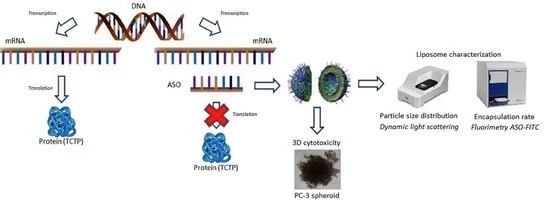
Enhanced Antisense Oligonucleotide Delivery Using Cationic Liposomes Grafted with Trastuzumab: A Proof-of-Concept Study in Prostate Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. Drugs and Chemicals
2.3. ASO Stability in Solvents
2.4. Pegylated Liposome Preparation
2.5. Pegylated Immunoliposome Preparation: Encapsulation Strategies
2.6. Pegylated Immunoliposome Preparation: Antibody Engraftment
2.7. Size and Polydispersity Study
2.8. ASO Encapsulation Rate
2.9. Quantification of HER2 on Cells
2.10. In Vitro Assays
2.11. Statistical Analysis
3. Results
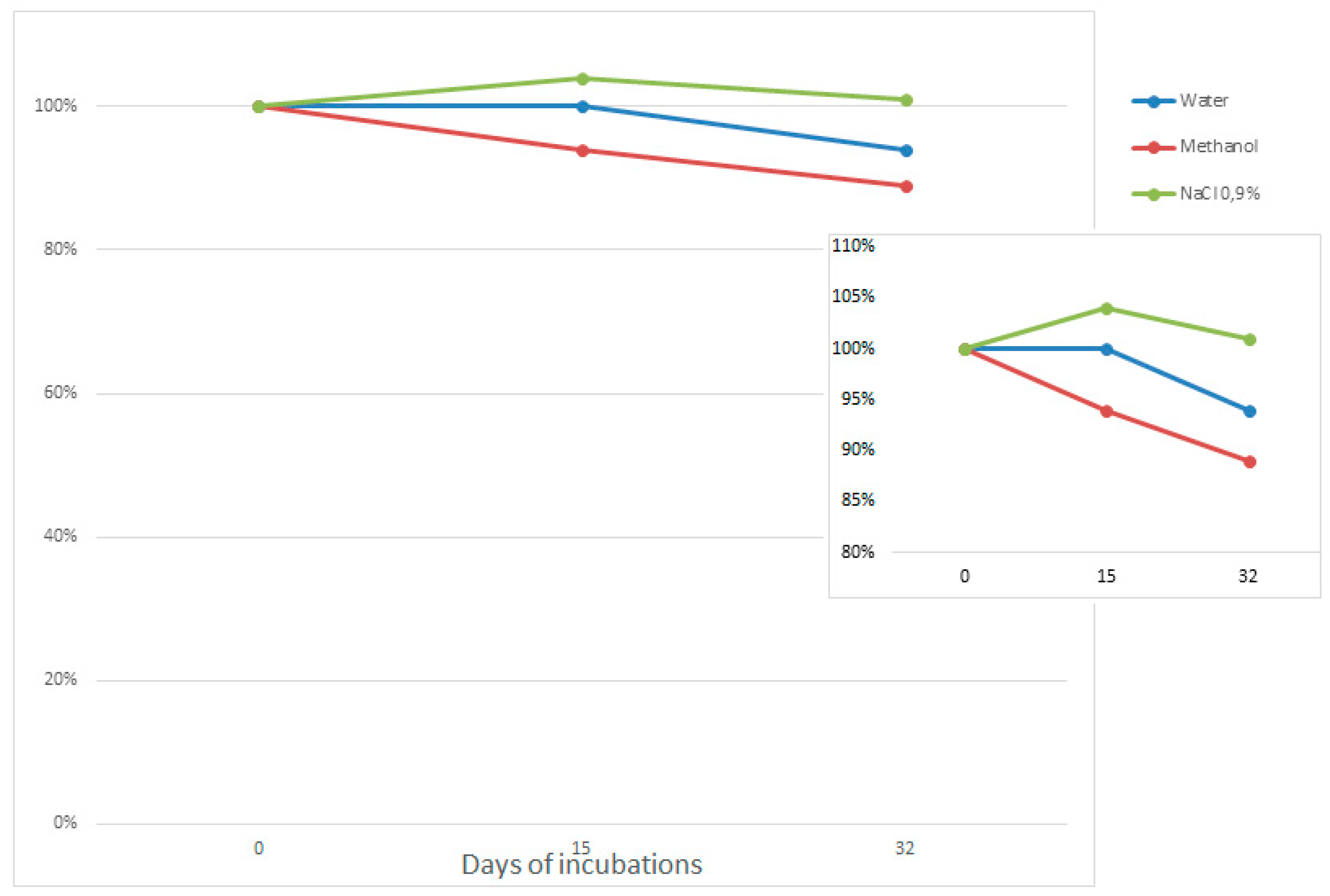
3.1. ASO Stability in Solvents
3.2. Size and Polydispersity
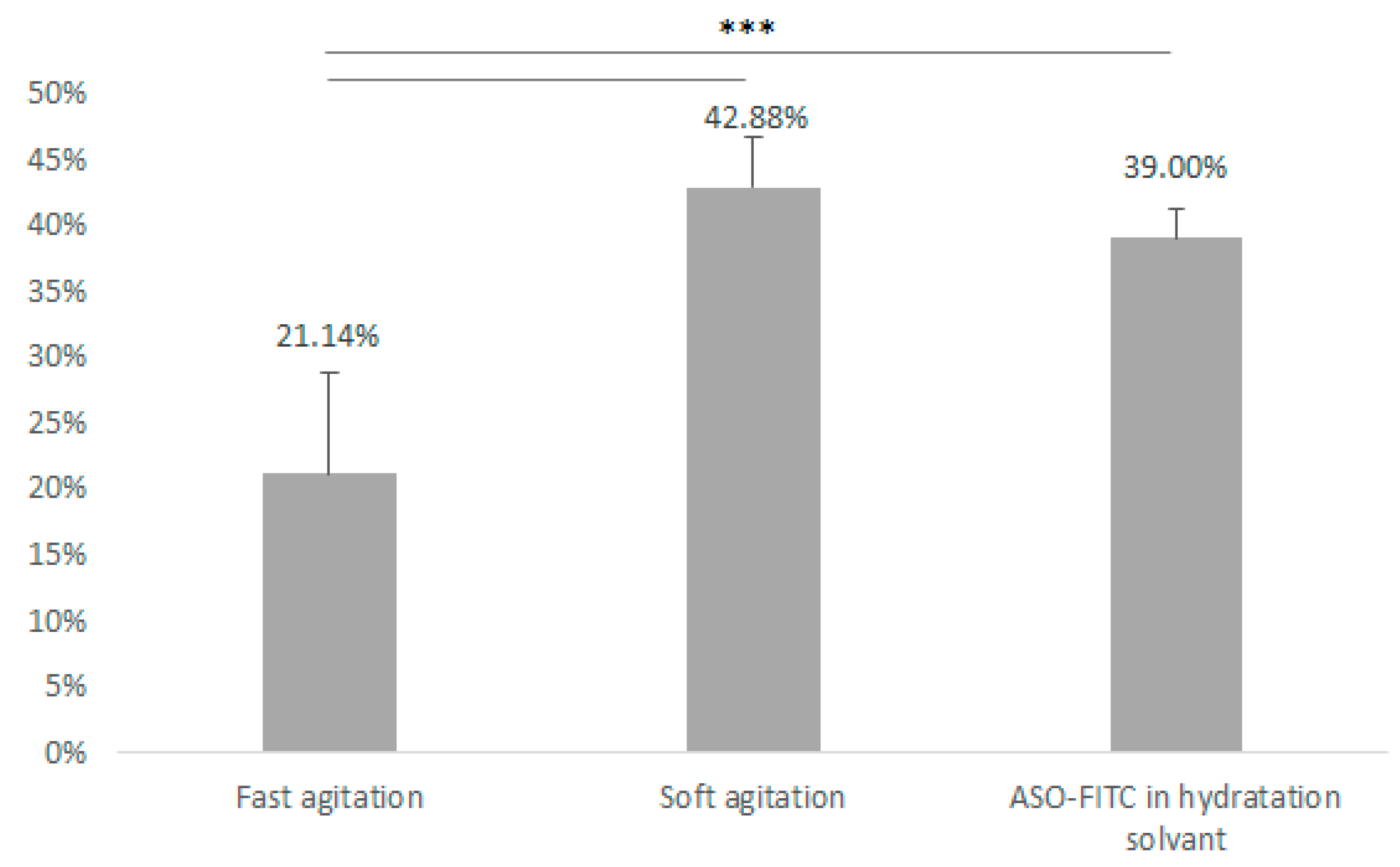
3.3. Pegylated Immunoliposome Preparation: Encapsulation Strategies
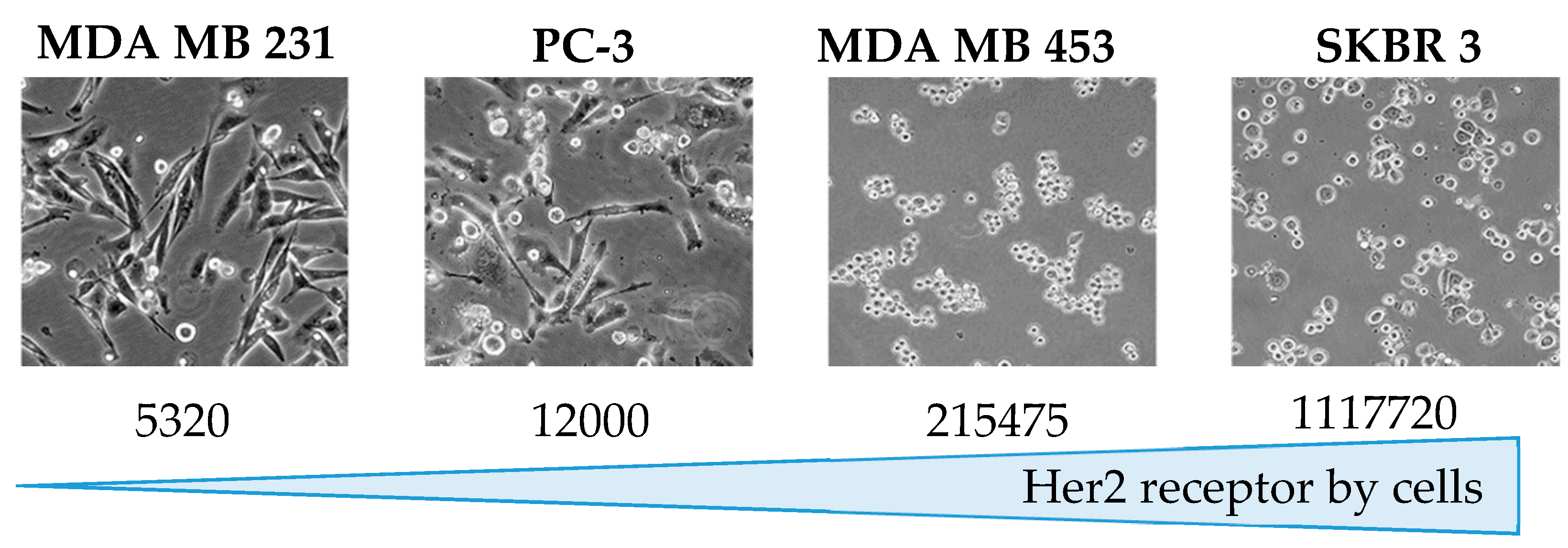
3.4. Quantification of Her2 on Cells
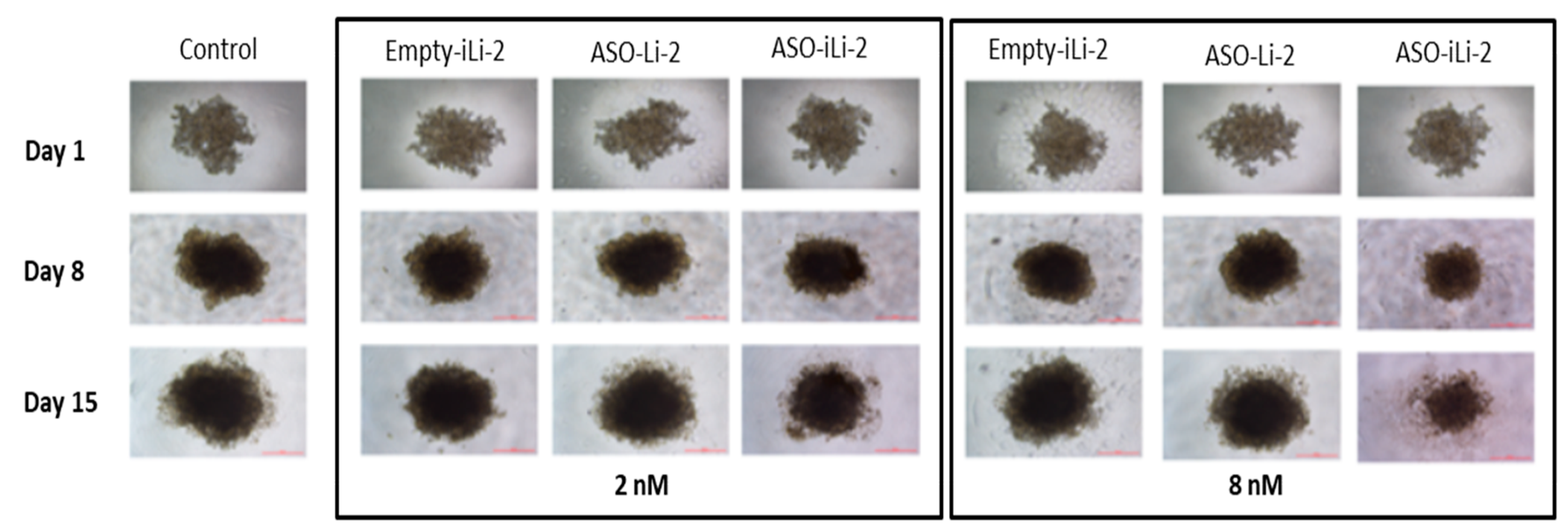
3.5. 3D In Vitro
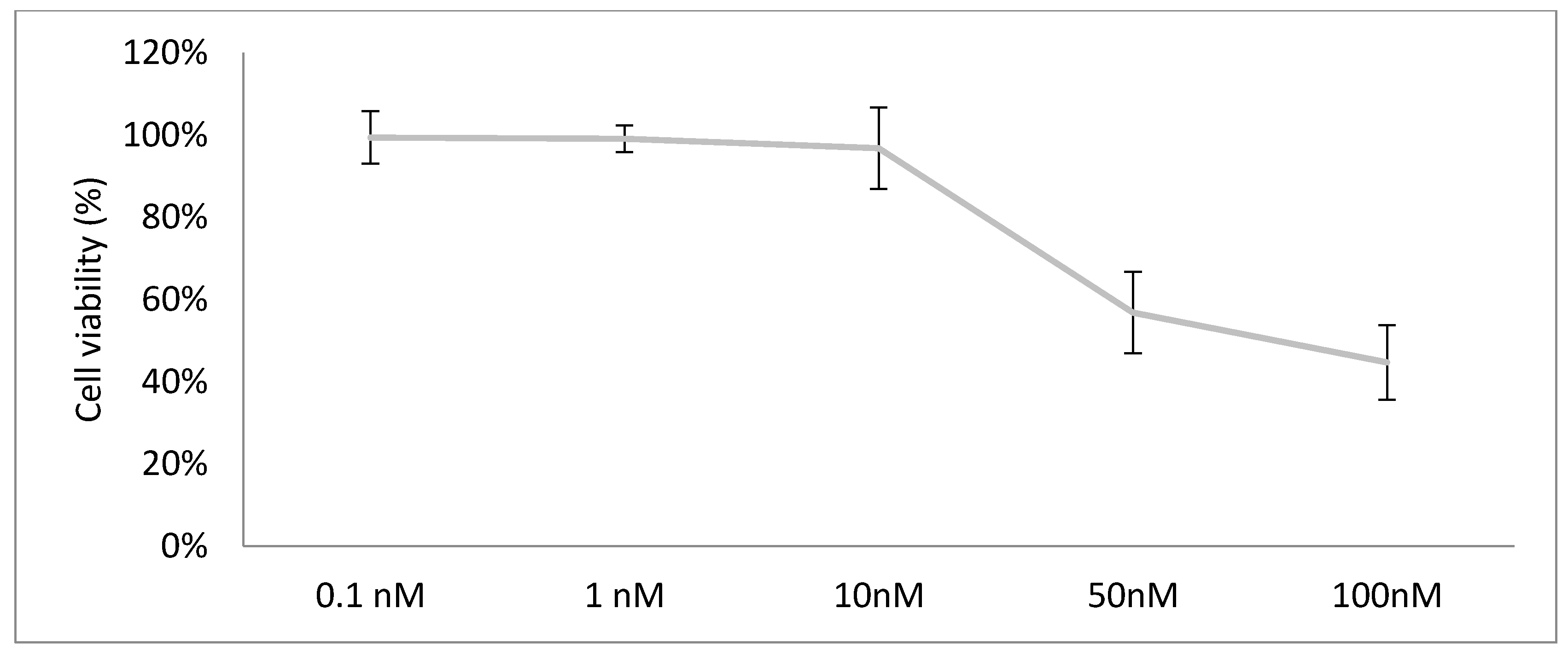
3.5.1. Lipidic Antiproliferative Activity
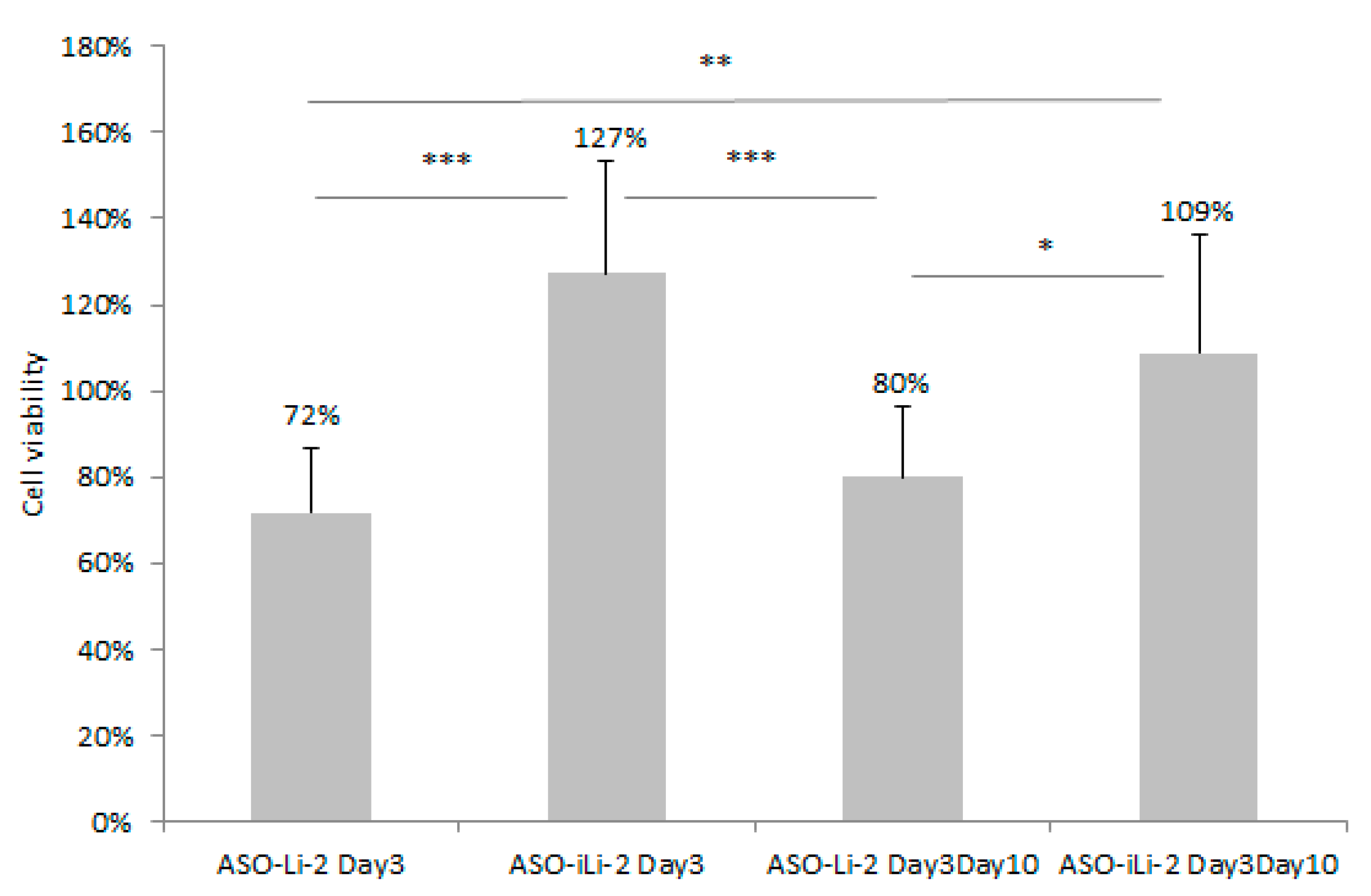
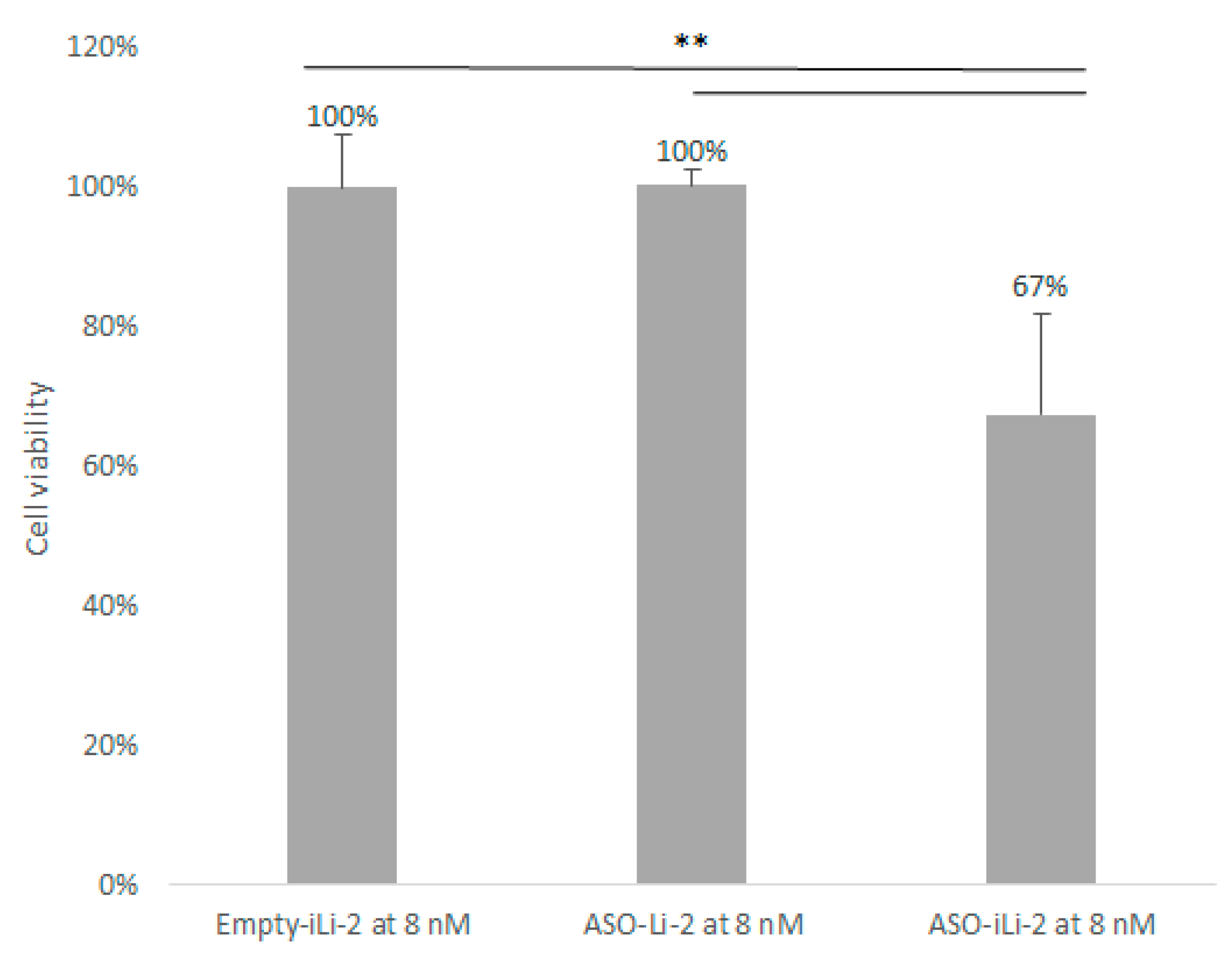
3.5.2. Liposomal Antiproliferative Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Litwin, M.S.; Tan, H.-J. The Diagnosis and Treatment of Prostate Cancer: A Review. JAMA 2017, 317, 2532–2542. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Epstein, J.I.; Zelefsky, M.J.; Sjoberg, D.D.; Nelson, J.B.; Egevad, L.; Magi-Galluzzi, C.; Andrew, J.; Parwani, A.V.; Reuter, V.E.; Fine, S.W.; et al. A Contemporary Prostate Cancer Grading System: A Validated Alternative to the Gleason Score. Eur. Urol. 2016, 69, 428–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jayaram, A.; Attard, G. Diagnostic Gleason score and castration-resistant prostate cancer. Ann. Oncol. 2016, 27, 962–964. [Google Scholar] [CrossRef]
