Sustained Calcium(II)-Release to Impart Bioactivity in Hybrid Glass Scaffolds for Bone Tissue Engineering
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
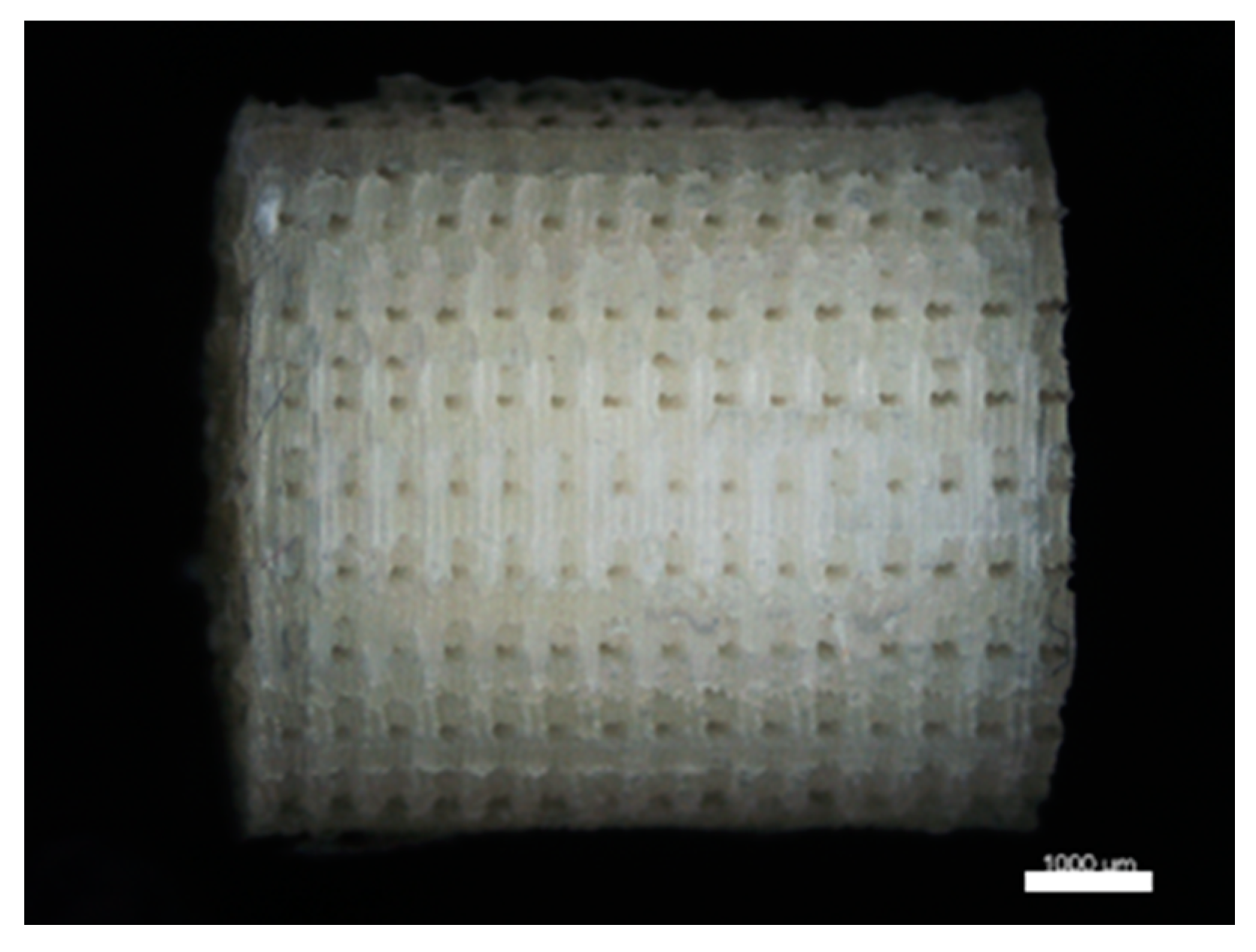
2.2.1. Template Fabrication
2.2.2. Silica Sol Synthesis
2.2.3. Cross-Linker Synthesis
2.2.4. Hybrid Scaffold Production
2.2.5. Hybrid Scaffold Production with Incorporation of Bioactive Glass (BG) Microparticles
2.2.6. Scaffold Characterization
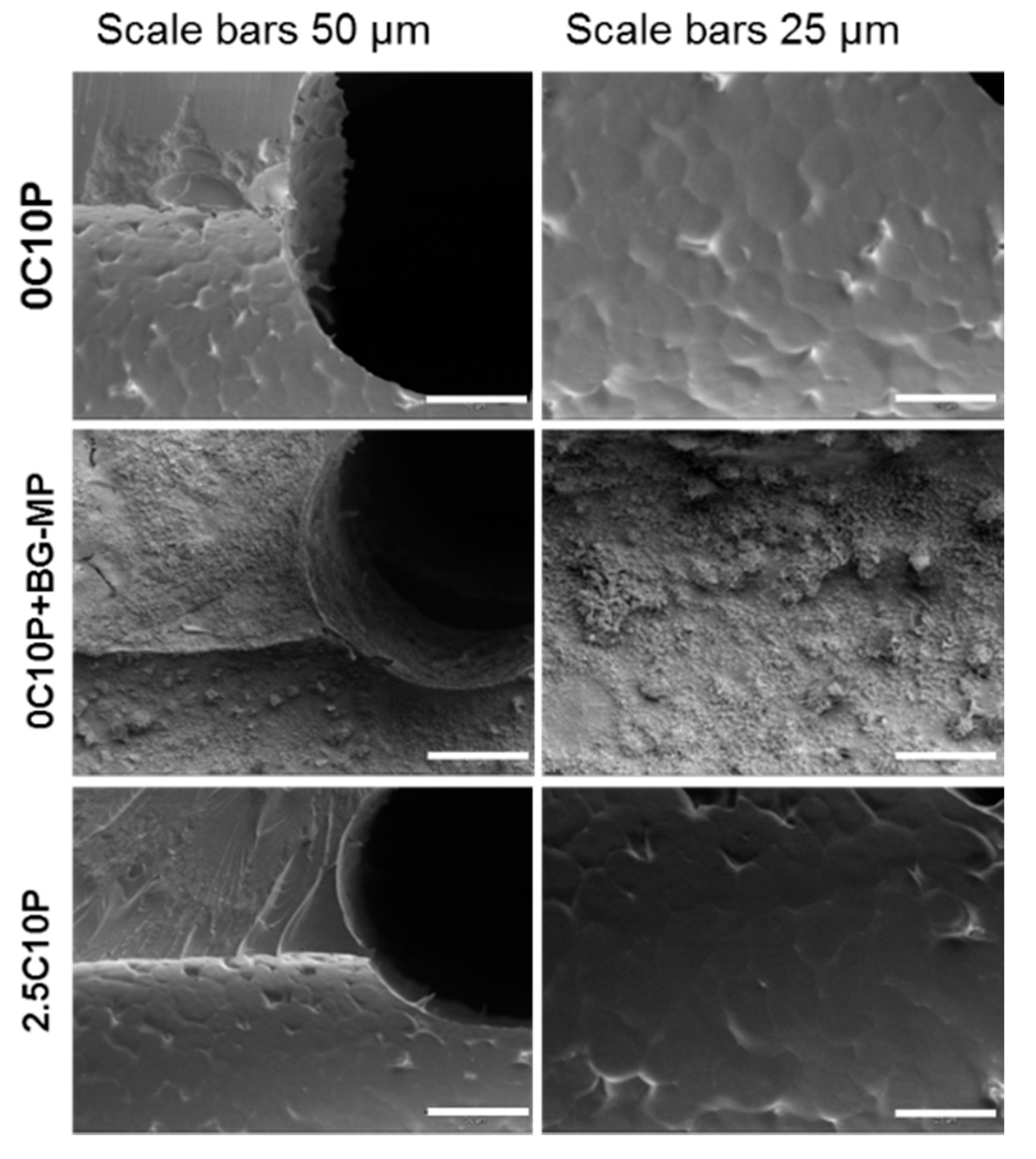
Scanning Electron Microscopy (SEM) and Energy Dispersive X-ray Spectroscopy (EDX)
Mechanical Testing
Characterization of Scaffold Degradation and Calcium(II) Release
Quantification of Released Calcium(II)
Bioactivity Testing in Simulated Body Fluid (SBF)
Cell Culture
2.2.7. Statistical Analysis
3. Results
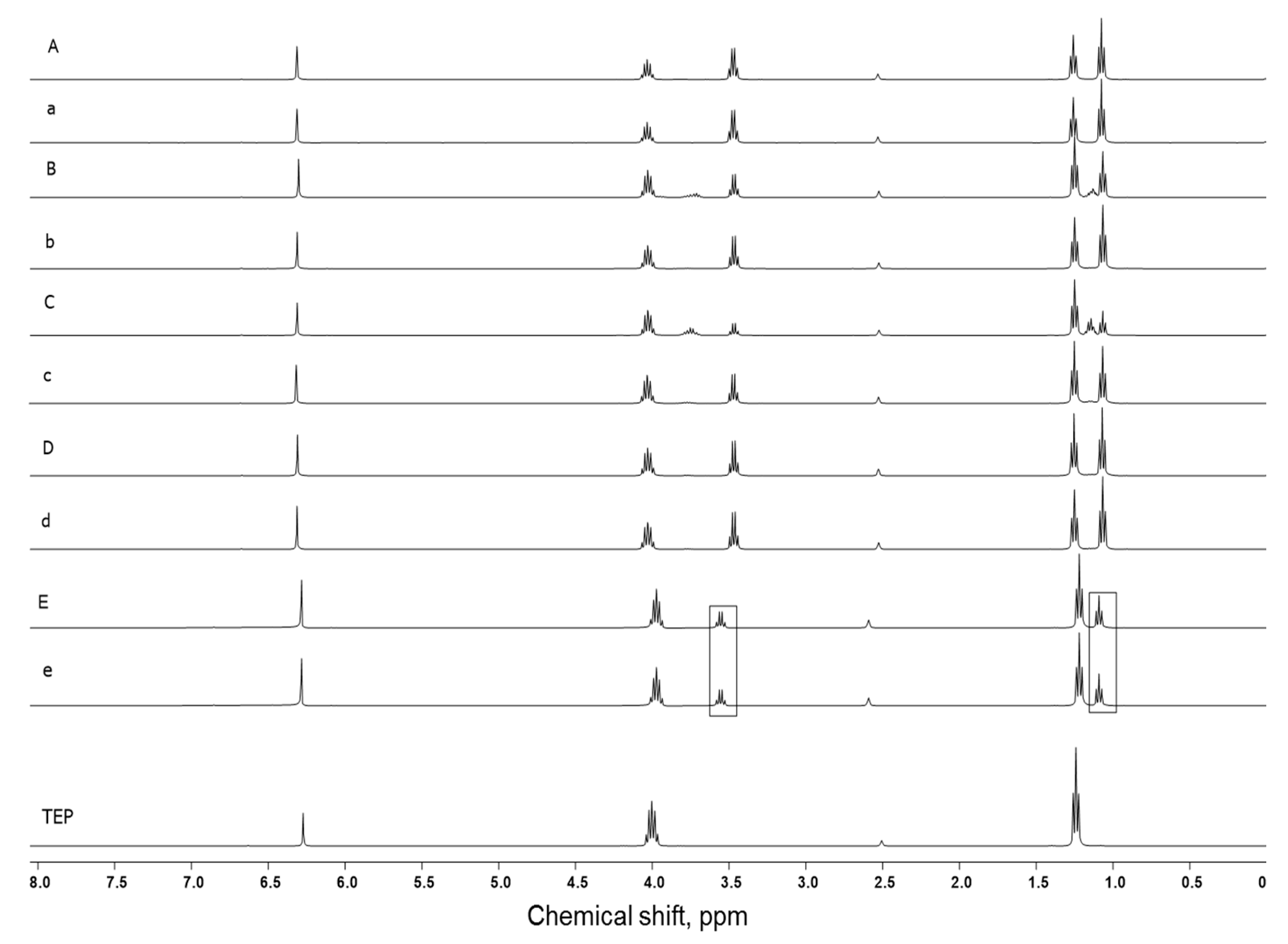
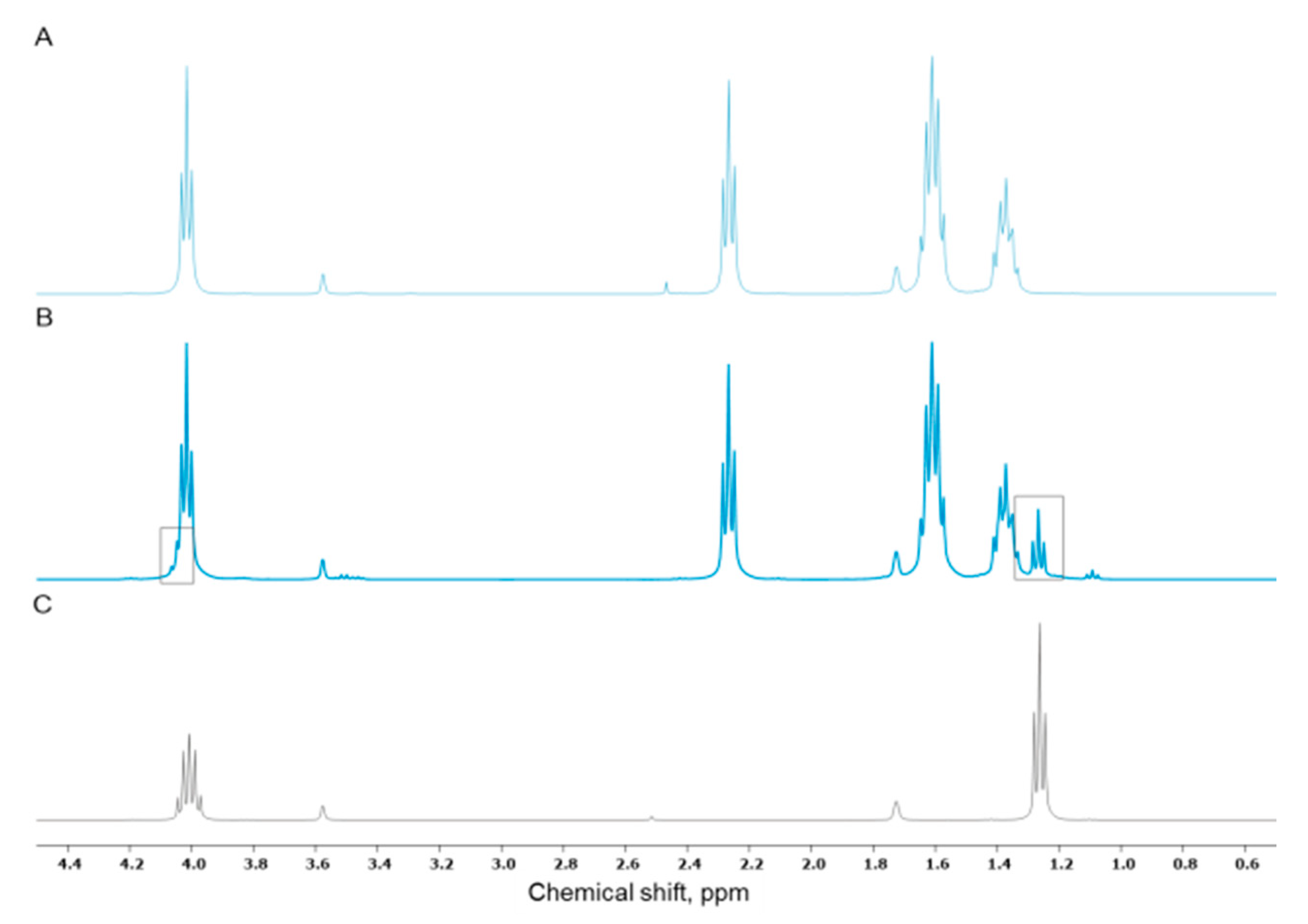
3.1. TEP as Solvent for Hybrid Sols
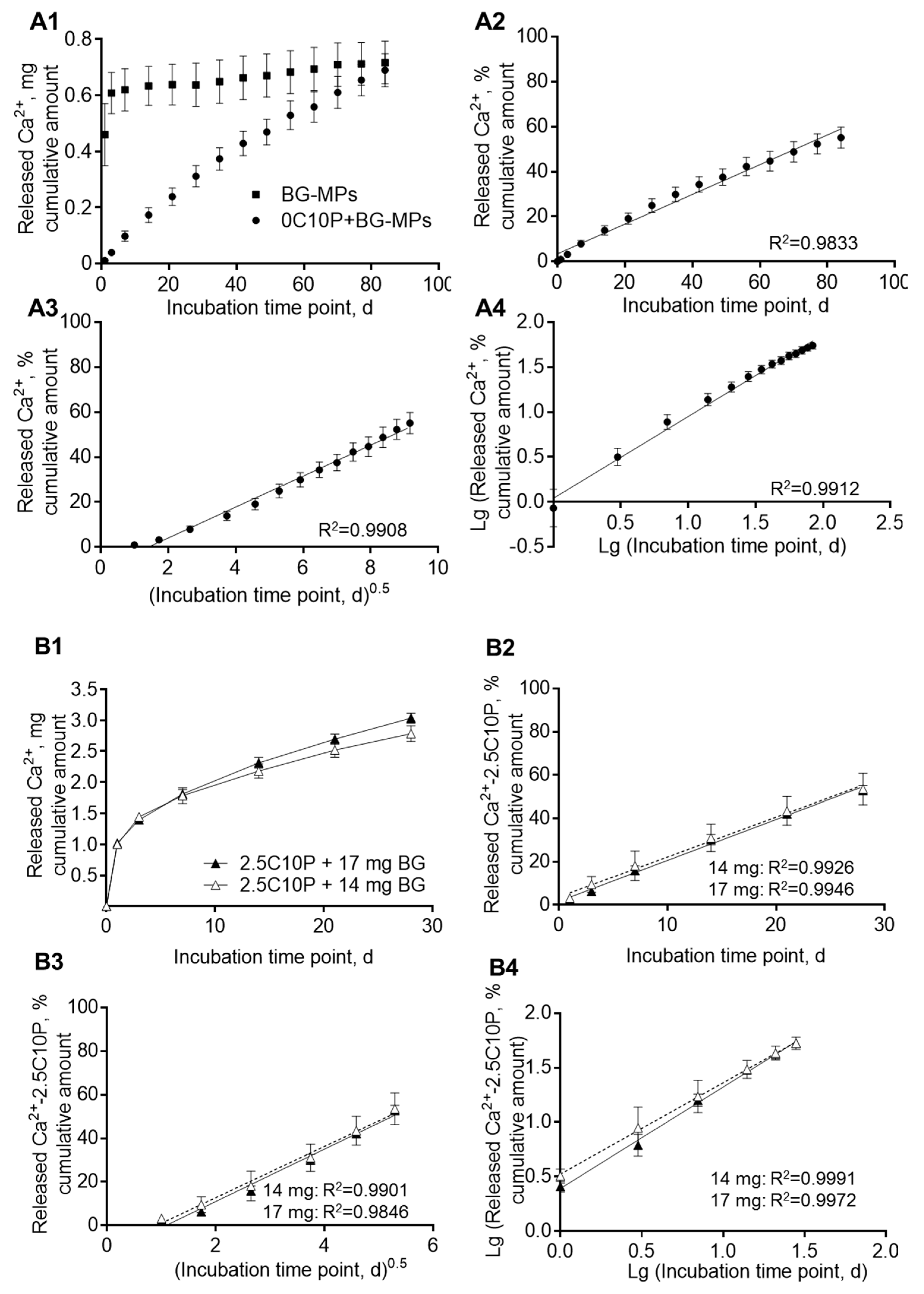
3.2. Incorporation of Calcium(II) from CaCl2 and Bioactive Glass Microparticles (Vitryxx®)
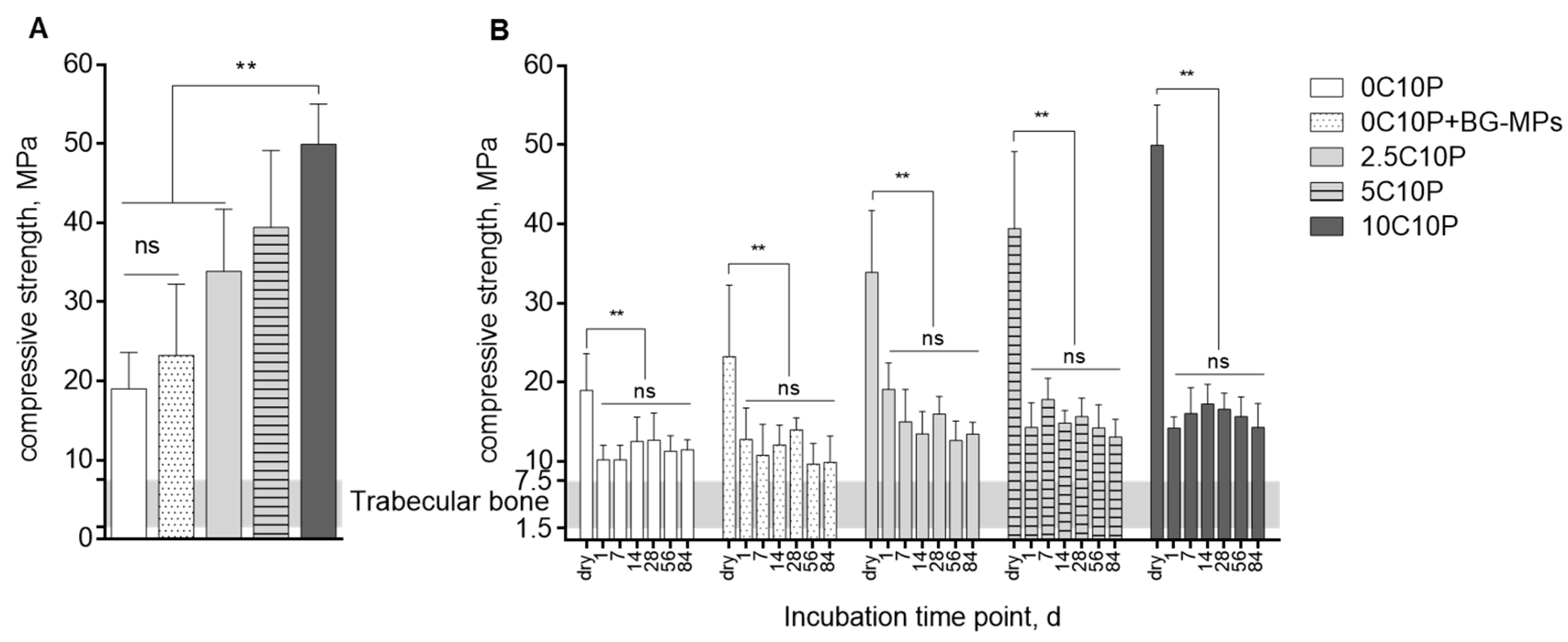
3.3. Scaffold Strength
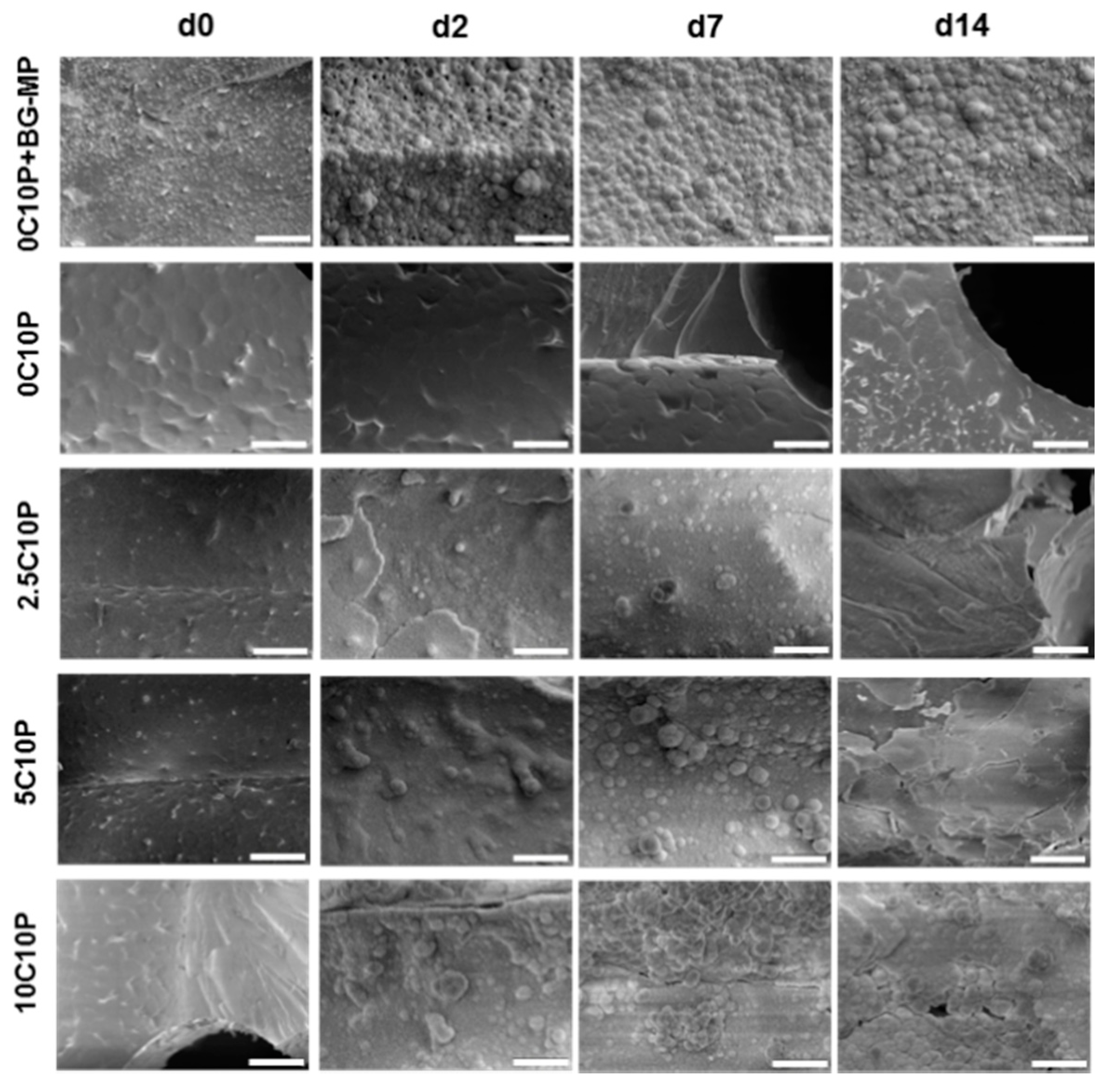
3.4. Scaffold Degradation upon Incubation in Aqueous Medium
3.5. Bioactivity Testing
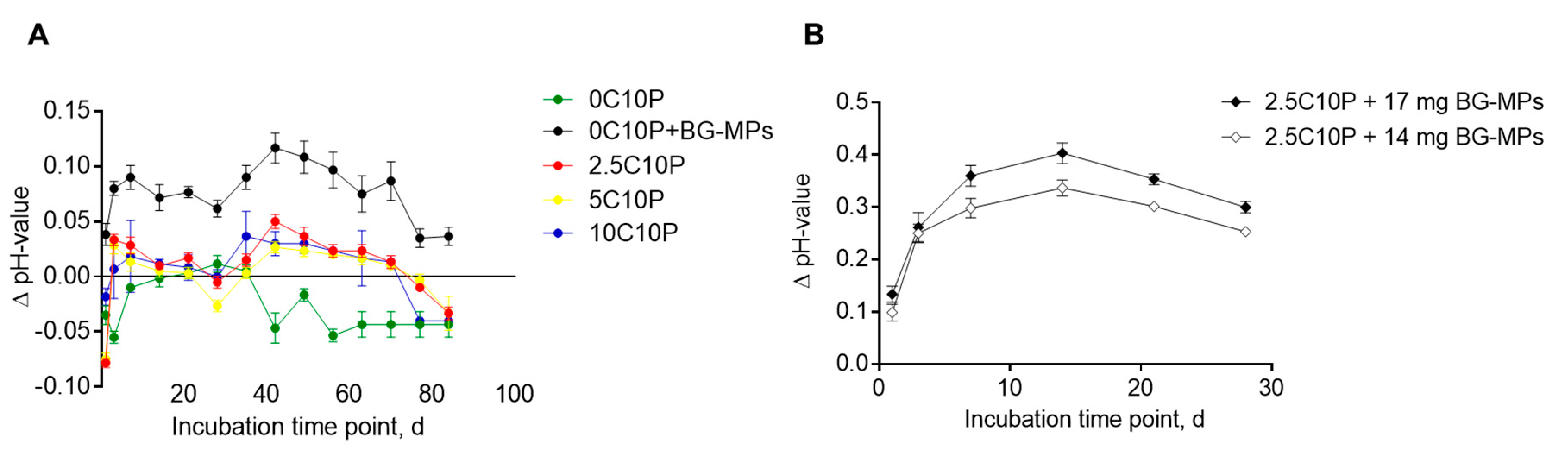
3.6. Calcium Ion Release and Media pH
3.7. Cell Culture Experiments
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A


| Formulation | Weight Loss, wt% |
| 10C10P | 26.9 ± 0.3% |
| 5C10P | 18.4 ± 1.3% |
| 2.5C10P | 14.4 ± 0.5% |
| 0C10P | 12.1 ± 0.3% |
| 0C10P + BG-MP | 11.3 ± 0.5% |

References
- Hutmacher, D.W. Scaffolds in tissue engineering bone and cartilage. Biomaterials 2000, 21, 2529–2543. [Google Scholar] [CrossRef]
- Jones, J.R.; Lee, P.D.; Hench, L.L. Hierarchical porous materials for tissue engineering. Philos. Trans. A Math. Phys. Eng. Sci. 2006, 364, 263–281. [Google Scholar] [CrossRef] [PubMed]
- Letaief, N.; Lucas-Girot, A.; Oudadesse, H.; Meleard, P.; Pott, T.; Jelassi, J.; Dorbez-Sridi, R. Effect of aging temperature on the structure, pore morphology and bioactivity of new sol–gel synthesized bioglass. J. Non-Cryst. Solids 2014, 402, 194–199. [Google Scholar] [CrossRef]
- Hench, L.L.; Paschall, H.A. Direct chemical bond of bioactive glass—Ceramic materials to bone and muscle. J. Biomed. Mater. Res. 1973, 7, 25–42. [Google Scholar] [CrossRef]
- Vogel, M.; Voigt, C.; Gross, U.M.; Müller-Mai, C.M. In vivo comparison of bioactive glass particles in rabbits. Biomaterials 2001, 22, 357–362. [Google Scholar] [CrossRef]
- Hench, L.L. Chronology of Bioactive Glass Development and Clinical Applications. New J. Glass Ceram. 2013, 3, 67–73. [Google Scholar] [CrossRef] [Green Version]
- Lindfors, N.C.; Hyvönen, P.; Nyyssönen, M.; Kirjavainen, M.; Kankare, J.; Gullichsen, E.; Salo, J. Bioactive glass S53P4 as bone graft substitute in treatment of osteomyelitis. Bone 2010, 47, 212–218. [Google Scholar] [CrossRef]
- Välimäki, V.V.; Aro, H.T. Molecular basis for action of bioactive glasses as bone graft substitute. Scand. J. Surg. 2006, 95, 95–102. [Google Scholar] [CrossRef] [Green Version]
- Zadpoor, A.A. Relationship between in vitro apatite-forming ability measured using simulated body fluid and in vivo bioactivity of biomaterials. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 35, 134–143. [Google Scholar] [CrossRef]
- Hench, L.L. The story of Bioglass®. J. Mater. Sci. Mater. Med. 2006, 17, 967–978. [Google Scholar] [CrossRef]
- Catteaux, R.; Grattepanche-Lebecq, I.; Désanglois, F.; Chai, F.; Hornez, J.-C.; Hampshire, S.; Follet-Houttemane, C. Synthesis, characterization and bioactivity of bioglasses in the Na2O–CaO–P2O5–SiO2 system prepared via sol gel processing. Chem. Eng. Res. Des. 2013, 91, 2420–2426. [Google Scholar] [CrossRef]
