Mechanisms of Resistance to Chemotherapy in Breast Cancer and Possible Targets in Drug Delivery Systems
Abstract
1. Introduction
2. Breast Cancer
3. Neoadjuvant Chemotherapy for Breast Cancer
4. Breast Cancer Molecular Mechanisms of Chemoresistance
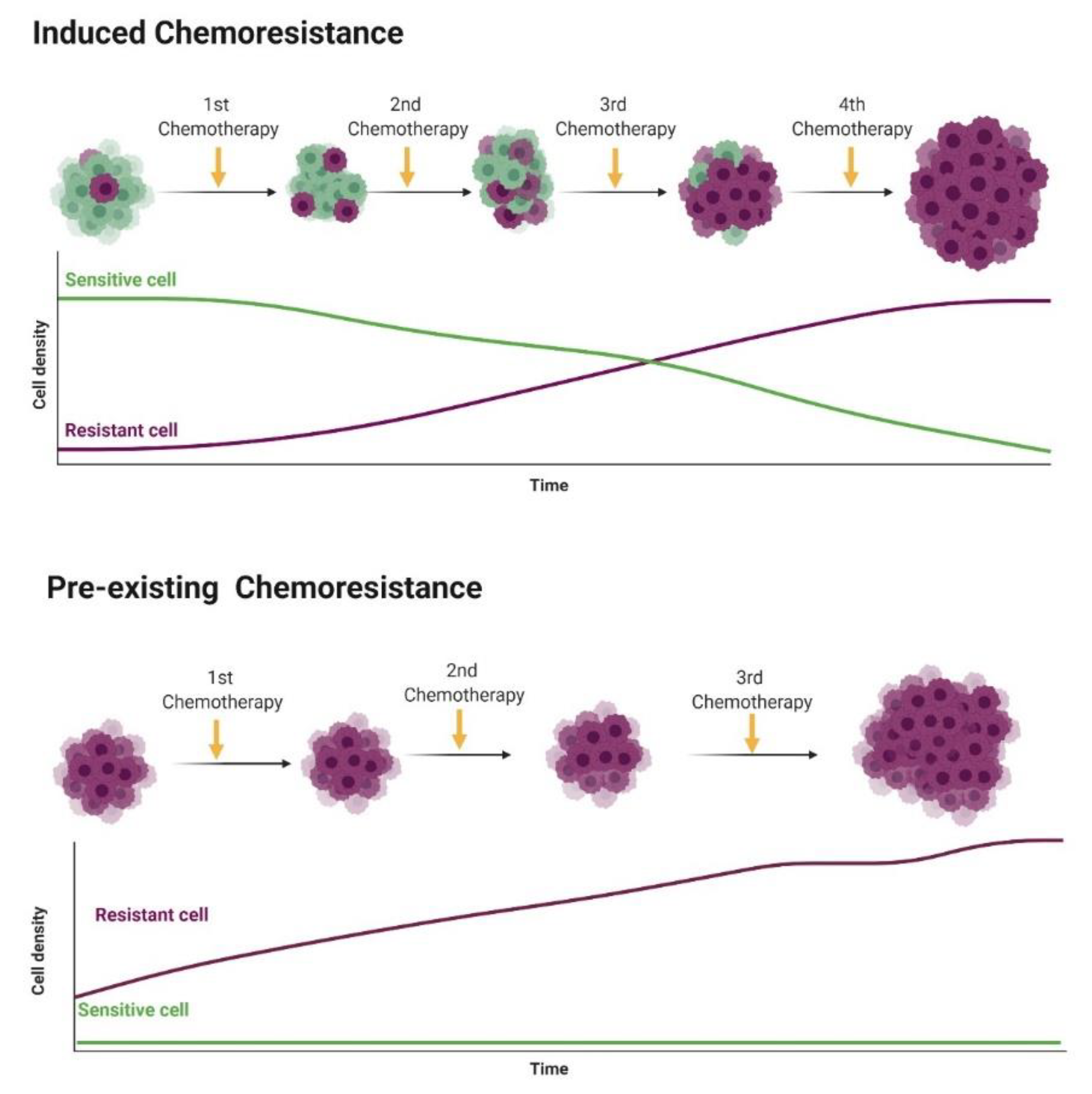
4.1. Multidrug Resistance (MDR)
4.2. Cancer Stem Cells
4.3. Signaling Pathways
4.4. Epithelial-Mesenchymal Transition
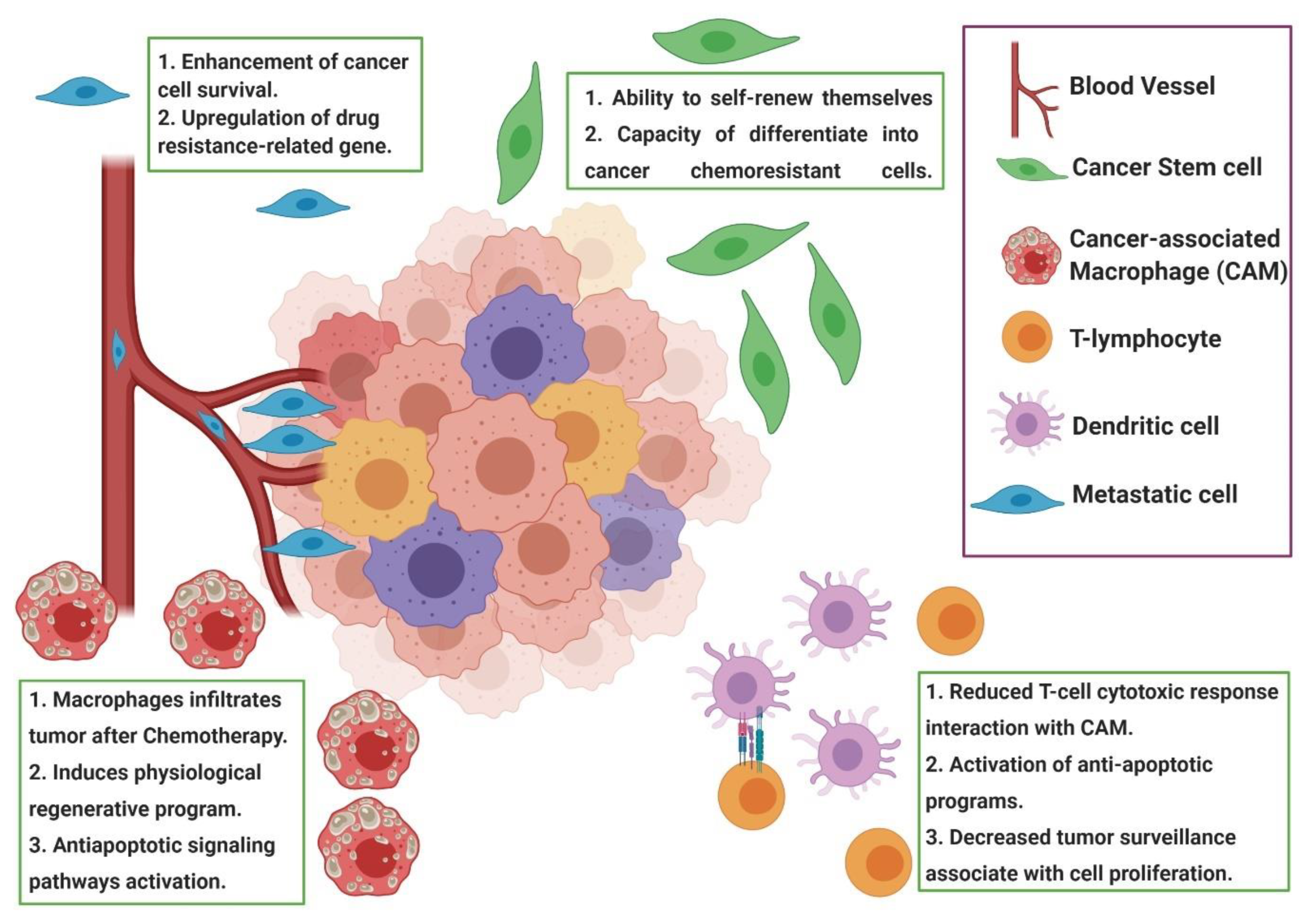
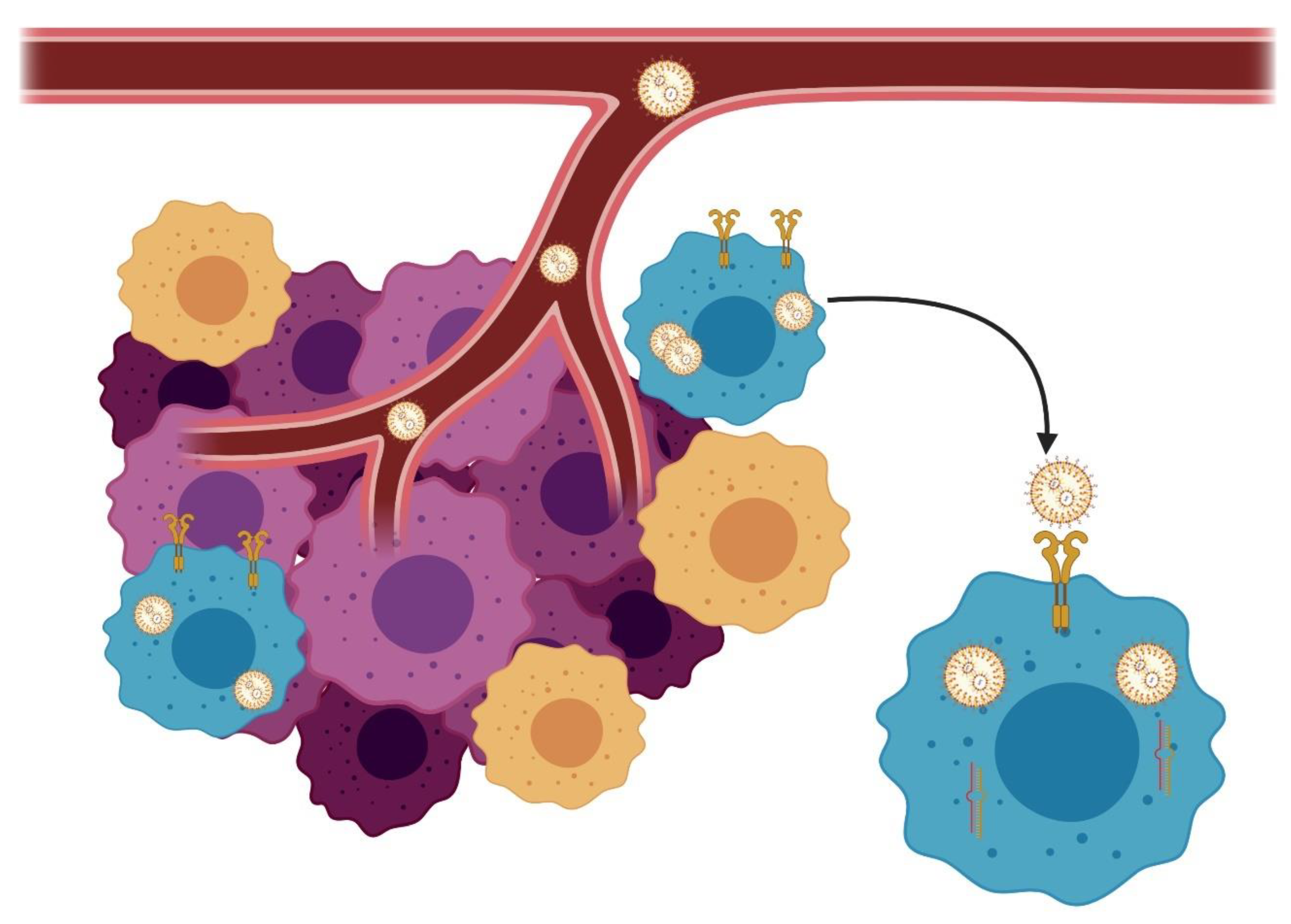
4.5. Tumor Microenvironment
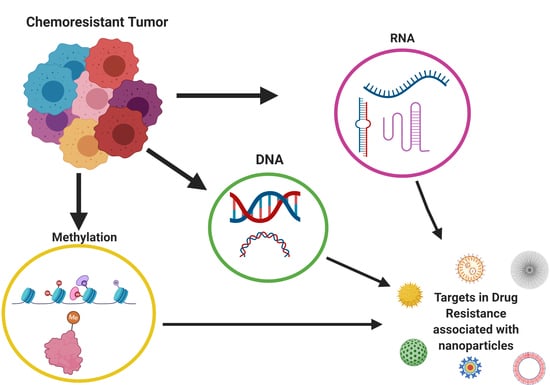
5. Epigenetics and Breast Cancer Chemoresistance
6. Markers Associated with the Chemotherapy Response
7. Drug Delivered Systems in Chemoresistance
8. Emerging Targets for Treatment
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Erratum: Global cancer statistics 2018: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2020, 70, 313. [Google Scholar] [CrossRef]
- Loboda, A.; Sr, I.S.; Orel, V.E.; Syvak, L.; Golovko, T.; Dosenko, I.; Lyashenko, A.; Smolanka, J.I.; Dasyukevich, O.; Tarasenko, T.; et al. Efficacy of combination neoadjuvant chemotherapy and regional inductive moderate hyperthermia in the treatment of patients with locally advanced breast cancer. Technol. Cancer Res. Treat. 2020, 19, 1–10. [Google Scholar] [CrossRef]
