Neutrophils and Macrophages as Targets for Development of Nanotherapeutics in Inflammatory Diseases
Abstract
1. Introduction
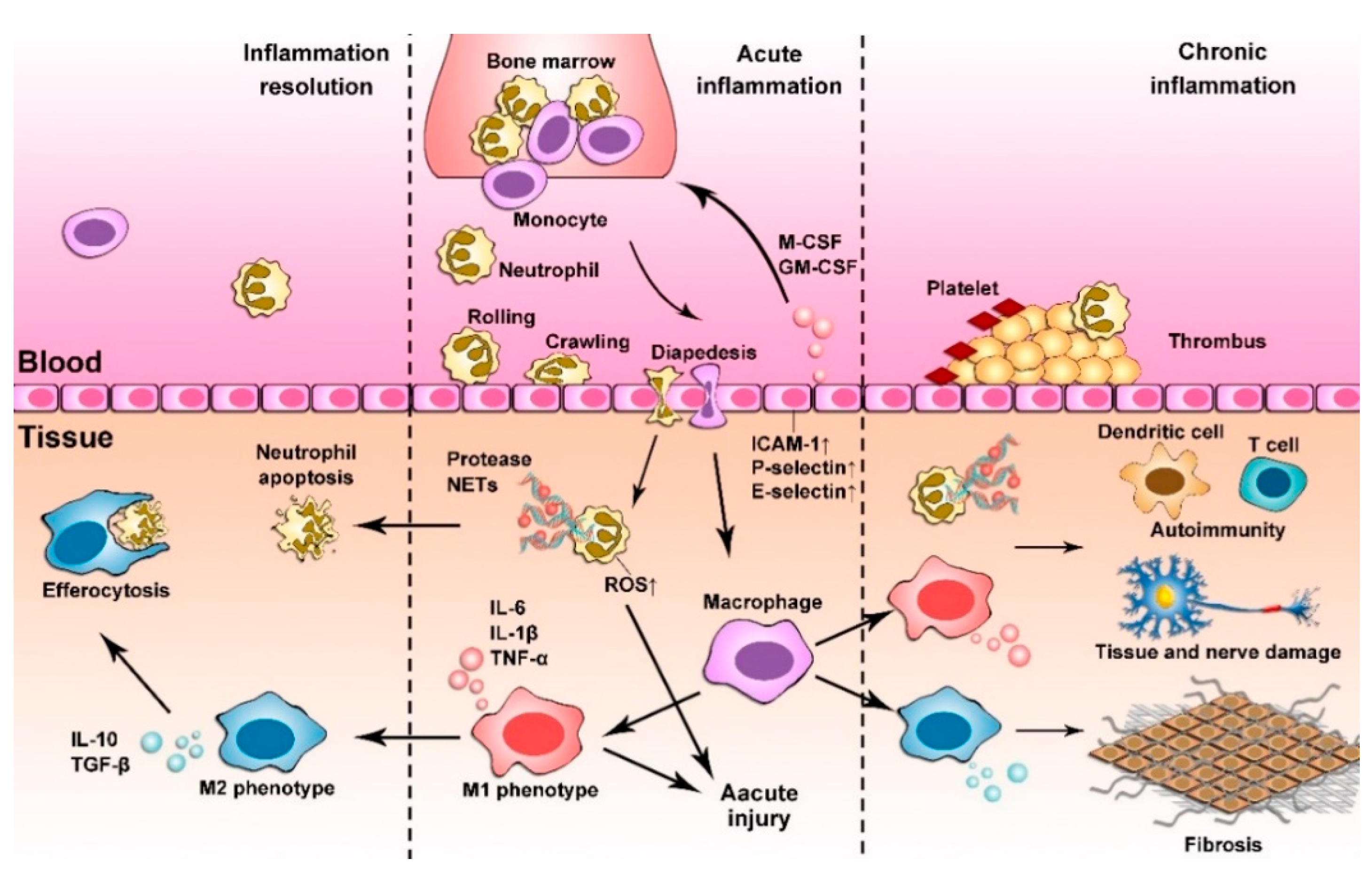
2. Inflammation Responses
2.1. Acute Inflammation
2.2. Chronic Inflammation
3. Immuno-Targeting by Nanoparticles
3.1. Nanoparticle Passive Targeting Immune Cells
3.2. Nanoparticle Active Targeting Immune Cells
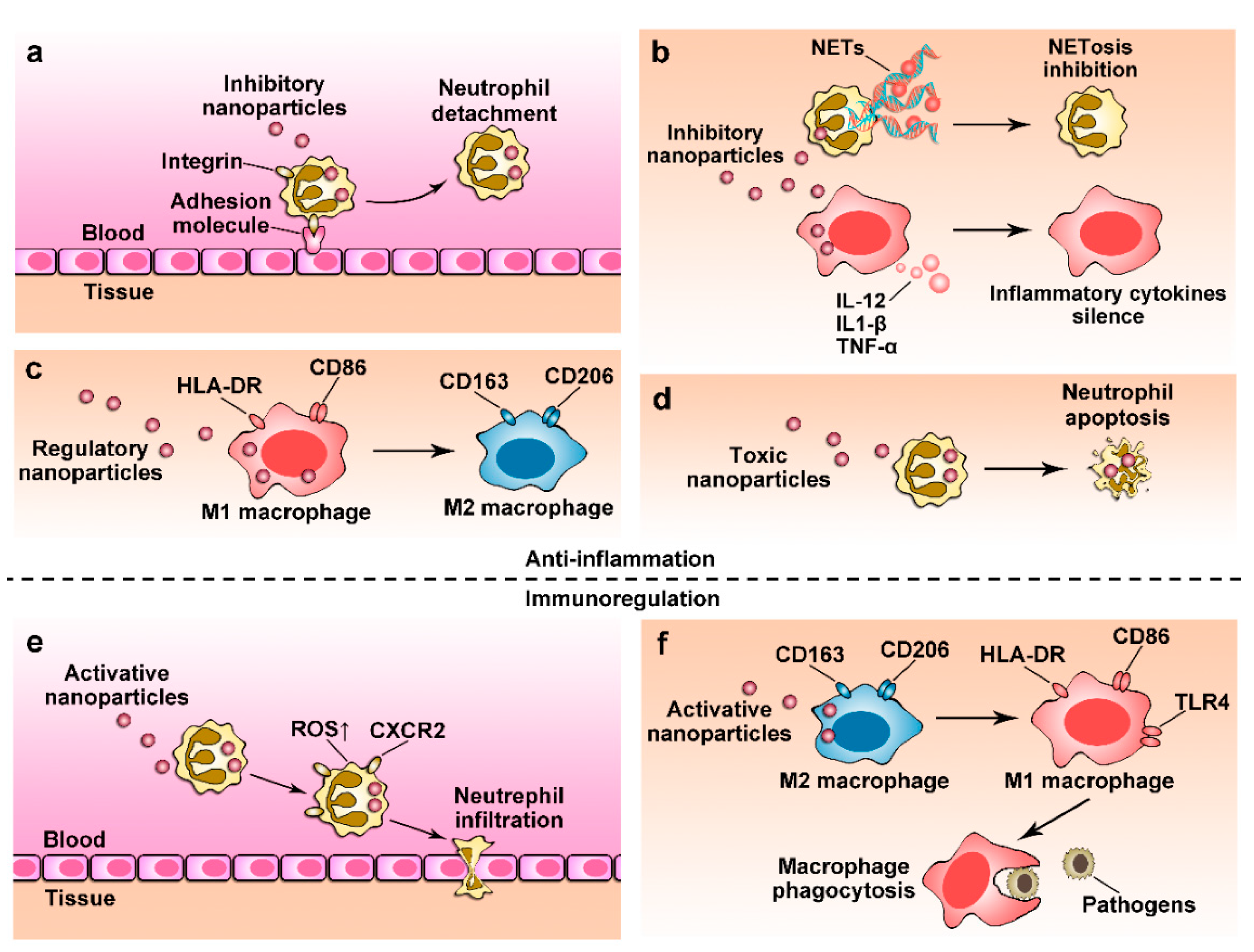
4. Nanoparticles for Anti-Inflammation Therapy
4.1. Regulating Immune Cell Transmigration
4.2. Depleting Immune Cells Using Nanoparticles
4.3. Controlling Cellular Phenotypes
4.4. Regulating Cellular Functions
5. Restoring Immune Functions Using Nanoparticles
5.1. Restoring Neutrophil Functions
5.2. Reactivating Macrophage Immunity
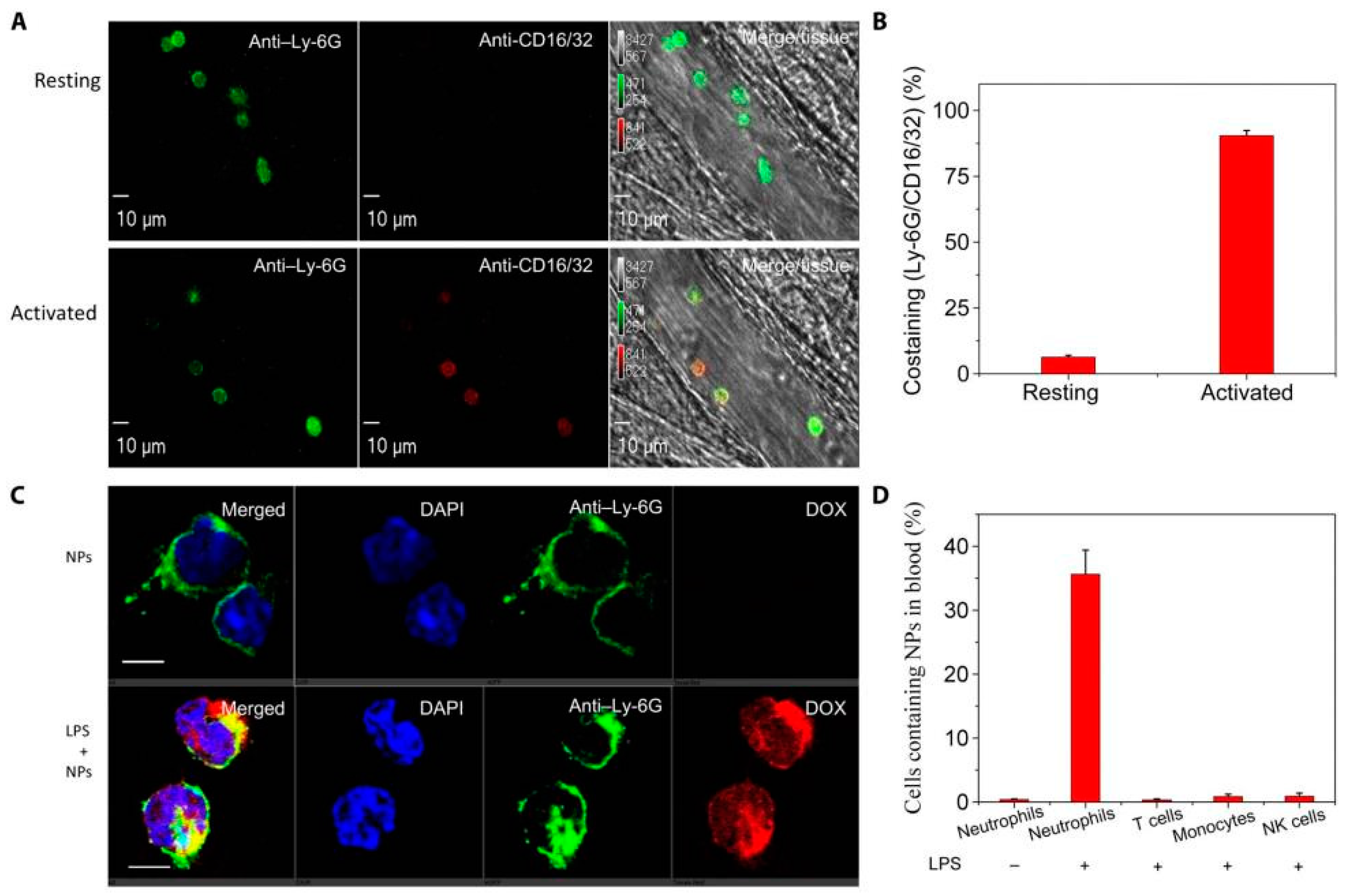
6. Delivery of Nanotherapeutics via Leukocytes
6.1. In Situ Hijacking of Leukocytes to Deliver Nanotherapeutics
6.2. Nanoparticle-Laden Leukocytes In Vitro
7. Immunotoxicity in Leukocytes of Nanoparticle Uptake
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef]
- Henson, P.M. Dampening inflammation. Nat. Immunol. 2005, 6, 1179–1181. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Wang, S.; Dong, X.; Leanse, L.G.; Dai, T.; Wang, Z. Co-delivery of resolvin d1 and antibiotics with nanovesicles to lungs resolves inflammation and clears bacteria in mice. Commun. Biol. 2020, 3, 680. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Gao, J.; Su, Y.; Wang, Z. Nanomedicine for ischemic stroke. Int. J. Mol. Sci. 2020, 21, 7600. [Google Scholar] [CrossRef] [PubMed]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef] [PubMed]
- Amulic, B.; Cazalet, C.; Hayes, G.L.; Metzler, K.D.; Zychlinsky, A. Neutrophil function: From mechanisms to disease. Annu. Rev. Immunol. 2012, 30, 459–489. [Google Scholar] [CrossRef] [PubMed]
- Summers, C.; Rankin, S.M.; Condliffe, A.M.; Singh, N.; Peters, A.M.; Chilvers, E.R. Neutrophil kinetics in health and disease. Trends Immunol. 2010, 31, 318–324. [Google Scholar] [CrossRef]
- Wright, H.L.; Moots, R.J.; Bucknall, R.C.; Edwards, S.W. Neutrophil function in inflammation and inflammatory diseases. Rheumatology 2010, 49, 1618–1631. [Google Scholar] [CrossRef]
- Lee, S.; Huen, S.; Nishio, H.; Nishio, S.; Lee, H.K.; Choi, B.S.; Ruhrberg, C.; Cantley, L.G. Distinct macrophage phenotypes contribute to kidney injury and repair. J. Am. Soc. Nephrol. JASN 2011, 22, 317–326. [Google Scholar] [CrossRef]
- Serhan, C.N.; Savill, J. Resolution of inflammation: The beginning programs the end. Nat. Immunol. 2005, 6, 1191–1197. [Google Scholar] [CrossRef]
- Soehnlein, O.; Steffens, S.; Hidalgo, A.; Weber, C. Neutrophils as protagonists and targets in chronic inflammation. Nat. Rev. Immunol. 2017, 17, 248–261. [Google Scholar] [CrossRef] [PubMed]
- Grieshaber-Bouyer, R.; Nigrovic, P.A. Neutrophil heterogeneity as therapeutic opportunity in immune-mediated disease. Front. Immunol. 2019, 10, 346. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Choi, E.Y. Macrophages and inflammation. J. Rheum. Dis. 2018, 25, 11–18. [Google Scholar] [CrossRef]
- Mejbri, M.; Theodoropoulou, K.; Hofer, M.; Cimaz, R. Interleukin-1 blockade in systemic juvenile idiopathic arthritis. Paediatr. Drugs 2020, 22, 251–262. [Google Scholar] [CrossRef]
- De Benedetti, F.; Brunner, H.I.; Ruperto, N.; Kenwright, A.; Wright, S.; Calvo, I.; Cuttica, R.; Ravelli, A.; Schneider, R.; Woo, P.; et al. Randomized trial of tocilizumab in systemic juvenile idiopathic arthritis. N. Engl. J. Med. 2012, 367, 2385–2395. [Google Scholar] [CrossRef]
- Boraschi, D.; Italiani, P.; Palomba, R.; Decuzzi, P.; Duschl, A.; Fadeel, B.; Moghimi, S.M. Nanoparticles and innate immunity: New perspectives on host defence. Semin. Immunol. 2017, 34, 33–51. [Google Scholar] [CrossRef]
- Fadeel, B. Clear and present danger? Engineered nanoparticles and the immune system. Swiss Med Wkly. 2012, 142, w13609. [Google Scholar] [CrossRef]
- Kolaczkowska, E.; Kubes, P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 2013, 13, 159–175. [Google Scholar] [CrossRef]
- Sheshachalam, A.; Srivastava, N.; Mitchell, T.; Lacy, P.; Eitzen, G. Granule protein processing and regulated secretion in neutrophils. Front. Immunol. 2014, 5, 448. [Google Scholar] [CrossRef]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef]
- Gomez Perdiguero, E.; Klapproth, K.; Schulz, C.; Busch, K.; Azzoni, E.; Crozet, L.; Garner, H.; Trouillet, C.; de Bruijn, M.F.; Geissmann, F.; et al. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature 2015, 518, 547–551. [Google Scholar] [CrossRef] [PubMed]
- Epelman, S.; Lavine, K.J.; Randolph, G.J. Origin and functions of tissue macrophages. Immunity 2014, 41, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Mowat, A.M.; Scott, C.L.; Bain, C.C. Barrier-tissue macrophages: Functional adaptation to environmental challenges. Nat. Med. 2017, 23, 1258–1270. [Google Scholar] [CrossRef]
- Sica, A.; Erreni, M.; Allavena, P.; Porta, C. Macrophage polarization in pathology. Cell. Mol. Life Sci. CMLS 2015, 72, 4111–4126. [Google Scholar] [CrossRef] [PubMed]
- Parisi, L.; Gini, E.; Baci, D.; Tremolati, M.; Fanuli, M.; Bassani, B.; Farronato, G.; Bruno, A.; Mortara, L. Macrophage polarization in chronic inflammatory diseases: Killers or builders? J. Immunol. Res. 2018, 2018, 8917804. [Google Scholar] [CrossRef] [PubMed]
- Adrover, J.M.; Nicolas-Avila, J.A.; Hidalgo, A. Aging: A temporal dimension for neutrophils. Trends Immunol. 2016, 37, 334–345. [Google Scholar] [CrossRef]
- Oishi, Y.; Manabe, I. Macrophages in age-related chronic inflammatory diseases. NPJ Aging Mech. Dis. 2016, 2, 16018. [Google Scholar] [CrossRef]
- Nemeth, T.; Sperandio, M.; Mocsai, A. Neutrophils as emerging therapeutic targets. Nat. Rev. Drug Discov. 2020, 19, 253–275. [Google Scholar] [CrossRef]
- Chawla, A.; Nguyen, K.D.; Goh, Y.P. Macrophage-mediated inflammation in metabolic disease. Nat. Rev. Immunol. 2011, 11, 738–749. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Gao, J.; Wang, Z. Bioresponsive nanoparticles targeted to infectious microenvironments for sepsis management. Adv. Mater. 2018, 30, e1803618. [Google Scholar] [CrossRef]
