BCS Class IV Oral Drugs and Absorption Windows: Regional-Dependent Intestinal Permeability of Furosemide
Abstract
:1. Introduction
2. Methods
2.1. Materials
2.2. Solubility Studies
2.3. Evaluation of Octanol-Buffer Partition Coefficients (Log D)
2.4. Physicochemical Analysis
2.5. Rat Single-Pass Intestinal Perfusion
2.6. Analytical Methods
2.7. Statistics
2.8. In-Silico Simulations
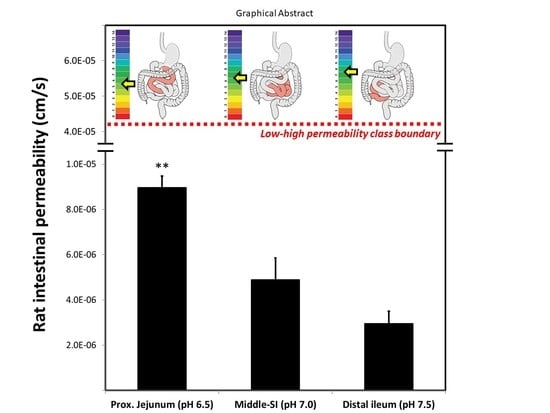
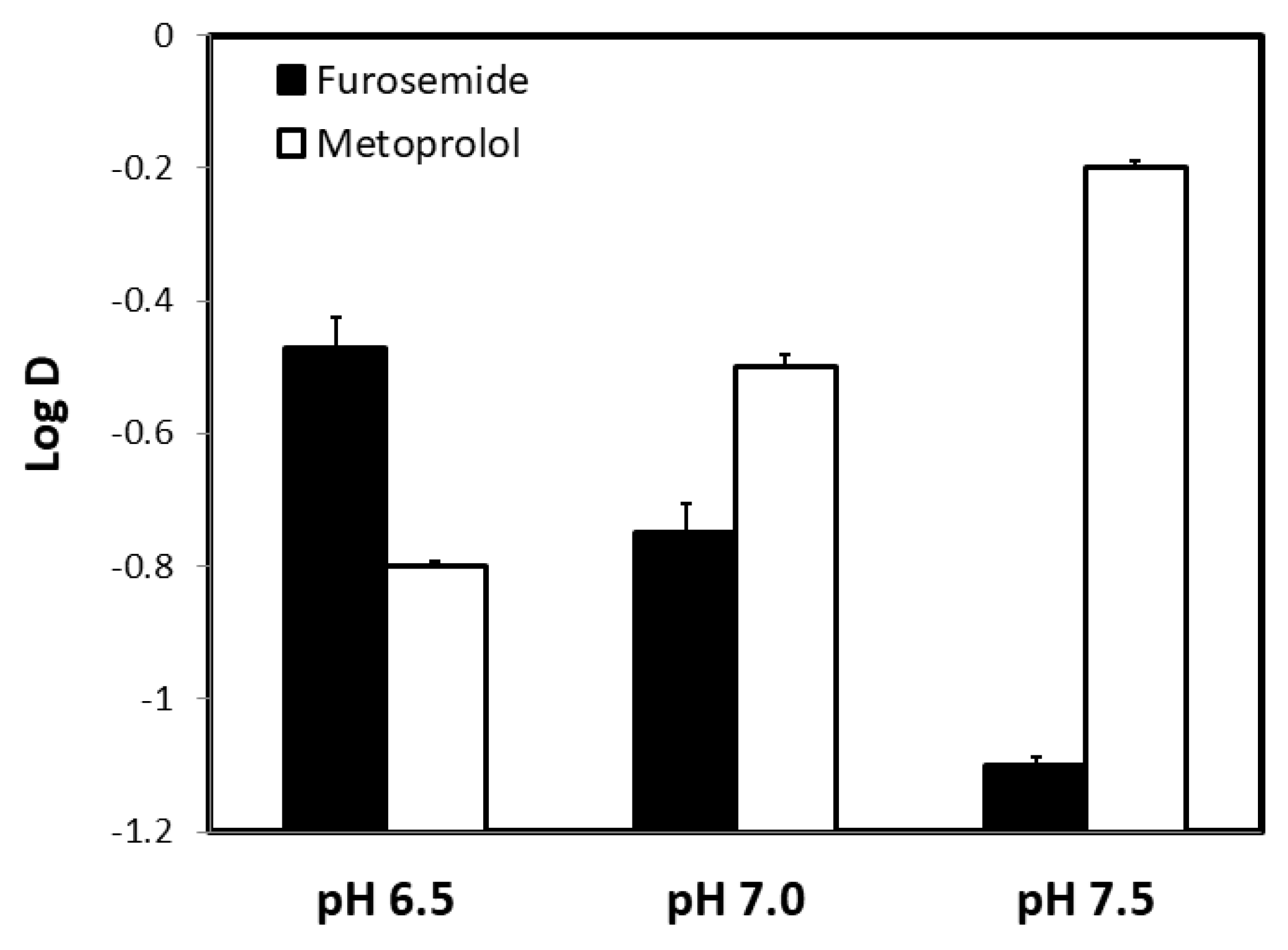
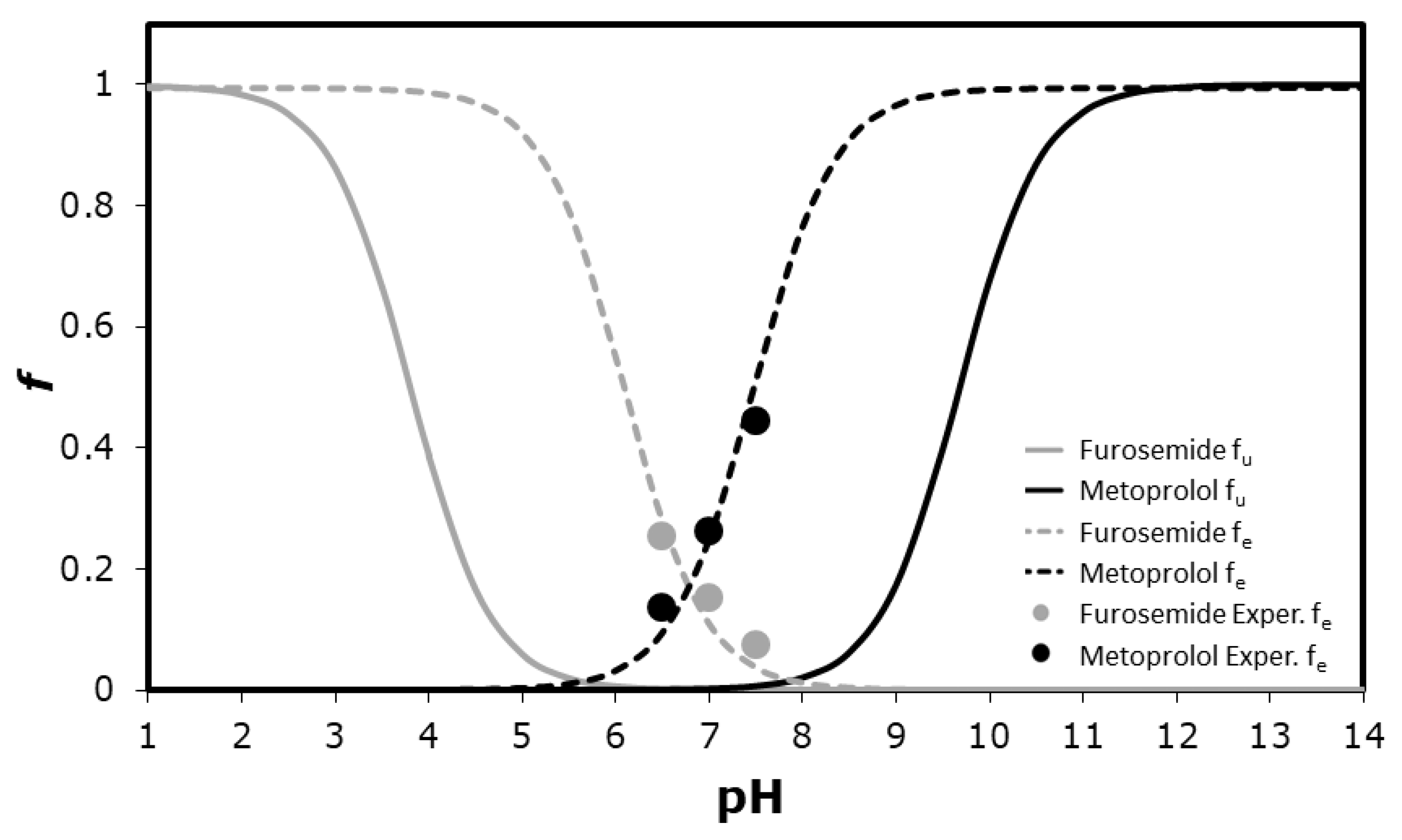
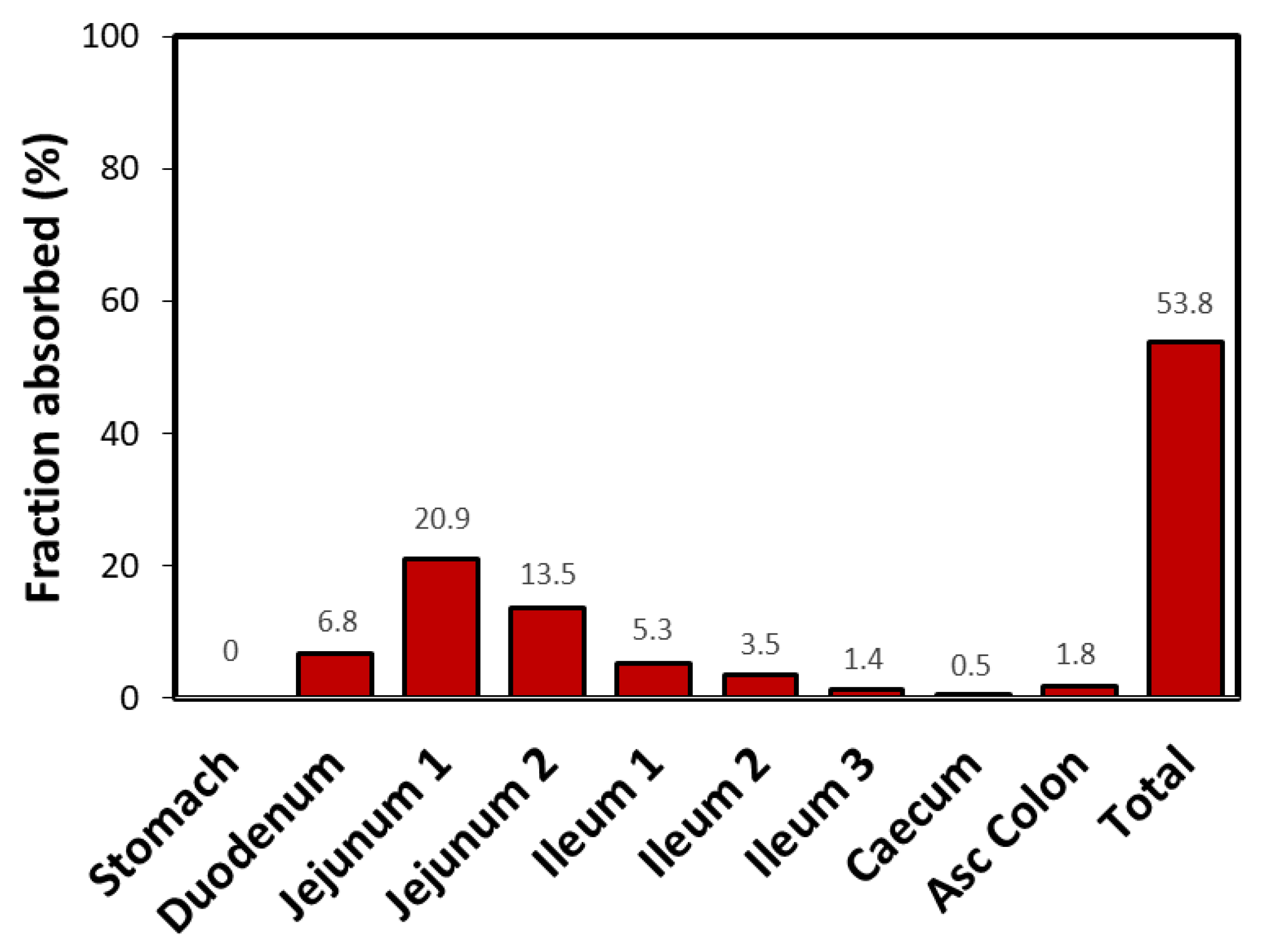
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Amidon, G.L.; Lennernäs, H.; Shah, V.P.; Crison, J.R. A theoretical basis for a biopharmaceutic drug classification: The correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm. Res. 1995, 12, 413–420. [Google Scholar] [CrossRef] [Green Version]
- Dahan, A.; Miller, J.M.; Amidon, G.L. Prediction of solubility and permeability class membership: Provisional BCS classification of the world’s top oral drugs. AAPS J. 2009, 11, 740–746. [Google Scholar] [CrossRef] [Green Version]
- Dahan, A.; Beig, A.; Lindley, D.; Miller, J.M. The solubility-permeability interplay and oral drug formulation design: Two heads are better than one. Adv. Drug Deliv. Rev. 2016, 101, 99–107. [Google Scholar] [CrossRef]
- Dahan, A.; Miller, J.M. The solubility-permeability interplay and its implications in formulation design and development for poorly soluble drugs. AAPS J. 2012, 14, 244–251. [Google Scholar] [CrossRef] [Green Version]
- Miller, J.M.; Beig, A.; Carr, R.A.; Webster, G.K.; Dahan, A. The solubility-permeability interplay when using cosolvents for solubilization: Revising the way we use solubility-enabling formulations. Mol. Pharm. 2012, 9, 581–590. [Google Scholar] [CrossRef]
- Dahan, A.; Amidon, G.L. Segmental dependent transport of low permeability compounds along the small intestine due to P-Glycoprotein: The role of efflux transport in the oral absorption of BCS class III drugs. Mol. Pharm. 2009, 6, 19–28. [Google Scholar] [CrossRef]
- Dahan, A.; West, B.T.; Amidon, G.L. Segmental-dependent membrane permeability along the intestine following oral drug administration: Evaluation of a triple single-pass intestinal perfusion (TSPIP) approach in the rat. Eur. J. Pharm. Sci. 2009, 36, 320–329. [Google Scholar] [CrossRef]
- Fairstein, M.; Swissa, R.; Dahan, A. Regional-dependent intestinal permeability and BCS classification: Elucidation of pH-related complexity in rats using pseudoephedrine. AAPS J. 2013, 15, 589–597. [Google Scholar] [CrossRef] [Green Version]