- Anders, C.K.; Carey, L.A. Biology, metastatic patterns, and treatment of patients with triple-negative breast cancer. Clin. Breast Cancer 2009, 9, S73–S81. [Google Scholar] [CrossRef] [PubMed]
- Cleator, S.; Heller, W.; Coombes, R.C. Triple-negative breast cancer: Therapeutic options. Lancet Oncol. 2007, 8, 235–244. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics. CA A Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Tan, P.H.; Ellis, I.; Allison, K.; Brogi, E.; Fox, S.B.; Lakhani, S.; Lazar, A.J.; Morris, E.A.; Sahin, A.; Salgado, R.; et al. The 2019 World Health Organization classification of tumours of the breast. Histopathology 2020, 77, 181–185. [Google Scholar] [CrossRef]
- Giuliano, A.E.; Connolly, J.L.; Edge, S.B.; Mittendorf, E.A.; Rugo, H.S.; Solin, L.J.; Weaver, D.L.; Winchester, D.J.; Hortobagyi, G.N. Breast cancer-major changes in the American joint committee on cancer eighth edition cancer staging manual. CA A Cancer J. Clin. 2017, 67, 290–303. [Google Scholar] [CrossRef]
- Cardoso, F.; Kyriakides, S.; Ohno, S.; Penault-Llorca, F.; Poortmans, P.; Rubio, I.; Zackrisson, S.; Senkus, E. Early breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2019, 30, 1194–1220. [Google Scholar] [CrossRef]
- Ruddy, K.J.; Ganz, P.A. Treatment of nonmetastatic breast cancer. JAMA 2019, 321, 1716–1717. [Google Scholar] [CrossRef]