- Hotchkiss, R.S.; Monneret, G.; Payen, D. Sepsis-induced immunosuppression: From cellular dysfunctions to immunotherapy. Nat. Rev. Immunol. 2013, 13, 862–874. [Google Scholar] [CrossRef] [PubMed]
- Sonego, F.; Castanheira, F.V.; Ferreira, R.G.; Kanashiro, A.; Leite, C.A.; Nascimento, D.C.; Colon, D.F.; Borges Vde, F.; Alves-Filho, J.C.; Cunha, F.Q. Paradoxical roles of the neutrophil in sepsis: Protective and deleterious. Front. Immunol. 2016, 7, 155. [Google Scholar] [CrossRef]
- Sonego, F.; Alves-Filho, J.C.; Cunha, F.Q. Targeting neutrophils in sepsis. Expert Rev. Clin. Immunol. 2014, 10, 1019–1028. [Google Scholar] [CrossRef] [PubMed]
- Porta, C.; Rimoldi, M.; Raes, G.; Brys, L.; Ghezzi, P.; Di Liberto, D.; Dieli, F.; Ghisletti, S.; Natoli, G.; De Baetselier, P.; et al. Tolerance and m2 (alternative) macrophage polarization are related processes orchestrated by p50 nuclear factor kappab. Proc. Natl. Acad. Sci. USA 2009, 106, 14978–14983. [Google Scholar] [CrossRef] [PubMed]
- Mizuma, A.; Yenari, M.A. Anti-inflammatory targets for the treatment of reperfusion injury in stroke. Front. Neurol. 2017, 8, 467. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Prieto, J.; Villena-Gutierrez, R.; Gomez, M.; Bernardo, E.; Pun-Garcia, A.; Garcia-Lunar, I.; Crainiciuc, G.; Fernandez-Jimenez, R.; Sreeramkumar, V.; Bourio-Martinez, R.; et al. Neutrophil stunning by metoprolol reduces infarct size. Nat. Commun. 2017, 8, 14780. [Google Scholar] [CrossRef] [PubMed]
- Anzai, A.; Choi, J.L.; He, S.; Fenn, A.M.; Nairz, M.; Rattik, S.; McAlpine, C.S.; Mindur, J.E.; Chan, C.T.; Iwamoto, Y.; et al. The infarcted myocardium solicits gm-csf for the detrimental oversupply of inflammatory leukocytes. J. Exp. Med. 2017, 214, 3293–3310. [Google Scholar] [CrossRef] [PubMed]
- Banati, R.B.; Gehrmann, J.; Schubert, P.; Kreutzberg, G.W. Cytotoxicity of microglia. Glia 1993, 7, 111–118. [Google Scholar] [CrossRef]
- Barone, F.C.; Arvin, B.; White, R.F.; Miller, A.; Webb, C.L.; Willette, R.N.; Lysko, P.G.; Feuerstein, G.Z. Tumor necrosis factor-α. A mediator of focal ischemic brain injury. Stroke 1997, 28, 1233–1244. [Google Scholar] [CrossRef]
- Han, D.; Liu, H.; Gao, Y. The role of peripheral monocytes and macrophages in ischemic stroke. Neurol. Sci. 2020, 41, 3589–3607. [Google Scholar] [CrossRef]
- Mouton, A.J.; Li, X.; Hall, M.E.; Hall, J.E. Obesity, hypertension, and cardiac dysfunction: Novel roles of immunometabolism in macrophage activation and inflammation. Circ. Res. 2020, 126, 789–806. [Google Scholar] [CrossRef]
- Kammoun, H.L.; Kraakman, M.J.; Febbraio, M.A. Adipose tissue inflammation in glucose metabolism. Rev. Endocr. Metab. Disord. 2014, 15, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Lackey, D.E.; Olefsky, J.M. Regulation of metabolism by the innate immune system. Nat. Rev. Endocrinol. 2016, 12, 15–28. [Google Scholar] [CrossRef]
- Elgazar-Carmon, V.; Rudich, A.; Hadad, N.; Levy, R. Neutrophils transiently infiltrate intra-abdominal fat early in the course of high-fat feeding. J. Lipid Res. 2008, 49, 1894–1903. [Google Scholar] [CrossRef] [PubMed]
- Talukdar, S.; Oh, D.Y.; Bandyopadhyay, G.; Li, D.; Xu, J.; McNelis, J.; Lu, M.; Li, P.; Yan, Q.; Zhu, Y.; et al. Neutrophils mediate insulin resistance in mice fed a high-fat diet through secreted elastase. Nat. Med. 2012, 18, 1407–1412. [Google Scholar] [CrossRef] [PubMed]
- Barbarin, V.; Xing, Z.; Delos, M.; Lison, D.; Huaux, F. Pulmonary overexpression of il-10 augments lung fibrosis and th2 responses induced by silica particles. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005, 288, L841–L848. [Google Scholar] [CrossRef]
- Lee, C.G.; Homer, R.J.; Zhu, Z.; Lanone, S.; Wang, X.; Koteliansky, V.; Shipley, J.M.; Gotwals, P.; Noble, P.; Chen, Q.; et al. Interleukin-13 induces tissue fibrosis by selectively stimulating and activating transforming growth factor β1. J. Exp. Med. 2001, 194, 809–821. [Google Scholar] [CrossRef]
- Naura, A.S.; Zerfaoui, M.; Kim, H.; Abd Elmageed, Z.Y.; Rodriguez, P.C.; Hans, C.P.; Ju, J.; Errami, Y.; Park, J.; Ochoa, A.C.; et al. Requirement for inducible nitric oxide synthase in chronic allergen exposure-induced pulmonary fibrosis but not inflammation. J. Immunol. 2010, 185, 3076–3085. [Google Scholar] [CrossRef]
- Laval, J.; Ralhan, A.; Hartl, D. Neutrophils in cystic fibrosis. Biol. Chem. 2016, 397, 485–496. [Google Scholar] [CrossRef]
- Bazzan, E.; Turato, G.; Tine, M.; Radu, C.M.; Balestro, E.; Rigobello, C.; Biondini, D.; Schiavon, M.; Lunardi, F.; Baraldo, S.; et al. Dual polarization of human alveolar macrophages progressively increases with smoking and copd severity. Respir. Res. 2017, 18, 40. [Google Scholar] [CrossRef]
- Khandpur, R.; Carmona-Rivera, C.; Vivekanandan-Giri, A.; Gizinski, A.; Yalavarthi, S.; Knight, J.S.; Friday, S.; Li, S.; Patel, R.M.; Subramanian, V.; et al. Nets are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci. Transl. Med. 2013, 5, 178ra140. [Google Scholar] [CrossRef]
- Lood, C.; Blanco, L.P.; Purmalek, M.M.; Carmona-Rivera, C.; De Ravin, S.S.; Smith, C.K.; Malech, H.L.; Ledbetter, J.A.; Elkon, K.B.; Kaplan, M.J. Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease. Nat. Med. 2016, 22, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Fulop, T.; Larbi, A.; Douziech, N.; Fortin, C.; Guerard, K.P.; Lesur, O.; Khalil, A.; Dupuis, G. Signal transduction and functional changes in neutrophils with aging. Aging Cell 2004, 3, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.W.; Liu, G.Y. Expanding roles of neutrophils in aging hosts. Curr. Opin. Immunol. 2014, 29, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Hearps, A.C.; Martin, G.E.; Angelovich, T.A.; Cheng, W.J.; Maisa, A.; Landay, A.L.; Jaworowski, A.; Crowe, S.M. Aging is associated with chronic innate immune activation and dysregulation of monocyte phenotype and function. Aging Cell 2012, 11, 867–875. [Google Scholar] [CrossRef] [PubMed]