- Katsogiannou, M.; Ziouziou, H.; Karaki, S.; Andrieu, C.; Henry de Villeneuve, M.; Rocchi, P. The hallmarks of castration-resistant prostate cancers. Cancer Treat. Rev. 2015, 41, 588–597. [Google Scholar] [CrossRef]
- Baylot, V.; Karaki, S.; Rocchi, P. TCTP Has a Crucial Role in the Different Stages of Prostate Cancer Malignant Progression. Results Probl. Cell Differ. 2017, 64, 255–261. [Google Scholar]
- Rho, S.B.; Lee, J.H.; Park, M.S.; Byun, H.-J.; Kang, S.; Seo, S.-S.; Kim, J.-Y.; Park, S.-Y. Anti-apoptotic protein TCTP controls the stability of the tumor suppressor p53. FEBS Lett. 2011, 585, 29–35. [Google Scholar] [CrossRef]
- Amson, R.; Pece, S.; Lespagnol, A.; Vyas, R.; Mazzarol, G.; Tosoni, D.; Colaluca, I.; Viale, G.; Rodrigues-Ferreira, S.; Wynendaele, J.; et al. Reciprocal repression between P53 and TCTP. Nat. Med. 2011, 18, 91–99. [Google Scholar] [CrossRef]
- Stahel, R.A.; Zangemeister-Wittke, U. Antisense oligonucleotides for cancer therapy—An overview. Lung Cancer 2003, 41, 81–88. [Google Scholar] [CrossRef]
- Geary, R.S.; Norris, D.; Yu, R.; Bennett, C.F. Pharmacokinetics, biodistribution and cell uptake of antisense oligonucleotides. Adv. Drug Deliv. Rev. 2015, 87, 46–51. [Google Scholar] [CrossRef] [Green Version]
- Baylot, V.; Katsogiannou, M.; Andrieu, C.; Taieb, D.; Acunzo, J.; Giusiano, S.; Fazli, L.; Gleave, M.; Garrido, C.; Rocchi, P. Targeting TCTP as a New Therapeutic Strategy in Castration-resistant Prostate Cancer. Mol. Ther. 2012, 20, 2244–2256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karaki, S.; Benizri, S.; Mejías, R.; Baylot, V.; Branger, N.; Nguyen, T.; Vialet, B.; Oumzil, K.; Barthélémy, P.; Rocchi, P. Lipid-oligonucleotide conjugates improve cellular uptake and efficiency of TCTP-antisense in castration-resistant prostate cancer. J. Control. Release 2017, 258, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Fanciullino, R.; Ciccolini, J. Liposome-encapsulated anticancer drugs: Still waiting for the magic bullet? Curr. Med. Chem. 2009, 16, 4361–4371. [Google Scholar] [CrossRef] [PubMed]
