Encapsulation of Activated Carbon into a Hollow-Type Spherical Bacterial Cellulose Gel and Its Indole-Adsorption Ability Aimed at Kidney Failure Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
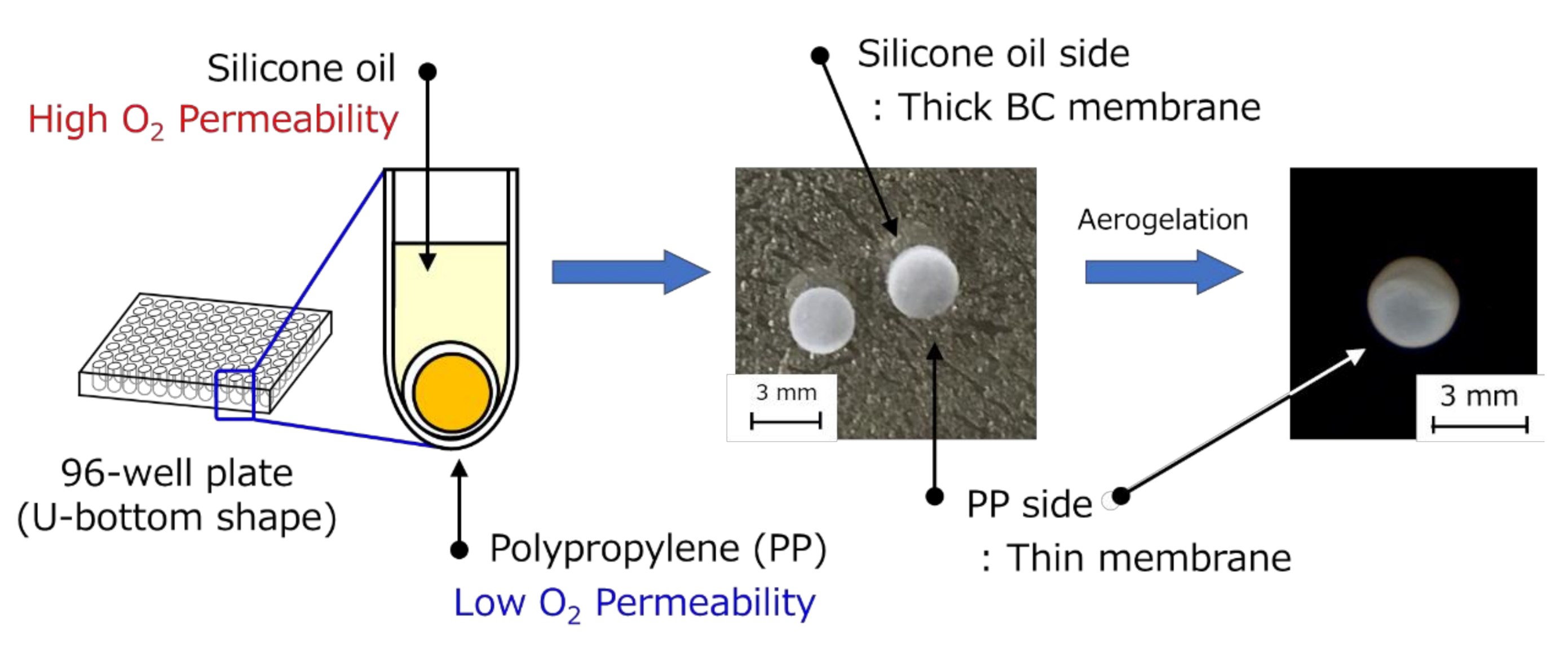
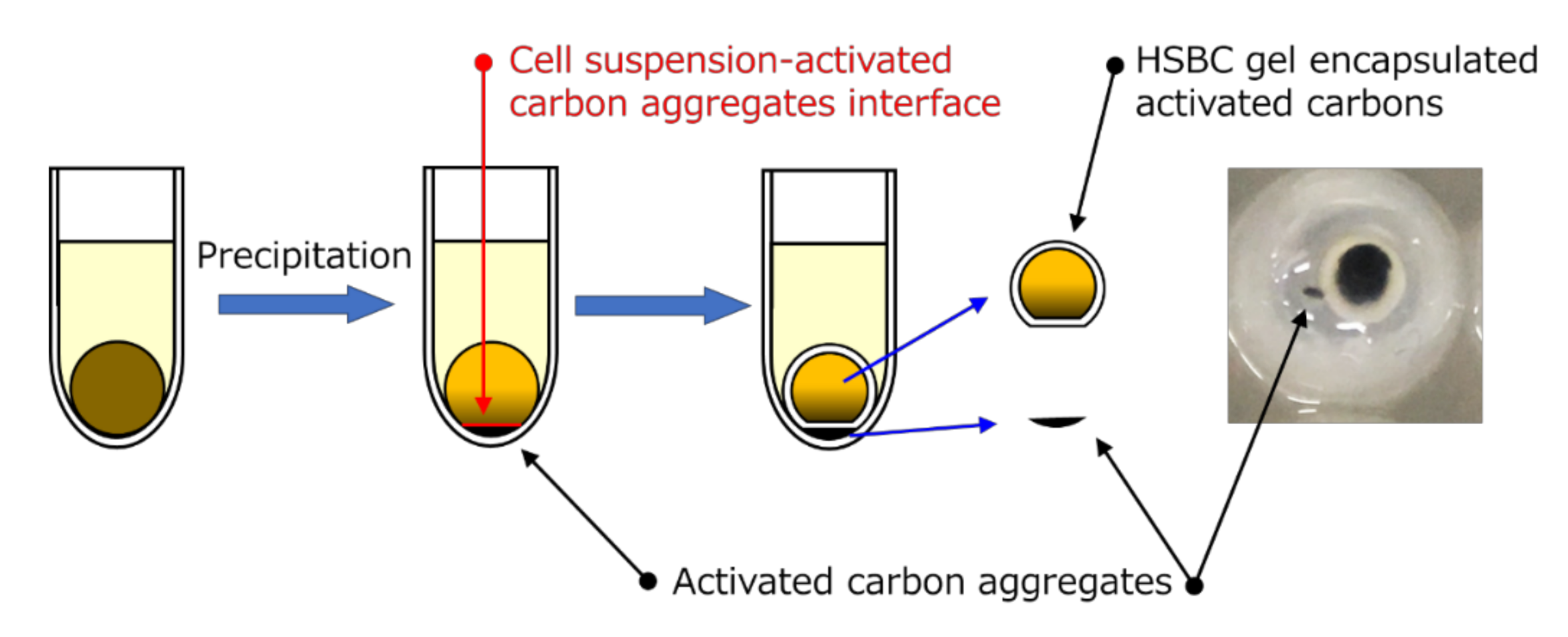