- Bonotis, K.; Krikki, E.; Holeva, V.; Aggouridaki, C.; Costa, V.; Baloyannis, S. Systemic immune aberrations in alzheimer’s disease patients. J. Neuroimmunol. 2008, 193, 183–187. [Google Scholar] [CrossRef]
- Fiala, M.; Lin, J.; Ringman, J.; Kermani-Arab, V.; Tsao, G.; Patel, A.; Lossinsky, A.S.; Graves, M.C.; Gustavson, A.; Sayre, J.; et al. Ineffective phagocytosis of amyloid-beta by macrophages of alzheimer’s disease patients. J. Alzheimer’s Dis. JAD 2005, 7, 221–232; discussion 255–262. [Google Scholar] [CrossRef]
- Zenaro, E.; Pietronigro, E.; Della Bianca, V.; Piacentino, G.; Marongiu, L.; Budui, S.; Turano, E.; Rossi, B.; Angiari, S.; Dusi, S.; et al. Neutrophils promote alzheimer’s disease-like pathology and cognitive decline via lfa-1 integrin. Nat. Med. 2015, 21, 880–886. [Google Scholar] [CrossRef]
- Torchilin, V.P. Multifunctional, stimuli-sensitive nanoparticulate systems for drug delivery. Nat. Rev. Drug Discov. 2014, 13, 813–827. [Google Scholar] [CrossRef]
- Wang, A.Z.; Langer, R.; Farokhzad, O.C. Nanoparticle delivery of cancer drugs. Annu. Rev. Med. 2012, 63, 185–198. [Google Scholar] [CrossRef]
- Wang, Z.; Tiruppathi, C.; Cho, J.; Minshall, R.D.; Malik, A.B. Delivery of nanoparticle: Complexed drugs across the vascular endothelial barrier via caveolae. IUBMB Life 2011, 63, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Dong, X.; Wang, Z. Generation, purification and engineering of extracellular vesicles and their biomedical applications. Methods 2020, 177, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Chu, D.; Wang, Z. Cell membrane-formed nanovesicles for disease-targeted delivery. J. Control Release 2016, 224, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Fromen, C.A.; Kelley, W.J.; Fish, M.B.; Adili, R.; Noble, J.; Hoenerhoff, M.J.; Holinstat, M.; Eniola-Adefeso, O. Neutrophil-particle interactions in blood circulation drive particle clearance and alter neutrophil responses in acute inflammation. ACS Nano 2017, 11, 10797–10807. [Google Scholar] [CrossRef] [PubMed]
- Moghimi, S.M.; Simberg, D. Complement activation turnover on surfaces of nanoparticles. Nano Today 2017, 15, 8–10. [Google Scholar] [CrossRef]
- Vu, V.P.; Gifford, G.B.; Chen, F.; Benasutti, H.; Wang, G.; Groman, E.V.; Scheinman, R.; Saba, L.; Moghimi, S.M.; Simberg, D. Immunoglobulin deposition on biomolecule corona determines complement opsonization efficiency of preclinical and clinical nanoparticles. Nat. Nanotechnol. 2019, 14, 260–268. [Google Scholar] [CrossRef]
- Davies, L.C.; Jenkins, S.J.; Allen, J.E.; Taylor, P.R. Tissue-resident macrophages. Nat. Immunol. 2013, 14, 986–995. [Google Scholar] [CrossRef]
- Lapenna, A.; De Palma, M.; Lewis, C.E. Perivascular macrophages in health and disease. Nat. Rev. Immunol. 2018, 18, 689–702. [Google Scholar] [CrossRef]
- Baer, C.; Squadrito, M.L.; Iruela-Arispe, M.L.; De Palma, M. Reciprocal interactions between endothelial cells and macrophages in angiogenic vascular niches. Exp. Cell Res. 2013, 319, 1626–1634. [Google Scholar] [CrossRef]
- Lewis, C.E.; Harney, A.S.; Pollard, J.W. The multifaceted role of perivascular macrophages in tumors. Cancer Cell 2016, 30, 18–25. [Google Scholar] [CrossRef]
- Mendes-Jorge, L.; Ramos, D.; Luppo, M.; Llombart, C.; Alexandre-Pires, G.; Nacher, V.; Melgarejo, V.; Correia, M.; Navarro, M.; Carretero, A.; et al. Scavenger function of resident autofluorescent perivascular macrophages and their contribution to the maintenance of the blood-retinal barrier. Investig. Ophthalmol. Vis. Sci. 2009, 50, 5997–6005. [Google Scholar] [CrossRef]
- Ferris, S.T.; Carrero, J.A.; Mohan, J.F.; Calderon, B.; Murphy, K.M.; Unanue, E.R. A minor subset of batf3-dependent antigen-presenting cells in islets of langerhans is essential for the development of autoimmune diabetes. Immunity 2014, 41, 657–669. [Google Scholar] [CrossRef] [PubMed]
- Stamatiades, E.G.; Tremblay, M.E.; Bohm, M.; Crozet, L.; Bisht, K.; Kao, D.; Coelho, C.; Fan, X.; Yewdell, W.T.; Davidson, A.; et al. Immune monitoring of trans-endothelial transport by kidney-resident macrophages. Cell 2016, 166, 991–1003. [Google Scholar] [CrossRef] [PubMed]
- Niemi, M.; Sharpe, R.M.; Brown, W.R. Macrophages in the interstitial tissue of the rat testis. Cell Tissue Res. 1986, 243, 337–344. [Google Scholar] [CrossRef]
- Dai, Q.; Wilhelm, S.; Ding, D.; Syed, A.M.; Sindhwani, S.; Zhang, Y.; Chen, Y.Y.; MacMillan, P.; Chan, W.C.W. Quantifying the ligand-coated nanoparticle delivery to cancer cells in solid tumors. ACS Nano 2018, 12, 8423–8435. [Google Scholar] [CrossRef] [PubMed]
- Bisso, P.W.; Gaglione, S.; Guimaraes, P.P.G.; Mitchell, M.J.; Langer, R. Nanomaterial interactions with human neutrophils. ACS Biomater. Sci. Eng. 2018, 4, 4255–4265. [Google Scholar] [CrossRef]
- Perry, J.L.; Reuter, K.G.; Luft, J.C.; Pecot, C.V.; Zamboni, W.; DeSimone, J.M. Mediating passive tumor accumulation through particle size, tumor type, and location. Nano Lett. 2017, 17, 2879–2886. [Google Scholar] [CrossRef]
- Tong, X.; Wang, Z.; Sun, X.; Song, J.; Jacobson, O.; Niu, G.; Kiesewetter, D.O.; Chen, X. Size dependent kinetics of gold nanorods in epr mediated tumor delivery. Theranostics 2016, 6, 2039–2051. [Google Scholar] [CrossRef]
- Safari, H.; Kelley, W.J.; Saito, E.; Kaczorowski, N.; Carethers, L.; Shea, L.D.; Eniola-Adefeso, O. Neutrophils preferentially phagocytose elongated particles-an opportunity for selective targeting in acute inflammatory diseases. Sci. Adv. 2020, 6, eaba1474. [Google Scholar] [CrossRef]
- Kelley, W.J.; Fromen, C.A.; Lopez-Cazares, G.; Eniola-Adefeso, O. Pegylation of model drug carriers enhances phagocytosis by primary human neutrophils. Acta Biomater. 2018, 79, 283–293. [Google Scholar] [CrossRef]
- Yu, S.S.; Lau, C.M.; Thomas, S.N.; Jerome, W.G.; Maron, D.J.; Dickerson, J.H.; Hubbell, J.A.; Giorgio, T.D. Size- and charge-dependent non-specific uptake of pegylated nanoparticles by macrophages. Int. J. Nanomed. 2012, 7, 799–813. [Google Scholar] [CrossRef]