- Lozoya-Agullo, I.; Zur, M.; Beig, A.; Fine, N.; Cohen, Y.; Gonzalez-Alvarez, M.; Merino-Sanjuan, M.; Gonzalez-Alvarez, I.; Bermejo, M.; Dahan, A. Segmental-dependent permeability throughout the small intestine following oral drug administration: Single-pass vs. Doluisio approach to in-situ rat perfusion. Int. J. Pharm. 2016, 515, 201–208. [Google Scholar] [CrossRef]
- Markovic, M.; Zur, M.; Dahan, A.; Cvijić, S. Biopharmaceutical characterization of rebamipide: The role of mucus binding in regional-dependent intestinal permeability. Eur. J. Pharm. Sci. 2020, 152, 105440. [Google Scholar] [CrossRef]
- Zur, M.; Hanson, A.S.; Dahan, A. The complexity of intestinal permeability: Assigning the correct BCS classification through careful data interpretation. Eur. J. Pharm. Sci. 2014, 61, 11–17. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services, Food and Drug Administration; Center for Drug Evaluation and Research (CDER). Waiver of In-Vivo Bioavailability and Bioequivalence Studies for Immediate-Release Solid Oral Dosage Forms Based on a Biopharmaceutics Classification System; Guidance for Industry; Center for Drug Evaluation and Research (CDER): Silver Spring, MD, USA, 2017.
- Dahan, A.; Wolk, O.; Kim, Y.H.; Ramachandran, C.; Crippen, G.M.; Takagi, T.; Bermejo, M.; Amidon, G.L. Purely in silico BCS classification: Science based quality standards for the world’s drugs. Mol. Pharm. 2013, 10, 4378–4390. [Google Scholar] [CrossRef]
- Takagi, T.; Ramachandran, C.; Bermejo, M.; Yamashita, S.; Yu, L.X.; Amidon, G.L. A provisional biopharmaceutical classification of the top 200 oral drug products in the United States, Great Britain, Spain, and Japan. Mol. Pharm. 2006, 3, 631–643. [Google Scholar] [CrossRef]
- Wolk, O.; Agbaria, R.; Dahan, A. Provisional in-silico biopharmaceutics classification (BCS) to guide oral drug product development. Drug Des. Dev. Ther. 2014, 8, 1563–1575. [Google Scholar] [CrossRef] [Green Version]
- Lindenberg, M.; Kopp, S.; Dressman, J.B. Classification of orally administered drugs on the World Health Organization model list of essential medicines according to the biopharmaceutics classification system. Eur. J. Pharm. Biopharm. 2004, 58, 265–278. [Google Scholar] [CrossRef]
- Furosemide Tablets, United States Pharmacopeia Label. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/018487s043lbl.pdf (accessed on 11 July 2020).
- Ellison, D.H.; Felker, G.M. Diuretic Treatment in Heart Failure. N. Engl. J. Med. 2017, 377, 1964–1975. [Google Scholar] [CrossRef]
- Hammarlund-Udenaes, M.; Benet, L.Z. Furosemide pharmacokinetics and pharmacodynamics in health and disease—An update. J. Pharmacokinet. Biopharm. 1989, 17, 1–46. [Google Scholar] [CrossRef]