2.2. Preparation of the Hollow-Type Spherical BC-Gel-Encapsulated Activated Carbon
2.2.1. Method A
2.2.2. Method B
2.3. Preparation of HSBC Aerogel Using Supercritical CO2
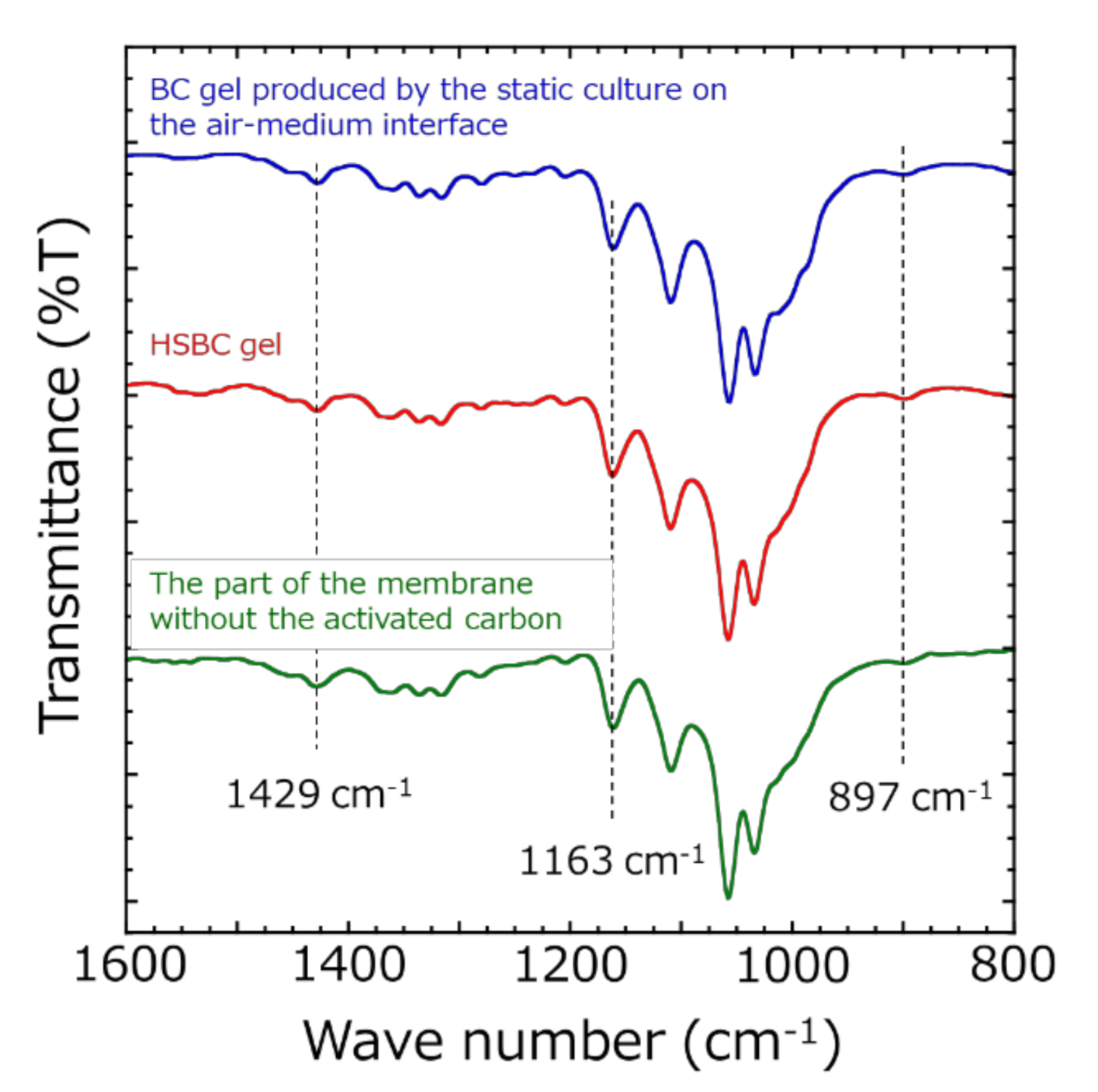
2.4. Characterization of HSBC Gels
2.5. Indole Adsorption Test of Activated Carbon Encapsulated in HSBC Gel