- Champion, J.A.; Mitragotri, S. Role of target geometry in phagocytosis. Proc. Natl. Acad. Sci. USA 2006, 103, 4930–4934. [Google Scholar] [CrossRef]
- Geng, Y.; Dalhaimer, P.; Cai, S.; Tsai, R.; Tewari, M.; Minko, T.; Discher, D.E. Shape effects of filaments versus spherical particles in flow and drug delivery. Nat. Nanotechnol. 2007, 2, 249–255. [Google Scholar] [CrossRef]
- Champion, J.A.; Walker, A.; Mitragotri, S. Role of particle size in phagocytosis of polymeric microspheres. Pharm. Res. 2008, 25, 1815–1821. [Google Scholar] [CrossRef]
- Saha, K.; Rahimi, M.; Yazdani, M.; Kim, S.T.; Moyano, D.F.; Hou, S.; Das, R.; Mout, R.; Rezaee, F.; Mahmoudi, M.; et al. Regulation of macrophage recognition through the interplay of nanoparticle surface functionality and protein corona. ACS Nano 2016, 10, 4421–4430. [Google Scholar] [CrossRef]
- Ergen, C.; Heymann, F.; Al Rawashdeh, W.; Gremse, F.; Bartneck, M.; Panzer, U.; Pola, R.; Pechar, M.; Storm, G.; Mohr, N.; et al. Targeting distinct myeloid cell populations in vivo using polymers, liposomes and microbubbles. Biomaterials 2017, 114, 106–120. [Google Scholar] [CrossRef]
- Zhang, L.W.; Monteiro-Riviere, N.A. Mechanisms of quantum dot nanoparticle cellular uptake. Toxicol. Sci. 2009, 110, 138–155. [Google Scholar] [CrossRef]
- Palomba, R.; Palange, A.L.; Rizzuti, I.F.; Ferreira, M.; Cervadoro, A.; Barbato, M.G.; Canale, C.; Decuzzi, P. Modulating phagocytic cell sequestration by tailoring nanoconstruct softness. ACS Nano 2018, 12, 1433–1444. [Google Scholar] [CrossRef]
- Hui, Y.; Yi, X.; Wibowo, D.; Yang, G.; Middelberg, A.P.J.; Gao, H.; Zhao, C.X. Nanoparticle elasticity regulates phagocytosis and cancer cell uptake. Sci. Adv. 2020, 6, eaaz4316. [Google Scholar] [CrossRef]
- Chu, D.; Dong, X.; Zhao, Q.; Gu, J.; Wang, Z. Photosensitization priming of tumor microenvironments improves delivery of nanotherapeutics via neutrophil infiltration. Adv. Mater. 2017, 29, 1701021. [Google Scholar] [CrossRef]
- Nakatani, K.; Takeshita, S.; Tsujimoto, H.; Kawamura, Y.; Kawase, H.; Sekine, I. Regulation of the expression of fc gamma receptor on circulating neutrophils and monocytes in kawasaki disease. Clin. Exp. Immunol. 1999, 117, 418–422. [Google Scholar] [CrossRef]
- Chen, K.; Nishi, H.; Travers, R.; Tsuboi, N.; Martinod, K.; Wagner, D.D.; Stan, R.; Croce, K.; Mayadas, T.N. Endocytosis of soluble immune complexes leads to their clearance by fcgammariiib but induces neutrophil extracellular traps via fcgammariia in vivo. Blood 2012, 120, 4421–4431. [Google Scholar] [CrossRef]
- Wang, Z.; Li, J.; Cho, J.; Malik, A.B. Prevention of vascular inflammation by nanoparticle targeting of adherent neutrophils. Nat. Nanotechnol. 2014, 9, 204–210. [Google Scholar] [CrossRef]
- Fabriek, B.O.; Dijkstra, C.D.; van den Berg, T.K. The macrophage scavenger receptor cd163. Immunobiology 2005, 210, 153–160. [Google Scholar] [CrossRef]
- Andersen, M.N.; Etzerodt, A.; Graversen, J.H.; Holthof, L.C.; Moestrup, S.K.; Hokland, M.; Moller, H.J. Stat3 inhibition specifically in human monocytes and macrophages by cd163-targeted corosolic acid-containing liposomes. Cancer Immunol. Immunother. CII 2019, 68, 489–502. [Google Scholar] [CrossRef]
- Suzuki, Y.; Shirai, M.; Asada, K.; Yasui, H.; Karayama, M.; Hozumi, H.; Furuhashi, K.; Enomoto, N.; Fujisawa, T.; Nakamura, Y.; et al. Macrophage mannose receptor, cd206, predict prognosis in patients with pulmonary tuberculosis. Sci. Rep. 2018, 8, 13129. [Google Scholar] [CrossRef]
- Song, Y.; Tang, C.; Yin, C. Combination antitumor immunotherapy with vegf and pigf sirna via systemic delivery of multi-functionalized nanoparticles to tumor-associated macrophages and breast cancer cells. Biomaterials 2018, 185, 117–132. [Google Scholar] [CrossRef]
- Puig-Kroger, A.; Sierra-Filardi, E.; Dominguez-Soto, A.; Samaniego, R.; Corcuera, M.T.; Gomez-Aguado, F.; Ratnam, M.; Sanchez-Mateos, P.; Corbi, A.L. Folate receptor beta is expressed by tumor-associated macrophages and constitutes a marker for m2 anti-inflammatory/regulatory macrophages. Cancer Res. 2009, 69, 9395–9403. [Google Scholar] [CrossRef]
- Soto, E.R.; Caras, A.C.; Kut, L.C.; Castle, M.K.; Ostroff, G.R. Glucan particles for macrophage targeted delivery of nanoparticles. J. Drug Deliv. 2012, 2012, 143524. [Google Scholar] [CrossRef]
- Song, X.; Wan, Z.; Chen, T.; Fu, Y.; Jiang, K.; Yi, X.; Ke, H.; Dong, J.; Yang, L.; Li, L.; et al. Development of a multi-target peptide for potentiating chemotherapy by modulating tumor microenvironment. Biomaterials 2016, 108, 44–56. [Google Scholar] [CrossRef]
- Tang, C.; Wang, C.; Zhang, Y.; Xue, L.; Li, Y.; Ju, C.; Zhang, C. Recognition, intervention, and monitoring of neutrophils in acute ischemic stroke. Nano Lett. 2019, 19, 4470–4477. [Google Scholar] [CrossRef]
- Dong, X.; Gao, J.; Zhang, C.Y.; Hayworth, C.; Frank, M.; Wang, Z. Neutrophil membrane-derived nanovesicles alleviate inflammation to protect mouse brain injury from ischemic stroke. ACS Nano 2019, 13, 1272–1283. [Google Scholar] [CrossRef]
- Leuschner, F.; Dutta, P.; Gorbatov, R.; Novobrantseva, T.I.; Donahoe, J.S.; Courties, G.; Lee, K.M.; Kim, J.I.; Markmann, J.F.; Marinelli, B.; et al. Therapeutic sirna silencing in inflammatory monocytes in mice. Nat. Biotechnol. 2011, 29, 1005–1010. [Google Scholar] [CrossRef] [PubMed]
- Robertson, J.D.; Ward, J.R.; Avila-Olias, M.; Battaglia, G.; Renshaw, S.A. Targeting neutrophilic inflammation using polymersome-mediated cellular delivery. J. Immunol. 2017, 198, 3596–3604. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Dong, X.; Gao, J.; Lin, W.; Liu, Z.; Wang, Z. Nanoparticle-induced neutrophil apoptosis increases survival in sepsis and alleviates neurological damage in stroke. Sci. Adv. 2019, 5, eaax7964. [Google Scholar] [CrossRef]
- Van Rooijen, N.; Sanders, A. Liposome mediated depletion of macrophages: Mechanism of action, preparation of liposomes and applications. J. Immunol. Methods 1994, 174, 83–93. [Google Scholar] [CrossRef]