- Dahan, A.; Wolk, O.; Zur, M.; Amidon, G.L.; Abrahamsson, B.; Cristofoletti, R.; Groot, D.W.; Kopp, S.; Langguth, P.; Polli, J.E.; et al. Biowaiver monographs for immediate-release solid oral dosage forms: Codeine phosphate. J. Pharm. Sci. 2014, 103, 1592–1600. [Google Scholar] [CrossRef] [Green Version]
- Markovic, M.; Zur, M.; Fine-Shamir, N.; Haimov, E.; González-Álvarez, I.; Dahan, A. Segmental-dependent solubility and permeability as key factors guiding controlled release drug product development. Pharmaceutics 2020, 12, 295. [Google Scholar] [CrossRef] [Green Version]
- Zur, M.; Cohen, N.; Agbaria, R.; Dahan, A. The biopharmaceutics of successful controlled release drug product: Segmental-dependent permeability of glipizide vs. metoprolol throughout the intestinal tract. Int. J. Pharm. 2015, 489, 304–310. [Google Scholar] [CrossRef]
- Zur, M.; Gasparini, M.; Wolk, O.; Amidon, G.L.; Dahan, A. The low/high BCS permeability class boundary: Physicochemical comparison of metoprolol and labetalol. Mol. Pharm. 2014, 11, 1707–1714. [Google Scholar] [CrossRef]
- Granero, G.E.; Longhi, M.R.; Mora, M.J.; Junginger, H.E.; Midha, K.K.; Shah, V.P.; Stavchansky, S.; Dressman, J.B.; Barends, D.M. Biowaiver monographs for immediate release solid oral dosage forms: Furosemide. J. Pharm. Sci. 2010, 99, 2544–2556. [Google Scholar] [CrossRef] [PubMed]
- Wagner, J.G.; Sedman, A.J. Quantitaton of rate of gastrointestinal and buccal absorption of acidic and basic drugs based on extraction theory. J. Pharmacokinet. Biopharm. 1973, 1, 23–50. [Google Scholar] [CrossRef] [Green Version]
- Winne, D. Shift of pH-absorption curves. J. Pharmacokinet. Biopharm. 1977, 5, 53–94. [Google Scholar] [CrossRef]
- Berthod, A.; Carda-Broch, S.; Garcia-Alvarez-Coque, M.C. Hydrophobicity of ionizable compounds. A theoretical study and measurements of diuretic octanol−water partition coefficients by countercurrent chromatography. Anal. Chem. 1999, 71, 879–888. [Google Scholar] [CrossRef]
- Henchoz, Y.; Guillarme, D.; Martel, S.; Rudaz, S.; Veuthey, J.L.; Carrupt, P.A. Fast log P determination by ultra-high-pressure liquid chromatography coupled with UV and mass spectrometry detections. Anal. Bioanal. Chem. 2009, 394, 1919–1930. [Google Scholar] [CrossRef] [Green Version]
- Teksin, Z.S.; Hom, K.; Balakrishnan, A.; Polli, J.E. Ion pair-mediated transport of metoprolol across a three lipid-component PAMPA system. J. Control. Release Off. J. Control. Release Soc. 2006, 116, 50–57. [Google Scholar] [CrossRef]