- Harbeck, N.; Penault-Llorca, F.; Cortes, J.; Gnant, M.; Houssami, N.; Poortmans, P.; Ruddy, K.; Tsang, J.; Cardoso, F. Breast cancer. Nat. Rev. Dis. Prim. 2019, 5, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Fragomeni, S.M.; Sciallis, A.; Jeruss, J.S. Molecular subtypes and local-regional control of breast cancer. Surg. Oncol. Clin. N. Am. 2018, 27, 95–120. [Google Scholar] [CrossRef] [PubMed]
- Blows, F.M.; Driver, K.E.; Schmidt, M.K.; Broeks, A.; Van Leeuwen, F.E.; Wesseling, J.; Cheang, M.C.; Gelmon, K.; Nielsen, T.O.; Blomqvist, C.; et al. Subtyping of breast cancer by immunohistochemistry to investigate a relationship between subtype and short and long term survival: A collaborative analysis of data for 10,159 cases from 12 studies. PLoS Med. 2010, 7, e1000279. [Google Scholar] [CrossRef] [PubMed]
- American Cancer Society. Treatment of Triple Negative Breast Cancer. Treating Breast Cancer. Available online: https://www.cancer.org/cancer/breast-cancer/treatment/treatment-of-triple-negative.html (accessed on 14 September 2020).
- Abdelmegeed, S.M.; Mohammed, S. Canine mammary tumors as a model for human disease (Review). Oncol. Lett. 2018, 15, 8195–8205. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute (NCI). Breast Cancer Treatment. Breast Cancer. Available online: https://www.cancer.gov/types/breast/hp/breast-treatment-pdq (accessed on 15 September 2020).