- Van Lent, P.L.; Holthuysen, A.E.; Van Rooijen, N.; Van De Putte, L.B.; Van Den Berg, W.B. Local removal of phagocytic synovial lining cells by clodronate-liposomes decreases cartilage destruction during collagen type ii arthritis. Ann. Rheum. Dis. 1998, 57, 408–413. [Google Scholar] [CrossRef]
- Barrera, P.; Blom, A.; van Lent, P.L.; van Bloois, L.; Beijnen, J.H.; van Rooijen, N.; de Waal Malefijt, M.C.; van de Putte, L.B.; Storm, G.; van den Berg, W.B. Synovial macrophage depletion with clodronate-containing liposomes in rheumatoid arthritis. Arthritis Rheum. 2000, 43, 1951–1959. [Google Scholar] [CrossRef]
- Waltl, I.; Kaufer, C.; Broer, S.; Chhatbar, C.; Ghita, L.; Gerhauser, I.; Anjum, M.; Kalinke, U.; Loscher, W. Macrophage depletion by liposome-encapsulated clodronate suppresses seizures but not hippocampal damage after acute viral encephalitis. Neurobiol. Dis. 2018, 110, 192–205. [Google Scholar] [CrossRef]
- Griesmann, H.; Drexel, C.; Milosevic, N.; Sipos, B.; Rosendahl, J.; Gress, T.M.; Michl, P. Pharmacological macrophage inhibition decreases metastasis formation in a genetic model of pancreatic cancer. Gut 2017, 66, 1278–1285. [Google Scholar] [CrossRef]
- Fink, K.; Ng, C.; Nkenfou, C.; Vasudevan, S.G.; van Rooijen, N.; Schul, W. Depletion of macrophages in mice results in higher dengue virus titers and highlights the role of macrophages for virus control. Eur. J. Immunol. 2009, 39, 2809–2821. [Google Scholar] [CrossRef] [PubMed]
- Chakravarthy, K.V.; Davidson, B.A.; Helinski, J.D.; Ding, H.; Law, W.C.; Yong, K.T.; Prasad, P.N.; Knight, P.R. Doxorubicin-conjugated quantum dots to target alveolar macrophages and inflammation. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Qindeel, M.; Khan, D.; Ahmed, N.; Khan, S.; Asim Ur, R. Surfactant-free, self-assembled nanomicelles-based transdermal hydrogel for safe and targeted delivery of methotrexate against rheumatoid arthritis. ACS Nano 2020, 14, 4662–4681. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Lobatto, M.E.; Hassing, L.; van der Staay, S.; van Rijs, S.M.; Calcagno, C.; Braza, M.S.; Baxter, S.; Fay, F.; Sanchez-Gaytan, B.L.; et al. Inhibiting macrophage proliferation suppresses atherosclerotic plaque inflammation. Sci. Adv. 2015, 1, e1400223. [Google Scholar] [CrossRef]
- Zhang, P.; Miska, J.; Lee-Chang, C.; Rashidi, A.; Panek, W.K.; An, S.; Zannikou, M.; Lopez-Rosas, A.; Han, Y.; Xiao, T.; et al. Therapeutic targeting of tumor-associated myeloid cells synergizes with radiation therapy for glioblastoma. Proc. Natl. Acad. Sci. USA 2019, 116, 23714–23723. [Google Scholar] [CrossRef]
- Zang, X.; Zhang, X.; Hu, H.; Qiao, M.; Zhao, X.; Deng, Y.; Chen, D. Targeted delivery of zoledronate to tumor-associated macrophages for cancer immunotherapy. Mol. Pharm. 2019, 16, 2249–2258. [Google Scholar] [CrossRef]
- Nagaraj, S.; Gabrilovich, D.I. Myeloid-derived suppressor cells. Adv. Exp. Med. Biol. 2007, 601, 213–223. [Google Scholar]
- Fridlender, Z.G.; Sun, J.; Kim, S.; Kapoor, V.; Cheng, G.; Ling, L.; Worthen, G.S.; Albelda, S.M. Polarization of tumor-associated neutrophil phenotype by TGF-β: “N1” versus “N2” tan. Cancer Cell 2009, 16, 183–194. [Google Scholar] [CrossRef]
- King, I.L.; Dickendesher, T.L.; Segal, B.M. Circulating ly-6C+ myeloid precursors migrate to the cns and play a pathogenic role during autoimmune demyelinating disease. Blood 2009, 113, 3190–3197. [Google Scholar] [CrossRef]
- Westendorf, A.M.; Fleissner, D.; Deppenmeier, S.; Gruber, A.D.; Bruder, D.; Hansen, W.; Liblau, R.; Buer, J. Autoimmune-mediated intestinal inflammation-impact and regulation of antigen-specific cd8+ T cells. Gastroenterology 2006, 131, 510–524. [Google Scholar] [CrossRef]
- Marhaba, R.; Vitacolonna, M.; Hildebrand, D.; Baniyash, M.; Freyschmidt-Paul, P.; Zoller, M. The importance of myeloid-derived suppressor cells in the regulation of autoimmune effector cells by a chronic contact eczema. J. Immunol. 2007, 179, 5071–5081. [Google Scholar] [CrossRef] [PubMed]
- Buckley, C.D.; Ross, E.A.; McGettrick, H.M.; Osborne, C.E.; Haworth, O.; Schmutz, C.; Stone, P.C.; Salmon, M.; Matharu, N.M.; Vohra, R.K.; et al. Identification of a phenotypically and functionally distinct population of long-lived neutrophils in a model of reverse endothelial migration. J. Leukoc. Biol. 2006, 79, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Fites, J.S.; Gui, M.; Kernien, J.F.; Negoro, P.; Dagher, Z.; Sykes, D.B.; Nett, J.E.; Mansour, M.K.; Klein, B.S. An unappreciated role for neutrophil-dc hybrids in immunity to invasive fungal infections. PLoS Pathog. 2018, 14, e1007073. [Google Scholar] [CrossRef]
- Singhal, S.; Bhojnagarwala, P.S.; O’Brien, S.; Moon, E.K.; Garfall, A.L.; Rao, A.S.; Quatromoni, J.G.; Stephen, T.L.; Litzky, L.; Deshpande, C.; et al. Origin and role of a subset of tumor-associated neutrophils with antigen-presenting cell features in early-stage human lung cancer. Cancer Cell 2016, 30, 120–135. [Google Scholar] [CrossRef] [PubMed]
- Andzinski, L.; Kasnitz, N.; Stahnke, S.; Wu, C.F.; Gereke, M.; von Kockritz-Blickwede, M.; Schilling, B.; Brandau, S.; Weiss, S.; Jablonska, J. Type I ifns induce anti-tumor polarization of tumor associated neutrophils in mice and human. Int. J. Cancer 2016, 138, 1982–1993. [Google Scholar] [CrossRef]
- Ma, Y.; Yabluchanskiy, A.; Iyer, R.P.; Cannon, P.L.; Flynn, E.R.; Jung, M.; Henry, J.; Cates, C.A.; Deleon-Pennell, K.Y.; Lindsey, M.L. Temporal neutrophil polarization following myocardial infarction. Cardiovasc. Res. 2016, 110, 51–61. [Google Scholar] [CrossRef]
- Pillay, J.; Ramakers, B.P.; Kamp, V.M.; Loi, A.L.; Lam, S.W.; Hietbrink, F.; Leenen, L.P.; Tool, A.T.; Pickkers, P.; Koenderman, L. Functional heterogeneity and differential priming of circulating neutrophils in human experimental endotoxemia. J. Leukoc. Biol. 2010, 88, 211–220. [Google Scholar] [CrossRef]
- Pillay, J.; Kamp, V.M.; van Hoffen, E.; Visser, T.; Tak, T.; Lammers, J.W.; Ulfman, L.H.; Leenen, L.P.; Pickkers, P.; Koenderman, L. A subset of neutrophils in human systemic inflammation inhibits T cell responses through mac-1. J. Clin. Investig. 2012, 122, 327–336. [Google Scholar] [CrossRef]
