Cardiovascular Paediatric Medicines Development: Have Paediatric Investigation Plans Lost Heart?
Abstract
1. Introduction
2. Materials and Methods
2.1. Overall PIP Activity
2.2. PIP Activity in Specific Therapeutic Areas
2.3. PIPs Addressing Paediatric Cardiovascular Needs
2.4. Study Limitations
3. Results
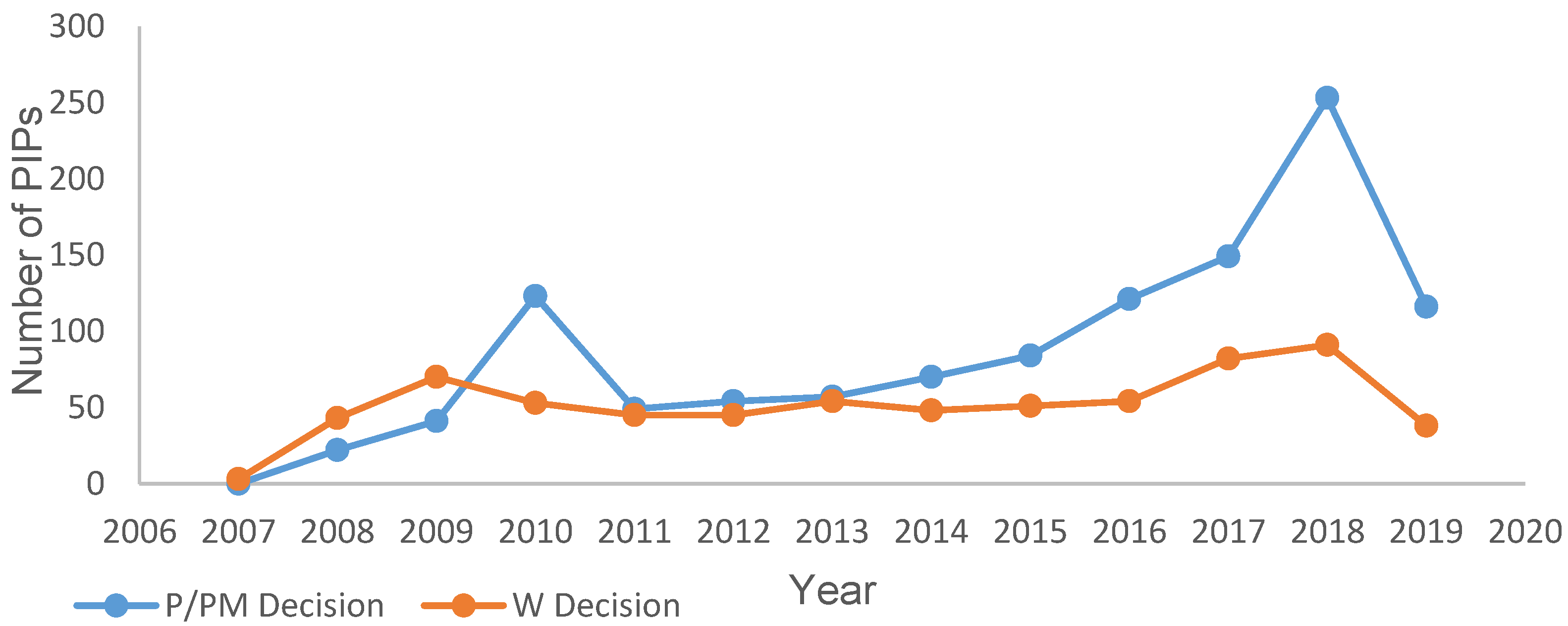
3.1. Overall PIP Activity
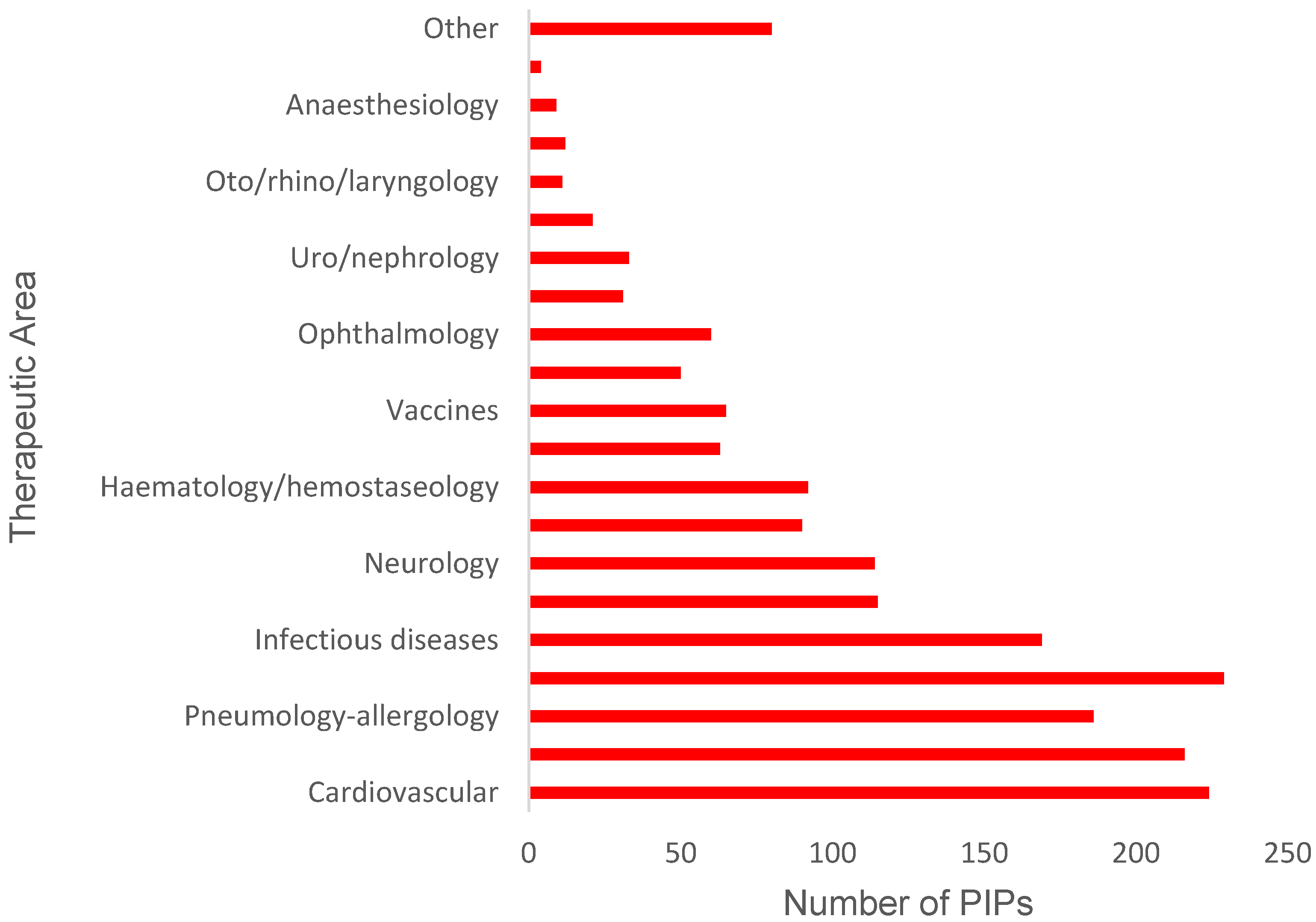
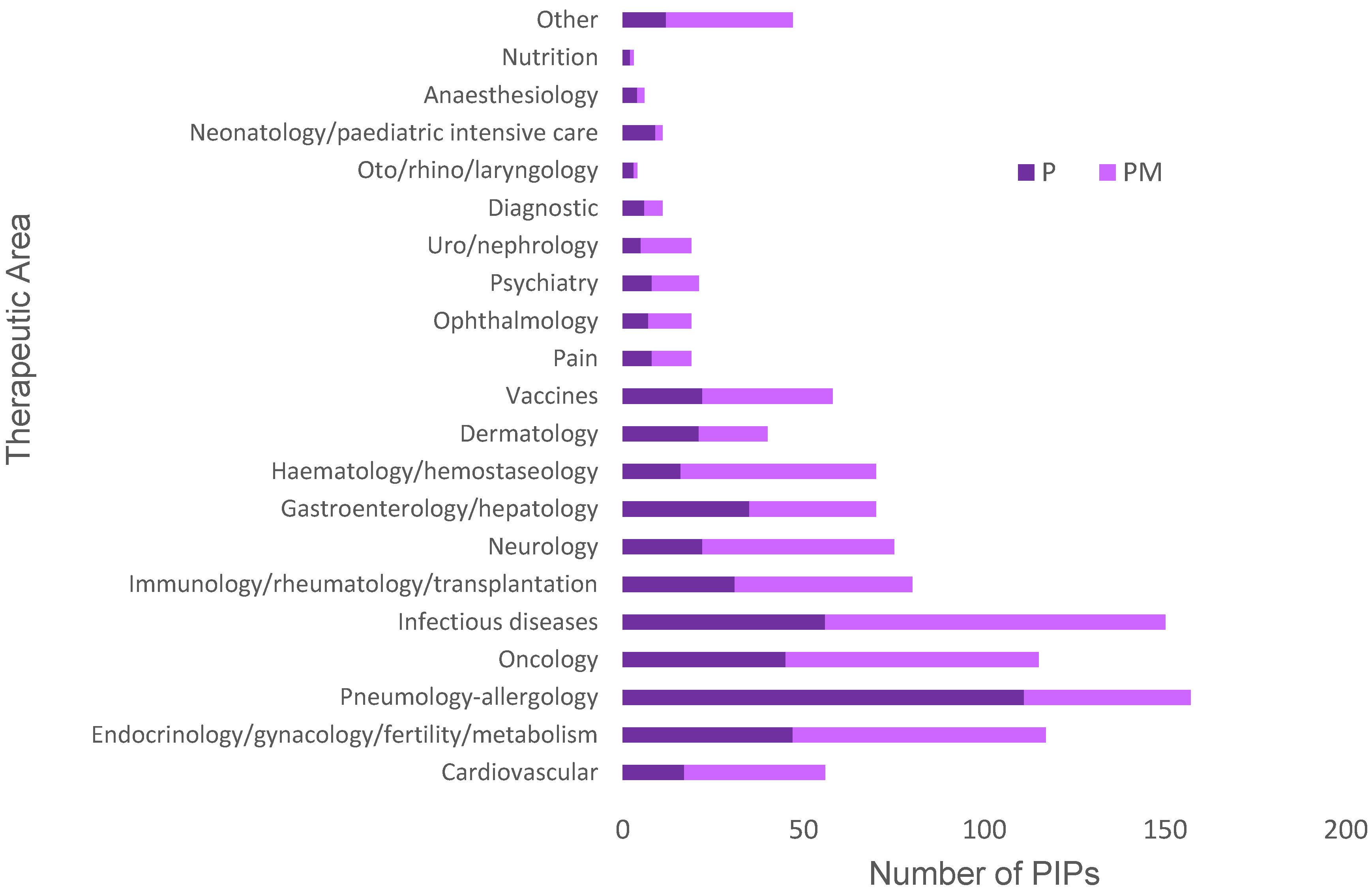
3.2. PIP Activity in Specific Therapeutic Areas
3.3. PIPs Addressing Paediatric Cardiovascular Needs
4. Discussion
