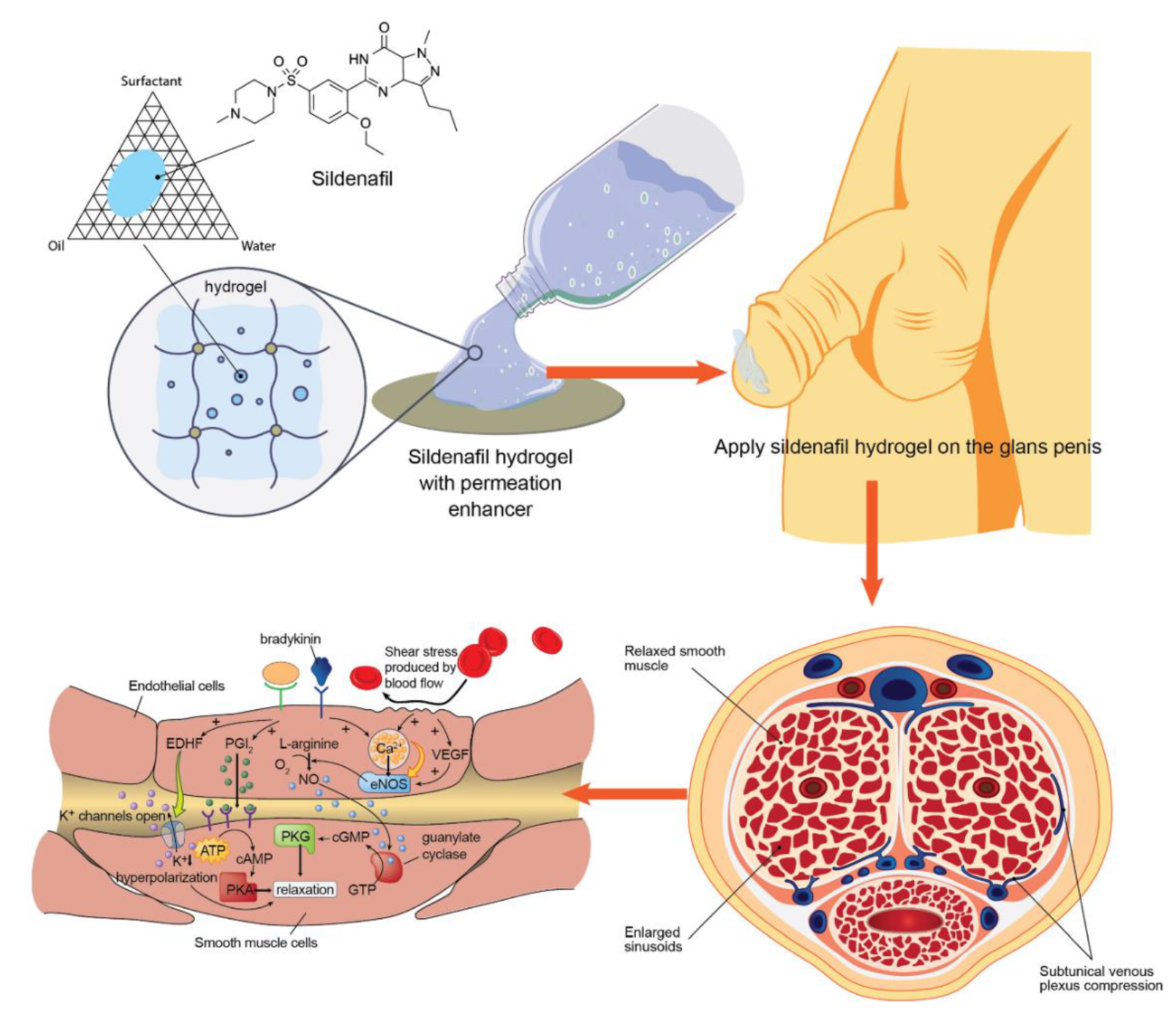
Development of a Sildenafil Citrate Microemulsion-Loaded Hydrogel as a Potential System for Drug Delivery to the Penis and Its Cellular Metabolic Mechanism
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Solubility Studies
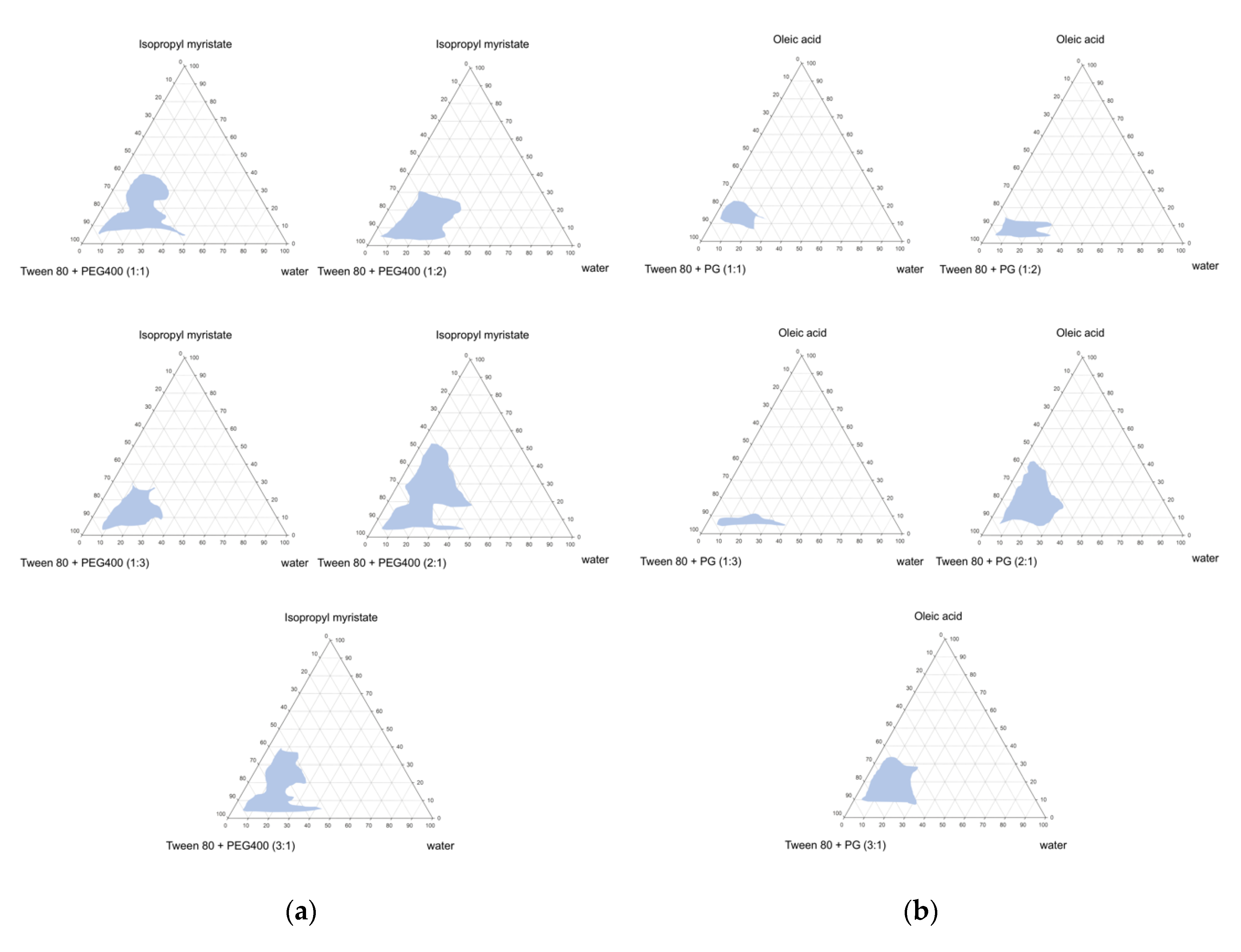
2.3. Construction of Pseudo-Ternary Phase Diagram
2.4. Preparation of the Sildenafil Citrate Microemulsion-Loaded Hydrogel
2.5. Physical Properties of the Sildenafil Citrate Microemulsion-Loaded Hydrogel
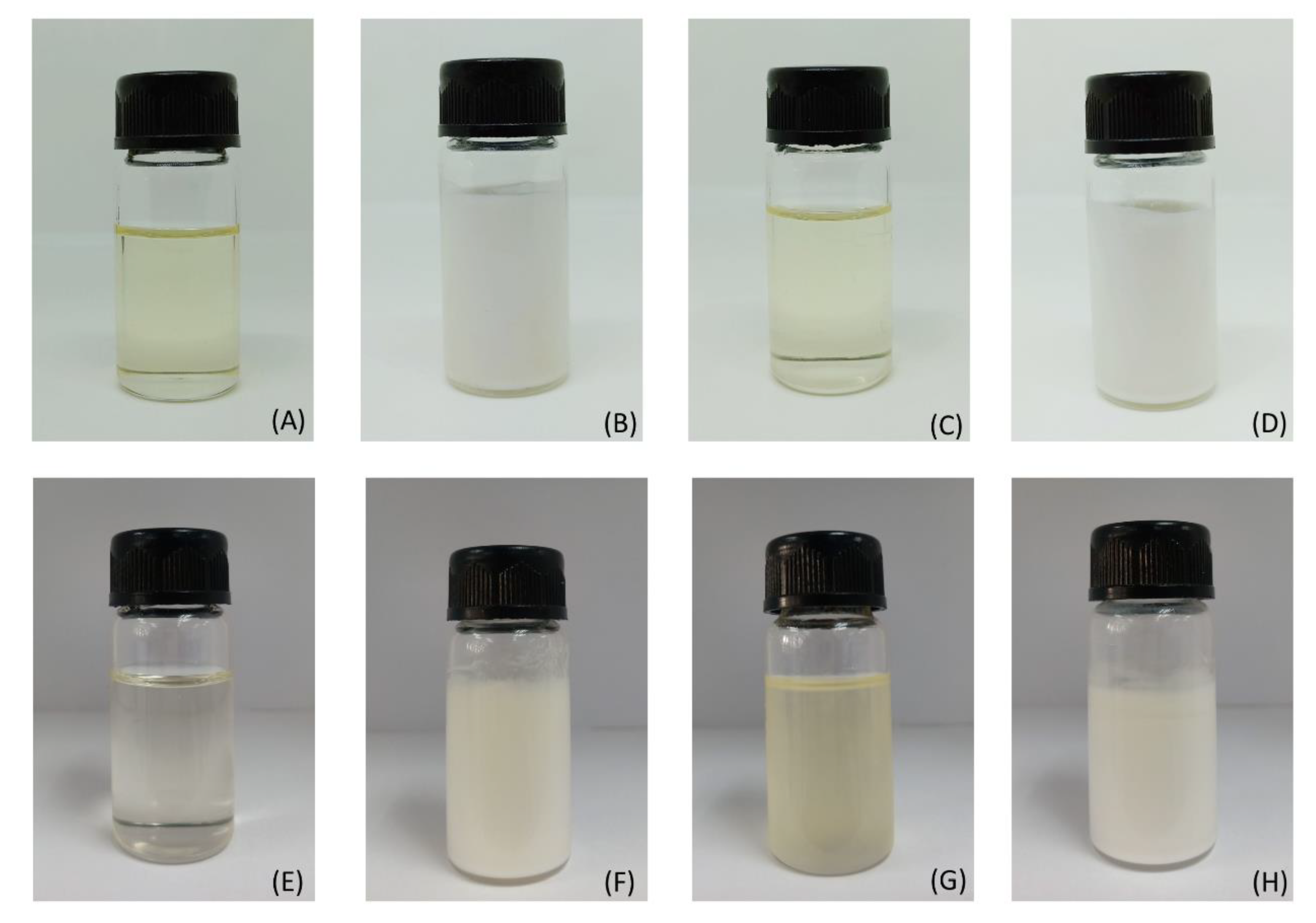
2.5.1. Physical Appearance and Clarity
2.5.2. Determination of Droplet Size
2.5.3. pH Determination
2.5.4. Spreadability Test
2.5.5. Swelling Index
2.6. Viscosity and Viscoelastic Measurements
2.7. Fourier Transform Infrared Spectroscopy (FTIR)
2.8. Sildenafil Citrate Content in the Microemulsion-Loaded Hydrogel
2.9. Stability Studies
2.10. Cell Viability Assay
2.11. Skin Permeation Studies Using Franz Diffusion Cells
2.12. In Vitro Drug Metabolism
2.12.1. Cell Culture Condition
2.12.2. Liquid-Liquid Extraction Procedure