- Hacbarth, E.; Kajdacsy-Balla, A. Low density neutrophils in patients with systemic lupus erythematosus, rheumatoid arthritis, and acute rheumatic fever. Arthritis Rheum. 1986, 29, 1334–1342. [Google Scholar] [CrossRef]
- Yang, H.; Fung, S.Y.; Xu, S.; Sutherland, D.P.; Kollmann, T.R.; Liu, M.; Turvey, S.E. Amino acid-dependent attenuation of toll-like receptor signaling by peptide-gold nanoparticle hybrids. ACS Nano 2015, 9, 6774–6784. [Google Scholar] [CrossRef]
- Yang, H.; Kozicky, L.; Saferali, A.; Fung, S.Y.; Afacan, N.; Cai, B.; Falsafi, R.; Gill, E.; Liu, M.; Kollmann, T.R.; et al. Endosomal ph modulation by peptide-gold nanoparticle hybrids enables potent anti-inflammatory activity in phagocytic immune cells. Biomaterials 2016, 111, 90–102. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Fung, S.Y.; Bao, A.; Li, Q.; Turvey, S.E. Screening bioactive nanoparticles in phagocytic immune cells for inhibitors of toll-like receptor signaling. J. Vis. Exp. JoVE 2017, 125, 56075. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, H.; Sun, L.; Gao, W.; Xiong, Y.; Ma, A.; Liu, X.; Shen, L.; Li, Q.; Yang, H. Manipulation of macrophage polarization by peptide-coated gold nanoparticles and its protective effects on acute lung injury. J. Nanobiotechnol. 2020, 18, 38. [Google Scholar] [CrossRef] [PubMed]
- Raimondo, T.M.; Mooney, D.J. Functional muscle recovery with nanoparticle-directed m2 macrophage polarization in mice. Proc. Natl. Acad. Sci. USA 2018, 115, 10648–10653. [Google Scholar] [CrossRef]
- Alvarado-Vazquez, P.A.; Bernal, L.; Paige, C.A.; Grosick, R.L.; Moracho Vilrriales, C.; Ferreira, D.W.; Ulecia-Moron, C.; Romero-Sandoval, E.A. Macrophage-specific nanotechnology-driven cd163 overexpression in human macrophages results in an m2 phenotype under inflammatory conditions. Immunobiology 2017, 222, 900–912. [Google Scholar] [CrossRef]
- Kang, H.; Zhang, K.; Wong, D.S.H.; Han, F.; Li, B.; Bian, L. Near-infrared light-controlled regulation of intracellular calcium to modulate macrophage polarization. Biomaterials 2018, 178, 681–696. [Google Scholar] [CrossRef]
- Kraynak, C.A.; Yan, D.J.; Suggs, L.J. Modulating inflammatory macrophages with an apoptotic body-inspired nanoparticle. Acta Biomater. 2020, 108, 250–260. [Google Scholar] [CrossRef]
- Gao, Q.; Zhang, J.; Chen, C.; Chen, M.; Sun, P.; Du, W.; Zhang, S.; Liu, Y.; Zhang, R.; Bai, M.; et al. In situ mannosylated nanotrinity-mediated macrophage remodeling combats candida albicans infection. ACS Nano 2020, 14, 3980–3990. [Google Scholar] [CrossRef]
- Sollberger, G.; Choidas, A.; Burn, G.L.; Habenberger, P.; Di Lucrezia, R.; Kordes, S.; Menninger, S.; Eickhoff, J.; Nussbaumer, P.; Klebl, B.; et al. Gasdermin d plays a vital role in the generation of neutrophil extracellular traps. Sci. Immunol. 2018, 3, eaar6689. [Google Scholar] [CrossRef]
- Chen, K.W.; Monteleone, M.; Boucher, D.; Sollberger, G.; Ramnath, D.; Condon, N.D.; von Pein, J.B.; Broz, P.; Sweet, M.J.; Schroder, K. Noncanonical inflammasome signaling elicits gasdermin d-dependent neutrophil extracellular traps. Sci. Immunol. 2018, 3, eaar6676. [Google Scholar] [CrossRef]
- Jorch, S.K.; Kubes, P. An emerging role for neutrophil extracellular traps in noninfectious disease. Nat. Med. 2017, 23, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Daniel, C.; Leppkes, M.; Munoz, L.E.; Schley, G.; Schett, G.; Herrmann, M. Extracellular DNA traps in inflammation, injury and healing. Nat. Rev. Nephrol. 2019, 15, 559–575. [Google Scholar] [CrossRef]
- Campbell, A.M.; Kashgarian, M.; Shlomchik, M.J. Nadph oxidase inhibits the pathogenesis of systemic lupus erythematosus. Sci. Transl. Med. 2012, 4, 157ra141. [Google Scholar] [CrossRef] [PubMed]
- Cedervall, J.; Zhang, Y.; Huang, H.; Zhang, L.; Femel, J.; Dimberg, A.; Olsson, A.K. Neutrophil extracellular traps accumulate in peripheral blood vessels and compromise organ function in tumor-bearing animals. Cancer Res. 2015, 75, 2653–2662. [Google Scholar] [CrossRef] [PubMed]
- Okeke, E.B.; Louttit, C.; Fry, C.; Najafabadi, A.H.; Han, K.; Nemzek, J.; Moon, J.J. Inhibition of neutrophil elastase prevents neutrophil extracellular trap formation and rescues mice from endotoxic shock. Biomaterials 2020, 238, 119836. [Google Scholar] [CrossRef]
- Spence, S.; Greene, M.K.; Fay, F.; Hams, E.; Saunders, S.P.; Hamid, U.; Fitzgerald, M.; Beck, J.; Bains, B.K.; Smyth, P.; et al. Targeting siglecs with a sialic acid-decorated nanoparticle abrogates inflammation. Sci. Transl. Med. 2015, 7, 303ra140. [Google Scholar] [CrossRef]
- Bornhofft, K.F.; Viergutz, T.; Kuhnle, A.; Galuska, S.P. Nanoparticles equipped with α2,8-linked sialic acid chains inhibit the release of neutrophil extracellular traps. Nanomaterials 2019, 9, 610. [Google Scholar] [CrossRef] [PubMed]
- Laroui, H.; Theiss, A.L.; Yan, Y.; Dalmasso, G.; Nguyen, H.T.; Sitaraman, S.V.; Merlin, D. Functional TNFα gene silencing mediated by polyethyleneimine/ TNFα sirna nanocomplexes in inflamed colon. Biomaterials 2011, 32, 1218–1228. [Google Scholar] [CrossRef]
- Xiao, B.; Laroui, H.; Ayyadurai, S.; Viennois, E.; Charania, M.A.; Zhang, Y.; Merlin, D. Mannosylated bioreducible nanoparticle-mediated macrophage-specific TNF-α RNA interference for IBD therapy. Biomaterials 2013, 34, 7471–7482. [Google Scholar] [CrossRef]
- Kosovrasti, V.Y.; Nechev, L.V.; Amiji, M.M. Peritoneal macrophage-specific TNF-α gene silencing in LPS-induced acute inflammation model using CD44 targeting hyaluronic acid nanoparticles. Mol. Pharm. 2016, 13, 3404–3416. [Google Scholar] [CrossRef]
- Jiang, Y.; Hardie, J.; Liu, Y.; Ray, M.; Luo, X.; Das, R.; Landis, R.F.; Farkas, M.E.; Rotello, V.M. Nanocapsule-mediated cytosolic sirna delivery for anti-inflammatory treatment. J. Control. Release 2018, 283, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Duan, B.; Li, M.; Sun, Y.; Zou, S.; Xu, X. Orally delivered antisense oligodeoxyribonucleotides of TNF-α via polysaccharide-based nanocomposites targeting intestinal inflammation. Adv. Healthc. Mater. 2019, 8, e1801389. [Google Scholar] [CrossRef] [PubMed]