- Lozoya-Agullo, I.; Gonzalez-Alvarez, I.; Zur, M.; Fine-Shamir, N.; Cohen, Y.; Markovic, M.; Garrigues, T.M.; Dahan, A.; Gonzalez-Alvarez, M.; Merino-Sanjuán, M.; et al. Closed-loop doluisio (colon, small intestine) and single-pass intestinal perfusion (colon, jejunum) in rat—Biophysical model and predictions based on Caco-2. Pharm. Res. 2017, 35, 2. [Google Scholar] [CrossRef]
- Lozoya-Agullo, I.; Zur, M.; Fine-Shamir, N.; Markovic, M.; Cohen, Y.; Porat, D.; Gonzalez-Alvarez, I.; Gonzalez-Alvarez, M.; Merino-Sanjuan, M.; Bermejo, M.; et al. Investigating drug absorption from the colon: Single-pass vs. Doluisio approaches to in-situ rat large-intestinal perfusion. Int. J. Pharm. 2017, 527, 135–141. [Google Scholar] [CrossRef]
- Lozoya-Agullo, I.; Zur, M.; Wolk, O.; Beig, A.; Gonzalez-Alvarez, I.; Gonzalez-Alvarez, M.; Merino-Sanjuan, M.; Bermejo, M.; Dahan, A. In-situ intestinal rat perfusions for human Fabs prediction and BCS permeability class determination: Investigation of the single-pass vs. the Doluisio experimental approaches. Int. J. Pharm. 2015, 480, 1–7. [Google Scholar] [CrossRef]
- Dahan, A.; Miller, J.M.; Hilfinger, J.M.; Yamashita, S.; Yu, L.X.; Lennernas, H.; Amidon, G.L. High-permeability criterion for BCS classification: Segmental/pH dependent permeability considerations. Mol. Pharm. 2010, 7, 1827–1834. [Google Scholar] [CrossRef]
- Wolk, O.; Markovic, M.; Porat, D.; Fine-Shamir, N.; Zur, M.; Beig, A.; Dahan, A. Segmental-dependent intestinal drug permeability: Development and model validation of in silico predictions guided by in vivo permeability values. J. Pharm. Sci. 2019, 108, 316–325. [Google Scholar] [CrossRef] [Green Version]
- Kelly, M.R.; Cutler, R.E.; Forrey, A.W.; Kimpel, B.M. Pharmacokinetics of orally administered furosemide. Clin. Pharmacol. Ther. 1974, 15, 178–186. [Google Scholar] [CrossRef]
- Benet, L.Z. Pharmacokinetics/pharmacodynamics of furosemide in man: A review. J. Pharmacokinet. Biopharm. 1979, 7, 1–27. [Google Scholar] [CrossRef]
- Hammarlund, M.M.; Paalzow, L.K.; Odlind, B. Pharmacokinetics of furosemide in man after intravenous and oral administration. Application of moment analysis. Eur. J. Clin. Pharmacol. 1984, 26, 197–207. [Google Scholar] [CrossRef]
- Agoram, B.; Woltosz, W.S.; Bolger, M.B. Predicting the impact of physiological and biochemical processes on oral drug bioavailability. Adv. Drug Deliv. Rev. 2001, 50, S41–S67. [Google Scholar] [CrossRef]
- Lin, L.; Wong, H. Predicting oral drug absorption: Mini review on physiologically-based pharmacokinetic models. Pharmaceutics 2017, 9, 41. [Google Scholar] [CrossRef] [Green Version]