2.12.3. Determination of Sildenafil and Its Metabolite Using Liquid Chromatography with Tandem Mass Spectrometry
2.13. Statistical Analysis
3. Results and Discussion
3.1. Microemulsion Systems
3.2. Sildenafil Citrate Microemulsion-Loaded Hydrogel
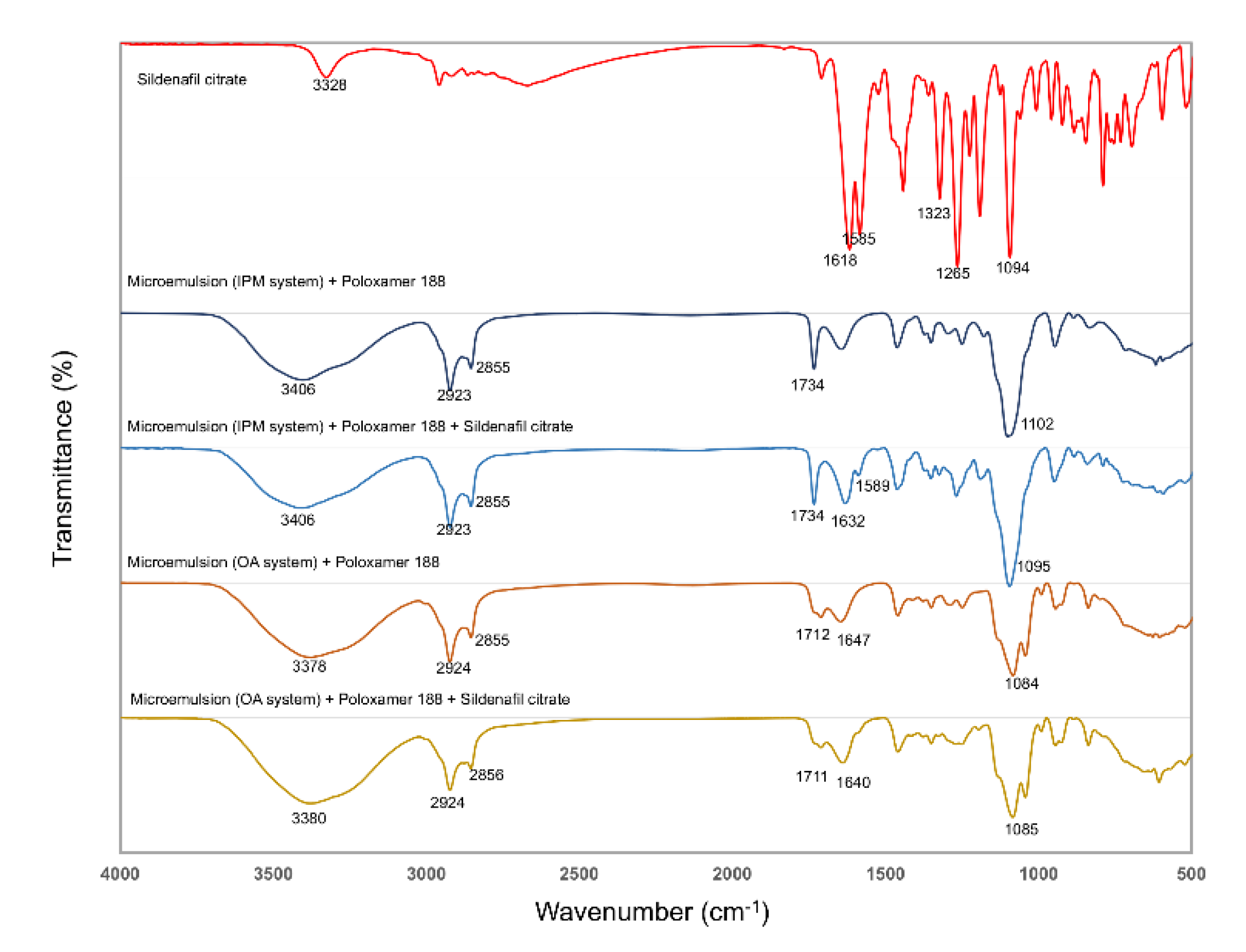
3.3. FTIR Analysis
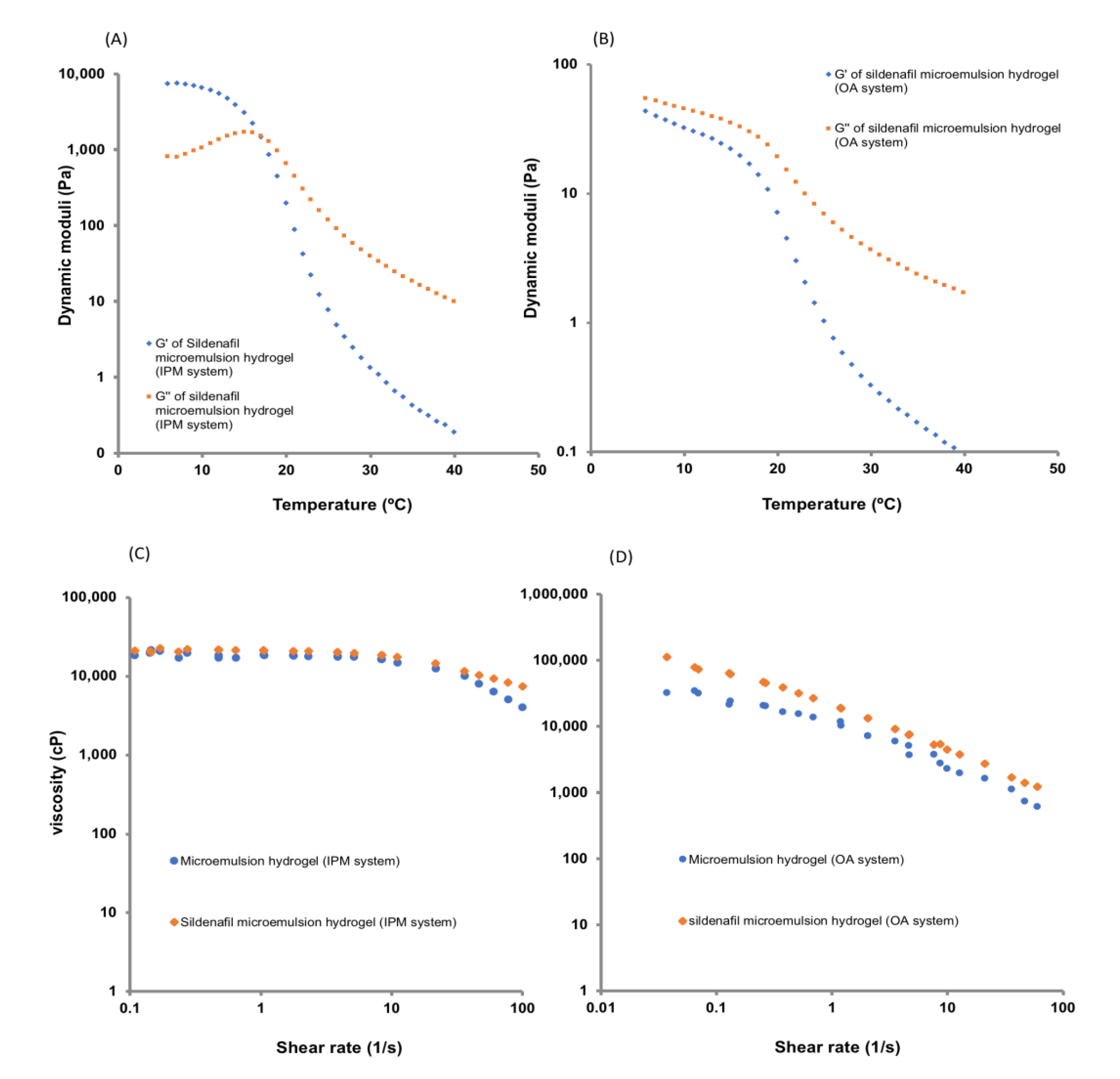
3.4. Rheological Analysis
3.5. Analytical Validation of Sildenafil Citrate in the Formulation
3.6. Stability
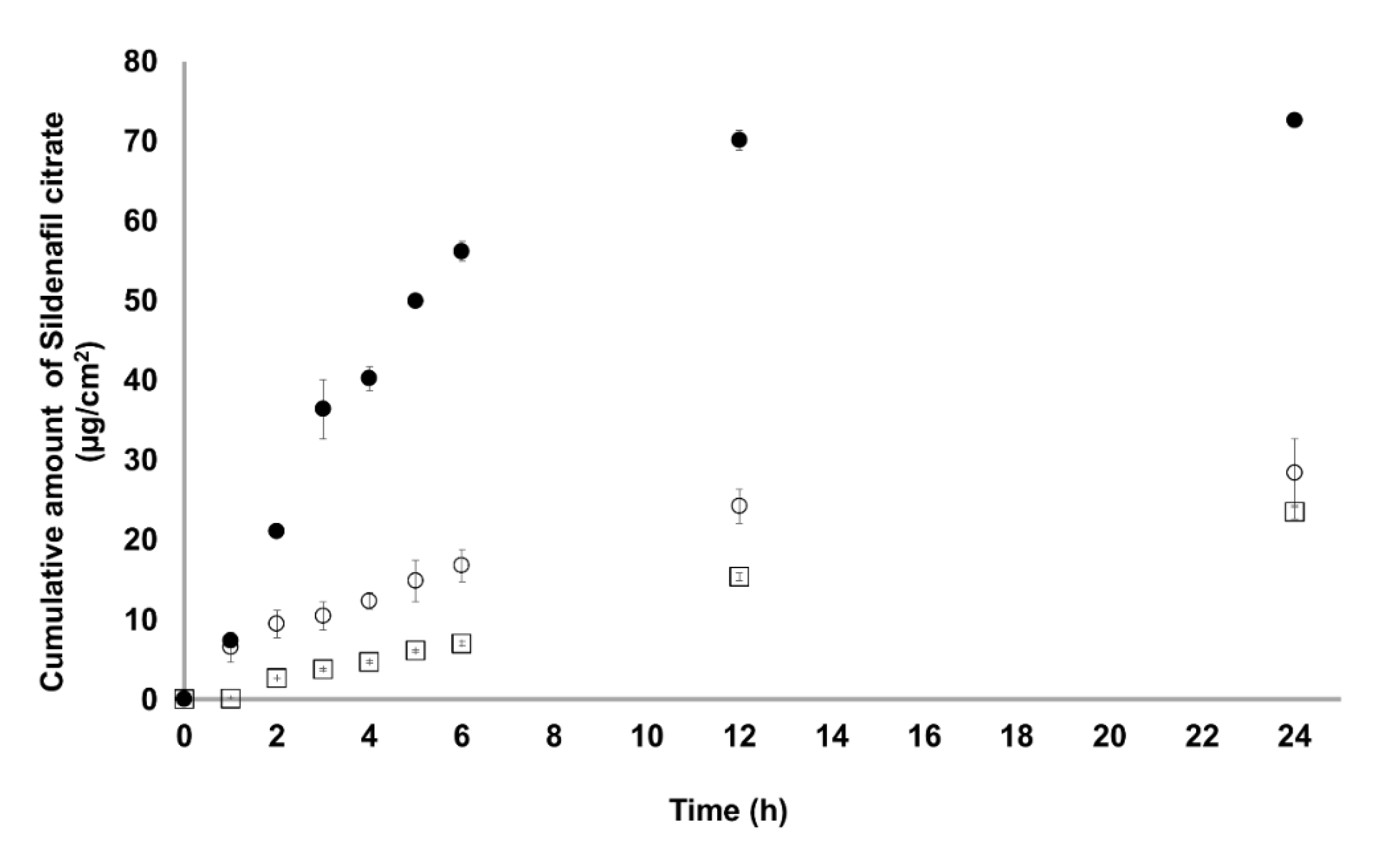
3.7. In Vitro Skin Permeation Studies
3.8. Cytotoxicity of the Sildenafil Citrate Microemulsion-Loaded Hydrogel to Human Skin Epithelial Cells (BJ Cells)
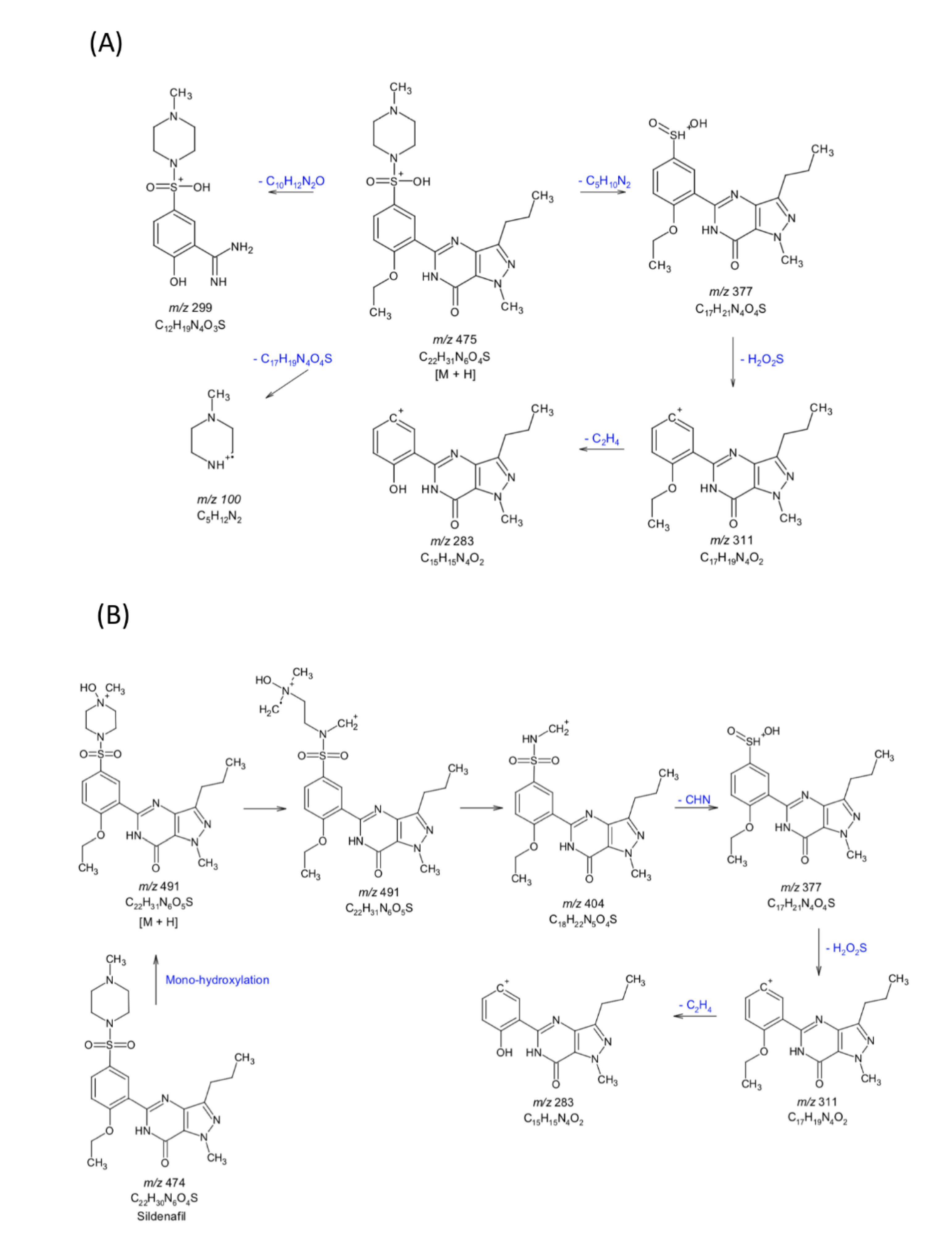
3.9. Drug Metabolism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| PDE5 | phosphodiesterase type 5 |
| EDHF | endothelium derived hyperpolarizing factor |
| PGI2 | prostacyclin |
| cAMP | cyclic adenosine monophosphate |
| cGMP | cyclic guanosine monophosphate |
| NO | nitric oxide |
| eNOS | endothelial nitric oxide synthase |
| VEGF | vascular endothelial growth factor |