4.1. Overall PIP Activity
4.2. PIPs Approved for Specific Therapeutic Areas
4.3. PIPs for Paediatric Cardiovascular Needs
4.4. Case Study: Valsartan
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hsien, L.; Breddemann, A.; Frobel, A.K.; Heusch, A.; Schmidt, K.G.; Läer, S. Off-label drug use among hospitalised children: Identifying areas with the highest need for research. Pharm. World Sci. 2008, 30, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Definition: “Off-Label Use” The European Medicines Agency. Available online: https://www.ema.europa.eu/en/glossary/label-use (accessed on 7 November 2019).
- Conroy, S.; Choonara, I.; Impicciatore, P.; Mohn, A.; Arnell, H.; Rane, A.; Knoeppel, C.; Seyberth, H.; Pandolfini, C.; Raffaelli, M.P.; et al. Survey of unlicensed and off label drug use in paediatric wards in European countries. Br. Med. J. 2000, 320, 79–82. [Google Scholar] [CrossRef]
- Evidence of Harm from Off-Label or Unlicensed Medicines in Children. The European Medicines Agency EMEA/126327/2004. Available online: https://www.ema.europa.eu/en/documents/other/evidence-harm-label-unlicensed-medicines-children_en.pdf (accessed on 7 November 2019).