- Bitounis, D.; Pourchez, J.; Forest, V.; Boudard, D.; Cottier, M.; Klein, J.-P. Detection and analysis of nanoparticles in patients: A critical review of the status quo of clinical nanotoxicology. Biomaterials 2016, 76, 302–312. [Google Scholar] [CrossRef] [Green Version]
- Pasut, G.; Veronese, F.M. State of the art in PEGylation: The great versatility achieved after forty years of research. J. Control. Release 2012, 161, 461–472. [Google Scholar] [CrossRef]
- Montero, A.J.; Adams, B.; Diaz-Montero, C.M.; Glück, S. Nab-paclitaxel in the treatment of metastatic breast cancer: A comprehensive review. Expert Rev. Clin Pharmacol. 2011, 4, 329–334. [Google Scholar] [CrossRef]
- Prabhu, S.; Boswell, C.A.; Leipold, D.D.; A Khawli, L.; Li, D.; Lu, D.; Theil, F.-P.; Joshi, A.; Lum, B.L. Antibody delivery of drugs and radionuclides: Factors influencing clinical pharmacology. Ther. Deliv. 2011, 2, 769–791. [Google Scholar] [CrossRef]
- Adair, J.R.; Howard, P.W.; Hartley, J.A.; Williams, D.G.; Chester, K.A. Antibody-drug conjugates—A perfect synergy. Expert Opin. Biol. Ther. 2012, 12, 1191–1206. [Google Scholar] [CrossRef]
- Greish, K. Enhanced permeability and retention (EPR) effect for anticancer nanomedicine drug targeting. Methods Mol. Biol. 2010, 624, 25–37. [Google Scholar]
- Mamot, C.; Ritschard, R.; Wicki, A.; Stehle, G.; Dieterle, T.; Bubendorf, L.; Hilker, C.; Deuster, S.; Herrmann, R.; Rochlitz, C. Tolerability, safety, pharmacokinetics, and efficacy of doxorubicin-loaded anti-EGFR immunoliposomes in advanced solid tumours: A phase 1 dose-escalation study. Lancet Oncol. 2012, 13, 1234–1241. [Google Scholar] [CrossRef]