| PKA | protein kinase A |
| PKG | protein kinase G |
| PEG400 | polyethylene glycol 400 |
| HPLC | high-performance liquid chromatography |
| FTIR | Fourier transform infrared spectroscopy |
| FBS | foetal bovine serum |
| MTT | 3-(4,5-dimethylthiazolyl-2-yl)-diphenyl-tetrazolium bromide |
| RH | relative humidity |
| LC-MS/MS | liquid chromatography with tandem mass spectrometry |
References
- Elnaggar, Y.S.R.; El-Massik, M.A.; Abdallah, O.Y. Fabrication, appraisal, and transdermal permeation of sildenafil citrate-loaded nanostructured lipid carriers versus solid lipid nanoparticles. Int. J. Nanomed. 2011, 6, 3196–3205. [Google Scholar] [CrossRef] [PubMed]
- Atan, A.; Basa, M.M.; Tuncel, A.; Ferhat, M.; Agras, K.; Tekdogan, U. Comparison of efficacy of sildenafil-only, sildenafil plus topical emla cream, and topical emla-cream-only in treatment of premature ejaculation. Urology 2006, 67, 388–391. [Google Scholar] [CrossRef] [PubMed]
- Yonessi, M.; Saeedi, M. A double-blind placebo-controlled evaluation of the effect of topical sildenafil on erectile dysfunction. J. Appl. Res. 2005, 5, 289–294. [Google Scholar]
