranol. J. Drug Target. 2013, 21, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Kalhapure, R.S.; Akamanchi, K.G. Oleodendrimers: A novel class of multicephalous heterolipids as chemical penetration enhancers for transdermal drug delivery. Int. J. Pharm. 2013, 454, 158–166. [Google Scholar] [CrossRef]
- Hayder, M.; Fruchon, S.; Fournié, J.J.; Poupot, M.; Poupot, R. Anti-inflammatory properties of dendrimers per se. Sci. World J. 2011, 11, 1367–1382. [Google Scholar] [CrossRef]
- Fruchon, S.; Poupot, R. Pro-inflammatory versus anti-inflammatory effects of dendrimers: The two faces of immuno-modulatory nanoparticles. Nanomaterials 2017, 7, 251. [Google Scholar] [CrossRef]
- Poupot, R.; Goursat, C.; Fruchon, S. Multivalent nanosystems: Targeting monocyres/macrophages. Int. J. Nanomedicine 2018, 13, 5511–5521. [Google Scholar] [CrossRef] [Green Version]
- Buhleier, E.; Wehner, W.; Vögtle, F. Cascade-chain-like and nonskid-chain-like syntheses of molecular cavity topologies. Synthesis 1978, 2, 155–158. [Google Scholar] [CrossRef]
- D’Emanuele, A.; Attwood, D. Dendrimer-drug interactions. Adv. Drug Deliv. Rev. 2005, 57, 2146–2162. [Google Scholar] [CrossRef]
- Gardikis, K.; Hatziantoniou, S.; Viras, K.; Wagner, M.; Demetzos, C. A DSC and Raman spectroscopy study on the effect of PAMAM dendrimer on DPPC model lipid membranes. Int. J. Pharm. 2006, 318, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Bielinska, A.U.; Mecke, A.; Keszler, B.; Beals, J.L.; Shi, X.; Balogh, L.; Orr, B.G.; Baker, J.R., Jr.; Holl, M.M.B. Interaction of Poly(amidoamine) dendrimers with supported lipid bilayers and cells: Hole formation and the relation to transport. Bioconjugate Chem. 2004, 15, 774–782. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Leroueil, P.R.; Janus, E.K.; Peters, J.L.; Kober, M.M.; Islam, M.T.; Orr, B.G.; Baker, J.R., Jr.; Holl, M.M.B. Interaction of polycationic polymers with supported lipid bilayers and cells: Nanoscale hole formation and enhanced membrane permeability. Bioconjugate Chem. 2006, 17, 728–734. [Google Scholar] [CrossRef]
- Dave, K.; Venuganti, V.V.K. Dendritic polymers for dermal drug delivery. Ther. Deliv. 2017, 8, 1077–1096. [Google Scholar] [CrossRef]
- Venuganti, V.V.K.; Sahdev, P.; Hildreth, M.; Guan, X.; Perumal, O. Structure-skin permeability relationship of dendrimers. Pharm. Res. 2011, 28, 2246–2260. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Sunoqrot, S.; Stowell, C.; Ji, J.; Lee, C.W.; Kim, J.W.; Khan, S.A.; Hong, S. Effect of size, surface charge, and hydrophobicity of poly(amidoamine) dendrimers on their skin penetration. Biomacromolecules 2012, 13, 2154–2162. [Google Scholar] [CrossRef] [Green Version]
- Sun, M.; Fan, A.; Wang, Z.; Zhao, Y. Dendrimer-mediated drug delivery to the skin. Soft Matter 2012, 8, 4301–4305. [Google Scholar] [CrossRef]
- Pandi, P.; Jain, A.; Kommineni, M.; Ionov, M.; Bryszewska, M.; Khan, W. Dendrimer as a new potential carrier for topical delivery of siRNA: A comparative study of dendriplex vs. lipoplex for delivery of TNF-α siRNA. Int. J. Pharm. 2018, 550, 240–250. [Google Scholar] [CrossRef]
- Kim, M.J.; Doh, H.J.; Choi, M.K.; Chung, S.J.; Shim, C.K.; Kim, D.D.; Kim, J.S.; Yong, C.S.; Choi, H.G. Skin permeation enhancement of diclofenac by fatty acids. Drug Deliv. 2008, 15, 373–379. [Google Scholar] [CrossRef]
- Chacko, I.A.; Ghate, V.M.; Dsouza, L.; Lewis, S.A. Lipid vesicles: A versatile drug delivery platform for dermal and transdermal applications. Colloids Surf. B Biointerfaces 2020, 195, 111262. [Google Scholar] [CrossRef]
- Garg, V.; Singh, H.; Bimbrawh, S.; Singh, S.K.; Gulati, M.; Vaidya, Y.; Kaur, P. Ethosomes and transfersomes: Principles, perspectives and practices. Curr. Drug Deliv. 2017, 14, 613–633. [Google Scholar] [CrossRef]