- Lehtinen, J.; Raki, M.; Bergström, K.A.; Uutela, P.; Lehtinen, K.; Hiltunen, A.; Pikkarainen, J.; Liang, H.; Pitkänen, S.; Määttä, A.; et al. Pre-Targeting and Direct Immunotargeting of Liposomal Drug Carriers to Ovarian Carcinoma. PLoS ONE 2012, 7, e41410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodallec, A.; Brunel, J.-M.; Giacometti, S.; Maccario, H.; Correard, F.; Mas, E.; Ornetto, C.; Savina, A.; Bouquet, F.; Lacarelle, B.; et al. Docetaxel-trastuzumab stealth immunoliposome: Development and in vitro proof of concept studies in breast cancer. Int. J. Nanomed. 2018, 13, 3451–3465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bangham, A.D.; Standish, M.M.; Watkins, J.C. Diffusion of univalent ions across the lamellae of swollen phospholipids. J. Mol. Biol. 1965, 13, 238–252. [Google Scholar] [CrossRef]
- Fanciullino, R.; Mollard, S.; Correard, F.; Giacometti, S.; Serdjebi, C.; Iliadis, A.; Ciccolini, J. Biodistribution, tumor uptake and efficacy of 5-FU-loaded liposomes: Why size matters. Pharm. Res. 2014, 31, 2677–2684. [Google Scholar] [CrossRef]
- Eg, B.; Wj, D. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar]
- Soema, P.C.; Willems, G.-J.; Jiskoot, W.; Amorij, J.-P.; Kersten, G.F. Predicting the influence of liposomal lipid composition on liposome size, zeta potential and liposome-induced dendritic cell maturation using a design of experiments approach. Eur. J. Pharm. Biopharm. 2015, 94, 427–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oude Blenke, E.; Evers, M.J.W.; Baumann, V.; Winkler, J.; Storm, G.; Mastrobattista, E. Critical evaluation of quantification methods for oligonucleotides formulated in lipid nanoparticles. Int. J. Pharm. 2018, 548, 793–802. [Google Scholar] [CrossRef]