- Baek, J.; Cho, C. Transdermal delivery of tadalafil using a novel formulation. Drug Deliv. 2016, 23, 1571–1577. [Google Scholar] [CrossRef]
- Kulshrestha, S.; Chawla, R.; Alam, M.T.; Adhikari, J.S.; Basu, M. Efficacy and dermal toxicity analysis of sildenafil citrate based topical hydrogel formulation against traumatic wounds. Biomed. Pharmacother. 2019, 112, 108571. [Google Scholar] [CrossRef]
- Osman, S.K. Pharmaceutical studies on the availability of sildenafil citrate from certain topical delivery systems. Int. J. Pharm. Pharm. Res. 2016, 7, 199–221. [Google Scholar]
- Patel, H.; Panchai, M.S.; Shah, S.; Vadalia, K.R. Formulation and evaluation of transdermal gel of sildenafil citrate. Int. J. Pharm. Res. Allied Sci. 2012, 1, 102–118. [Google Scholar]
- Rosenthal, B.D.; May, N.R.; Metro, M.J.; Harkaway, R.C.; Ginberg, P.C. Adjunctive use of androgel (testosterone gel) with sildenafil o treat erectile dysfunction in men with acquired androgen deficiency syndrome after failure using sildenafil alone. Urology 2006, 67, 571–574. [Google Scholar] [CrossRef] [PubMed]