- Yu, B.; Turdean-Ionescu, C.A.; Martin, R.A.; Newport, R.J.; Hanna, J.V.; Smith, M.E.; Jones, J.R. Effect of calcium source on structure and properties of sol-gel derived bioactive glasses. Langmuir 2012, 28, 17465–17476. [Google Scholar] [CrossRef] [PubMed]
- Bossard, C.; Granel, H.; Jallot, É.; Montouillout, V.; Fayon, F.; Soulié, J.; Drouet, C.; Wittrant, Y.; Lao, J. Mechanism of Calcium Incorporation Inside Sol–Gel Silicate Bioactive Glass and the Advantage of Using Ca(OH) 2 over Other Calcium Sources. ACS Biomater. Sci. Eng. 2019, 5, 5906–5915. [Google Scholar] [CrossRef] [Green Version]
- Greasley, S.L.; Page, S.J.; Sirovica, S.; Chen, S.; Martin, R.A.; Riveiro, A.; Hanna, J.V.; Porter, A.E.; Jones, J.R. Controlling particle size in the Stöber process and incorporation of calcium. J. Colloid Interface Sci. 2016, 469, 213–223. [Google Scholar] [CrossRef] [Green Version]
- Olmo, N. Bioactive sol–gel glasses with and without a hydroxycarbonate apatite layer as substrates for osteoblast cell adhesion and proliferation. Biomaterials 2003, 24, 3383–3393. [Google Scholar] [CrossRef]
- Wang, D.; Poologasundarampillai, G.; van den Bergh, W.; Chater, R.J.; Kasuga, T.; Jones, J.R.; McPhail, D.S. Strategies for the chemical analysis of highly porous bone scaffolds using secondary ion mass spectrometry. Biomed. Mater. 2014, 9, 15013. [Google Scholar] [CrossRef]
- Jones, J.R. Review of bioactive glass: From Hench to hybrids. Acta Biomater. 2013, 9, 4457–4486. [Google Scholar] [CrossRef]
- Ding, Y.; Roether, J.A.; Boccaccini, A.R.; Schubert, D.W. Fabrication of electrospun poly (3-hydroxybutyrate)/poly (ε-caprolactone)/silica hybrid fibermats with and without calcium addition. Eur. Polym. J. 2014, 55, 222–234. [Google Scholar] [CrossRef]
- Ciraldo, F.E.; Boccardi, E.; Melli, V.; Westhauser, F.; Boccaccini, A.R. Tackling bioactive glass excessive in vitro bioreactivity: Preconditioning approaches for cell culture tests. Acta Biomater. 2018, 75, 3–10. [Google Scholar] [CrossRef]
- Hoppe, A.; Boccaccini, A.R. Biological impact of bioactive glasses and their dissolution products. Front. Oral Biol. 2015, 17, 22–32. [Google Scholar] [CrossRef]
- Gholami, S.; Labbaf, S.; Houreh, A.B.; Ting, H.-K.; Jones, J.R.; Esfahani, M.-H.N. Long term effects of bioactive glass particulates on dental pulp stem cells in vitro. Biomed. Glasses 2017, 3, 96–103. [Google Scholar] [CrossRef] [Green Version]
- Houreh, A.B.; Labbaf, S.; Ting, H.-K.; Ejeian, F.; Jones, J.R.; Esfahani, M.-H.N. Influence of calcium and phosphorus release from bioactive glasses on viability and differentiation of dental pulp stem cells. J. Mater. Sci. 2017, 52, 8928–8941. [Google Scholar] [CrossRef]
- Lao, J.; Dieudonné, X.; Fayon, F.; Montouillout, V.; Jallot, E. Bioactive glass–gelatin hybrids: Building scaffolds with enhanced calcium incorporation and controlled porosity for bone regeneration. J. Mater. Chem. B 2016, 4, 2486–2497. [Google Scholar] [CrossRef] [PubMed]
- Bossard, C.; Granel, H.; Wittrant, Y.; Jallot, É.; Lao, J.; Vial, C.; Tiainen, H. Polycaprolactone / bioactive glass hybrid scaffolds for bone regeneration. Biomed. Glasses 2018, 4, 108–122. [Google Scholar] [CrossRef]
- Granel, H.; Bossard, C.; Collignon, A.-M.; Wauquier, F.; Lesieur, J.; Rochefort, G.Y.; Jallot, E.; Lao, J.; Wittrant, Y. Bioactive Glass/Polycaprolactone Hybrid with a Dual Cortical/Trabecular Structure for Bone Regeneration. ACS Appl. Bio Mater. 2019, 2, 3473–3483. [Google Scholar] [CrossRef]