- Lu, A.T.K.; Frisella, M.E.; Johnson, K.C. Dissolution modeling: Factors affecting the dissolution rates of polydisperse powders. Pharm. Res. 1993, 10, 1308–1314. [Google Scholar] [CrossRef]
- Grahnén, A.; Hammarlund, M.; Lundqvist, T. Implications of intraindividual variability in bioavailability studies of furosemide. Eur. J. Clin. Pharmacol. 1984, 27, 595–602. [Google Scholar] [CrossRef]
- Waller, E.S.; Hamilton, S.F.; Massarella, J.W.; Sharanevych, M.A.; Smith, R.V.; Yakatan, G.J.; Doluisio, J.T. Disposition and absolute bioavailability of furosemide in healthy males. J. Pharm. Sci. 1982, 71, 1105–1108. [Google Scholar] [CrossRef]
- Beermann, B.; Midskov, C. Reduced bioavailability and effect of furosemide given with food. Eur. J. Clin. Pharmacol. 1986, 29, 725–727. [Google Scholar] [CrossRef]
- Dahan, A.; Lennernäs, H.; Amidon, G.L. The fraction dose absorbed, in humans, and high jejunal human permeability relationship. Mol. Pharm. 2012, 9, 1847–1851. [Google Scholar] [CrossRef]
- Lee, M.G.; Chiou, W.L. Evaluation of potential causes for the incomplete bioavailability of furosemide: Gastric first-pass metabolism. J. Pharm. Biopharm. 1983, 11, 623–640. [Google Scholar] [CrossRef]
- Dahlgren, D.; Lennernas, H. Intestinal permeability and drug absorption: Predictive experimental, computational and in vivo approaches. Pharmaceutics 2019, 11, 411. [Google Scholar] [CrossRef] [Green Version]
- Ghadi, R.; Dand, N. BCS class IV drugs: Highly notorious candidates for formulation development. J. Control. Release Off. J. Control. Release Soc. 2017, 248, 71–95. [Google Scholar] [CrossRef]
- Flanagan, S.D.; Cummins, C.L.; Susanto, M.; Liu, X.; Takahashi, L.H.; Benet, L.Z. Comparison of furosemide and vinblastine secretion from cell lines overexpressing multidrug resistance protein (P-glycoprotein) and multidrug resistance-associated proteins (MRP1 and MRP2). Pharmacology 2002, 64, 126–134. [Google Scholar] [CrossRef]
- Takahashi, M.; Washio, T.; Suzuki, N.; Igeta, K.; Fujii, Y.; Hayashi, M.; Shirasaka, Y.; Yamashita, S. Characterization of gastrointestinal drug absorption in cynomolgus monkeys. Mol. Pharm. 2008, 5, 340–348. [Google Scholar] [CrossRef]
- Cao, X.; Yu, L.X.; Barbaciru, C.; Landowski, C.P.; Shin, H.C.; Gibbs, S.; Miller, H.A.; Amidon, G.L.; Sun, D. Permeability dominates in vivo intestinal absorption of P-gp substrate with high solubility and high permeability. Mol. Pharm. 2005, 2, 329–340. [Google Scholar] [CrossRef]
- Englund, G.; Rorsman, F.; Rönnblom, A.; Karlbom, U.; Lazorova, L.; Gråsjö, J.; Kindmark, A.; Artursson, P. Regional levels of drug transporters along the human intestinal tract: Co-expression of ABC and SLC transporters and comparison with Caco-2 cells. Eur. J. Pharm. Sci. 2006, 29, 269–277. [Google Scholar] [CrossRef]