- Elshafeey, A.H.; Bendas, E.R.; Mohamed, O.H. Intranasal microemulsion of sildenafil citrate: In vitro evaluation and in vivo pharmacokinetic study in rabbits. AAPS Pharmscitech 2009, 10, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.; Maniyar, A.; Patel, H. Use of transdermal gel of sildenafil citrate in sexual dysfunction. Int. J. Pharm. Sci. Res. 2012, 3, 4131–4134. [Google Scholar]
- Meena, A.K.; Sharma, K.; Kandaswamy, M.; Rajagopal, S.; Mullangi, R. Formulation development of an albendazole self-emulsifying drug delivery system (SEDDS) with enhanced systemic exposure. Acta Pharm. 2012, 62, 563–580. [Google Scholar] [PubMed]
- Yadev, V.; Jadhav, P.; Kanase, K.; Bodhe, A.; Dombe, S. Preparation and evaluation of microemulsion containg antihypertensive drug. Int. J. Appl. Pharm 2018, 10, 138–146. [Google Scholar] [CrossRef]
- Biswa, G.R.; Majee, S.B.; Roy, A. Combination of synthetic and natural polymers in hydrogel: An impact on drug permeation. J. Appl. Pharm. 2016, 6, 158–164. [Google Scholar] [CrossRef][Green Version]