- Burstein, H.; Curigliano, G.; Loibl, S.; Dubsky, P.; Gnant, M.; Poortmans, P.; Colleoni, M.; Denkert, C.; Piccart-Gebhart, M.; Regan, M.; et al. Estimating the benefits of therapy for early-stage breast cancer: The St. Gallen International Consensus Guidelines for the primary therapy of early breast cancer. Ann. Oncol. 2019, 30, 1541–1557. [Google Scholar] [CrossRef] [PubMed]
- Waks, A.G.; Winer, E.P. Breast cancer treatment: A review. JAMA 2019, 321, 288–300. [Google Scholar] [CrossRef]
- Curigliano, G.; Burstein, H.; Winer, E.; Gnant, M.; Dubsky, P.; Loibl, S.; Colleoni, M.; Regan, M.; Piccart-Gebhart, M.; Senn, H.-J.; et al. De-escalating and escalating treatments for early-stage breast cancer: The St. Gallen International Expert Consensus Conference on the primary therapy of early breast cancer. Ann. Oncol. 2017, 28, 1700–1712. [Google Scholar] [CrossRef] [PubMed]
- Balic, M.; Thomssen, C.; Würstlein, R.; Gnant, M.; Harbeck, N. St. Gallen/Vienna 2019: A brief summary of the consensus discussion on the optimal primary breast cancer treatment. Breast Care 2019, 14, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Rangarao, R.; Smruti, B.K.; Singh, K.; Gupta, A.; Batra, S.; Choudhary, R.K.; Sahani, S.; Kabra, V.; Parikh, P.M.; Aggarwal, S. Practical consensus recommendations on management of triple-negative metastatic breast cancer. South Asian J. Cancer 2018, 7, 127–131. [Google Scholar] [CrossRef]
- Gottesman, M.M. Mechanisms of cancer drug resistance. Annu. Rev. Med. 2002, 53, 615–627. [Google Scholar] [CrossRef] [PubMed]
- Nikolaou, M.; Pavlopoulou, A.; Cetin, Z.; Kyrodimos, E. The challenge of drug resistance in cancer treatment: A current overview. Clin. Exp. Metastasis 2018, 35, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Vasan, N.; Baselga, J.; Hyman, D.M. A view on drug resistance in cancer. Nat. Cell Biol. 2019, 575, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Mansoori, B.; Mohammadi, A.; Davudian, S.; Shirjang, S.; Baradaran, B. The different mechanisms of cancer drug resistance: A brief review. Adv. Pharm. Bull. 2017, 7, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Min-Ji, W.; Lu, Y.; Tian, H.; Meng, X.; Wei, M.; Cho, W.C. Chemoresistance mechanisms of breast cancer and their countermeasures. Biomed. Pharmacother. 2019, 114, 108800. [Google Scholar] [CrossRef]
- Hernandez-Aya, L.F.; Gonzalez-Angulo, A.M. Adjuvant systemic therapies in breast cancer. Surg. Clin. N. Am. 2013, 93, 473–491. [Google Scholar] [CrossRef]
- Nedeljković, M.; Damjanović, A. Mechanisms of chemotherapy resistance in triple-negative breast cancer—How we can rise to the challenge. Cells 2019, 8, 957. [Google Scholar] [CrossRef]
- Yeldag, G.; Rice, A.; Del Hernández, A.R. Chemoresistance and the self-maintaining tumor microenvironment. Cancers 2018, 10, 471. [Google Scholar] [CrossRef]
- Ma, D.; Wu, L.; Li, S.; Sun, Z.; Wang, K. Vasohibin2 promotes adriamycin resistance of breast cancer cells through regulating ABCG2 via AKT signaling pathway. Mol. Med. Rep. 2017, 16, 9729–9734. [Google Scholar] [CrossRef][Green Version]
- Zheng, H.-C. The molecular mechanisms of chemoresistance in cancers. Oncotarget 2017, 8, 59950–59964. [Google Scholar] [CrossRef]
- Chen, S.; Wang, H.; Li, Z.; You, J.; Wu, Q.-W.; Zhao, C.; Tzeng, C.-M.; Zhang, Z. Interaction of WBP2 with ERα increases doxorubicin resistance of breast cancer cells by modulating MDR1 transcription. Br. J. Cancer 2018, 119, 182–192. [Google Scholar] [CrossRef]
- Kuşoğlu, A.; Avcı, Ç.B. Cancer stem cells: A brief review of the current status. Gene 2019, 681, 80–85. [Google Scholar] [CrossRef]
- Moulder, S.L. Intrinsic resistance to chemotherapy in breast cancer. Women’s Health 2010, 6, 821–830. [Google Scholar] [CrossRef] [PubMed]
- Guanizo, A.C.; Fernando, C.D.; Garama, D.J.; Gough, D.J. STAT3: A multifaceted oncoprotein. Growth Factors 2018, 36, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.H.T.; Munusamy, P.; Loke, S.Y.; Koh, G.L.; Wong, E.S.Y.; Law, H.Y.; Yoon, C.S.; Tan, M.-H.; Yap, Y.S.; Ang, P.; et al. Identification of novel breast cancer risk loci. Cancer Res. 2017, 77, 5428–5437. [Google Scholar] [CrossRef] [PubMed]
- Harkness, E.F.; Astley, S.M.; Evans, D. Risk-based breast cancer screening strategies in women. Best Pract. Res. Clin. Obstet. Gynaecol. 2020, 65, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Pop, L.A.; Cojocneanu-Petric, R.; Pileczki, V.; Morar-Bolba, G.; Irimie, A.; Lazar, V.; Lombardo, C.; Paradiso, A.V.; Berindan-Neagoe, I. Genetic alterations in sporadic triple negative breast cancer. Breast 2018, 38, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Takada, M.; Nagai, S.; Haruta, M.; Sugino, R.P.; Tozuka, K.; Takei, H.; Ohkubo, F.; Inoue, K.; Kurosumi, M.; Miyazaki, M.; et al. BRCA1alterations with additional defects in DNA damage response genes may confer chemoresistance to BRCA-like breast cancers treated with neoadjuvant chemotherapy. Genes Chromosom. Cancer 2017, 56, 405–420. [Google Scholar] [CrossRef]
- Paoletti, C.; Cani, A.K.; Larios, J.M.; Hovelson, D.H.; Aung, K.; Darga, E.P.; Cannell, E.M.; Baratta, P.J.; Liu, C.-J.; Chu, D.; et al. Comprehensive mutation and copy number profiling in archived circulating breast cancer tumor cells documents heterogeneous resistance mechanisms. Cancer Res. 2017, 78, 1110–1122. [Google Scholar] [CrossRef]