- Poologasundarampillai, G.; Yu, B.; Jones, J.R.; Kasuga, T. Electrospun silica/PLLA hybrid materials for skeletal regeneration. Soft Matter 2011, 7, 10241–10251. [Google Scholar] [CrossRef]
- Poologasundarampillai, G.; Yu, B.; Tsigkou, O.; Wang, D.; Romer, F.; Bhakhri, V.; Giuliani, F.; Stevens, M.M.; McPhail, D.S.; Smith, M.E.; et al. Poly(γ-glutamic acid)/silica hybrids with calcium incorporated in the silica network by use of a calcium alkoxide precursor. Chemistry 2014, 20, 8149–8160. [Google Scholar] [CrossRef] [Green Version]
- Li, A.; Shen, H.; Ren, H.; Wang, C.; Wu, D.; Martin, R.A.; Qiu, D. Bioactive organic/inorganic hybrids with improved mechanical performance. J. Mater. Chem. B 2015, 3, 1379–1390. [Google Scholar] [CrossRef] [Green Version]
- Fernando, D.; Attik, N.; Cresswell, M.; Mokbel, I.; Pradelle-Plasse, N.; Jackson, P.; Grosgogeat, B.; Colon, P. Influence of network modifiers in an acetate based sol-gel bioactive glass system. Microporous Mesoporous Mater. 2018, 257, 99–109. [Google Scholar] [CrossRef]
- Maçon, A.L.B.; Jacquemin, M.; Page, S.J.; Li, S.; Bertazzo, S.; Stevens, M.M.; Hanna, J.V.; Jones, J.R. Lithium-silicate sol–gel bioactive glass and the effect of lithium precursor on structure–property relationships. J. Sol-Gel. Sci. Technol. 2017, 81, 84–94. [Google Scholar] [CrossRef] [Green Version]
- Hendrikx, S.; Kascholke, C.; Flath, T.; Schumann, D.; Gressenbuch, M.; Schulze, F.P.; Hacker, M.C.; Schulz-Siegmund, M. Indirect rapid prototyping of sol-gel hybrid glass scaffolds for bone regeneration—Effects of organic crosslinker valence, content and molecular weight on mechanical properties. Acta Biomater. 2016, 35, 318–329. [Google Scholar] [CrossRef] [PubMed]
- Mondal, D.; Dixon, S.J.; Mequanint, K.; Rizkalla, A.S. Bioactivity, Degradation and Mechanical Properties of Poly(vinylpyrrolidone-co-triethoxyvinylsilane)/Tertiary Bioactive Glass Hybrids. ACS Appl. Bio Mater. 2018, 1, 1369–1381. [Google Scholar] [CrossRef]
- Kascholke, C.; Hendrikx, S.; Flath, T.; Kuzmenka, D.; Dörfler, H.-M.; Schumann, D.; Gressenbuch, M.; Schulze, F.P.; Schulz-Siegmund, M.; Hacker, M.C. Biodegradable and adjustable sol-gel glass based hybrid scaffolds from multi-armed oligomeric building blocks. Acta Biomater. 2017, 63, 336–349. [Google Scholar] [CrossRef] [PubMed]
- Hendrikx, S.; Kuzmenka, D.; Köferstein, R.; Flath, T.; Uhlig, H.; Enke, D.; Schulze, F.P.; Hacker, M.C.; Schulz-Siegmund, M. Effects of curing and organic content on bioactivity and mechanical properties of hybrid sol–gel glass scaffolds made by indirect rapid prototyping. J. Sol-Gel. Sci. Technol. 2017, 83, 143–154. [Google Scholar] [CrossRef]
- Messori, M.; Toselli, M.; Pilati, F.; Fabbri, E.; Fabbri, P.; Pasquali, L.; Nannarone, S. Prevention of plasticizer leaching from PVC medical devices by using organic–inorganic hybrid coatings. Polymer 2004, 45, 805–813. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef]
- Maçon, A.L.B.; Kim, T.B.; Valliant, E.M.; Goetschius, K.; Brow, R.K.; Day, D.E.; Hoppe, A.; Boccaccini, A.R.; Kim, I.Y.; Ohtsuki, C.; et al. A unified in vitro evaluation for apatite-forming ability of bioactive glasses and their variants. J. Mater. Sci. Mater. Med. 2015, 26, 115. [Google Scholar] [CrossRef] [Green Version]
- Lieb, E.; Vogel, T.; Milz, S.; Dauner, M.; Schulz, M.B. Effects of transforming growth factor beta1 on bonelike tissue formation in three-dimensional cell culture. II: Osteoblastic differentiation. Tissue Eng. 2004, 10, 1414–1425. [Google Scholar] [CrossRef]
- Schröck, K.; Schneider, H.; Lutz, J.; Hacker, M.C.; Mändl, S.; Kamprad, M.; Schulz-Siegmund, M. Cytocompatibility of nitrogen plasma ion immersed medical cobalt-chromium alloys. J. Biomed. Mater. Res. A 2014, 102, 1744–1754. [Google Scholar] [CrossRef]