- Rodallec, A.; Franco, C.; Robert, S.; Sicard, G.; Giacometti, S.; Lacarelle, B.; Bouquet, F.; Savina, A.; Lacroix, R.; Dignat-George, F.; et al. Prototyping Trastuzumab Docetaxel Immunoliposomes with a New FCM-Based Method to Quantify Optimal Antibody Density on Nanoparticles. Sci. Rep. 2020, 10, 4147. [Google Scholar] [CrossRef] [Green Version]
- Panke, C.; Weininger, D.; Haas, A.; Schelter, F.; Schlothauer, T.; Bader, S.; Sircar, R.; Josel, H.P.; Baer, U.; Burtscher, H.; et al. Quantification of cell surface proteins with bispecific antibodies. Protein Eng. Des. Sel. 2013, 26, 645–654. [Google Scholar] [CrossRef]
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 3rd ed.; Sage Publications: Thousand Oaks, CA, USA, 2019. [Google Scholar]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous inference in general parametric models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef] [Green Version]
- R: The R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 13 June 2020).
- Gray, G.D.; Basu, S.; Wickstrom, E. Transformed and immortalized cellular uptake of oligodeoxynucleoside phosphorothioates, 3′-Alkylamino oligodeoxynucleotides, 2′-o-methyl oligoribonucleotides, oligodeoxynucleoside methylphosphonates, and peptide nucleic acids. Biochem. Pharmacol. 1997, 53, 1465–1476. [Google Scholar] [CrossRef]
- Moreno, P.M.D.; Pêgo, A.P. Therapeutic antisense oligonucleotides against cancer: Hurdling to the clinic. Front. Chem. 2014, 2, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winkler, J. Oligonucleotide conjugates for therapeutic applications. Ther. Deliv. 2013, 4, 791–809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Godeau, G.; Arnion, H.; Brun, C.; Staedel, C.; Barthélémy, P. Fluorocarbon oligonucleotide conjugates for nucleic acids delivery. MedChemComm 2010, 1, 76–78. [Google Scholar] [CrossRef]