- Praticò, A.D.; Longo, L.; Mansueto, S.; Gozzo, L.; Barberi, I.; Tiralongo, V.; Salvo, V.; Falsaperla, R.; Vitaliti, G.; La Rosa, M.; et al. Off-label use of drugs and adverse drug reactions in pediatric units: A prospective, multicenter study. Curr. Drug Saf. 2018, 13, 200–207. [Google Scholar] [CrossRef]
- Schrier, L.; Hadjipanayis, A.; Stiris, T.; Ross-Russell, R.I.; Valiulis, A.; Turner, M.A.; Zhao, W.; De Cock, P.; de Wildt, S.N.; Allegaert, K.; et al. Off-label use of medicines in neonates, infants, children, and adolescents: A joint policy statement by the European Academy of Paediatrics and the European Society for Developmental Perinatal and Pediatric Pharmacology. Eur. J. Pediatr. 2020, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Regulation (EC) No 1901/2006 of the European Parliament and of the Council. 2006. Available online: https://ec.europa.eu/health//sites/health/files/files/eudralex/vol-1/reg_2006_1901/reg_2006_1901_en.pdf (accessed on 7 November 2019).
- Nordenmalm, S.; Tomasi, P.; Pallidis, C. More medicines for children: Impact of the EU paediatric regulation. Arch. Dis. Child. 2018, 103, 557–564. [Google Scholar] [CrossRef]
- Paediatric-Use Marketing Authorisations. The European Medicines Agency. Available online: https://www.ema.europa.eu/en/human-regulatory/marketing-authorisation/paediatric-medicines/paediatric-use-marketing-authorisations (accessed on 24 November 2020).
- Rose, K.; Grant-Kels, J.M. Pediatric melanoma and drug development. Children 2018, 5, 43. [Google Scholar] [CrossRef] [PubMed]
- Rose, K. The challenges of pediatric drug development. Curr. Ther. Res. 2019, 90, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Rose, K.; Kopp, M.V. Pediatric investigation plans for specific immunotherapy: Questionable contributions to childhood health. Pediatr. Allergy Immunol. 2015, 26, 695–701. [Google Scholar] [CrossRef] [PubMed]
- SOP/H/3452 Paediatric Investigation Plan or a Waiver from Start of Procedure to Clock-Stop or PDCO Opinion. The European Medicines Agency. Available online: https://www.ema.europa.eu/en/documents/sop/standard-operating-procedure-paediatric-investigation-plan-waiver-start-procedure-clock-stop-pdco_en.pdf (accessed on 7 November 2019).
- Tomasi, P.A.; Egger, G.F.; Pallidis, C.; Saint-Raymond, A. Enabling development of paediatric medicines in Europe: 10 Years of the EU Paediatric Regulation. Pediatr. Drugs 2017, 19, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Report on the Survey of All Paediatric Uses of Medicinal Products in Europe. The European Medicines Agency, 2010. Available online: https://www.ema.europa.eu/en/documents/report/report-survey-all-paediatric-uses-medicinal-products-europe_en.pdf (accessed on 20 January 2020).
- Needs for Pediatric Medicines. The European Medicines Agency. Available online: https://www.ema.europa.eu/en/human-regulatory/research-development/paediatric-medicines/needs-paediatric-medicines#:~:text=the%20Paediatric%20Committee%20%28PDCO%29%20to%20judge%20the%20need,their%20decisions%20as%20to%20which%20medicines%20to%20choose (accessed on 10 November 2020).
- Revised Priority List for Studies on Off-Patent Paediatric Medicinal Products. The European Medicines Agency. Available online: https://www.ema.europa.eu/en/documents/other/revised-priority-list-studies-patent-paediatric-medicinal-products_en.pdf (accessed on 10 November 2020).
- Inventory of Pediatric Medicines. The European Medicines Agency. Available online: https://www.ema.europa.eu/en/documents/other/draft-inventory-paediatric-medicines-cardiovascular-therapeutic-area_en.pdf (accessed on 10 November 2020).