- Sedaghati, B.; Jahroomishirazi, R.; Starke, A.; Hacker, M.C.; Schulz-Siegmund, M. Rat osteosarcoma cells as a therapeutic target model for osteoregeneration via sclerostin knockdown. Cells Tissues Organs 2016, 201, 366–379. [Google Scholar] [CrossRef]
- García, A.; Cicuéndez, M.; Izquierdo-Barba, I.; Arcos, D.; Vallet-Regí, M. Essential role of calcium phosphate heterogeneities in 2D-hexagonal and 3D-cubic SiO2−CaO−P2O5 mesoporous bioactive glasses. Chem. Mater. 2009, 21, 5474–5484. [Google Scholar] [CrossRef]
- de Laia, A.G.S.; Barrioni, B.R.; Valverde, T.M.; de Goes, A.M.; de Sá, M.A.; Pereira, M.D.M. Therapeutic cobalt ion incorporated in poly(vinyl alcohol)/bioactive glass scaffolds for tissue engineering. J. Mater. Sci. 2020, 55, 8710–8727. [Google Scholar] [CrossRef]
- de Barros Coelho, M.; Magalhães Pereira, M. Sol-gel synthesis of bioactive glass scaffolds for tissue engineering: Effect of surfactant type and concentration. J. Biomed. Mater. Res. Part B Appl. Biomater. 2005, 75, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Lemos, E.M.F.; Patrício, P.S.O.; Pereira, M.M. 3D nanocomposite chitosan/bioactive glass scaffolds obtained using two different routes: An evaluation of the porous structure and mechanical properties. Química Nova 2016, 39, 462–466. [Google Scholar] [CrossRef]
- Lyznicki, E.P., Jr.; Oyama, K.; Tidwell, T.T. Reactivity of organophosphates. IV. Acid-catalyzed hydrolysis of triethyl phosphate: A comparison with ethyl acetate. Can. J. Chem. 1974, 52, 1066–1071. [Google Scholar] [CrossRef]
- Westheimer, F.H.; Huang, S.; Covitz, F. Rates and mechanisms of hydrolysis of esters of phosphorous acid. J. Am. Chem. Soc. 1988, 110, 181–185. [Google Scholar] [CrossRef]
- Anastasescu, M.; Gartner, M.; Ghita, A.; Predoana, L.; Todan, L.; Zaharescu, M.; Vasiliu, C.; Grigorescu, C.; Negrila, C. Loss of phosphorous in silica-phosphate sol-gel films. J. Sol-Gel. Sci. Technol. 2006, 40, 325–333. [Google Scholar] [CrossRef]
- Fernández-Lorenzo, C.; Esquivias, L.; Barboux, P.; Maquet, J.; Taulelle, F. Sol-gel synthesis of SiO2-P2O5 glasses. J. Non-Cryst. Solids 1994, 176, 189–199. [Google Scholar] [CrossRef]
- Todan, L.; Andronescu, C.; Vuluga, D.M.; Culita, D.C.; Zaharescu, M. Thermal behavior of silicophosphate gels obtained from different precursors. J. Therm. Anal. Calorim. 2013, 114, 91–99. [Google Scholar] [CrossRef]
- Owens, G.J.; Singh, R.K.; Foroutan, F.; Alqaysi, M.; Han, C.-M.; Mahapatra, C.; Kim, H.-W.; Knowles, J.C. Sol–gel based materials for biomedical applications. Prog. Mater. Sci. 2016, 77, 1–79. [Google Scholar] [CrossRef] [Green Version]
- Hench, L.L.; Wang, S.H. The sol-gel glass transformation of silica. Phase Transit. 1990, 24-26, 785–834. [Google Scholar] [CrossRef]
- Fu, Q.; Saiz, E.; Rahaman, M.N.; Tomsia, A.P. Bioactive glass scaffolds for bone tissue engineering: State of the art and future perspectives. Mater. Sci. Eng. C Mater. Biol. Appl. 2011, 31, 1245–1256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balasubramanian, P.; Grünewald, A.; Detsch, R.; Hupa, L.; Jokic, B.; Tallia, F.; Solanki, A.K.; Jones, J.R.; Boccaccini, A.R. Ion Release, Hydroxyapatite Conversion and Cytotoxicity of Boron-Containing Bioactive Glass Scaffolds. Int. J. Appl. Glass. Sci. 2016, 7, 206–215. [Google Scholar] [CrossRef]
- Maçon, A.L.B.; Li, S.; Chung, J.J.; Nommeots-Nomm, A.; Solanki, A.K.; Stevens, M.M.; Jones, J.R. Ductile silica/methacrylate hybrids for bone regeneration. J. Mater. Chem. B 2016, 4, 6032–6042. [Google Scholar] [CrossRef]
- Hench, L.; West, J.K. The Sol-Gel Process. Chem. Rev. 1990, 33–72. [Google Scholar] [CrossRef]