2.5.1. Pseudo-First-Order Model
2.5.2. Pseudo-Second-Order Model
2.5.3. Intra-Particle Diffusion Study
3. Results and Discussion
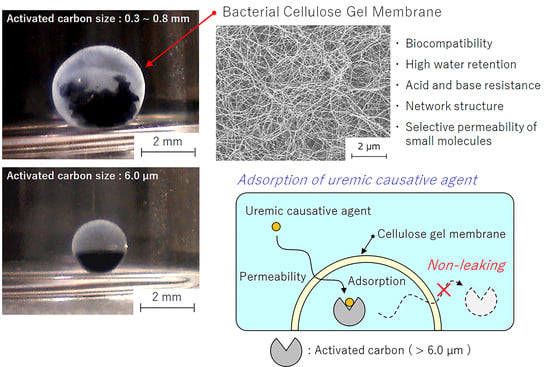
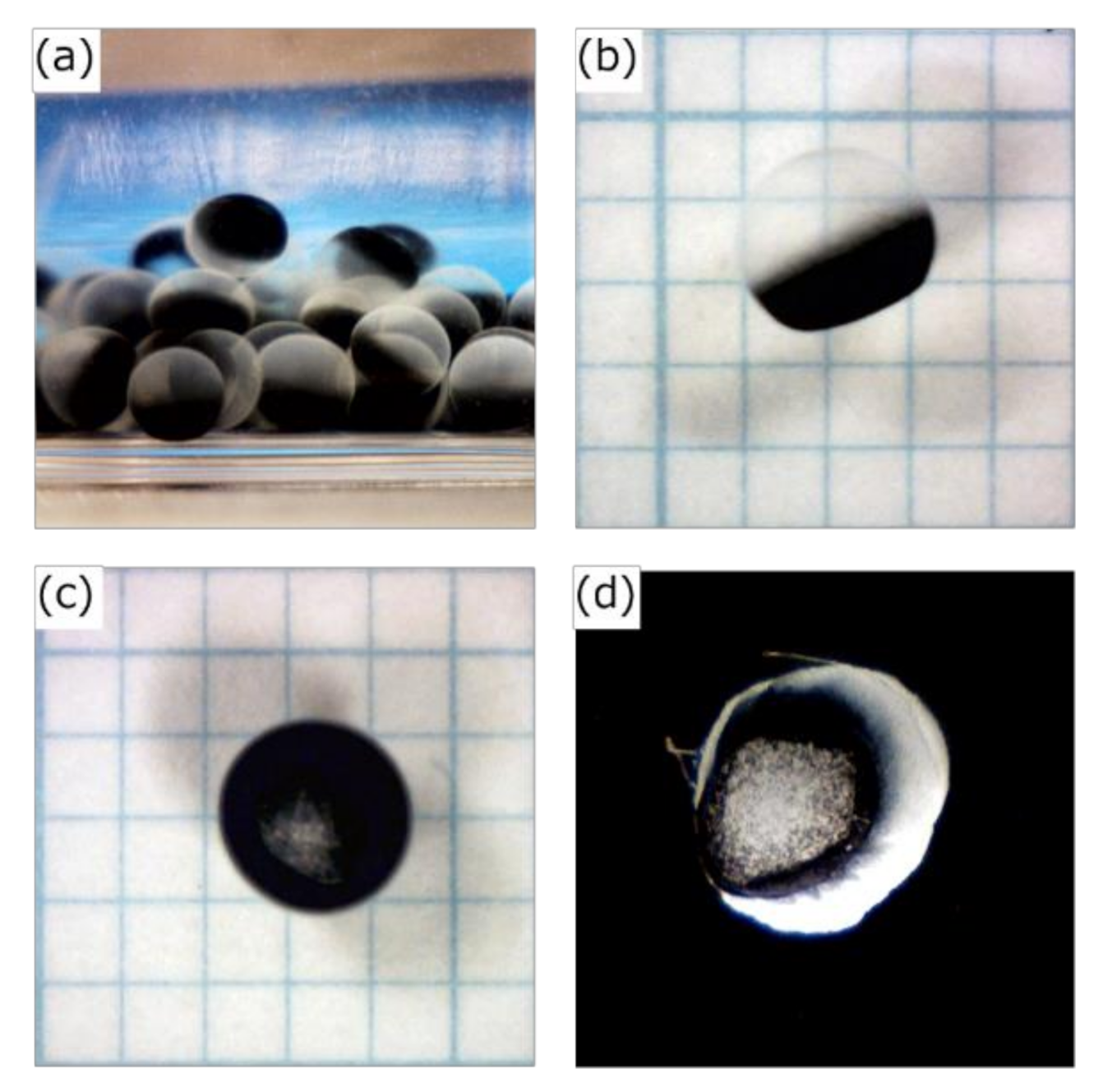
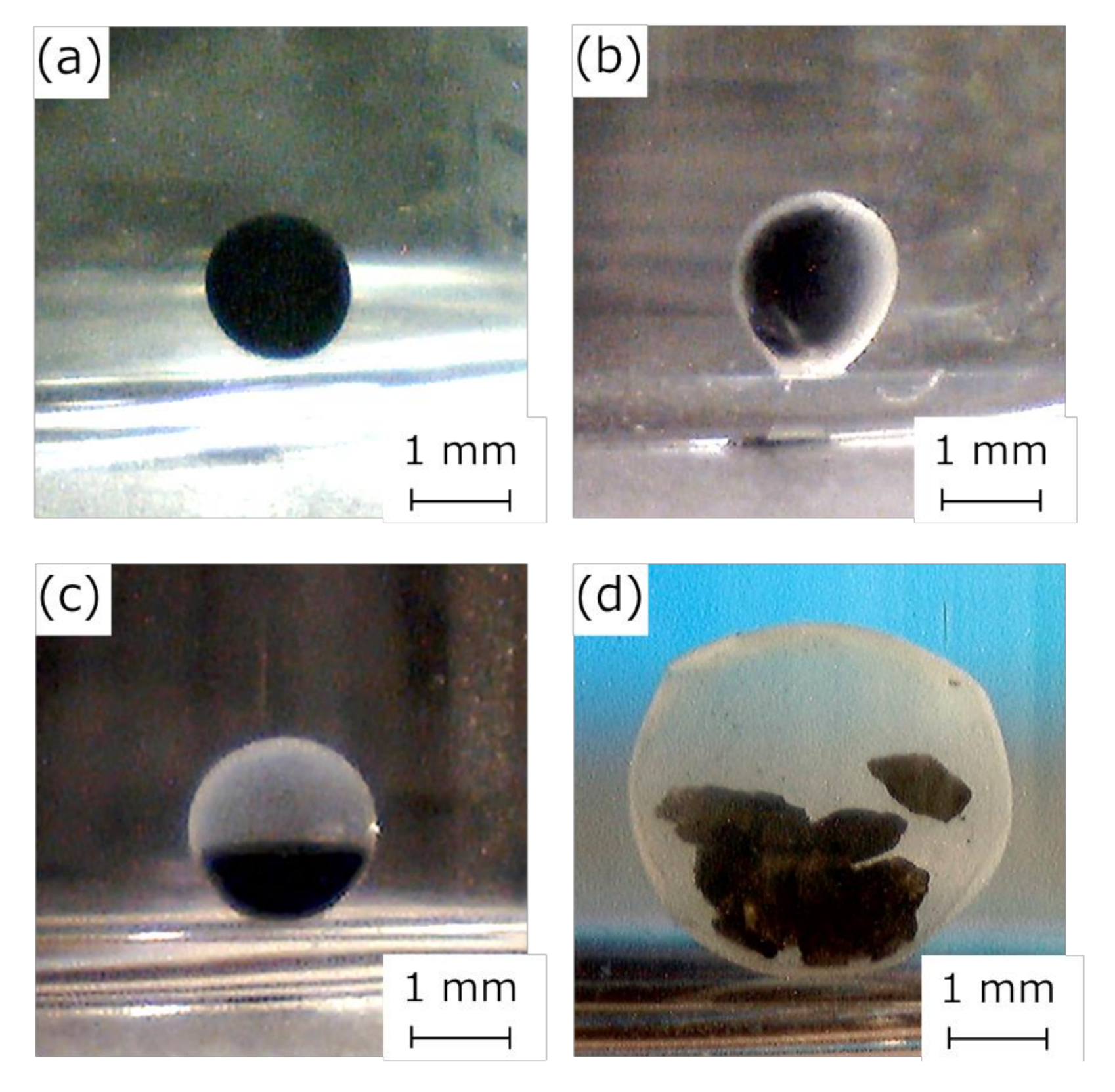
3.1. Preparation of the HSBC Gel Encapsulating Activated Carbon Using Method A