- Coté, D.; Eustace, A.; Toomey, S.; Cremona, M.; Milewska, M.; Furney, S.; Carr, A.; Fay, J.; Kay, E.; Kennedy, S.; et al. Germline single nucleotide polymorphisms in ERBB3 and BARD1 genes result in a worse relapse free survival response for HER2-positive breast cancer patients treated with adjuvant based docetaxel, carboplatin and trastuzumab (TCH). PLoS ONE 2018, 13, e0200996. [Google Scholar] [CrossRef]
- Kim, C.; Gao, R.; Sei, E.; Brandt, R.; Hartman, J.; Hatschek, T.; Crosetto, N.; Foukakis, T.; Navin, N.E. Chemoresistance evolution in triple-negative breast cancer delineated by single-cell sequencing. Cell 2018, 173, 879–893.e13. [Google Scholar] [CrossRef]
- Magnani, L.; Brunelle, M.; Gévry, N.; Lupien, M. Chromatin landscape and endocrine response in breast cancer. Epigenomics 2012, 4, 675–683. [Google Scholar] [CrossRef]
- Amorim, M.; Salta, S.; De Sousa, S.P.; Henrique, R. Predicting resistance to endocrine therapy in breast cancer: It’s time for epigenetic biomarkers (Review). Oncol. Rep. 2019, 41, 1431–1438. [Google Scholar] [CrossRef]
- Stone, A.; Zotenko, E.; Locke, W.J.; Korbie, D.; Millar, E.K.A.; Pidsley, R.; Stirzaker, C.; Graham, P.; Trau, M.; Musgrove, E.A.; et al. DNA methylation of oestrogen-regulated enhancers defines endocrine sensitivity in breast cancer. Nat. Commun. 2015, 6, 7758. [Google Scholar] [CrossRef] [PubMed]
- Achinger-Kawecka, J.; Valdés-Mora, F.; Luu, P.-L.; Giles, K.A.; Caldon, C.E.; Qu, W.; Nair, S.; Soto, S.; Locke, W.J.; Yeo-Teh, N.S.; et al. Epigenetic reprogramming at estrogen-receptor binding sites alters 3D chromatin landscape in endocrine-resistant breast cancer. Nat. Commun. 2020, 11, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Lustberg, M.; Ramaswamy, B. Epigenetic targeting in breast cancer: Therapeutic impact and future direction. Drug News Perspect. 2009, 22, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Lapidus, R.G.; Ferguson, A.T.; Ottaviano, Y.L.; Parl, F.F.; Smith, H.S.; Weitzman, S.A.; Baylin, S.B.; Issa, J.P.; Davidson, N.E. Methylation of estrogen and progesterone receptor gene 5′ CpG islands correlates with lack of estrogen and progesterone receptor gene expression in breast tumors. Clin. Cancer Res. 1996, 2, 805–810. [Google Scholar]
- Verde, G.; Cucalon, L.I.D.L.; Wright, R.H.G.; Quilez, J.; Peiró, S.; LeDily, F.; Beato, M. Unliganded progesterone receptor governs estrogen receptor gene expression by regulating DNA methylation in breast cancer cells. Cancers 2018, 10, 371. [Google Scholar] [CrossRef]
- Drago, J.Z.; Formisano, L.; Juric, D.; Niemierko, A.; Servetto, A.; Wander, S.A.; Spring, L.M.; Vidula, N.; Younger, J.; Peppercorn, J.; et al. FGFR1 amplification mediates endocrine resistance but retains TORC sensitivity in metastatic hormone receptor–positive (HR+) breast cancer. Clin. Cancer Res. 2019, 25, 6443–6451. [Google Scholar] [CrossRef]
- Bianchini, G.; Balko, J.M.; Mayer, I.A.; Sanders, M.E.; Gianni, L. Triple-negative breast cancer: Challenges and opportunities of a heterogeneous disease. Nat. Rev. Clin. Oncol. 2016, 13, 674–690. [Google Scholar] [CrossRef]
- Jang, H.S.; Woo, S.R.; Song, K.-H.; Cho, H.; Chay, D.B.; Hong, S.-O.; Lee, H.-J.; Oh, S.J.; Chung, J.-Y.; Kim, J.-H.; et al. API5 induces cisplatin resistance through FGFR signaling in human cancer cells. Exp. Mol. Med. 2017, 49, e374. [Google Scholar] [CrossRef]
- Li, S.; Payne, S.; Wang, F.; Claus, P.; Su, Z.; Groth, J.; Geradts, J.; De Ridder, G.; Álvarez, R.; Marcom, P.K.; et al. Nuclear basic fibroblast growth factor regulates triple-negative breast cancer chemo-resistance. Breast Cancer Res. 2015, 17, 91. [Google Scholar] [CrossRef]
- Hu, Y.; Qiu, Y.; Yagüe, E.; Ji, W.; Liu, J.; Zhang, J. miRNA-205 targets VEGFA and FGF2 and regulates resistance to chemotherapeutics in breast cancer. Cell Death Dis. 2016, 7, e2291. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Wu, W.; Fu, B.; Shi, L.; Wang, X.; Kuca, K. JNK signaling in cancer cell survival. Med. Res. Rev. 2019, 39, 2082–2104. [Google Scholar] [CrossRef] [PubMed]
- Xiang, H.; Zhang, J.; Lin, C.; Zhang, L.; Liu, B.; Ouyang, L. Targeting autophagy-related protein kinases for potential therapeutic purpose. Acta Pharm. Sin. B 2020, 10, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-W.; Zhang, W.; Ma, R. Bioinformatic identification of chemoresistance-associated microRNAs in breast cancer based on microarray data. Oncol. Rep. 2018, 39, 1003–1010. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, Z. The emerging role of microRNAs in breast cancer. J. Oncol. 2020, 2020, 1–7. [Google Scholar] [CrossRef]
- Wang, Y.; Rathinam, R.; Walch, A.; Alahari, S.K. ST14 (suppression of tumorigenicity 14) gene is a target for miR-27b, and the inhibitory effect of ST14 on cell growth is independent of miR-27b regulation. J. Biol. Chem. 2009, 284, 23094–23106. [Google Scholar] [CrossRef]
- Yu, S.-J.; Hu, J.-Y.; Kuang, X.-Y.; Luo, J.-M.; Hou, Y.-F.; Di, G.-H.; Wu, J.; Shen, Z.-Z.; Song, H.-Y.; Shao, Z.-M. MicroRNA-200a promotes anoikis resistance and metastasis by targeting YAP1 in human breast cancer. Clin. Cancer Res. 2013, 19, 1389–1399. [Google Scholar] [CrossRef]
- Zhang, C.-M.; Zhao, Y.; Deng, H.-Y. MiR-155 promotes proliferation of human breast cancer MCF-7 cells through targeting tumor protein 53-induced nuclear protein. J. Biomed. Sci. 2013, 20, 79. [Google Scholar] [CrossRef]