- Wimmer, S.; Rascher, W.; McCarthy, S.; Neubert, A. The EU Paediatric Regulation: Still a large discrepancy between therapeutic needs and approved Paediatric Investigation Plans. Pediatr. Drugs 2014, 16, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Search the Website. The European Medicines Agency. Available online: https://www.ema.europa.eu/en/search/search (accessed on 10 November 2020).
- The Electronic Medicines Compendium. Available online: https://www.medicines.org.uk/emc/ (accessed on 10 November 2020).
- Medicines under Evaluation. The European Medicines Agency. Available online: https://www.ema.europa.eu/en/medicines/medicines-under-evaluation (accessed on 20 January 2020).
- Egger, G.F.; Wharton, G.T.; Malli, S.; Temeck, J.; Murphy, M.D.; Tomasi, P. A comparative review of waivers granted in pediatric drug development by FDA and EMA from 2007–2013. Ther. Innov. Regul. Sci. 2016, 50, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Hwang, T.J.; Tomasi, P.A.; Bourgeois, F.T. Delays in completion and results reporting of clinical trials under the Paediatric Regulation in the European Union: A cohort study. PLoS Med. 2018, 15, e1002520. [Google Scholar] [CrossRef] [PubMed]
- Ekins-Daukes, S.; Helms, P.J.; Taylor, M.W.; McLay, J.S. Off-label prescribing to children: Attitudes and experience of general practitioners. Br. J. Clin. Pharmacol. 2005, 60, 145–149. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, J.S.; Cohen-Wolkowiez, M.; Pasquali, S.K. Pediatric cardiovascular drug trials, lessons learned. J. Cardiovasc. Pharmacol. 2011, 58, 4–8. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency Decision on the Acceptance of a Modification of an Agreed Paediatric Investigation Plan for Valsartan (Diovan), (EMEA-000005-PIP01-07-M01) in Accordance with Regulation (EC) No 1901/2006 of the European Parliament and of the Council as Amended. The European Medicines Agency. Available online: https://www.ema.europa.eu/en/documents/pip-decision/p/125/2009-european-medicines-agency-decision-26-june-2009-acceptance-modification-agreed-paediatric_en.pdf (accessed on 23 January 2020).
- Diovan Artical 29 Paediatrics Referral Annex I,II,III. The European Medicines Agency. Available online: https://www.ema.europa.eu/en/documents/referral/diovan-emea/h/a/29-pad/1220-artical-29-paediatrics-referral-annex-i-ii-iii_en.pdf (accessed on 20 January 2020).
- Hypertension in Children and Adolescents. National Institute for Health and Care Excellence. Available online: https://bnfc.nice.org.uk/treatment-summary/hypertension.html (accessed on 23 January 2020).
- Valsartan. Medicinal Forms. Medicines Complete BNF for Children. Available online: https://www-medicinescomplete-com (accessed on 27 November 2020).
- Special Orders Manufacturers. Medicines Complete BNF for Children. Available online: https://www-medicinescomplete-com (accessed on 27 November 2020).
- Drug Tariff Part VIIIA Products, V. NHS Business Services Authority. Available online: http://www.drugtariff.nhsbsa.nhs.uk/#/00770298-DC/DC00770293/Home (accessed on 20 January 2020).