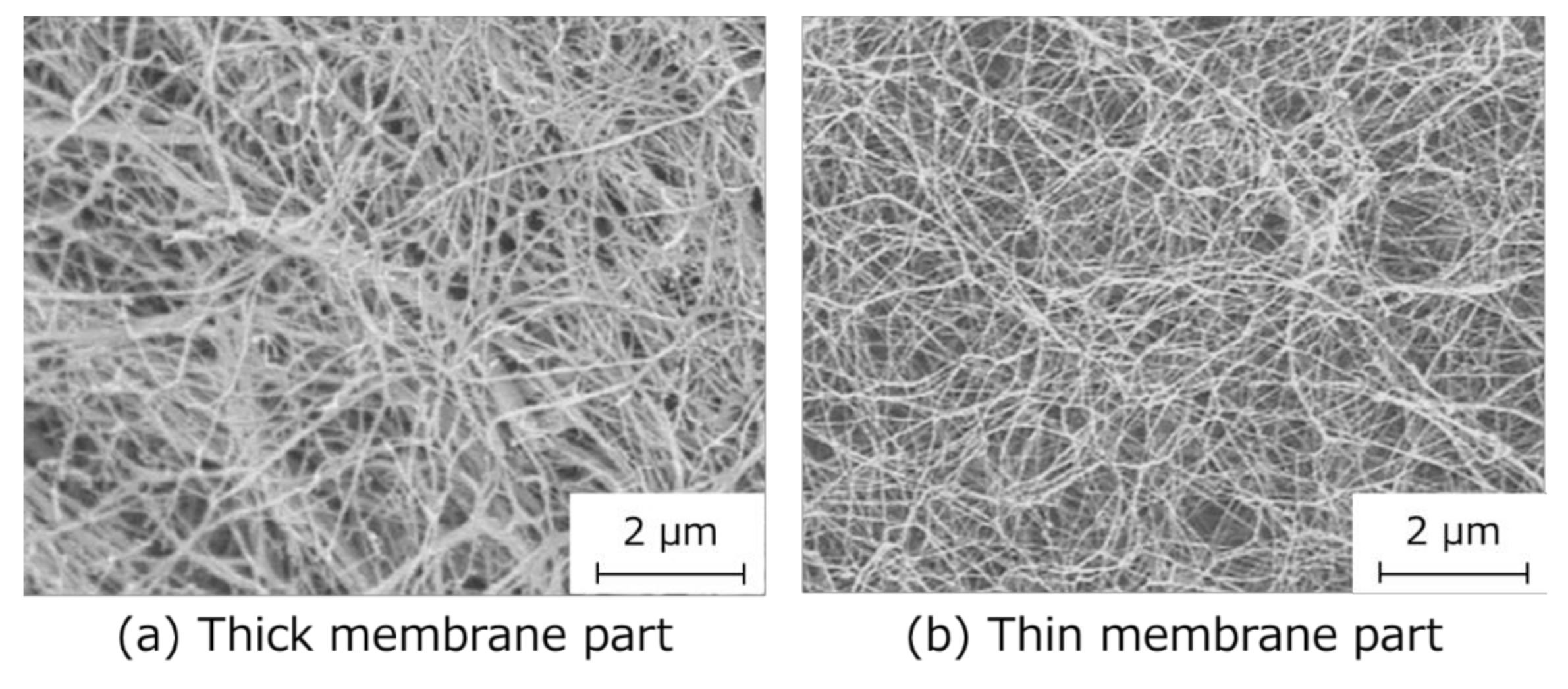
3.2. Preparation of the HSBC Gel Encapsulating Activated Carbon Using Method B
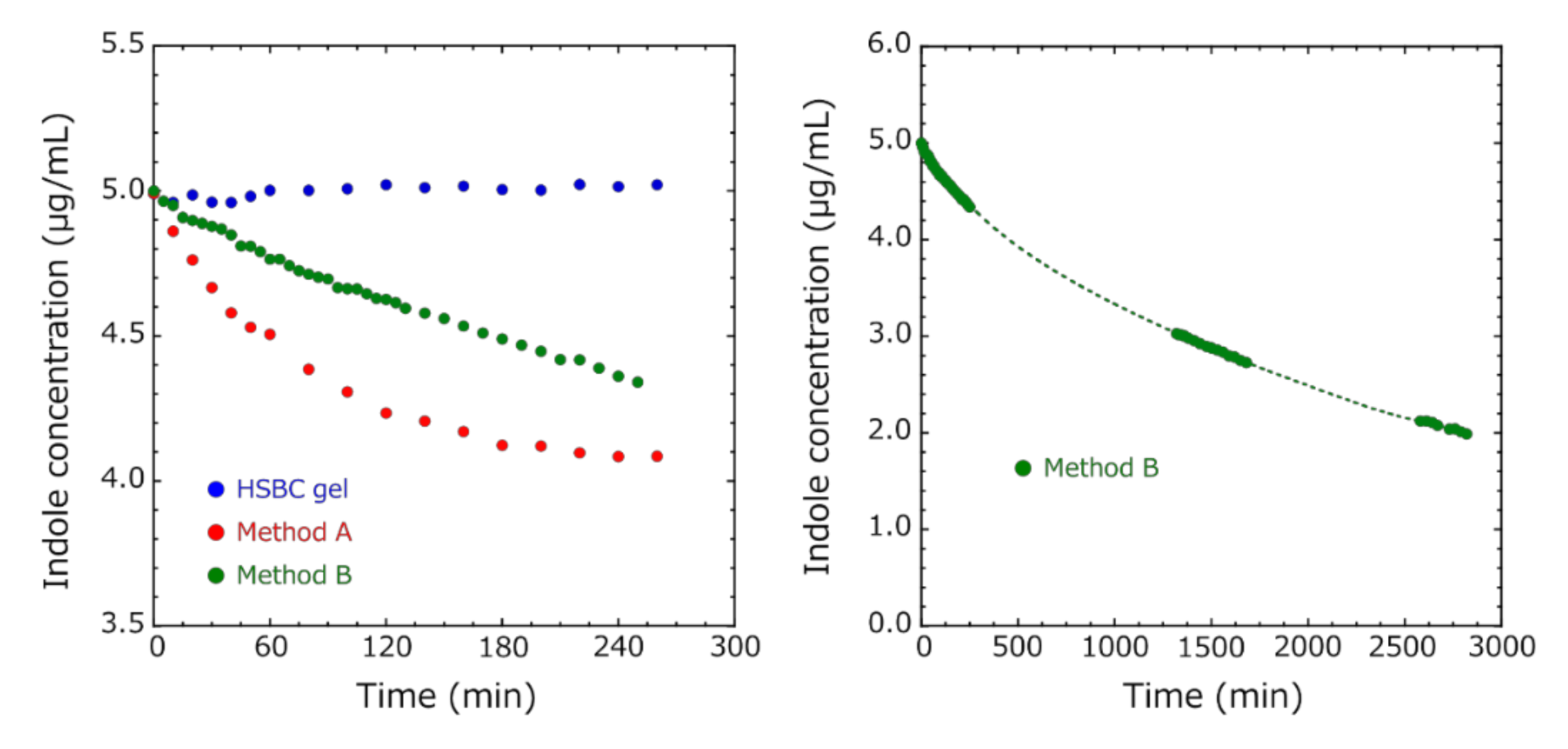
3.3. Indole Adsorption Test
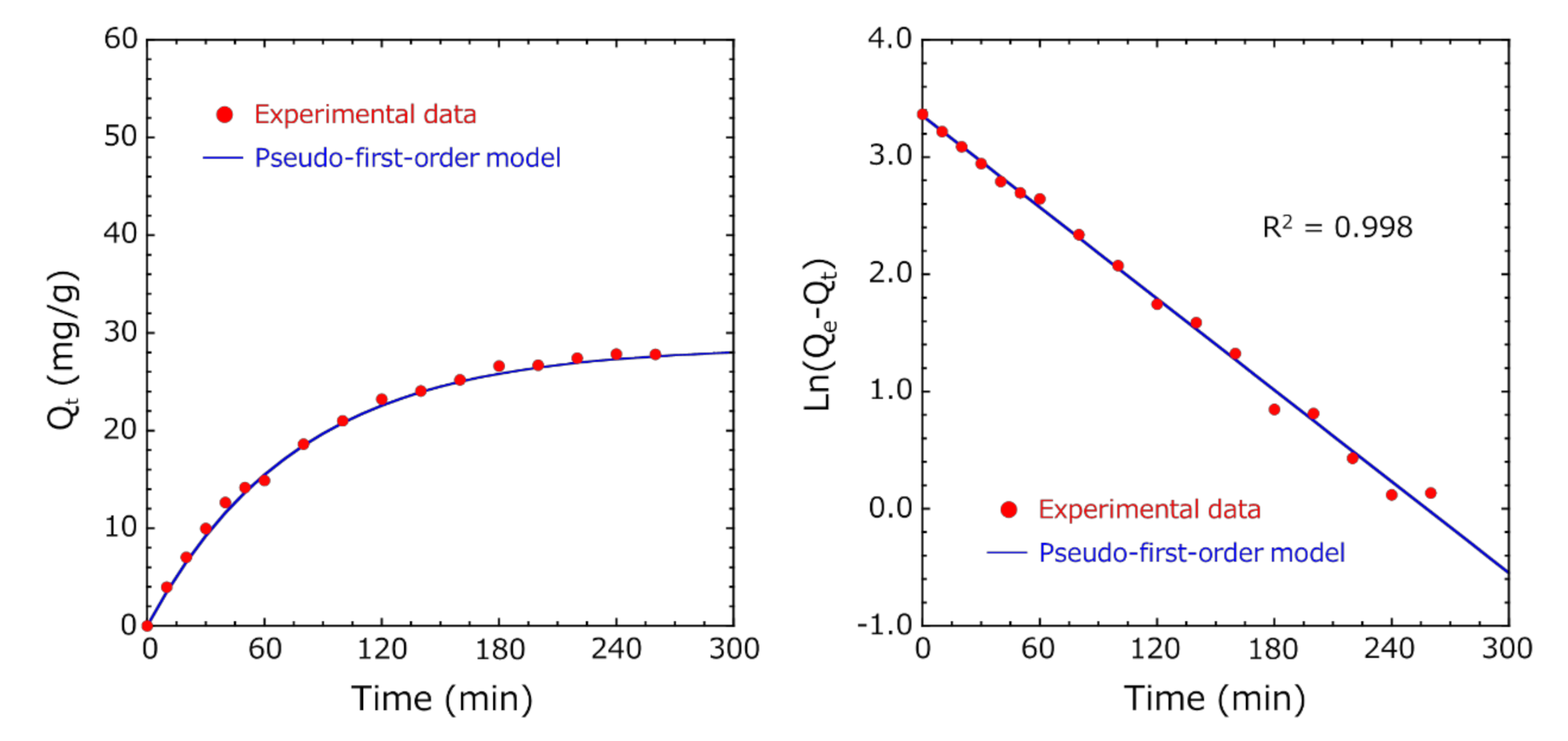
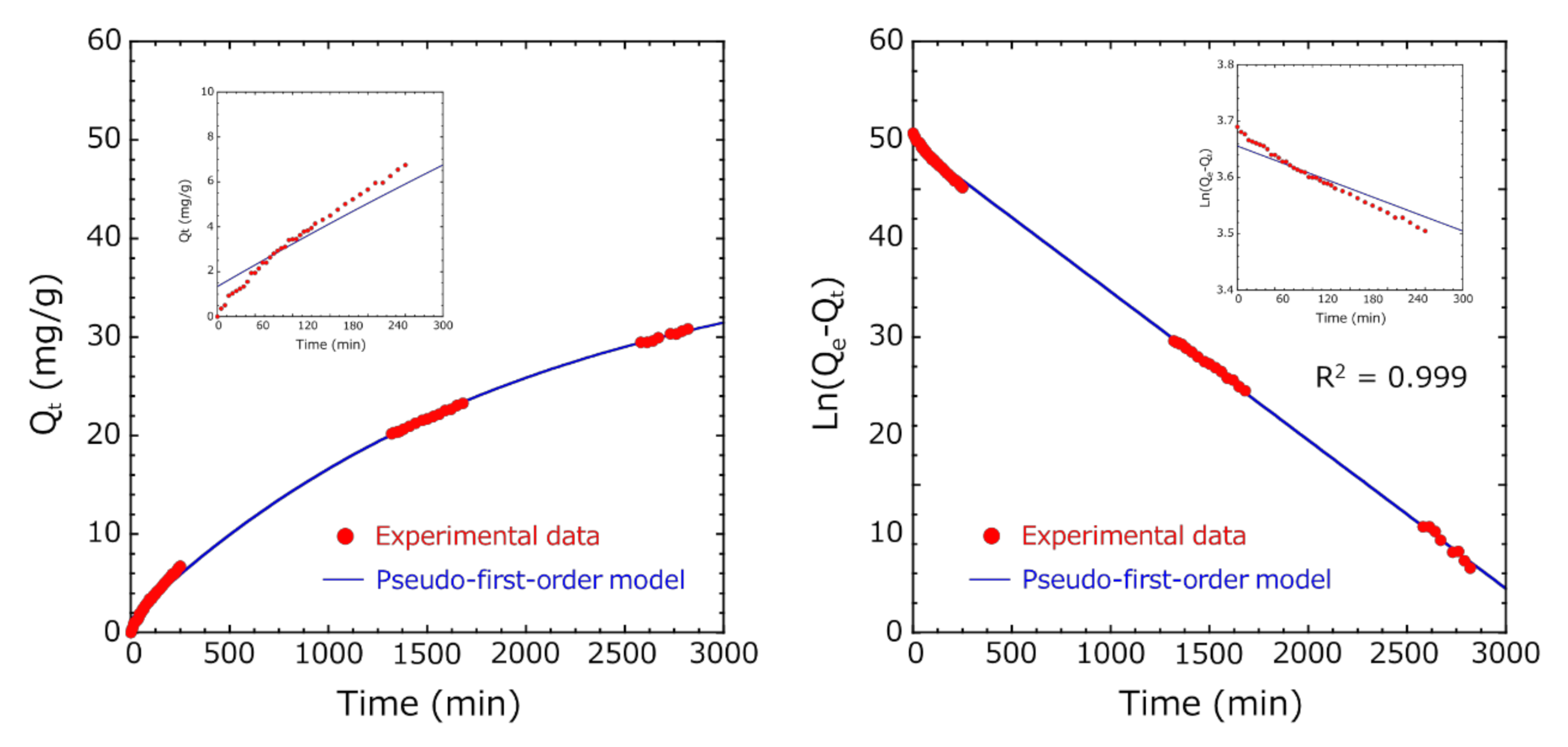