- Rodrigues-Ferreira, S.; Nehlig, A.; Kacem, M.; Nahmias, C. ATIP3 deficiency facilitates intracellular accumulation of paclitaxel to reduce cancer cell migration and lymph node metastasis in breast cancer patients. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef]
- Gong, Y.; Hu, N.; Ma, L.; Li, W.; Cheng, X.; Zhang, Y.; Zhu, Y.; Yang, Y.; Peng, X.; Zou, D.; et al. ABTB2 regulatory variant as predictor of epirubicin-based neoadjuvant chemotherapy in luminal a breast cancer. Front. Oncol. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Al Amri, W.S.; Baxter, D.E.; Hanby, A.M.; Stead, L.F.; Verghese, E.T.; Thorne, J.L.; Hughes, T.A. Identification of candidate mediators of chemoresponse in breast cancer through therapy-driven selection of somatic variants. Breast Cancer Res. Treat. 2020, 183, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Ren, L.; Chen, H.; Pan, J.; Zhang, Z.; Kuang, X.; Chen, X.; Bao, W.; Lin, C.; Zhou, Z.; et al. NCAPG confers trastuzumab resistance via activating SRC/STAT3 signaling pathway in HER2-positive breast cancer. Cell Death Dis. 2020, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Yang, H.; Zhu, X.; Xiong, S.; Chi, H.; Xu, W. Integrative analysis of the doxorubicin-associated lncRNA–mRNA network identifies chemoresistance-associated lnc-TRDMT1-5 as a biomarker of breast cancer progression. Front. Genet. 2020, 11, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Demir, S.; Müslümanoğlu, M.H.; Müslümanoğlu, M.; Başaran, S.; Çalay, Z.Z.; Aydıner, A.; Vogt, U.; Çakır, T.; Kadıoğlu, H.; Artan, S. TWIST1 gene expression as a biomarker for predicting primary doxorubicin resistance in breast cancer. Balk. J. Med. Genet. 2019, 22, 25–30. [Google Scholar] [CrossRef]
- Zhao, Y.; Wei, L.; Liu, J.; Li, F. Chemoresistance was correlated with elevated expression and activity of indoleamine 2,3-dioxygenase in breast cancer. Cancer Chemother. Pharmacol. 2019, 85, 77–93. [Google Scholar] [CrossRef]
- Xing, M.; Wang, J.; Yang, Q.; Wang, Y.; Li, J.; Xiong, J.; Zhou, S. FKBP12 is a predictive biomarker for efficacy of anthracycline-based chemotherapy in breast cancer. Cancer Chemother. Pharmacol. 2019, 84, 861–872. [Google Scholar] [CrossRef]
- Wang, Y.; Zeng, J.; Wu, W.; Xie, S.; Yu, H.; Li, G.; Zhu, T.; Li, F.; Lu, J.; Wang, G.Y.; et al. Nicotinamide N-methyltransferase enhances chemoresistance in breast cancer through SIRT1 protein stabilization. Breast Cancer Res. 2019, 21, 1–17. [Google Scholar] [CrossRef]
- Lainetti, P.; Zuliani, F.; Leis-Filho, A.F.; Alves, R.H.F.; Fonseca-Alves, C.E. Controlled drug delivery vehicles in veterinary oncology: State-of-the-art and future directions. Processes 2020, 8, 541. [Google Scholar] [CrossRef]
- Toh, T.-B.; Lee, D.-K.; Hou, W.; Abdullah, L.N.; Nguyen, J.; Ho, D.; Chow, E.K.-H. Nanodiamond–mitoxantrone complexes enhance drug retention in chemoresistant breast cancer cells. Mol. Pharm. 2014, 11, 2683–2691. [Google Scholar] [CrossRef]
- Abou-El-Naga, A.M.; Mutawa, G.; El-Sherbiny, I.M.; Mousa, S.A. Activation of polymeric nanoparticle intracellular targeting overcomes chemodrug resistance in human primary patient breast cancer cells. Int. J. Nanomed. 2018, 13, 8153–8164. [Google Scholar] [CrossRef]
- Dunford, J.E.; Thompson, K.; Coxon, F.P.; Luckman, S.P.; Hahn, F.M.; Poulter, C.D.; Ebetino, F.H.; Rogers, M.J. Structure-activity relationships for inhibition of farnesyl diphosphate synthase in vitro and inhibition of bone resorption in vivo by nitrogen-containing bisphosphonates. J. Pharmacol. Exp. Ther. 2001, 296, 235–242. [Google Scholar]
- Kopecka, J.; Porto, S.; Lusa, S.; Gazzano, E.; Salzano, G.; Pinzon-Daza, M.L.; Giordano, A.; Desiderio, V.; Ghigo, D.; De Rosa, G.; et al. Zoledronic acid-encapsulating self-assembling nanoparticles and doxorubicin: A combinatorial approach to overcome simultaneously chemoresistance and immunoresistance in breast tumors. Oncotarget 2016, 7, 20753–20772. [Google Scholar] [CrossRef] [PubMed]
- Schiraldi, C.; Zappavigna, S.; Agostino, A.D.; Porto, S.; Gaito, O.; Lusa, S.; Lamberti, M.; De Rosa, M.; De Rosa, G.; Caraglia, M. Nanoparticles for the delivery of zoledronic acid to prostate cancer cells: A comparative analysis through time lapse video-microscopy technique. Cancer Biol. Ther. 2014, 15, 1524–1532. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jones, S.K.; Merkel, O.M. Tackling breast cancer chemoresistance with nano-formulated siRNA. Gene Ther. 2016, 23, 821–828. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, J.; Li, X.; Huang, L. Non-viral nanocarriers for siRNA delivery in breast cancer. J. Control. Release 2014, 190, 440–450. [Google Scholar] [CrossRef]
- Hamurcu, Z.; Ashour, A.; Kahraman, N.; Ozpolat, B. FOXM1 regulates expression of eukaryotic elongation factor 2 kinase and promotes proliferation, invasion and tumorgenesis of human triple negative breast cancer cells. Oncotarget 2016, 7, 16619–16635. [Google Scholar] [CrossRef]
- Kren, B.T.; Unger, G.M.; Abedin, J.; Vogel, R.I.; Henzler, C.M.; Ahmed, K.; Trembley, J.H. Preclinical evaluation of cyclin dependent kinase 11 and casein kinase 2 survival kinases as RNA interference targets for triple negative breast cancer therapy. Breast Cancer Res. 2015, 17, 1–21. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, Y.-H.; Mao, C.-Q.; Dou, S.; Shen, S.; Tan, Z.-B.; Wang, J. Triple negative breast cancer therapy with CDK1 siRNA delivered by cationic lipid assisted PEG-PLA nanoparticles. J. Control. Release 2014, 192, 114–121. [Google Scholar] [CrossRef]