- Biswal, B.; Karna, N.; Nayak, J.; Joshi, V. Formulation and evaluation of microemulsion based topical hydrogel containing lornoxicam. J. Appl. Pharm. 2014, 4, 77–84. [Google Scholar]
- Boonlai, W.; Tantishaiyakul, V.; Hirun, N.; Sangfai, T.; Suknuntha, K. Thermosensitive poloxamer407/poly(acrylic acid) hydrogels with potential application as injectable drug delivery system. AAPS Pharmscitech 2018, 19, 2103–2117. [Google Scholar] [CrossRef]
- Sawatdee, S.; Phetmung, H.; Srichana, T. Sildenafil citrate monohydrate-cyclodextrin nanosuspension complexes for use in metered-dose inhalers. Int. J. Pharm. 2013, 455, 248–258. [Google Scholar] [CrossRef]
- Sawatdee, S.; Atipairin, A.; Sae Yoon, A.; Srichana, T.; Changsan, N. Enhanced dissolution of sildenafil citrate as dry foam tablets. Pharm. Dev. Technol. 2019, 24, 1–11. [Google Scholar] [CrossRef]
- Asean Guideline on Stability Study of Drug Product. Available online: https://asean.org/wp-content/uploads/2018/01/25PPWG-ANNEX-7-iv-Final-ASEAN-Guideline-on-Stability-Study-Drug-Product-R2.pdf (accessed on 7 April 2018).