- Zimmermann, C.; Gutmann, H.; Hruz, P.; Gutzwiller, J.-P.; Beglinger, C.; Drewe, J. Mapping of multidrug resistance gene 1 and multidrug resistance-associated protein isoform 1 to 5 mRNA expression along the human intestinal tract. Drug Metab. Dispos. 2005, 33, 219. [Google Scholar] [CrossRef] [Green Version]
- Winiwarter, S.; Bonham, N.M.; Ax, F.; Hallberg, A.; Lennernäs, H.; Karlén, A. Correlation of human jejunal permeability (in vivo) of drugs with experimentally and theoretically derived parameters. A multivariate data analysis approach. J. Med. Chem. 1998, 41, 4939–4949. [Google Scholar] [CrossRef]
- Clark, D.E. Rapid calculation of polar molecular surface area and its application to the prediction of transport phenomena. 1. Prediction of intestinal absorption. J. Pharm. Sci. 1999, 88, 807–814. [Google Scholar] [CrossRef]
- Palm, K.; Luthman, K.; Ungell, A.-L.; Strandlund, G.; Beigi, F.; Lundahl, P.; Artursson, P. Evaluation of dynamic polar molecular surface area as predictor of drug absorption: Comparison with other computational and experimental predictors. J. Med. Chem. 1998, 41, 5382–5392. [Google Scholar] [CrossRef]
- Ertl, P.; Rohde, B.; Selzer, P. Fast calculation of molecular polar surface area as a sum of fragment-based contributions and its application to the prediction of drug transport properties. J. Med. Chem. 2000, 43, 3714–3717. [Google Scholar] [CrossRef]
- Koziolek, M.; Grimm, M.; Becker, D.; Iordanov, V.; Zou, H.; Shimizu, J.; Wanke, C.; Garbacz, G.; Weitschies, W. Investigation of pH and temperature profiles in the GI tract of fasted human subjects using the intellicap(®) system. J. Pharm. Sci. 2015, 104, 2855–2863. [Google Scholar] [CrossRef]
- Davis, S.S.; Hardy, J.G.; Fara, J.W. Transit of pharmaceutical dosage forms through the small intestine. Gut 1986, 27, 886–892. [Google Scholar] [CrossRef] [Green Version]
- Streubel, A.; Siepmann, J.; Bodmeier, R. Drug delivery to the upper small intestine window using gastroretentive technologies. Curr. Opin. Pharmacol. 2006, 6, 501–508. [Google Scholar] [CrossRef]
- Clear, N.J.; Milton, A.; Humphrey, M.; Henry, B.T.; Wulff, M.; Nichols, D.J.; Anziano, R.J.; Wilding, I. Evaluation of the Intelisite capsule to deliver theophylline and frusemide tablets to the small intestine and colon. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2001, 13, 375–384. [Google Scholar] [CrossRef]