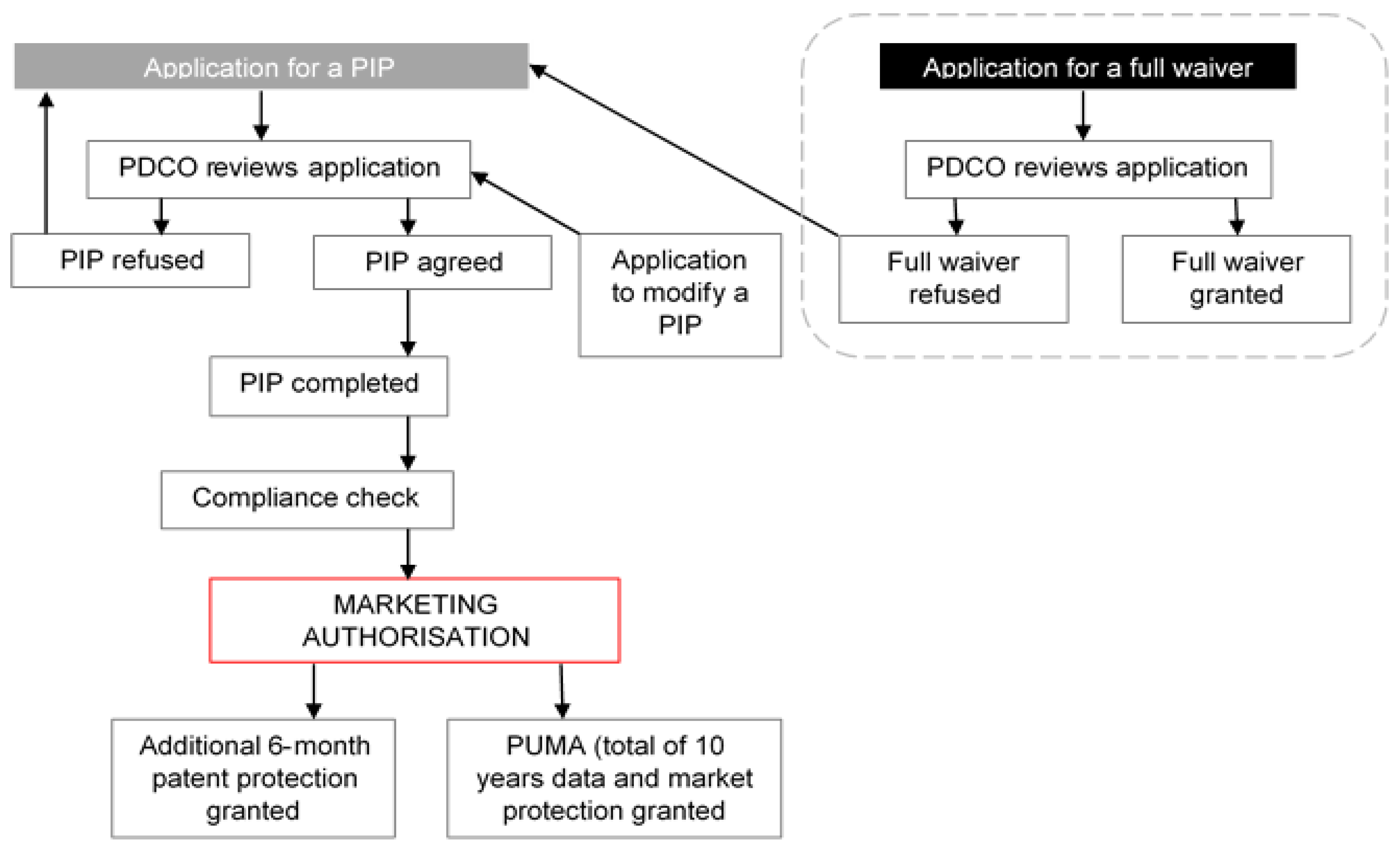
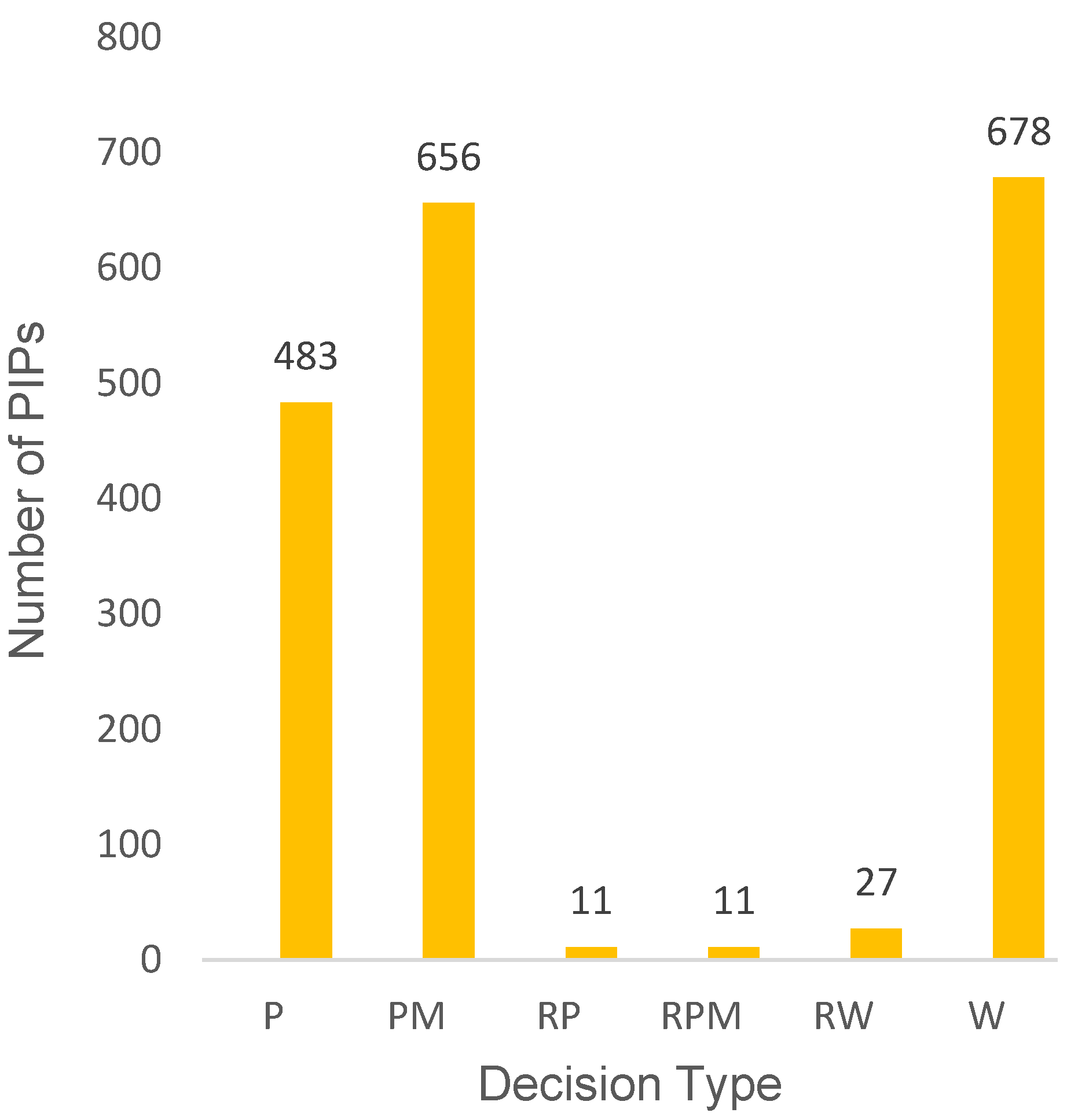

| Decision | Definition |
|---|---|
| P | Decision agreeing on an investigation plan with or without partial waiver(s) and or deferral(s) |
| PM | Decision on the application for modification of an agreed PIP |
| RP | Decision refers to a refusal on a proposed paediatric investigation plan |
| RPM | Decision refers to a refusal on the application for modification of an agreed PIP |
| RW | Decision refers to a refusal on a request for waiver in all age groups for the listed conditions |
| W | Decision granting a waiver in all age groups for all conditions/indications |
| Document | Year | Purpose |
|---|---|---|
| Assessment of the paediatric needs (APN) | 2007 | To identify active substances which require further development and/or research in paediatrics. Includes off-patent and new substances. |
| Off-patent priority list (OPPL) | 2013 | To identify priorities for development of off-patent products (INN) with the view of the developers submitting a PUMA. |
| Inventory of paediatric medicines (IPM) | N/A * | To detail development priorities for active substances in order to help product developers choose where to direct research |
| Indication | Active Substances | Positive PIP Agreed |
|---|---|---|
| Acute hypotension | Norepinephrine | N/A |
| Arrhythmias | Adenosine triphosphate, amiodarone, atenolol, esmolol, flecainide, lidocaine, metoprolol, propafenone, propranolol, sotalol, verapamil | N/A |
| Cardiogenic shock | Arginine-vasopressin, dobutamine, dopamine, epinephrine | Dopamine (PM), dobutamine (PM) |
| Dyslipidaemia | Atorvastatin, colesevelam, fluvastatin, lovastatin, pravastatin, simvastatin, | N/A |