- Iqbal, N.; Iqbal, N. Human Epidermal Growth Factor Receptor 2 (HER2) in Cancers: Overexpression and Therapeutic Implications. Mol. Biol. Int. 2014, 2014, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tai, S.; Sun, Y.; Squires, J.M.; Zhang, H.; Oh, W.K.; Liang, C.-Z.; Huang, J. PC3 is a cell line characteristic of prostatic small cell carcinoma. Prostate 2011, 71, 1668–1679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bladou, F.; E Gleave, M.; Penault-Llorca, F.; Serment, G.; Lange, P.H.; Vessella, R.L. In vitro and in vivo models developed from human prostatic cancer. Prog. Urol. 1997, 7, 384–396. [Google Scholar] [PubMed]
- Maeda, H. Polymer therapeutics and the EPR effect. J. Drug Target. 2017, 25, 781–785. [Google Scholar] [CrossRef]
- Sharifi, N.; Salmaninejad, A.; Ferdosi, S.; Bajestani, A.N.; Khaleghiyan, M.; Estiar, M.A.; Jamali, M.; Nowroozi, M.R.; Shakoori, A. HER2 gene amplification in patients with prostate cancer: Evaluating a CISH-based method. Oncol. Lett. 2016, 12, 4651–4658. [Google Scholar] [CrossRef]
- Andersson, J.; Rosestedt, M.; Asplund, V.; Yavari, N.; Orlova, A. In vitro modeling of HER2-targeting therapy in disseminated prostate cancer. Int. J. Oncol. 2014, 45, 2153–2158. [Google Scholar] [CrossRef] [Green Version]
- Uehara, H. Angiogenesis of prostate cancer and antiangiogenic therapy. J. Med. Investig. 2003, 50, 146–153. [Google Scholar]
- Kluetz, P.G.; Figg, W.D.; Dahut, W.L. Angiogenesis Inhibitors in the treatment of Prostate Cancer. Expert Opin. Pharmacother. 2010, 11, 233–247. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cheng, Q.; Jiang, Q.; Huang, Y.; Liu, H.; Zhao, Y.; Cao, W.; Ma, G.; Dai, F.; Liang, X.