- Darandale, S.S.; Vavia, P.R. Design of a gastroretentive mucoadhesive dosage form of furosemide for controlled release. Acta Pharm. Sin. B 2012, 2, 509–517. [Google Scholar] [CrossRef] [Green Version]
- Kagan, L.; Lapidot, N.; Afargan, M.; Kirmayer, D.; Moor, E.; Mardor, Y.; Friedman, M.; Hoffman, A. Gastroretentive accordion pill: Enhancement of riboflavin bioavailability in humans. J. Control. Release Off. J. Control. Release Soc. 2006, 113, 208–215. [Google Scholar] [CrossRef]
- Klausner, E.A.; Eyal, S.; Lavy, E.; Friedman, M.; Hoffman, A. Novel levodopa gastroretentive dosage form: In-vivo evaluation in dogs. J. Control. Release 2003, 88, 117–126. [Google Scholar] [CrossRef]
- Israel, S.; Elinav, H.; Elazary, R.; Porat, D.; Gibori, R.; Dahan, A.; Azran, C.; Horwitz, E. Case report of increased exposure to antiretrovirals following sleeve gastrectomy. Antimicrob. Agents Chemother. 2020, 64. [Google Scholar] [CrossRef]
- Porat, D.; Dahan, A. Medication management after bariatric surgery: Providing optimal patient care. J. Clin. Med. 2020, 9, 1511. [Google Scholar] [CrossRef]
- Porat, D.; Markovic, M.; Zur, M.; Fine-Shamir, N.; Azran, C.; Shaked, G.; Czeiger, D.; Vaynshtein, J.; Replyanski, I.; Sebbag, G.; et al. Increased paracetamol bioavailability after sleeve gastrectomy: A crossover pre- vs. post-operative clinical trial. J. Clin. Med. 2019, 8, 1949. [Google Scholar] [CrossRef] [Green Version]




| At 37 °C | ||
| pH | Solubility (µg/mL) | Corresponding D0 |
| 1 | 19.4 ± 3.7 | 16.5 |
| 4 | 65.5 ± 9.0 | 4.8 |
| 7.5 | 8340.1 ± 81.6 | 0.04 |
| At 25 °C | ||
| pH | Solubility (µg/mL) | Corresponding D0 |
| 1 | 40.3 ± 16.2 | 7.9 |
| 4 | 56.7 ± 12.2 | 5.6 |
| 7.5 | 8550.6 ± 149.4 | 0.04 |
| Drug | Chemical Structure | pKa | Log P | PSA |
|---|---|---|---|---|
| Furosemide |  | 3.8 | 2.3 | 127.7 |
| Metoprolol |  | 9.7 | 2.2 | 53.2 |
| Parameter | Value | Source |
|---|---|---|
| Molecular weight (g/mol) | 330.75 | / |
| Log D (pH 7.5) | −1.0818 | experimental values |
| Solubility at 37 °C (µg/mL) | 19.4 (pH 1.0) | |
| 65.5 (pH 4.0) | ||
| 8340.1 (pH 7.5) | ||
| pKa (acid) | 3.8 | [24] |
| Human effective permeability, Peff (cm/s) | 0.4043 × 10−4 (duodenum, jejunum) | values converted using GastroPlus™ integrated “permeability converter” based on experimental rat perfusion data |
| 0.2246 × 10−4 (ileum 1 and 2) | ||
| 0.1392 × 10−4 (ileum 3, caecum, colon) | ||
| Diffusion coefficient (cm2/s) | 0.7289 × 10−5 | GastroPlus™ calculated value (based on molecular weight) |
| Mean precipitation time (s) | 900 | GastroPlus™ default values |
| Particle density (g/mL) | 1.2 | |
| Particle radius (µm) | 25 | |
| Blood/plasma concentration ratio | 1 | |