- Zang, Z.J.; Michniak-Kohn, B. Flavosomes, novel deformable liposomes for the co-delivery of anti-inflammatory compounds to skin. Int. J. Pharm. 2020, 585, 119500. [Google Scholar] [CrossRef]
- Babaie, S.; Bakhshayesh, A.R.D.; Ha, J.W.; Hamishehkar, H.; Kim, K.H. Invasome: A novel nanocarrier for transdermal delivery. Nanomaterials 2020, 10, 341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.; Hanning, S.; Falconer, J.; Locke, M.; Wen, J. Recent advances in non-ionic surfactant vesicles (niosomes): Fabrication, characterization, pharmaceutical and cosmetic applications. Eur. J. Pharm. Biopharm. 2019, 144, 18–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Consola, S.; Blanzat, M.; Perez, E.; Garrigues, J.C.; Bordat, P.; Rico-Lattes, I. Design of original bioactive formulations based on sugar-surfactant/non-steroidal anti-inflammatory catanionic self-assemblies: A new way of dermal drug delivery. Chemistry 2007, 13, 3039–3047. [Google Scholar] [CrossRef] [PubMed]
- Guterres, S.S.; Alves, M.P.; Pohlmann, A.R. Polymeric nanoparticles, nanospheres and nanocapsules, for cutaneous applications. Drug Target Insights 2007, 2, 147–157. [Google Scholar] [CrossRef] [Green Version]
- Muzzalupo, R.; Tavano, L. Niosomal drug delivery for transdermal targeting: Recent advances. Res. Rep. Transdermal Drug Deliv. 2015, 4, 23–33. [Google Scholar] [CrossRef] [Green Version]
- Cevc, G.; Vierl, U. Nanotechnology and the transdermal route: A state of the art review and critical appraisal. J. Control. Release 2010, 141, 277–299. [Google Scholar] [CrossRef]
- Campos, E.V.R.; Proença, P.L.F.; Doretto-Silva, L.; Andrade-Oliveira, V.; Fraceto, L.F.; de Araujo, D.R. Trends in nanoformulations for atopic dermatitis treatment. Expert Opin. Drug Deliv. 2020, 17, 1615–1630. [Google Scholar] [CrossRef]
- Dadwal, A.; Mishra, N.; Narang, R.K. Novel topical nanocarriers for treatment of psoriasis: An overview. Curr. Pharm. Des. 2018, 24, 3934–3950. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic. Bioeng. Transl. Med. 2016, 1, 10–29. [Google Scholar] [CrossRef] [PubMed]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic: An update. Bioeng. Transl. Med. 2019, 4, e10143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janaszewska, A.; Lazniewska, J.; Trzepiński, P.; Marcinkowska, M.; Klajnert-Maculewicz, B. Cytotoxicity of dendrimers. Biomolecules 2019, 9, 330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poupot, M.; Turrin, C.O.; Caminade, A.M.; Fournié, J.J.; Attal, M.; Poupot, R.; Fruchon, S. Poly(phosphorhydrazone) dendrimers: Yin and yang of monocyte activation for human NK cell amplification applied to immunotherapy against Multiple Myeloma. Nanomedicine 2016, 12, 2321–2330. [Google Scholar] [CrossRef]
- Winnicka, K.; Wroblewska, M.; Sosnowska, K.; Car, H.; Kasacka, I. Evaluation of cationic polyamidoamine dendrimers’ dermal toxicity in the rat skin model. Drug Des. Dev. Ther. 2015, 9, 1367–1377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fruchon, S.; Mouriot, S.; Thiollier, T.; Grandin, C.; Caminade, A.M.; Turrin, C.O.; Contamin, H.; Poupot, R. Repeated intravenous injections in non-human primates demonstrate preclinical safety of an anti-inflammatory phosphorus-based dendrimer. Nanotoxicology 2015, 9, 933–941. [Google Scholar] [CrossRef]
- Fruchon, S.; Bellard, E.; Beton, N.; Goursat, C.; Oukhrib, A.; Caminade, A.M.; Blanzat, M.; Turrin, C.O.; Golzio, M.; Poupot, R. Biodistribution and biosafety of a poly(phosphorhydrazone) dendrimer, an anti-inflammatory drug candidate. Biomolecules 2019, 9, 475. [Google Scholar] [CrossRef] [Green Version]