- Henry, F.; Devassine, M.; Guerin, P.; Costa, L.C.; Briand, X. Biodegradable Polymer Studied by Physical Properties Measurements. MSF 2005, 480-481, 165–168. [Google Scholar] [CrossRef]
- Siemann, U. The influence of water on the glass transition of poly(dl-lactic acid). Thermochim. Acta 1985, 85, 513–516. [Google Scholar] [CrossRef]
- Alamri, H.; Low, I.M. Effect of water absorption on the mechanical properties of nano-filler reinforced epoxy nanocomposites. Mater. Des. 2012, 42, 214–222. [Google Scholar] [CrossRef] [Green Version]
- Lin, S.; Ionescu, C.; Pike, K.J.; Smith, M.E.; Jones, J.R. Nanostructure evolution and calcium distribution in sol–gel derived bioactive glass. J. Mater. Chem. 2009, 19, 1276–1282. [Google Scholar] [CrossRef]
- He, Q.; Shi, J.; Zhu, M.; Chen, Y.; Chen, F. The three-stage in vitro degradation behavior of mesoporous silica in simulated body fluid. Microporous Mesoporous Mater. 2010, 131, 314–320. [Google Scholar] [CrossRef]
- Kolan, K.C.R.; Leu, M.C.; Hilmas, G.E.; Velez, M. Effect of material, process parameters and simulated body fluids on mechanical properties of 13-93 bioactive glass porous constructs made by selective laser sintering. J. Mech. Behav. Biomed. Mater. 2012, 13, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Ohtsuki, C.; Kokubo, T.; Nakanishi, K.; Soga, N.; de Groot, K. The role of hydrated silica, titania and alumina in inducing apatite on implants. J. Biomed. Mater. Res. 1994, 28, 7–15. [Google Scholar] [CrossRef]
- International Organization for Standardization. Implants for Surgery—In Vitro Evaluation for Apatite-Forming Ability of Implant Materials; International Organization for Standardization: Geneva, Switzerland, 2014. [Google Scholar]
- Santos, S.C.; Barreto, L.S.; dos Santos, E.A. Nanocrystalline apatite formation on bioactive glass in a sol–gel synthesis. J. Non-Cryst. Solids 2016, 439, 30–37. [Google Scholar] [CrossRef]
- Akbari Dourbash, F.; Alizadeh, P. Organosilane modified bioactive glass/poly (amido amine) generation 5 hybrids: Effect of solvent and synthesis route on structural properties, thermal stability and apatite formation. Mater. Chem. Phys. 2017, 202, 104–113. [Google Scholar] [CrossRef]
- Sepulveda, P.; Jones, J.R.; Hench, L.L. In vitro dissolution of melt-derived 45S5 and sol-gel derived 58S bioactive glasses. J. Biomed. Mater. Res. 2002, 61, 301–311. [Google Scholar] [CrossRef]
- Arcos, D.; Vallet-Regí, M. Sol-gel silica-based biomaterials and bone tissue regeneration. Acta Biomater. 2010, 6, 2874–2888. [Google Scholar] [CrossRef]
- Yan, H.; Zhang, K.; Blanford, C.F.; Francis, L.F.; Stein, A. In vitro hydroxycarbonate apatite mineralization of CaO−SiO2 sol−gel glasses with a three-dimensionally ordered macroporous structure. Chem. Mater. 2001, 13, 1374–1382. [Google Scholar] [CrossRef]
- López-Noriega, A.; Arcos, D.; Izquierdo-Barba, I.; Sakamoto, Y.; Terasaki, O.; Vallet-Regí, M. Ordered Mesoporous Bioactive Glasses for Bone Tissue Regeneration. Chem. Mater. 2006, 18, 3137–3144. [Google Scholar] [CrossRef]
- Izquierdo-Barba, I.; Salinas, A.J.; Vallet-Reg, M. In vitro calcium phosphate layer formation on sol-gel glasses of the CaO-SiO2 system. J. Biomed. Mater. Res. 1999, 47, 243–250. [Google Scholar] [CrossRef]
- Sepulveda, P.; Jones, J.R.; Hench, L.L. Characterization of melt-derived 45S5 and sol-gel-derived 58S bioactive glasses. J. Biomed. Mater. Res. 2001, 58, 734–740. [Google Scholar] [CrossRef]
- Catauro, M.; Bollino, F.; Renella, R.A.; Papale, F. Sol–gel synthesis of SiO2–CaO–P2O5 glasses: Influence of the heat treatment on their bioactivity and biocompatibility. Ceram. Int. 2015, 41, 12578–12588. [Google Scholar] [CrossRef]