- Xiao, B.; Chen, Q.; Zhang, Z.; Wang, L.; Kang, Y.; Denning, T.; Merlin, D. Tnfα gene silencing mediated by orally targeted nanoparticles combined with interleukin-22 for synergistic combination therapy of ulcerative colitis. J. Control. Release 2018, 287, 235–246. [Google Scholar] [CrossRef]
- Peng, L.; Chen, X. Antibody-drug conjugates. Bioconjug. Chem. 2015, 26, 2169. [Google Scholar] [CrossRef]
- Luo, Y.L.; Xu, C.F.; Li, H.J.; Cao, Z.T.; Liu, J.; Wang, J.L.; Du, X.J.; Yang, X.Z.; Gu, Z.; Wang, J. Macrophage-specific in vivo gene editing using cationic lipid-assisted polymeric nanoparticles. ACS Nano 2018, 12, 994–1005. [Google Scholar] [CrossRef]
- Coffelt, S.B.; Kersten, K.; Doornebal, C.W.; Weiden, J.; Vrijland, K.; Hau, C.S.; Verstegen, N.J.M.; Ciampricotti, M.; Hawinkels, L.; Jonkers, J.; et al. IL-17-producing γβ T cells and neutrophils conspire to promote breast cancer metastasis. Nature 2015, 522, 345–348. [Google Scholar] [CrossRef]
- Wu, P.; Wu, D.; Ni, C.; Ye, J.; Chen, W.; Hu, G.; Wang, Z.; Wang, C.; Zhang, Z.; Xia, W.; et al. Gammadeltat17 cells promote the accumulation and expansion of myeloid-derived suppressor cells in human colorectal cancer. Immunity 2014, 40, 785–800. [Google Scholar] [CrossRef] [PubMed]
- Nywening, T.M.; Belt, B.A.; Cullinan, D.R.; Panni, R.Z.; Han, B.J.; Sanford, D.E.; Jacobs, R.C.; Ye, J.; Patel, A.A.; Gillanders, W.E.; et al. Targeting both tumour-associated CXCR2+ neutrophils and CCR2+ macrophages disrupts myeloid recruitment and improves chemotherapeutic responses in pancreatic ductal adenocarcinoma. Gut 2018, 67, 1112–1123. [Google Scholar] [CrossRef]
- Schott, A.F.; Goldstein, L.J.; Cristofanilli, M.; Ruffini, P.A.; McCanna, S.; Reuben, J.M.; Perez, R.P.; Kato, G.; Wicha, M. Phase ib pilot study to evaluate reparixin in combination with weekly paclitaxel in patients with her-2-negative metastatic breast cancer. Clin. Cancer Res. 2017, 23, 5358–5365. [Google Scholar] [CrossRef]
- Jaillon, S.; Ponzetta, A.; Di Mitri, D.; Santoni, A.; Bonecchi, R.; Mantovani, A. Neutrophil diversity and plasticity in tumour progression and therapy. Nat. Rev. Cancer 2020, 20, 485–503. [Google Scholar] [CrossRef]
- Chu, D.; Dong, X.; Shi, X.; Zhang, C.; Wang, Z. Neutrophil-based drug delivery systems. Adv. Mater. 2018, 30, e1706245. [Google Scholar] [CrossRef]
- Dong, X.; Chu, D.; Wang, Z. Leukocyte-mediated delivery of nanotherapeutics in inflammatory and tumor sites. Theranostics 2017, 7, 751–763. [Google Scholar] [CrossRef]
- Dong, X.; Chu, D.; Wang, Z. Neutrophil-mediated delivery of nanotherapeutics across blood vessel barrier. Ther. Deliv. 2018, 9, 29–35. [Google Scholar] [CrossRef]
- Sapey, E.; Greenwood, H.; Walton, G.; Mann, E.; Love, A.; Aaronson, N.; Insall, R.H.; Stockley, R.A.; Lord, J.M. Phosphoinositide 3-kinase inhibition restores neutrophil accuracy in the elderly: Toward targeted treatments for immunosenescence. Blood 2014, 123, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Alves-Filho, J.C.; Sonego, F.; Souto, F.O.; Freitas, A.; Verri, W.A., Jr.; Auxiliadora-Martins, M.; Basile-Filho, A.; McKenzie, A.N.; Xu, D.; Cunha, F.Q.; et al. Interleukin-33 attenuates sepsis by enhancing neutrophil influx to the site of infection. Nat. Med. 2010, 16, 708–712. [Google Scholar] [CrossRef] [PubMed]
- Yen, C.L.; Liao, Y.C.; Chen, R.F.; Huang, Y.F.; Chung, W.C.; Lo, P.C.; Chang, C.F.; Wu, P.C.; Shieh, D.B.; Jiang, S.T.; et al. Targeted delivery of curcumin rescues endoplasmic reticulum-retained mutant nox2 protein and avoids leukocyte apoptosis. J. Immunol. 2019, 202, 3394–3403. [Google Scholar] [CrossRef]
- DeNardo, D.G.; Ruffell, B. Macrophages as regulators of tumour immunity and immunotherapy. Nat. Rev. Immunol. 2019, 19, 369–382. [Google Scholar] [CrossRef]
- Ramesh, A.; Kumar, S.; Nandi, D.; Kulkarni, A. Csf1r- and shp2-inhibitor-loaded nanoparticles enhance cytotoxic activity and phagocytosis in tumor-associated macrophages. Adv. Mater. 2019, 31, e1904364. [Google Scholar] [CrossRef]
- Bose, R.J.C.; Tharmalingam, N.; Garcia Marques, F.J.; Sukumar, U.K.; Natarajan, A.; Zeng, Y.; Robinson, E.; Bermudez, A.; Chang, E.; Habte, F.; et al. Reconstructed apoptotic bodies as targeted “nano decoys” to treat intracellular bacterial infections within macrophages and cancer cells. ACS Nano 2020, 14, 5818–5835. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.; Gao, J.; Wang, Z. Neutrophil-mediated delivery of therapeutic nanoparticles across blood vessel barrier for treatment of inflammation and infection. ACS Nano 2015, 9, 11800–11811. [Google Scholar] [CrossRef]
- Zhang, C.; Ling, C.L.; Pang, L.; Wang, Q.; Liu, J.X.; Wang, B.S.; Liang, J.M.; Guo, Y.Z.; Qin, J.; Wang, J.X. Direct macromolecular drug delivery to cerebral ischemia area using neutrophil-mediated nanoparticles. Theranostics 2017, 7, 3260–3275. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Yang, X.; Li, S.; Cheng, Z.; Wang, Y.; Zhao, J.; Zhang, C.; Li, Y.; Luo, M.; Ren, H.; et al. Accessing neuroinflammation sites: Monocyte/neutrophil-mediated drug delivery for cerebral ischemia. Sci. Adv. 2019, 5, eaau8301. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, M.; Tang, W.; Wen, R.; Zhou, S.; Lee, C.; Wang, H.; Jiang, W.; Delahunty, I.M.; Zhen, Z.; et al. Nanoparticle-laden macrophages for tumor-tropic drug delivery. Adv. Mater. 2018, 30, e1805557. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.A.; Zheng, Y.R.; Gadde, S.; Pfirschke, C.; Zope, H.; Engblom, C.; Kohler, R.H.; Iwamoto, Y.; Yang, K.S.; Askevold, B.; et al. Tumour-associated macrophages act as a slow-release reservoir of nano-therapeutic pt(IV) pro-drug. Nat. Commun. 2015, 6, 8692. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Wu, Y.; Wang, Q.; Xue, L.; Su, Z.; Zhang, C. Cellular vehicles based on neutrophils enable targeting of atherosclerosis. Mol. Pharm. 2019, 16, 3109–3120. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Luo, X.; Zhou, S.; Zhu, J.; Xiao, M.; Li, C.; Zheng, H.; Qiu, Q.; Lai, C.; Liu, X.; et al. Neutrophil-mediated delivery of dexamethasone palmitate-loaded liposomes decorated with a sialic acid conjugate for rheumatoid arthritis treatment. Pharm. Res. 2019, 36, 97. [Google Scholar] [CrossRef] [PubMed]