- Fruchon, S.; Caminade, A.M.; Abadie, C.; Davignon, J.L.; Combette, J.M.; Turrin, C.O.; Poupot, R. An azabisphosphonate-capped poly(phosphorhydrazone) dendrimer for the treatment of endotoxin-induced uveitis. Molecules 2013, 18, 9305–9316. [Google Scholar] [CrossRef] [Green Version]
- Hayder, M.; Varilh, M.; Turrin, C.O.; Saoudi, A.; Caminade, A.M.; Poupot, R.; Liblau, R.S. Phosphorus-based dendrimer ABP treats neuroinflammation by promoting IL-10-producing CD4+ T cells. Biomacromolecules 2015, 16, 3425–3433. [Google Scholar] [CrossRef]
- Hayder, M.; Garzoni, M.; Bochicchio, D.; Caminade, A.M.; Couderc, F.; Ong-Meang, V.; Davignon, J.L.; Turrin, C.O.; Pavan, G.M.; Poupot, R. Three-dimensional directionality is a pivotal structural feature for the bioactivity of azabisphosphonate-capped poly(phosphorhydrazone) nanodrug dendrimers. Biomacromolecules 2018, 19, 712–720. [Google Scholar] [CrossRef]
- Jebbawi, R.; Oukhrib, A.; Clement, E.; Blanzat, M.; Turrin, C.O.; Caminade, A.M.; Lacoste, E.; Fruchon, S.; Poupot, R. An anti-inflammatory poly(phosphorhydrazone) dendrimer capped with azabisphosphonate groups to treat psoriasis. Biomolecules 2020, 10, 949. [Google Scholar] [CrossRef] [PubMed]
- Shaunak, S. Perspective: Dendrimer drugs for infection and inflammation. Biochem. Biophys. Res. Commun. 2015, 468, 435–441. [Google Scholar] [CrossRef] [PubMed]

| API | Nanocarriers | Permeability Coefficient, Kp (cm/h) | Steady State Flux, JSS (µg/cm2/h) | Reference |
|---|---|---|---|---|
| minoxidil | nanoemulsion | Nd 1 | 1.9 2/18.1 | [20] |
| betamethasone dipropionate | microemulsion | nd | 3.95 | [22] |
| NLC | nd | 1.53 | ||
| SLN | nd | 0.79 | ||
| adapalene | tyrospheres | nd | 1.4 | [33] |
| mometasone furoate | drug-loaded gel | 9.82 × 10−3 | 3.27 | [43] |
| NLC dispersion | 5.76 × 10−3 | 1.93 | ||
| NLC-based hydrogel | 4.47 × 10−3 | 1.49 | ||
| marketed formulation | 10.51 × 10−3 | 3.50 | ||
| 5-fluorouracyl | PAMAM dendrimers | 1.86 | 67.0 | [45] dendrimers are used as pretreatment |
| G2-cationic | 5.39 | 194.4 | ||
| G4-cationic 0.1/1/10 mM | 3.48/4.65/6.54 | 125.5/167.5/235.6 | ||
| G6-cationic | 2.67 | 96.4 | ||
| G4-neutral | 3.62 | 130.5 | ||
| G3.5-anionic | 2.79 | 100.5 | ||
| indomethacin | PAMAM dendrimers: | 61.2 × 10−3 | 1.53 | [46] |
| G4.5-anionic | 15.0 × 10−3 | 1.83 | ||
| G4-neutral | 6.7 × 10−3 | 2.17 | ||
| G4-cationic | 10.1 × 10−3 | 3.77 | ||
| salicylic acid | PAMAM dendrimers | nd | 38.5 | [47] dendrimers are used as pretreatment |
| G2-cationic (1 and 10 mM) | nd | 38.1/66.3 | ||
| G3-cationic | nd | 34.3 | ||
| dithranol | PPI dendrimers | nd | 2.72 | [48] |
| G5-cationic | nd | 11.61 | ||
| diclofenac sodium | Oleodendrimers (PETIM dend. + oleic acid) | 0.58 × 10−4 | 0.87 | [49] |
| oleic acid as enhancer | 1.09 × 10−4 | 1.63 | ||
| oleodendrimer E1E | 1.94 × 10−4 | 2.90 | ||
| oleodendrimer E2E | 1.97 × 10−4 | 2.95 | ||
| oleodendrimer A1E | 1.17 × 10−4 | 1.76 | ||
| oleodendrimer A2E | 1.50 × 10−4 | 2.24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jebbawi, R.; Fruchon, S.; Turrin, C.-O.; Blanzat, M.; Poupot, R. Supramolecular and Macromolecular Matrix Nanocarriers for Drug Delivery in Inflammation-Associated Skin Diseases. Pharmaceutics 2020, 12, 1224. https://doi.org/10.3390/pharmaceutics12121224
Jebbawi R, Fruchon S, Turrin C-O, Blanzat M, Poupot R. Supramolecular and Macromolecular Matrix Nanocarriers for Drug Delivery in Inflammation-Associated Skin Diseases. Pharmaceutics. 2020; 12(12):1224. https://doi.org/10.3390/pharmaceutics12121224
Chicago/Turabian StyleJebbawi, Ranime, Séverine Fruchon, Cédric-Olivier Turrin, Muriel Blanzat, and Rémy Poupot. 2020. "Supramolecular and Macromolecular Matrix Nanocarriers for Drug Delivery in Inflammation-Associated Skin Diseases" Pharmaceutics 12, no. 12: 1224. https://doi.org/10.3390/pharmaceutics12121224