| Heart failure | Bisoprolol, captopril, carvedilol, dobutamine, dopamine, enalapril, enoximone, furosemide, hydrochlorothiazide/chlorothiazide, levosimendan, milrinone, nitroprusside, ramipril, spironolactone | Captopril (P), enalapril (PM), furosemide (discontinued) |
| Hypertension | Acebutol, amlodipine, atenolol, bosentan, candesartan, captopril, carvedilol, clonidine, dihydralazine, enalapril, esmolol, furosemide, hydrochlorothiazide/chlorothiazide, inhaled nitric oxide, irbesartan, labetalol, metoprolol, nicardipine, nifedipine, nitroprusside, prazosin, propranolol, prostacyclin, ramipril, sildenafil, spironolactone, telmisartan, valsartan | Valsartan (PM), bosentan (PM), sildenafil (PM), prostacyclin (P) |
| Prevention and treatment of thromboembolic events | Alteplase, aspirin, clopidogrel, dalteparin, dipyridamole, enoxaparin, heparin, low molecular weight heparin, urokinase, warfarin | Clopidogrel (PM) |
| Indication | Agreed PIP | Not Agreed PIP |
|---|---|---|
| Heart failure | Captopril, dobutamine, dopamine, eleclazine *, enalapril, furosemide *, ivabradine, mavacamten, omecamtiv mecarbil, rolofylline *, sacubitril and valsartan, serelaxin *, vericiguat | Arginine-vasopressin, bisoprolol, carvedilol, enoximone, hydrochlorothiazide/chlorothiazide, levosimendan, milrinone, nitroprusside, ramipril, spironolactone |
| Hypertension | Aliskiren, ambrisentan azilsartan medoxomil, benzo derivative *, bosentan, clevidipine butyrate, diethanolamine, enalapril, furosemide *, imatinib, losartan potassium, macitentan, prostacyclin (and related analogues), riociguat, sildenafil, tadalafil, treprostinil, valsartan | Acebutol, amlodipine, atenolol, candesartan, captopril, carvedilol, clonidine, dihydralazine, esmolol, hydrochlorothiazide/chlorothiazide, iloprost, inhaled nitric oxide, irbesartan, labetalol, metoprolol, nicardipine, nifedipine, prazosin, propranolol, ramipril, spironolactone, telmisartan |