- Viitala, R.; Jokinen, M.; Peltola, T.; Gunnelius, K.; Rosenholm, J.B. Surface properties of in vitro bioactive and non-bioactive sol–gel derived materials. Biomaterials 2002, 23, 3073–3086. [Google Scholar] [CrossRef]
- Myszka, B.; Schodder, P.I.; Leupold, S.; Barr, M.K.S.; Hurle, K.; Schüßler, M.; Demmert, B.; Biggemann, J.; Fey, T.; Boccaccini, A.R.; et al. Shape Matters: Crystal Morphology and Surface Topography Alter Bioactivity of Bioceramics in Simulated Body Fluid. Adv. Eng. Mater. 2020, 2000044. [Google Scholar] [CrossRef]
- Aguiar, H.; Solla, E.L.; Serra, J.; González, P.; León, B.; Almeida, N.; Cachinho, S.; Davim, E.J.C.; Correia, R.; Oliveira, J.M.; et al. Orthophosphate nanostructures in SiO2–P2O5–CaO–Na2O–MgO bioactive glasses. J. Non-Cryst. Solids 2008, 354, 4075–4080. [Google Scholar] [CrossRef]
- Hoppe, A.; Güldal, N.S.; Boccaccini, A.R. A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials 2011, 32, 2757–2774. [Google Scholar] [CrossRef] [PubMed]
- Gentleman, E.; Stevens, M.M.; Hill, R.G.; Brauer, D.S. Surface properties and ion release from fluoride-containing bioactive glasses promote osteoblast differentiation and mineralization in vitro. Acta Biomater. 2013, 9, 5771–5779. [Google Scholar] [CrossRef] [Green Version]
- Maeno, S.; Niki, Y.; Matsumoto, H.; Morioka, H.; Yatabe, T.; Funayama, A.; Toyama, Y.; Taguchi, T.; Tanaka, J. The effect of calcium ion concentration on osteoblast viability, proliferation and differentiation in monolayer and 3D culture. Biomaterials 2005, 26, 4847–4855. [Google Scholar] [CrossRef]
- Miguez-Pacheco, V.; De Ligny, D.; Schmidt, J.; Detsch, R.; Boccaccini, A.R. Development and characterization of niobium-releasing silicate bioactive glasses for tissue engineering applications. J. Eur. Ceram. Soc. 2018, 38, 871–876. [Google Scholar] [CrossRef]
- Bingel, L.; Groh, D.; Karpukhina, N.; Brauer, D.S. Influence of dissolution medium pH on ion release and apatite formation of Bioglass® 45S5. Materials Letters 2015, 143, 279–282. [Google Scholar] [CrossRef]
- Gronbach, M.; Mitrach, F.; Lidzba, V.; Müller, B.; Möller, S.; Rother, S.; Salbach-Hirsch, J.; Hofbauer, L.C.; Schnabelrauch, M.; Hintze, V.; et al. Scavenging of Dickkopf-1 by macromer-based biomaterials covalently decorated with sulfated hyaluronan displays pro-osteogenic effects. Acta Biomater. 2020, 114, 76–89. [Google Scholar] [CrossRef]
- Fu, Q.; Saiz, E.; Tomsia, A.P. Bioinspired srong and highly porous glass scaffolds. Adv. Funct. Mater. 2011, 21, 1058–1063. [Google Scholar] [CrossRef] [Green Version]
- Cattalini, J.P.; García, J.; Boccaccini, A.R.; Lucangioli, S.; Mouriño, V. A new calcium releasing nano-composite biomaterial for bone tissue engineering scaffolds. Procedia Eng. 2013, 59, 78–84. [Google Scholar] [CrossRef] [Green Version]
- Zeng, Q.; Han, Y.; Li, H.; Chang, J. Bioglass/alginate composite hydrogel beads as cell carriers for bone regeneration. J. Biomed. Mater. Res. 2014, 102, 42–51. [Google Scholar] [CrossRef]
- Han, Y.; Zeng, Q.; Li, H.; Chang, J. The calcium silicate/alginate composite: Preparation and evaluation of its behavior as bioactive injectable hydrogels. Acta Biomater. 2013, 9, 9107–9117. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Matsugaki, A.; Kasuga, T.; Nakano, T. Development of bifunctional oriented bioactive glass/poly(lactic acid) composite scaffolds to control osteoblast alignment and proliferation. J. Biomed. Mater. Res. A 2019, 107, 1031–1041. [Google Scholar] [CrossRef] [PubMed]
- Peppas, N.A.; Colombo, P. Analysis of drug release behavior from swellable polymer carriers using the dimensionality index. J. Control. Release 1997, 45, 35–40. [Google Scholar] [CrossRef]