- Xu, J.; Liu, Y.; Li, Y.; Wang, H.; Stewart, S.; Van Der Jeught, K.; Agarwal, P.; Zhang, Y.; Liu, S.; Zhao, G.; et al. Precise targeting of POLR2A as a therapeutic strategy for human triple negative breast cancer. Nat. Nanotechnol. 2019, 14, 388–397. [Google Scholar] [CrossRef]
- Wang, S.-M.; Dai, Q.; Zhou, H.; Wang, L.; Cheang, T.; Wei, J.; Luo, J. CaCO3/CaIP6 composite nanoparticles effectively deliver AKT1 small interfering RNA to inhibit human breast cancer growth. Int. J. Nanomed. 2015, 10, 4255–4266. [Google Scholar] [CrossRef]
- Vaidya, A.M.; Sun, Z.; Ayat, N.; Schilb, A.; Liu, X.; Jiang, H.; Sun, D.; Scheidt, J.; Qian, V.; He, S.; et al. Systemic delivery of tumor-targeting siRNA nanoparticles against an oncogenic lncRNA facilitates effective triple-negative breast cancer therapy. Bioconjugate Chem. 2019, 30, 907–919. [Google Scholar] [CrossRef] [PubMed]
| MicroRNA | Expression | Fold Change |
|---|---|---|
| hsa-miR-195a-5p | upregulated | 5.44 |
| hsa-miR-4266 | upregulated | 3.45 |
| hsa-miR-200b-3p | upregulated | 3.13 |
| hsa-miR-214-3p | upregulated | 3.00 |
| hsa-miR-107 | upregulated | 2.96 |
| hsa-miR-4454 | upregulated | 2.88 |
| hsa-miR-5100 | upregulated | 2.41 |
| hsa-miR-23a-3p | upregulated | 2.30 |
| hsa-miR-23b-3p | upregulated | 2.29 |
| hsa-miR-16-5p | upregulated | 2.09 |
| hsa-miR-4707-5p | downregulated | 0.49 |
| hsa-miR-3656 | downregulated | 0.46 |
| hsa-miR-1233-1-5p | downregulated | 0.46 |
| hsa-miR-3621 | downregulated | 0.44 |
| hsa-miR-3141 | downregulated | 0.44 |
| hsa-miR-489 | downregulated | 0.41 |
| hsa-miR-1227-5p | downregulated | 0.41 |
| hsa-miR-1275 | downregulated | 0.39 |
| hsa-miR-1268b | downregulated | 0.36 |
| hsa-miR-572 | downregulated | 0.30 |
| hsa-miR-4467 | downregulated | 0.29 |
| hsa-miR-4472 | downregulated | 0.18 |
| Reference | Drug | Number of Petients | Markers | Expression | BC * Subtype |
|---|---|---|---|---|---|
| Rodrigues-Ferreira et al. [61] | Paclitaxel | 133 | ATIP3 | Overexpression | |
| Gong et al. [62] | Epirubicin and Docetaxel | 421 | ABTB2 | Overexpression | |
| Amri et al. [63] | Epirubicin/cyclophosphamide | 6 | TCHH, MUC17, ARAP2, FLG2, ABL1, CENPF, COL6A3, DMBT1, ITGA7, PLXNA1, S100PBP, SYNE1, ZFHX4, and CACNA1C | Somatic variance | Estrogen receptor-positive/HER2-negative |
| Jiang et al. [64] | Trastuzumab | 12 | NCAPG | Overexpression | HER2-positive |
| Chen et al. [65] | doxorubicin | 20 | lnc-TRDMT1-5 | Overexpression | Not informed |
| Demir et al. [66] | doxorubicin | 26 | TWIST1 | Overexpression | Not informed |
| Zhao et al. [67] | TEC (paclitaxel 135 ~ 175 mg/m2 or docetaxel 75 mg/m2, epirubicin 60 mg/m2, cyclophosphamide 600 mg/m2) | 53 | Indoleamine 2,3-dioxygenase | Overexpression | All subtypes ** |
| Xing et al. [68] | Anthracycline-based chemotherapy | 524 | FKBP12 | Downexpression | Luminal, HER-2 overexpressing and TNBC *** |
| Wang et al. [69] | CMF (cyclophosphamide + methotrexate + fluorouracil) and FEC-P (fluorouracil + epirubicin + cyclophosphamide + paclitaxel) | 165 | NNMT | Overexpression | All subtypes ** |
| Target | Function | Drug Delivery System | Reference |
|---|---|---|---|
| FoxM1 | Cell cycle regulator | Liposomal lipid nanoparticles | Hamurcu et al. [78] |
| CDK11 | Cell grwoth and survival | Polyamine-based micelles | Kren et al. [79] |
| CDK1 | Cyclin-dependent kinase | Cationic lipid-based nanoparticle made of polylactic acid and polyethylene glycol system | Liu et al. [80] |
| POLR2A | Catalytic component of RNA polymerase II | pH-activated nanoparticles | Xu et al. [81] |
| AKT1 | Regulator of mTOR signaling pathway | Inorganic amorphous calcium carbonate (ACC) hybrid nanospheres functionalized with CaIP6 (ACC/CaIP6) nanoparticles | Zhou et al. [82] |
| onco-lncRNAs | Influences gene signature | 1-aminoethylimino[bis(N-oleoylcysteinyl-aminoethyl)propionamide]- polyethylene glycol-RGD/siRNA nanoparticles | Vaidya et al. [83] |
| Status | Brief Description | Interventions/Treatment | Phase | Study Title |
|---|---|---|---|---|
| Active, not recruiting | This phase I trial studies the side effects and best dose of APN401 in treating patients with pancreatic cancer, colorectal cancer or other solid tumors that have spread to other places in the body or have come back. APN401 may stop the growth of tumor cells by blocking some of the enzymes needed for cell growth. | siRNA-transfected Peripheral Blood Mononuclear Cells APN401 | I | APN401 in Treating Patients With Recurrent or Metastatic Pancreatic Cancer, Colorectal Cancer, or Other Solid Tumors That Cannot Be Removed by Surgery |
| Completed | This phase I trial studies the side effects and best dose of small-interfering ribonucleic acid (siRNA)-transfected peripheral blood mononuclear cells APN401 (APN401) in treating patients with melanoma, kidney or pancreatic cancer or other solid tumors that have spread to other parts of the body or that cannot be removed by surgery. There are factors in immune cells in the blood that inhibit their ability to kill cancers. Treating white blood cells with one of these factors in the laboratory may help the white blood cells kill more cancer cells when they are put back in the body. | Biological: siRNA-transfected peripheral blood mononuclear cells APN401 | I | APN401 in Treating Patients With Melanoma, Kidney Cancer, Pancreatic Cancer, or Other Solid Tumors That Are Metastatic or Cannot Be Removed By Surgery |