| SmPC Section | 4.2 | 4.3 | 4.4 | 4.5 | 4.8 | 5.1 | 5.2 | 5.2 |
|---|---|---|---|---|---|---|---|---|
| Number of products | 25 * | 1 | 4 | 6 | 6 | 23 | 12 | 9 |
| A Product Can Be Granted a Full Waiver If: | |
|---|---|
| (a) | The specific medicinal product or class of medicinal products is likely to be ineffective or unsafe in part or all of the paediatric population. |
| (b) | The disease or condition for which the specific medicinal product or class is intended occurs only in adult populations. |
| (c) | The specific medicinal product does not represent a significant therapeutic benefit over existing treatments for paediatric patients. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faulkner, B.; Delgado-Charro, M.B. Cardiovascular Paediatric Medicines Development: Have Paediatric Investigation Plans Lost Heart? Pharmaceutics 2020, 12, 1176. https://doi.org/10.3390/pharmaceutics12121176
Faulkner B, Delgado-Charro MB. Cardiovascular Paediatric Medicines Development: Have Paediatric Investigation Plans Lost Heart? Pharmaceutics. 2020; 12(12):1176. https://doi.org/10.3390/pharmaceutics12121176
Chicago/Turabian StyleFaulkner, Bethany, and M. Begoña Delgado-Charro. 2020. "Cardiovascular Paediatric Medicines Development: Have Paediatric Investigation Plans Lost Heart?" Pharmaceutics 12, no. 12: 1176. https://doi.org/10.3390/pharmaceutics12121176
APA StyleFaulkner, B., & Delgado-Charro, M. B. (2020). Cardiovascular Paediatric Medicines Development: Have Paediatric Investigation Plans Lost Heart? Pharmaceutics, 12(12), 1176. https://doi.org/10.3390/pharmaceutics12121176