- Koennings, S.; Tessmar, J.; Blunk, T.; Göpferich, A. Confocal microscopy for the elucidation of mass transport mechanisms involved in protein release from lipid-based matrices. Pharm. Res. 2007, 24, 1325–1335. [Google Scholar] [CrossRef] [PubMed]
- Pryce, R.S.; Hench, L.L. Dissolution Characteristics of Bioactive Glasses. KEM 2003, 240-242, 201–204. [Google Scholar] [CrossRef]
- Thavornyutikarn, B.; Feltis, B.; Wright, P.F.A.; Turney, T.W. Effect of pre-treatment of crystallized bioactive glass with cell culture media on structure, degradability and biocompatibility. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 97, 188–197. [Google Scholar] [CrossRef]
- Ting, H.-K.; Page, S.J.; Poologasundarampillai, G.; Chen, S.; Yu, B.; Hanna, J.V.; Jones, J.R. Phosphate content affects structure and bioactivity of sol-gel silicate bioactive glasses. Int. J. Appl. Glass Sci. 2017, 8, 372–382. [Google Scholar] [CrossRef]
- Gentile, P.; Mattioli-Belmonte, M.; Chiono, V.; Ferretti, C.; Baino, F.; Tonda-Turo, C.; Vitale-Brovarone, C.; Pashkuleva, I.; Reis, R.L.; Ciardelli, G. Bioactive glass/polymer composite scaffolds mimicking bone tissue. J. Biomed. Mater. Res. A 2012, 100, 2654–2667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lossdörfer, S.; Schwartz, Z.; Lohmann, C.H.; Greenspan, D.C.; Ranly, D.M.; Boyan, B.D. Osteoblast response to bioactive glasses in vitro correlates with inorganic phosphate content. Biomaterials 2004, 25, 2547–2555. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.R.; Tsigkou, O.; Coates, E.E.; Stevens, M.M.; Polak, J.M.; Hench, L.L. Extracellular matrix formation and mineralization on a phosphate-free porous bioactive glass scaffold using primary human osteoblast (HOB) cells. Biomaterials 2007, 28, 1653–1663. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zhou, Y.; Xu, M.; Han, P.; Chen, L.; Chang, J.; Xiao, Y. Copper-containing mesoporous bioactive glass scaffolds with multifunctional properties of angiogenesis capacity, osteostimulation and antibacterial activity. Biomaterials 2013, 34, 422–433. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Dai, X.; Lu, M.; Hüser, N.; Taccardi, N.; Boccaccini, A.R. Synthesis of copper-containing bioactive glass nanoparticles using a modified Stöber method for biomedical applications. Colloids Surf. B Biointerfaces 2017, 150, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Detsch, R.; Alles, S.; Hum, J.; Westenberger, P.; Sieker, F.; Heusinger, D.; Kasper, C.; Boccaccini, A.R. Osteogenic differentiation of umbilical cord and adipose derived stem cells onto highly porous 45S5 Bioglass®-based scaffolds. J. Biomed. Mater. Res. A 2015, 103, 1029–1037. [Google Scholar] [CrossRef]
- Lakhkar, N.J.; Lee, I.-H.; Kim, H.-W.; Salih, V.; Wall, I.B.; Knowles, J.C. Bone formation controlled by biologically relevant inorganic ions: Role and controlled delivery from phosphate-based glasses. Adv. Drug Deliv. Rev. 2013, 65, 405–420. [Google Scholar] [CrossRef]
- Yang, X.B.; Webb, D.; Blaker, J.; Boccaccini, A.R.; Maquet, V.; Cooper, C.; Oreffo, R.O.C. Evaluation of human bone marrow stromal cell growth on biodegradable polymer/bioglass composites. Biochem. Biophys. Res. Commun. 2006, 342, 1098–1107. [Google Scholar] [CrossRef]
- Reilly, G.C.; Radin, S.; Chen, A.T.; Ducheyne, P. Differential alkaline phosphatase responses of rat and human bone marrow derived mesenchymal stem cells to 45S5 bioactive glass. Biomaterials 2007, 28, 4091–4097. [Google Scholar] [CrossRef] [Green Version]