| Plasma fraction unbound (%) | 1 | [24] |
| Clearance, CL (L/h/kg) | 0.121 | calculated using GastroPlus™ PKPlus module, based on the i.v. data [35] |
| Volume of distribution, Vd (L/kg) | 0.043 | |
| Distribution constant k12 (1/h) | 0.964 | |
| Distribution constant k21 (1/h) | 1.614 | |
| Distribution constant k13 (1/h) | 0.925 | |
| Distribution constant k32 (1/h) | 0.708 | |
| Regional pH in the GIT | 1.3; 6.0; 6.2; 6.4; 6.6; 6.9; 7.4; 6.4; 6.8 | GastroPlus™ default values for stomach, duodenum, jejunum 1, jejunum 2, ileum 1, ileum 2, ileum 3, caecum, and ascendant colon |
| Regional volume of fluid in the GIT (mL) | 46.56; 40.54; 150.00; 119.30; 91.71; 68.88; 48.57; 46.44; 49.21 | |
| Regional transit time in the GIT (h) | 0.25; 0.26; 0.93; 0.74; 0.58; 0.42; 0.29; 4.13; 12.38 |
| 40 mg p.o. Dose | |||||
|---|---|---|---|---|---|
| Parameter | In-Vivo I a | In-Vivo II b | Predicted | PE(%) I | PE(%) II |
| Cmax (µg/mL) | 0.61 | 0.75 | 0.71 | −17.14 | 5.54 |
| tmax (h) | 1.5 | 1.12 | 1.36 | 9.33 | −22.22 |
| AUC0→∞ (µg∙h/mL) | 2.13 | 2.44 | 3.66 | −71.25 | −50.06 |
| AUC0→24 h (µg∙h/mL) | 2.11 | 2.33 | 2.52 | −19.25 | −8.15 |
| F (%) | NA | NA | 52.2 | NA | NA |
| Dissolution | Cmax (µg/mL) | tmax (h) | AUC0→∞ (µg∙h/mL) | F (%) |
|---|---|---|---|---|
| 85% in 15 min | 0.71 | 1.36 | 3.65 | 51.91 |
| 85% in 1 h | 0.64 | 1.76 | 3.71 | 46.35 |
| 85% in 6 h | 0.15 | 2.80 | 0.80 | 16.64 |
| 85% in 8 h | 0.11 | 2.80 | 0.61 | 12.73 |
| 85% in 12 h | 0.08 | 2.80 | 0.41 | 8.65 |
| 85% in 24 h | 0.04 | 2.80 | 0.21 | 4.36 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Markovic, M.; Zur, M.; Ragatsky, I.; Cvijić, S.; Dahan, A. BCS Class IV Oral Drugs and Absorption Windows: Regional-Dependent Intestinal Permeability of Furosemide. Pharmaceutics 2020, 12, 1175. https://doi.org/10.3390/pharmaceutics12121175
Markovic M, Zur M, Ragatsky I, Cvijić S, Dahan A. BCS Class IV Oral Drugs and Absorption Windows: Regional-Dependent Intestinal Permeability of Furosemide. Pharmaceutics. 2020; 12(12):1175. https://doi.org/10.3390/pharmaceutics12121175
Chicago/Turabian StyleMarkovic, Milica, Moran Zur, Inna Ragatsky, Sandra Cvijić, and Arik Dahan. 2020. "BCS Class IV Oral Drugs and Absorption Windows: Regional-Dependent Intestinal Permeability of Furosemide" Pharmaceutics 12, no. 12: 1175. https://doi.org/10.3390/pharmaceutics12121175
APA StyleMarkovic, M., Zur, M., Ragatsky, I., Cvijić, S., & Dahan, A. (2020). BCS Class IV Oral Drugs and Absorption Windows: Regional-Dependent Intestinal Permeability of Furosemide. Pharmaceutics, 12(12), 1175. https://doi.org/10.3390/pharmaceutics12121175