-J.; et al. Enhanced endosomal/lysosomal escape by distearoyl phosphoethanolamine-polycarboxybetaine lipid for systemic delivery of siRNA. J. Control. Release 2014, 176, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Zahednezhad, F.; Saadat, M.; Valizadeh, H.; Zakeri-Milani, P.; Baradaran, B. Liposome and immune system interplay: Challenges and potentials. J. Control. Release 2019, 305, 194–209. [Google Scholar] [CrossRef] [PubMed]
- Bennett, C.F.; Chiang, M.Y.; Chan, H.; Shoemaker, J.E.; Mirabelli, C.K. Cationic lipids enhance cellular uptake and activity of phosphorothioate antisense oligonucleotides. Mol. Pharmacol. 1992, 41, 1023–1033. [Google Scholar] [PubMed]
- Anderson, M.; Omri, A. The Effect of Different Lipid Components on the In Vitro Stability and Release Kinetics of Liposome Formulations. Drug Deliv. 2004, 11, 33–39. [Google Scholar] [CrossRef]
- Coderch, L.; Fonollosa, J.; De Pera, M.; Estelrich, J.; De La Maza, A.; Parra, J.L. Influence of cholesterol on liposome fluidity by EPR: Relationship with percutaneous absorption. J. Control. Release 2000, 68, 85–95. [Google Scholar] [CrossRef]
- Mourtas, S.; Fotopoulou, S.; Duraj, S.; Sfika, V.; Tsakiroglou, C.; Antimisiaris, S.G. Liposomal drugs dispersed in hydrogels: Effect of liposome, drug and gel properties on drug release kinetics. Colloids Surf. B Biointerfaces 2007, 55, 212–221. [Google Scholar] [CrossRef]
- Rodallec, A.; Sicard, G.; Giacometti, S.; Carré, M.; Pourroy, B.; Bouquet, F.; Savina, A.; Lacarelle, B.; Ciccolini, J.; Fanciullino, R. From 3D spheroids to tumor bearing mice: Efficacy and distribution studies of trastuzumab-docetaxel immunoliposome in breast cancer. Int. J. Nanomed. 2018, 13, 6677–6688. [Google Scholar] [CrossRef] [Green Version]




| Formulation | Size (nm) ± SD | PDI ± SD |
|---|---|---|
| ASO-Li-1 | 127.7 ± 4.8 | 0.161 ± 0.03 |
| ASO-iLi-1 | 139.6 ± 1.8 | 0.183 ± 0.06 |
| ASO-Li-2 | 145.6 ± 4.1 | 0.080 ± 0.01 |
| ASO-iLi-2 | 154.1 ± 9.4 | 0.090 ± 0.03 |