- Badr-Eldin, S.M.; Ahmed, O.A.A. Optimixed nano-transfersomal films for enhanced sildenafil citrate transdermal delivery: Ex vivo and in vivo evaluation. Drug Des. Dev. Ther. 2016, 10, 1323–1333. [Google Scholar] [CrossRef]
- Serrano, D.R.; Gordo, M.J.; Matji, A.; González, S.; Lalatsa, A.; Torrado, J.J. Tuning the transdermal delivery of hydroquinone upon formulation with novel permeation enhancers. Pharmaceutics 2019, 11, 167. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Chen, R.; Sheu, M.; Chang, C.; Chou, P.; Ho, H. Rapid-onset sildenafil nasal spray carried by microemulsion systems: In vitro evaluation and in vivo pharmacokinetic studies in rabbits. Xenobiotica 2011, 41, 567–577. [Google Scholar] [CrossRef]
- Jung, S.; Seo, Y.; Kim, G.; Woo, J.; Yong, C.; Choi, H. Comparison of the solubility and pharmacokinetics of sildenafil salts. Arch. Pharm. Res. 2011, 34, 451–454. [Google Scholar] [CrossRef]
- Elnaggar, Y.S.R.; El-massik, M.A.; Abdallah, O.Y. Sildenfil citrate nanoemulsion vs. self-nanoemulsifying delivery systems; rational development and transdermal permeation. Int. J. Nanotechnol. 2011, 8, 749–763. [Google Scholar] [CrossRef]
- Haq, A.; Michniak-Kohn, B. Effects of solvents and penetration enhancers on transdermal delivery of thymoquinone: Permeability and skin deposition study. Drug Deliv. 2018, 25, 1943–1949. [Google Scholar] [CrossRef]
- Melnikov, P.; Corbi, P.P.; Cuin, A.; Cavicchioli, M.; Guimarães, W.R. Physical properties of sildenafil citrate (Viagra) and sildenafil base. J. Pharm. Sci. 2003, 92, 2140–2143. [Google Scholar] [CrossRef] [PubMed]
- Sawatdee, S.; Pakawatchai, C.; Nitichai, K.; Srichana, T.; Phetmung, H. Why sildenafil and sildenafil citrate monohydrate crystals are not stable? Saudi Pharm. J. 2015, 23, 504–514. [Google Scholar] [CrossRef] [PubMed]
- Pavia, D.L.; Lampman, G.M.; Kriz, G.S. Introduction to Spectroscopy: A Guide for Students of Organic Chemistry, 3rd ed.; Thomson Learning: Boston, MA, USA, 2001. [Google Scholar]
- Russo, E.; Villa, C. Poloxamer hydrogels for biomedical applications. Pharmaceutics 2019, 11, 671. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, R.; Li, L.; Li, B.; Zhang, X.; Su, J. Mechanical, rheological and release behaviors of a poloxamer 407/poloxamer 188/Carbopol 940 thermosensitive composite hydrogel. Molecules 2013, 18, 12415–12425. [Google Scholar] [CrossRef]
- Chen, H.; Chang, X.; Du, D.; Li, J.; Xu, H.; Yang, X. Microemulsion-based hydrogel formulation of ibuprofen for topical delivery. Int. J. Pharm. 2006, 315, 52–58. [Google Scholar] [CrossRef]
- Prasanthi, D.; Lakshmi, P.K. Effect of chemical enhancers in transdermal permeation of alfuzosin hydrochloride. Int. Sch. Res. Netw. ISRN Pharm. 2012, 2012, 965280. [Google Scholar] [CrossRef][Green Version]
- Badwin, A.A.; Nabulsi, L.; Al-Omari, M.M.; Daraghmeh, N.; Ashour, M. Sildenafil citrate. Anal. Profiles Drug Subst. 2001, 27, 339–376. [Google Scholar]
| Ingredients | Formulations and Amount (g) | |
|---|---|---|
| Isopropyl Myristate-Based System | Oleic Acid-Based System | |
| Sildenafil citrate | 0.24 | 0.24 |
| Isopropyl myristate | 0.60 | - |
| Oleic acid | - | 0.44 |
| Tween 80 | 0.80 | 0.87 |
| Polyethylene glycol 400 | 0.40 | - |
| Propylene glycol | - | 0.43 |
| Purified water | 0.20 | 0.26 |
| Poloxamer 188 (10% solution) | 0.50 | 0.50 |
| Total weight | 2.74 | 2.74 |
| Component | Solvent | Saturated Solubility (mg/mL) |
|---|---|---|
| Oils | Oleic acid | 7.63 ± 0.48 |
| Isopropyl myristate | 4.21 ± 0.65 | |
| Palm oil | 3.65 ± 0.19 | |
| Sesame oil | 3.16 ± 0.73 | |
| Olive oil | 2.54 ± 0.22 | |
| Castor oil | 1.89 ± 0.62 | |
| Surfactants | Tween 80 | 12.14 ± 0.45 |
| Cremophore RH40 | 10.21 ± 0.32 | |
| Tween 40 | 8.15 ± 0.13 | |
| Tween 20 | 7.22 ± 0.43 | |
| Co-surfactants | PEG400 | 5.16 ± 0.22 |
| Propylene glycol | 4.10 ± 0.58 | |
| Transcutol | 3.88 ± 0.15 | |
| Water | Distilled water | 3.20 ± 0.11–4.1 ± 1.3 * |
| Composition of Microemulsion Systems (%) | Droplet Size (nm) | Solubility of Sildenafil Citrate (mg/mL) | ||||
|---|---|---|---|---|---|---|
| Isopropyl Myristate | Tween 80/PEG 400 | Oleic Acid | Tween 80/Propylene Glycol | Purified Water | ||
| 30 | 25:25 (1:1) | - | - | 20 | 35 ± 10 | 72 ± 12 |
| 20 | 20:40 (1:2) | - | - | 20 | 62 ± 12 | 58 ± 4 |
| 20 | 16:49 (1:3) | - | - | 15 | 45 ± 8 | 82 ± 16 |
| 30 | 40:20 (2:1) | - | - | 10 | 30 ± 6 | 122 ± 28 |
| 20 | 49:16 (3:1) | - | - | 15 | 110 ± 20 | 108 ± 14 |
| - | - | 15 | 37.5:37.5 (1:1) | 10 | 593 ± 126 | 108 ± 12 |
| - | - | 10 | 25:50 (1:2) | 15 | 385 ± 81 | 103 ± 9 |
| - | - | 8 | 18:54 (1:3) | 20 | 600 ± 125 | 95 ± 24 |
| - | - | 22 | 43:22 (2:1) | 13 | 320 ± 45 | 128 ± 8 |
| - | - | 15 | 52.5:17.5 (3:1) | 15 | 465 ± 114 | 112 ± 16 |
| Test | Sildenafil Citrate Microemulsion | Sildenafil Citrate Microemulsion-Loaded Hydrogel | ||
|---|---|---|---|---|