- Monfoulet, L.-E.; Becquart, P.; Marchat, D.; Vandamme, K.; Bourguignon, M.; Pacard, E.; Viateau, V.; Petite, H.; Logeart-Avramoglou, D. The pH in the microenvironment of human mesenchymal stem cells is a critical factor for optimal osteogenesis in tissue-engineered constructs. Tissue Eng. Part A 2014, 20, 1827–1840. [Google Scholar] [CrossRef] [Green Version]
- Leach, J.K.; Kaigler, D.; Wang, Z.; Krebsbach, P.H.; Mooney, D.J. Coating of VEGF-releasing scaffolds with bioactive glass for angiogenesis and bone regeneration. Biomaterials 2006, 27, 3249–3255. [Google Scholar] [CrossRef] [PubMed]



| Sol Formulation | TEOS | Calcium(II) | TEP |
|---|---|---|---|
| 0C10P | 90 | 0 | 10 |
| 2.5C10P | 87.5 | 2.5 | 10 |
| 5C10P | 85 | 5 | 10 |
| 10C10P | 80 | 10 | 10 |
| Step | |
|---|---|
| 1 | heating from room temperature to 60 °C, dwell at 60 °C for 96 h |
| 2 | heating from 60 °C to 90 °C, dwell at 90 °C for 24 h |
| 3 | heating from 90 °C to 130 °C, dwell at 130 °C for 48 h |
| Ca(II) Release, [%/d] | ||||
|---|---|---|---|---|
| Incubation Time, [d] | 10C10P | 5C10P | 2.5C10P | 1C10P |
| 0–1 | 98.37 ± 3.88 | 85.87 ± 3.89 | 72.36 ± 1.61 | 74.02 ± 3.47 |
| 1–3 | 0.09 ± 0.62 | 1.58 ± 1.59 | 4.65 ± 1.93 | 5.73 ± 0.45 |
| 3–7 | 0.30 ± 0.04 | 1.43 ± 0.18 | 2.09 ± 0.29 | 2.32 ± 0.05 |
| 7–14 | 0.04 ± 0.02 | 0.45 ± 0.09 | 0.73 ± 0.08 | 0.37 ± 0.05 |
| 14–21 | 0.00 | 0.13 ± 0.05 | 0.24 ± 0.09 | 0.36 ± 0.14 |
| 21–28 | 0.00 | 0.09 ± 0.04 | 0.15 ± 0.04 | 0.02 ± 0.04 |
| A | Calcium(II) Release [µg/5mL/d] | |||||
|---|---|---|---|---|---|---|
| time, [d] | 7 mg BG-MP | 0C10P + 7 mg BG-MP | 2.5C10P + 14 mg BG‑MP | 2.5C10P + 17 mg BG-MP | (2.5C10P + 14 mg BG-MP)-2.5C10P | (2.5C10P + 17 mg BG-MP)-2.5C10P |
| 0–1 | 459.4 ± 110.1 | 11.9 ± 6.2 | 1003.1 ± 35.6 | 1023.1 ± 37.3 | 81.1 ± 11.6 | 77.5 ± 8.5 |
| 1–3 | 74.3 ± 36.5 | 14.3 ± 1.8 | 220.7 ± 14.6 | 186.0 ± 19.6 | 78.5 ± 43.2 | 55.5 ± 24.0 |
| 3–7 | 2.8 ± 1.0 | 14.6 ± 2.50 | 84.8 ± 21.7 | 103.7 ± 7.4 | 53.9 ± 24.3 | 72.8 ± 6.7 |
| 7–14 | 2.0 ± 1.3 | 10.7 ± 1.2 | 56.8 ± 2.4 | 71.9 ± 5.1 | 46.1 ± 2.1 | 61.1 ± 4.6 |
| 14–21 | 0.9 ± 0.9 | 9.3 ± 1.1 | 48.1 ± 1.0 | 54.7 ± 3.6 | 44.5 ± 1.6 | 51.1 ± 3.8 |
| 21–28 | 0.3 ± 0.5 | 10.4 ± 1.5 | 38.0 ± 2.7 | 48.3 ± 6.7 | 35.9 ± 3.1 | 46.2 ± 6.6 |
| B | Formulation | Average Calcium(II) Release Rate [µg/5mL/d] | ||||
| 0C10P + 7 mg BG-MP | 11.9 | |||||
| (2.5C10P + 14 mg BG-MP)–2.5C10P | 42.1 * | |||||
| (2.5C10P + 17 mg BG-MP)–2.5C10P | 52.8 * | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuzmenka, D.; Sewohl, C.; König, A.; Flath, T.; Hahnel, S.; Schulze, F.P.; Hacker, M.C.; Schulz-Siegmund, M. Sustained Calcium(II)-Release to Impart Bioactivity in Hybrid Glass Scaffolds for Bone Tissue Engineering. Pharmaceutics 2020, 12, 1192. https://doi.org/10.3390/pharmaceutics12121192
Kuzmenka D, Sewohl C, König A, Flath T, Hahnel S, Schulze FP, Hacker MC, Schulz-Siegmund M. Sustained Calcium(II)-Release to Impart Bioactivity in Hybrid Glass Scaffolds for Bone Tissue Engineering. Pharmaceutics. 2020; 12(12):1192. https://doi.org/10.3390/pharmaceutics12121192
Chicago/Turabian StyleKuzmenka, Dzmitry, Claudia Sewohl, Andreas König, Tobias Flath, Sebastian Hahnel, Fritz Peter Schulze, Michael C. Hacker, and Michaela Schulz-Siegmund. 2020. "Sustained Calcium(II)-Release to Impart Bioactivity in Hybrid Glass Scaffolds for Bone Tissue Engineering" Pharmaceutics 12, no. 12: 1192. https://doi.org/10.3390/pharmaceutics12121192
APA StyleKuzmenka, D., Sewohl, C., König, A., Flath, T., Hahnel, S., Schulze, F. P., Hacker, M. C., & Schulz-Siegmund, M. (2020). Sustained Calcium(II)-Release to Impart Bioactivity in Hybrid Glass Scaffolds for Bone Tissue Engineering. Pharmaceutics, 12(12), 1192. https://doi.org/10.3390/pharmaceutics12121192