| Formulation | Day 7 | Day 14 | Day 28 | |||
|---|---|---|---|---|---|---|
| Size (nm) ± SD | PDI ± SD | Size (nm) ± SD | PDI ± SD | Size (nm) ± SD | PDI ± SD | |
| ASO-Li-1 | 150.7 ± 6.1 | 0.16 ± 0.03 | not performed | |||
| ASO-iLi-1 | 166.9± 6.4 | 0.18 ± 0.07 | not performed | |||
| ASO-Li-2 | 148.3 ± 8.4 | 0.07 ± 0.01 | 148.2 ± 5.3 | 0.08 ± 0.03 | 150.4 ± 4.7 | 0.11 ± 0.05 |
| ASO-iLi-2 | 151.6 ± 1.0 | 0.06 ± 0.01 | 154.4 ± 3.4 | 0.08 ± 0.03 | 162.3 ± 1.0 | 0.10 ± 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sicard, G.; Paris, C.; Giacometti, S.; Rodallec, A.; Ciccolini, J.; Rocchi, P.; Fanciullino, R. Enhanced Antisense Oligonucleotide Delivery Using Cationic Liposomes Grafted with Trastuzumab: A Proof-of-Concept Study in Prostate Cancer. Pharmaceutics 2020, 12, 1166. https://doi.org/10.3390/pharmaceutics12121166
Sicard G, Paris C, Giacometti S, Rodallec A, Ciccolini J, Rocchi P, Fanciullino R. Enhanced Antisense Oligonucleotide Delivery Using Cationic Liposomes Grafted with Trastuzumab: A Proof-of-Concept Study in Prostate Cancer. Pharmaceutics. 2020; 12(12):1166. https://doi.org/10.3390/pharmaceutics12121166
Chicago/Turabian StyleSicard, Guillaume, Clément Paris, Sarah Giacometti, Anne Rodallec, Joseph Ciccolini, Palma Rocchi, and Raphaëlle Fanciullino. 2020. "Enhanced Antisense Oligonucleotide Delivery Using Cationic Liposomes Grafted with Trastuzumab: A Proof-of-Concept Study in Prostate Cancer" Pharmaceutics 12, no. 12: 1166. https://doi.org/10.3390/pharmaceutics12121166
APA StyleSicard, G., Paris, C., Giacometti, S., Rodallec, A., Ciccolini, J., Rocchi, P., & Fanciullino, R. (2020). Enhanced Antisense Oligonucleotide Delivery Using Cationic Liposomes Grafted with Trastuzumab: A Proof-of-Concept Study in Prostate Cancer. Pharmaceutics, 12(12), 1166. https://doi.org/10.3390/pharmaceutics12121166