| (IPM System) | (OA System) | (IPM System) | (OA System) | |
| Appearance | White liquid | White liquid | White liquid | White liquid |
| Clarity | Not clear | Not clear | Not clear | Not clear |
| Droplet size (nm) | 450 ± 120 | 525 ± 45 | 461 ± 108 | 619 ± 68 |
| pH | 5.14 ± 0.02 | 4.54 ± 0.03 | 5.27 ± 0.04 | 4.67 ± 0.07 |
| Viscosity (mPa s) | 4569 ± 76 | 1256 ± 55 | 11,997 ± 1465 | 3231 ± 69 |
| Spreadability (g cm s−1) | 12.28 ± 2.35 | 17.65 ± 1.23 | 16.44 ± 2.12 | 19.45 ± 1.62 |
| Swelling index (%) | - | - | 11.0 ± 1.1 | 14.5 ± 1.2 |
| Sample | Frequency (cm−1) | Interpretation | Compound class |
|---|---|---|---|
| Sildenafil citrate | 3328 | N–H stretch | |
| 1618 | N–H bend | ||
| 1585 | Carboxylic acid | (citrate) | |
| 1265 | SO2 | (symmetric) | |
| 1323 | SO2 | (asymmetric) | |
| 1094 | C-N | ||
| Microemulsion (IPM system) + poloxamer 188 | 3406 | OH | (H2O), poloxamer, PEG400 |
| 2923, 2855 | C–H alkane | ||
| 1734 | COO–R | ||
| 1102 | C–O | ||
| Microemulsion (IPM system) + poloxamer 188 + Sildenafil citrate | 3406 | OH | |
| 2923, 2855 | C–H alkane | ||
| 1734 | COO–R | ||
| 1632 | N–H bend | (sildenafil) | |
| 1589 | Carboxylic acid | (citrate) | |
| Microemulsion (OA system) + poloxamer 188 | 3378 | OH | (H2O), poloxamer, PG |
| 2924, 2855 | C–H alkane | ||
| 1712 | COOH | (oleic acid) | |
| 1647 | C=C alkene | (oleic acid) | |
| 1102 | C–O | ||
| Microemulsion (OA system) + poloxamer 188 + Sildenafil citrate | 3380 | OH | (H2O), poloxamer, PG |
| 2924, 2856 | C–H alkane | ||
| 1711 | COOH | ||
| 1640 | N–H bend | (sildenafil) | |
| 1085 | C–O |
| Formulation | Test | Storage Conditions | ||||||
|---|---|---|---|---|---|---|---|---|
| Initial | 30 °C/75% RH | 40 °C/75% RH | ||||||
| 1 Month | 3 Month | 6 Month | 1 Month | 3 Month | 6 Month | |||
| Sildenafil microemulsion (IPM system) | Appearance | White colour liquid | White colour liquid | White colour liquid | White colour liquid | White colour liquid | White colour liquid | White colour liquid |
| Assay sildenafil (%) | 104.5 ± 0.001 | 107.4 ± 0.28 | 106.5 ± 0.11 | 105.4 ± 0.13 | 99.1 ± 0.19 | 102.3 ± 0.25 | 101.1 ± 0.34 | |
| pH | 5.27 ± 0.04 | 4.91 ± 0.06 | 5.02 ± 0.00 | 5.07 ± 0.02 | 4.79 ± 0.06 | 4.89 ± 0.03 | 4.97 ± 0.02 | |
| Viscosity (m Pa s) | 11,997 ± 1465 | 7909 ± 1988 | 10,025 ± 1222 | 9923 ± 2456 | 10,035 ± 465 | 12,214 ± 1165 | 11,234 ± 2086 | |
| Sildenafil microemulsion (OA system) | Appearance | White colour liquid | White colour liquid | White colour liquid | Phase separation | Phase separation | Phase separation | Phase separation |
| Assay sildenafil (%) | 97.0 ± 0.002 | 112.8 ± 1.26 | 105.2 ± 2.18 | 108.6 ± 4.52 | 112.7 ± 3.58 | NA | NA | |
| pH | 4.67 ± 0.07 | 4.73 ± 0.03 | 4.66 ± 0.02 | 4.55 ± 0.02 | 4.45 ± 0.04 | 4.65 ± 0.03 | 4.58 ± 0.02 | |
| Viscosity (m Pa s) | 3231 ± 69 | 3372 ± 189 | 3344 ± 256 | 3312 ± 175 | 3605 ± 70 | NA | NA | |
| Parameter | Sildenafil Microemulsion (IPM System) | Sildenafil Microemulsion (OA System) | Sildenafil Citrate Suspension |
|---|---|---|---|
| Cumulative amount at 24 h (μg/cm2) | 60.54 ± 0.62 | 28.42 ± 4.31 | 23.47 ± 0.86 |
| Steady-stat flux (Jss) (μg cm−2 h−1) | 2.11 ± 0.00 * | 0.98 ± 0.24 | 1.07 ± 0.18 |
| Permeability coefficient (Pc) (cm h−1) | 10.76 × 10−5 * | 5.03 × 10−5 | 10.47 × 10−5 |
| Diffusion coefficient (D) (cm2 h−1) | 1.07 × 10−6 * | 5.62 × 10−6 | 7.59 × 10−6 |
| Enhancement ratio (ER) | 1.97 | 0.92 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atipairin, A.; Chunhachaichana, C.; Nakpheng, T.; Changsan, N.; Srichana, T.; Sawatdee, S. Development of a Sildenafil Citrate Microemulsion-Loaded Hydrogel as a Potential System for Drug Delivery to the Penis and Its Cellular Metabolic Mechanism. Pharmaceutics 2020, 12, 1055. https://doi.org/10.3390/pharmaceutics12111055
Atipairin A, Chunhachaichana C, Nakpheng T, Changsan N, Srichana T, Sawatdee S. Development of a Sildenafil Citrate Microemulsion-Loaded Hydrogel as a Potential System for Drug Delivery to the Penis and Its Cellular Metabolic Mechanism. Pharmaceutics. 2020; 12(11):1055. https://doi.org/10.3390/pharmaceutics12111055
Chicago/Turabian StyleAtipairin, Apichart, Charisopon Chunhachaichana, Titpawan Nakpheng, Narumon Changsan, Teerapol Srichana, and Somchai Sawatdee. 2020. "Development of a Sildenafil Citrate Microemulsion-Loaded Hydrogel as a Potential System for Drug Delivery to the Penis and Its Cellular Metabolic Mechanism" Pharmaceutics 12, no. 11: 1055. https://doi.org/10.3390/pharmaceutics12111055
APA StyleAtipairin, A., Chunhachaichana, C., Nakpheng, T., Changsan, N., Srichana, T., & Sawatdee, S. (2020). Development of a Sildenafil Citrate Microemulsion-Loaded Hydrogel as a Potential System for Drug Delivery to the Penis and Its Cellular Metabolic Mechanism. Pharmaceutics, 12(11), 1055. https://doi.org/10.3390/pharmaceutics12111055