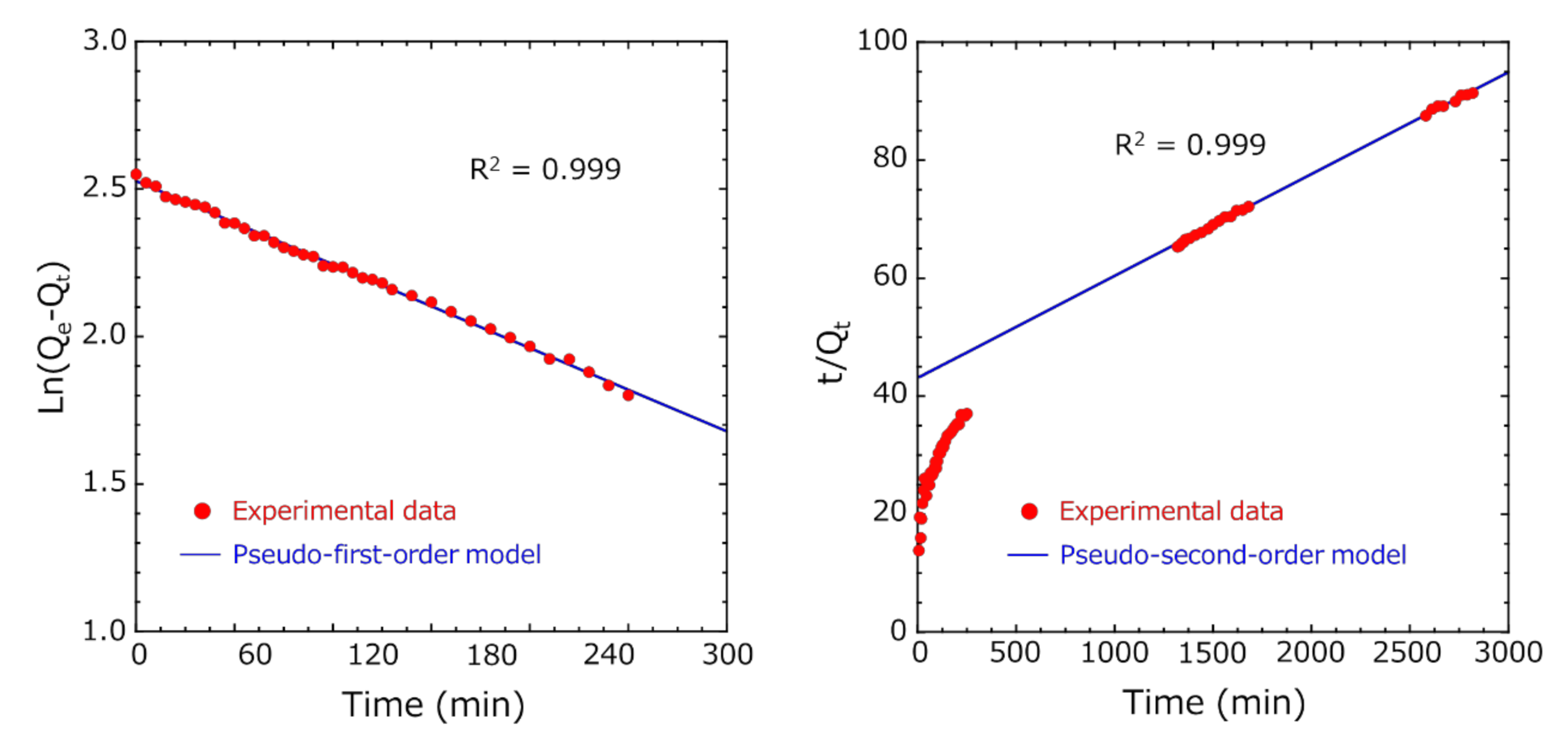
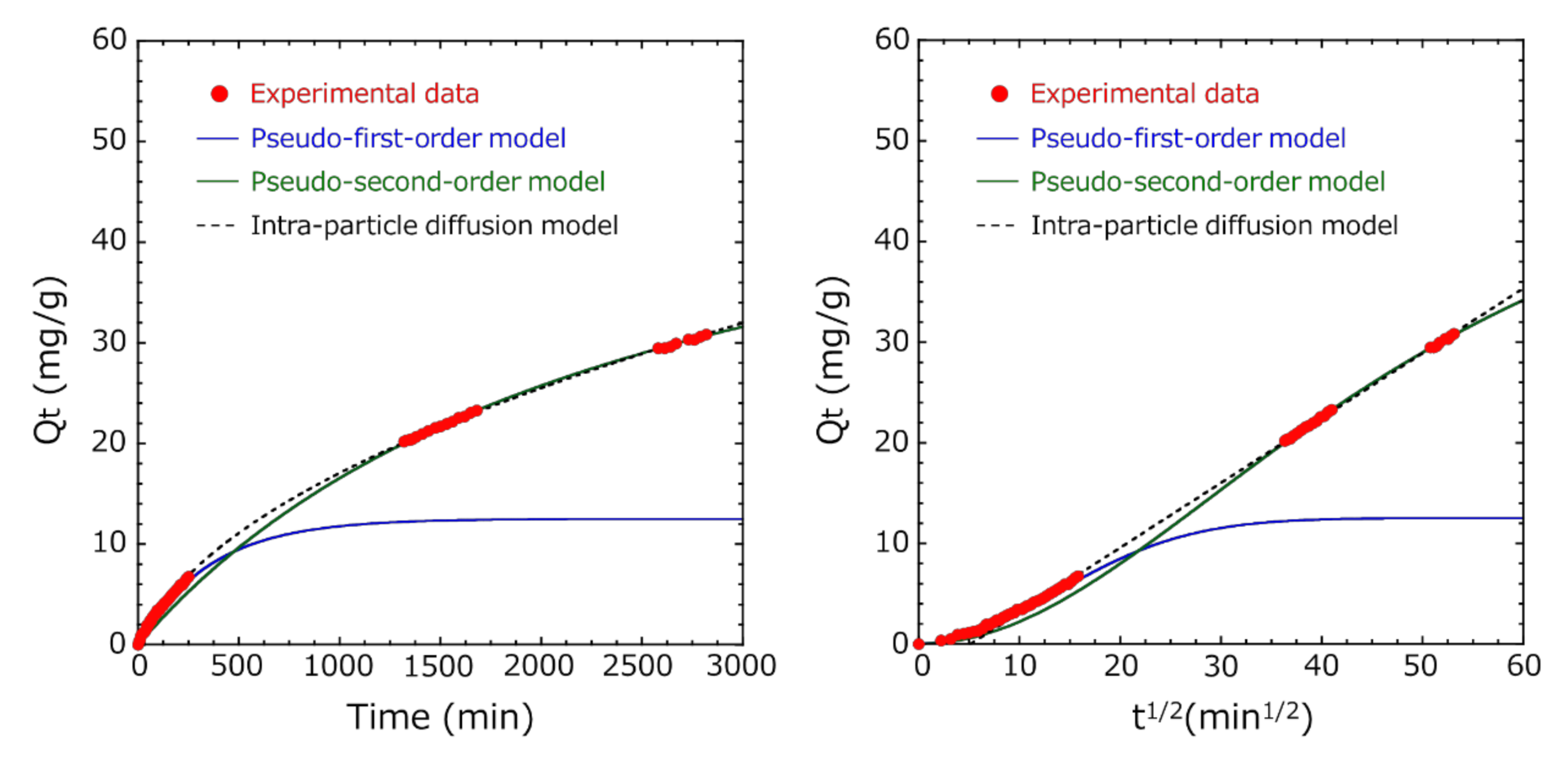
3.4. Adsorption Kinetic Study
3.5. Comparison of the Adsorption Capacity at Equilibrium of the Activated-Carbon-Encapsulated HSBC Gel Obtained by Methods A and B
3.6. Influence of BC Gelatinous Membrane on Indole Adsorption
- The HSBC gel without activated carbon did not adsorb indole molecules.
- In both Methods A and B, the diffusion of indole molecules on the outer surface of activated carbon was dominant at the initial stage of adsorption because these experimental data fitted well with the pseudo-first-order model.