- Doshi, N.; Swiston, A.J.; Gilbert, J.B.; Alcaraz, M.L.; Cohen, R.E.; Rubner, M.F.; Mitragotri, S. Cell-based drug delivery devices using phagocytosis-resistant backpacks. Adv. Mater. 2011, 23, H105–H109. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Gilbert, J.B.; Kumar, S.; Gupta, V.; Cohen, R.E.; Rubner, M.F.; Mitragotri, S. Monocyte-mediated delivery of polymeric backpacks to inflamed tissues: A generalized strategy to deliver drugs to treat inflammation. J. Control. Release 2015, 199, 29–36. [Google Scholar] [CrossRef]
- Klyachko, N.L.; Polak, R.; Haney, M.J.; Zhao, Y.; Gomes Neto, R.J.; Hill, M.C.; Kabanov, A.V.; Cohen, R.E.; Rubner, M.F.; Batrakova, E.V. Macrophages with cellular backpacks for targeted drug delivery to the brain. Biomaterials 2017, 140, 79–87. [Google Scholar] [CrossRef]
- Shields, C.W., 4th; Evans, M.A.; Wang, L.L.; Baugh, N.; Iyer, S.; Wu, D.; Zhao, Z.; Pusuluri, A.; Ukidve, A.; Pan, D.C.; et al. Cellular backpacks for macrophage immunotherapy. Sci. Adv. 2020, 6, eaaz6579. [Google Scholar] [CrossRef]
- Shirasuna, K.; Karasawa, T.; Takahashi, M. Exogenous nanoparticles and endogenous crystalline molecules as danger signals for the nlrp3 inflammasomes. J. Cell. Physiol. 2019, 234, 5436–5450. [Google Scholar] [CrossRef] [PubMed]
- Vita, A.A.; Royse, E.A.; Pullen, N.A. Nanoparticles and danger signals: Oral delivery vehicles as potential disruptors of intestinal barrier homeostasis. J. Leukoc. Biol. 2019, 106, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Gallud, A.; Fadeel, B. Keeping it small: Towards a molecular definition of nanotoxicology. Eur. J. Nanomed. 2015, 7, 143–151. [Google Scholar] [CrossRef]
- Mukherjee, S.P.; Bondarenko, O.; Kohonen, P.; Andon, F.T.; Brzicova, T.; Gessner, I.; Mathur, S.; Bottini, M.; Calligari, P.; Stella, L.; et al. Macrophage sensing of single-walled carbon nanotubes via toll-like receptors. Sci. Rep. 2018, 8, 1115. [Google Scholar] [CrossRef] [PubMed]
- Turabekova, M.; Rasulev, B.; Theodore, M.; Jackman, J.; Leszczynska, D.; Leszczynski, J. Immunotoxicity of nanoparticles: A computational study suggests that cnts and c60 fullerenes might be recognized as pathogens by toll-like receptors. Nanoscale 2014, 6, 3488–3495. [Google Scholar] [CrossRef] [PubMed]
- Fraser, J.A.; Kemp, S.; Young, L.; Ross, M.; Prach, M.; Hutchison, G.R.; Malone, E. Silver nanoparticles promote the emergence of heterogeneic human neutrophil sub-populations. Sci. Rep. 2018, 8, 7506. [Google Scholar] [CrossRef]
- Muzi, L.; Tardani, F.; La Mesa, C.; Bonincontro, A.; Bianco, A.; Risuleo, G. Interactions and effects of bsa-functionalized single-walled carbon nanotubes on different cell lines. Nanotechnology 2016, 27, 155704. [Google Scholar] [CrossRef] [PubMed]
- Desai, J.; Foresto-Neto, O.; Honarpisheh, M.; Steiger, S.; Nakazawa, D.; Popper, B.; Buhl, E.M.; Boor, P.; Mulay, S.R.; Anders, H.J. Particles of different sizes and shapes induce neutrophil necroptosis followed by the release of neutrophil extracellular trap-like chromatin. Sci. Rep. 2017, 7, 15003. [Google Scholar] [CrossRef]
- Munoz, L.E.; Bilyy, R.; Biermann, M.H.; Kienhofer, D.; Maueroder, C.; Hahn, J.; Brauner, J.M.; Weidner, D.; Chen, J.; Scharin-Mehlmann, M.; et al. Nanoparticles size-dependently initiate self-limiting netosis-driven inflammation. Proc. Natl. Acad. Sci. USA 2016, 113, E5856–E5865. [Google Scholar] [CrossRef]
- Getts, D.R.; Terry, R.L.; Getts, M.T.; Deffrasnes, C.; Muller, M.; van Vreden, C.; Ashhurst, T.M.; Chami, B.; McCarthy, D.; Wu, H.; et al. Therapeutic inflammatory monocyte modulation using immune-modifying microparticles. Sci. Transl. Med. 2014, 6, 219ra217. [Google Scholar] [CrossRef]
- Jeong, S.J.; Cooper, J.G.; Ifergan, I.; McGuire, T.L.; Xu, D.; Hunter, Z.; Sharma, S.; McCarthy, D.; Miller, S.D.; Kessler, J.A. Intravenous immune-modifying nanoparticles as a therapy for spinal cord injury in mice. Neurobiol. Dis. 2017, 108, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Saito, E.; Kuo, R.; Pearson, R.M.; Gohel, N.; Cheung, B.; King, N.J.C.; Miller, S.D.; Shea, L.D. Designing drug-free biodegradable nanoparticles to modulate inflammatory monocytes and neutrophils for ameliorating inflammation. J. Control. Release 2019, 300, 185–196. [Google Scholar] [CrossRef] [PubMed]
- MacParland, S.A.; Tsoi, K.M.; Ouyang, B.; Ma, X.Z.; Manuel, J.; Fawaz, A.; Ostrowski, M.A.; Alman, B.A.; Zilman, A.; Chan, W.C.; et al. Phenotype determines nanoparticle uptake by human macrophages from liver and blood. ACS Nano 2017, 11, 2428–2443. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z. Imaging nanotherapeutics in inflamed vasculature by intravital microscopy. Theranostics 2016, 6, 2431–2438. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, Y.; Gao, J.; Kaur, P.; Wang, Z. Neutrophils and Macrophages as Targets for Development of Nanotherapeutics in Inflammatory Diseases. Pharmaceutics 2020, 12, 1222. https://doi.org/10.3390/pharmaceutics12121222
Su Y, Gao J, Kaur P, Wang Z. Neutrophils and Macrophages as Targets for Development of Nanotherapeutics in Inflammatory Diseases. Pharmaceutics. 2020; 12(12):1222. https://doi.org/10.3390/pharmaceutics12121222
Chicago/Turabian StyleSu, Yujie, Jin Gao, Puneet Kaur, and Zhenjia Wang. 2020. "Neutrophils and Macrophages as Targets for Development of Nanotherapeutics in Inflammatory Diseases" Pharmaceutics 12, no. 12: 1222. https://doi.org/10.3390/pharmaceutics12121222
APA StyleSu, Y., Gao, J., Kaur, P., & Wang, Z. (2020). Neutrophils and Macrophages as Targets for Development of Nanotherapeutics in Inflammatory Diseases. Pharmaceutics, 12(12), 1222. https://doi.org/10.3390/pharmaceutics12121222