| Not yet recruiting | This phase I trial studies the best dose and side effects of mesenchymal stromal cell-derived exosomes with KrasG12D siRNA (iExosomes) in treating participants with pancreatic cancer with a KrasG12D mutation that has spread to other places in the body. iExosomes may work better at treating pancreatic cancer. | Mesenchymal Stromal Cells-derived Exosomes with KRAS G12D siRNA | I | iExosomes in Treating Participants With Metastatic Pancreas Cancer With KrasG12D Mutation |
| Completed | Cancer in the liver can start in the liver (e.g., primary liver cancer or hepatocellular cancer) or spread to the liver from cancers in other parts of the body (e.g., colon, pancreas, gastric, breast, ovarian, esophageal cancers and cancer with metastases to the liver). People who have tumors that can be removed by surgery live longer than those whose cancer cannot be removed. Chemotherapy can shrink some tumors in the liver, which also helps people to live longer, and sometimes, chemotherapy can shrink tumors enough that they can be removed by surgery. However, most chemotherapy drugs do not work well on tumors in the liver. In this study, we are testing a new drug, TKM-080301, given directly into the cancer blood supply in the liver circulation to see if it will cause tumors to shrink. | TKM-080301 | I | TKM 080301 for Primary or Secondary Liver Cancer |
| Completed | Phase I: This study is designed to investigate the safety of a siG12D LODER (Local Drug EluteR) in patients diagnosed with adenocarcinoma of the pancreas. The primary endpoint is to assess the efficacy of the siG12D LODER and local distribution in nonoperable patients by histopathology measurements and local distribution by RNA analysis. | siG12D LODER | I | Phase I - Escalating Dose Study of siG12D LODER (Local Drug EluteR) in Patients With Locally Advanced Adenocarcinoma of the Pancreas, and a Single Dose Study of siG12D LODER (Local Drug EluteR) in Patients With Non-operable Adenocarcinoma of the Pancreas |
| Terminated | The purpose of this study is to assess the safety and tolerability of the investigational anticancer drug DCR-MYC. DCR-MYC is a novel synthetic double-stranded RNA in a stable lipid particle suspension that targets the oncogene MYC. MYC oncogene activation is important to the growth of many hematologic and solid tumor malignancies. In this study, the sponsor proposes to study DCR-MYC and its ability to inhibit MYC and thereby inhibit cancer cell growth. | DCR-MYC | I | Phase I, Multicenter, Dose Escalation Study of DCR-MYC in Patients With Solid Tumors, Multiple Myeloma, or Lymphoma |
| Unknown | In this Phase II study, a dose of 2.8 mg (eight 0.35-mg siG12D-LODERs) will be administered in 12-week cycles to patients with unresectable locally advanced pancreatic cancer combined with chemotherapy treatment. | siG12D-LODER | II | A Phase 2 Study of siG12D LODER in Combination With Chemotherapy in Patients With Locally Advanced Pancreatic Cancer |
| Recruiting | This phase I trial studies the side effects and best dose of EphA2 siRNA in treating patients with solid tumors that have spread to other places in the body and usually cannot be cured or controlled with treatment (advanced) or have come back after a period of improvement (recurrent). EphA2-targeting 1,2-dioleoyl-sn-glycero-3-phosphatidylcholine -encapsulated siRNA may slow the growth of tumor cells by shutting down the activity of a gene that causes tumor growth. | EphA2-targeting DOPC-encapsulated siRNA | I | EphA2 siRNA in Treating Patients With Advanced or Recurrent Solid Tumors |
| Terminated | The purpose of this study is to assess the safety and tolerability of the investigational anticancer drug DCR-MYC. DCR-MYC is a novel synthetic double-stranded RNA in a stable lipid particle suspension that targets the oncogene MYC. MYC oncogene activation is important to the growth of many hematologic and solid tumor malignancies. In this study, the sponsor proposes to study DCR-MYC and its ability to inhibit MYC and thereby inhibit cancer cell growth. | DCR-MYC | II | Phase Ib/2, Multicenter, Dose Escalation Study of DCR-MYC in Patients With Hepatocellular Carcinoma |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lainetti, P.d.F.; Leis-Filho, A.F.; Laufer-Amorim, R.; Battazza, A.; Fonseca-Alves, C.E. Mechanisms of Resistance to Chemotherapy in Breast Cancer and Possible Targets in Drug Delivery Systems. Pharmaceutics 2020, 12, 1193. https://doi.org/10.3390/pharmaceutics12121193
Lainetti PdF, Leis-Filho AF, Laufer-Amorim R, Battazza A, Fonseca-Alves CE. Mechanisms of Resistance to Chemotherapy in Breast Cancer and Possible Targets in Drug Delivery Systems. Pharmaceutics. 2020; 12(12):1193. https://doi.org/10.3390/pharmaceutics12121193
Chicago/Turabian StyleLainetti, Patrícia de Faria, Antonio Fernando Leis-Filho, Renee Laufer-Amorim, Alexandre Battazza, and Carlos Eduardo Fonseca-Alves. 2020. "Mechanisms of Resistance to Chemotherapy in Breast Cancer and Possible Targets in Drug Delivery Systems" Pharmaceutics 12, no. 12: 1193. https://doi.org/10.3390/pharmaceutics12121193
APA StyleLainetti, P. d. F., Leis-Filho, A. F., Laufer-Amorim, R., Battazza, A., & Fonseca-Alves, C. E. (2020). Mechanisms of Resistance to Chemotherapy in Breast Cancer and Possible Targets in Drug Delivery Systems. Pharmaceutics, 12(12), 1193. https://doi.org/10.3390/pharmaceutics12121193