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nigam, S.K.; Bush, K.T. Uremic syndrome of chronic kidney disease: Altered remote sensing and signaling. lose. Nat. Rev. Nephrol. 2019, 15, 301–316. [Google Scholar] [CrossRef] [PubMed]
- Vanholder, R.; Massy, Z.; Argiles, A.; Spasovski, G.; Verbeke1, F.; Lameire, N. Chronic kidney disease as cause of cardiovascular morbidity and mortality. Nephr. Dial. Transpl. 2005, 20, 1048–1056. [Google Scholar] [CrossRef] [PubMed]
- Asai, M.; Kumakura, S.; Kikuchi, M. Review of the efficacy of AST-120 (KREMEZIN®) on renal function in chronic kidney disease patients. Renal Fail. 2019, 41, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Nakano, N.I.; Shimamori, Y.; Umehashi, M.; Nakano, M. Preparation and drug adsorption characteristics of activated carbon beads suitable for oral administration. Chem. Pharm. Bull. 1984, 32, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Aljohani, T.K.; Alshamrani, A.M.; Alzahrani, A.M.; Sairafi, R.A. A rare case of small bowel obstruction secondary to activated charcoal administration. J. Surg. Case Rep. 2019, 2, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Gomez, H.F.; Brent, J.A.; Munoz, D.C.; Mimmack, R.F.; Ritvo, J.; Phillips, S.; McKinney, P. Charcoal stercolith with intestinal perforation in a patient treated for amitriptyline ingestion. J. Emerg. Med. 1994, 12, 57–60. [Google Scholar] [CrossRef]
- Goulbourne, K.B.; Cisek, J.E. Small-bowel obstruction secondary to activated charcoal and adhesions. Ann. Emerg. Med. 1994, 24, 108–110. [Google Scholar] [CrossRef]
- Lim, F.S.; Sun, A.M. Microencapsulated islets as bioartificial endocrine pancreas. Science 1980, 210, 908–910. [Google Scholar] [CrossRef]
- O’Shea, G.M.; Goosen, M.F.; Sun, A.M. Prolonged survival of transplanted islets of Langerhans encapsulated in a biocompatible membrane. Biochim. Biophys. Acta 1984, 804, 133–136. [Google Scholar] [CrossRef]
- Klöck, G.; Frank, H.; Houben, R.; Zekorn, T.; Horcher, A.; Siebers, U.; Whörle, M.; Federlin, K.; Zimmermann, U. Production of purified alginates suitable for use in immunoisolated transplantation. Appl. Microbiol. Biotechnol. 1994, 40, 638–643. [Google Scholar] [CrossRef]
- Otterlei, M.; Ostgaard, K.; Skjåk-Braek, G.; Smidsrød, O.; Soon-Shiong, P.; Espevik, T. Induction of cytokine production from human monocytes stimulated with alginate. J. Immunother. 1991, 10, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Vaithilingam, V.; Bal, S.; Touch, B.E. Encapsulated Islet Transplantation: Where Do We Stand? Rev. Diabet. Stud. 2017, 14, 51–78. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Lacik, I.; Brissová, M.; Anilkumar, A.V.; Prokop, A.; Hunkeler, D.; Green, R.; Shahrokhi, K.; Powers, A.C. An encapsulation system for the immunoisolation of pancreatic islets. Nat. Biotechnol. 1997, 15, 358–362. [Google Scholar] [CrossRef] [PubMed]
- de Vos, P.; Lazarjani, H.A.; Poncelet, D.; Faas, M.M. Polymers in cell encapsulation from an enveloped cell perspective. Adv. Drug. Deliv. Rev. 2014, 67–68, 15–34. [Google Scholar] [CrossRef]
- Klemm, D.; Heublein, B.; Fink, H.-P.; Bohn, A. Cellulose: Fascinating Biopolymer and Sustainable Raw Material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef]
- Stoica-Guzun, A.; Stroescu, M.; Tache, F.; Zaharescu, T.; Grosu, E. Effect of electron beam irradiation on bacterial cellulose membranes used as transdermal drug delivery systems. Nucl. Instr. Meth. Phys. Res. B 2007, 265, 434–438. [Google Scholar] [CrossRef]
- Weyella, P.; Beekmanna, U.; Küpperb, C.; Dederichsb, M.; Thamma, J.; Fischera, D.; Kralischa, D. Tailor-made material characteristics of bacterial cellulose for drug delivery applications in dentistry. Carbohydr. Polym. 2019, 207, 1–10. [Google Scholar] [CrossRef]
- Wanga, B.; Lv, X.; Chen, S.; Lia, Z.; Yao, J.; Peng, X.; Feng, C.; Xu, C.; Wang, H. Use of heparinized bacterial cellulose based scaffold for improving angiogenesis in tissue regeneration. Carbohydr. Polym. 2018, 181, 948–956. [Google Scholar] [CrossRef]
- Esguerra, M.; Fink, H.; Laschke, M.W.; Jeppsson, A.; Delbro, D.; Gatenholm, P.; Menger, M.D.; Risberg, B. Intravital fluorescent microscopic evaluation of bacterial cellulose as scaffold for vascular grafts. J. Biomed. Mater. Res. Part A 2010, 93A, 140–149. [Google Scholar] [CrossRef]
- Hoshi, T.; Suzuki, M.; Ishikawa, M.; Endo, M.; Aoyagi, T. Encapsulation of micro- and milli-sized particles with a hollow-type spherical bacterial cellulose gel via particle-preloaded droplet cultivation. Int. J. Mol. Sci. 2019, 20, 4919. [Google Scholar] [CrossRef]
- Hestrin, S.; Schramm, M. Synthesis of cellulose by Acetobacter xylinum. 2. Preparation of freeze-dried cells capable of polymerizing glucose to cellulose. Biochem. J. 1954, 58, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Niwa, T.; Ise, M.; Miyazaki, T. Progression of glomerular sclerosis in experimental uremic rats by administration of indole, a precursor of indoxyl sulfate. Am. J. Nephrol. 1994, 14, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Buchtová, N.; Budtova, T. Cellulose aero-, cryo- and xerogels: Towards understanding of morphology control. Cellulose 2016, 23, 2585–2595. [Google Scholar] [CrossRef]
- Lagergren, S. Zur theorie der sogenannten adsorption gelöster stoffe. K. Sven. Vetensk. Handl. 1898, 24, 1–39. [Google Scholar]
- Ho., Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Kuroki, V.; Bosco, G.E.; Fadini, P.S.; Mozeto, A.A.; Cestart, A.R.; Carvalho, W.A. Use of a La(III)-modified bentonite for effective phosphate removal from aqueous media. J. Hazard. Mater. 2014, 274, 124–131. [Google Scholar] [CrossRef]
- Zhong, Z.; Yu, G.; Mo, W.; Zhang, C.; Huang, H.; Li, S.; Gao, M.; Lu, X.; Zhang, B.; Zhu, H. Enhanced phosphate sequestration by Fe(III) modified biochar derived from coconut shell. RSC Adv. 2019, 9, 10425–10436. [Google Scholar] [CrossRef]
- Ji, K.; Wang, W.; Zeng, B.; Chen, S.; Zhao, Q.; Chen, Y.; Li, G.; Ma, T. Bacterial cellulose synthesis mechanism of facultative anaerobe Enterobacter sp. FY-07. Sci. Rep. 2016, 6, 21863. [Google Scholar] [CrossRef]
- Yue, Y.; Han, G.; Wu, Q. Transitional Properties of Cotton Fibers from Cellulose I to Cellulose II Structure. BioResources 2013, 8, 6460–6471. [Google Scholar] [CrossRef]
- KREMEZIN® Tablets 500 mg. 2018 Interview Form; KUREHA Corporation: Tokyo, Japan, 2018; Available online: https://medical.mt-pharma.co.jp/di/file/if/f_krm_c.pdf (accessed on 27 September 2020).
- Mall, I.D.; Srivastava, V.C.; Agarwal, N.K.; Mishra, I.M. Adsorptive removal of malachite green dye from aqueous solution by bagasse fly ash and activated carbon-kinetic study and equilibrium isotherm analyses. Coll. Surf. A 2005, 264, 17–28. [Google Scholar] [CrossRef]
- Koubaissy, B.; Toufaily, J.; Yaseen, Z.; Daou, T.J.; Jradi, S.; Hamieh, T. Adsorption of uremic toxins over dealuminated zeolites. Adsorp. Sci. Technol. 2017, 35, 3–19. [Google Scholar] [CrossRef]
- Simonin, J.-P. On the compassion of pseudo-first order and pseudo-second order rate laws in the modeling od adsorption kinetics. Chem. Eng. J. 2016, 15, 254–263. [Google Scholar]
- He, S.; Han, C.; Wang, H.; Zhu, W.; He, S.; He, D.; Luo, Y. Uptake of arsenic(V) using alumina functionalized highly ordered mesoporous SBA-15(Alx-SBA-15) as an effective adsorbent. J. Chem. Eng. Data 2015, 60, 1300–1310. [Google Scholar] [CrossRef]
- Payne, J.M.; Sansom, B.F.; Garner, R.J.; Thomson, A.R.; Miles, B.J. Uptake of small resin particles (1–5 µ diameter) by the alimentary canal of the calf. Nature 1960, 188, 586–587. [Google Scholar] [CrossRef] [PubMed]

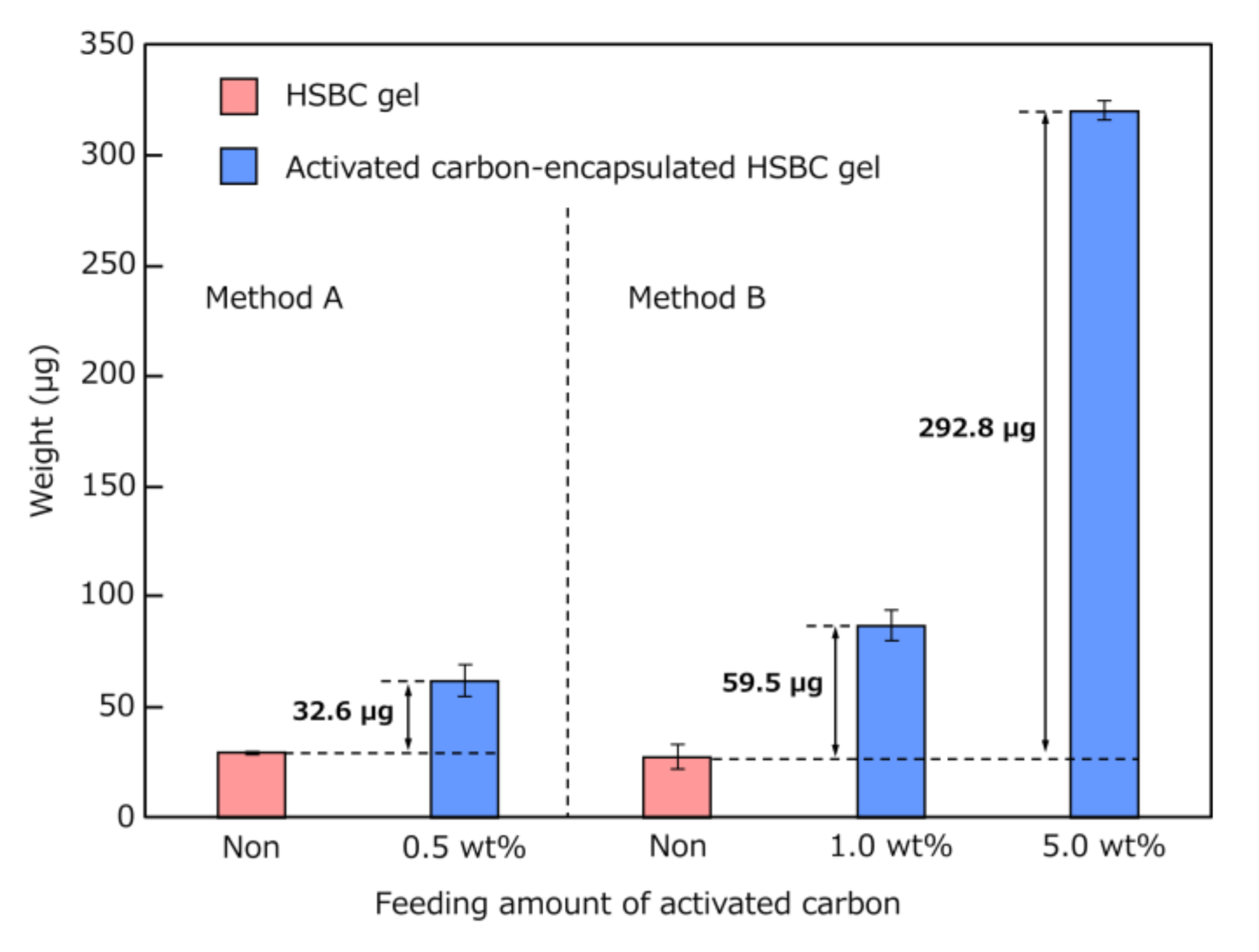
| Activated Carbon | Dropwise Volume | Encapsulated Activated Carbon | |
|---|---|---|---|
| (wt%) | (µL) | Calculated (µg) | Measured (µg) |
| 0.5 (Method A) | 10.0 * | 50.0 | 32.6 |
| 1.0 (Method B) | 5.0 ** | 50.0 | 59.5 |
| 5.0 (Method B) | 5.0 ** | 250.0 | 292.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoshi, T.; Endo, M.; Hirai, A.; Suzuki, M.; Aoyagi, T. Encapsulation of Activated Carbon into a Hollow-Type Spherical Bacterial Cellulose Gel and Its Indole-Adsorption Ability Aimed at Kidney Failure Treatment. Pharmaceutics 2020, 12, 1076. https://doi.org/10.3390/pharmaceutics12111076
Hoshi T, Endo M, Hirai A, Suzuki M, Aoyagi T. Encapsulation of Activated Carbon into a Hollow-Type Spherical Bacterial Cellulose Gel and Its Indole-Adsorption Ability Aimed at Kidney Failure Treatment. Pharmaceutics. 2020; 12(11):1076. https://doi.org/10.3390/pharmaceutics12111076
Chicago/Turabian StyleHoshi, Toru, Masahito Endo, Aya Hirai, Masashige Suzuki, and Takao Aoyagi. 2020. "Encapsulation of Activated Carbon into a Hollow-Type Spherical Bacterial Cellulose Gel and Its Indole-Adsorption Ability Aimed at Kidney Failure Treatment" Pharmaceutics 12, no. 11: 1076. https://doi.org/10.3390/pharmaceutics12111076
APA StyleHoshi, T., Endo, M., Hirai, A., Suzuki, M., & Aoyagi, T. (2020). Encapsulation of Activated Carbon into a Hollow-Type Spherical Bacterial Cellulose Gel and Its Indole-Adsorption Ability Aimed at Kidney Failure Treatment. Pharmaceutics, 12(11), 1076. https://doi.org/10.3390/pharmaceutics12111076