The Design of Anionic Surfactant-Based Amino-Functionalized Mesoporous Silica Nanoparticles and their Application in Transdermal Drug Delivery
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
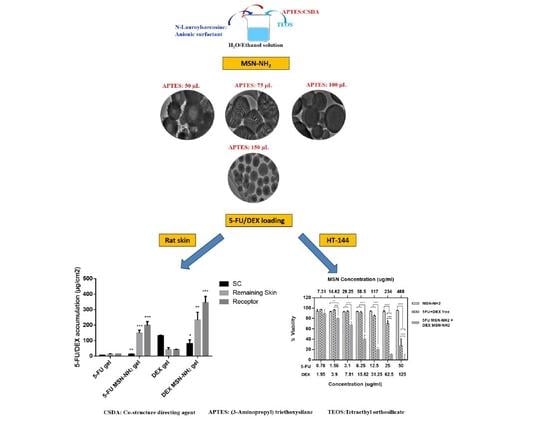
2.2. Synthesis of MSN-NH2
2.3. Characterization of the MSN-NH2
2.4. Preparation of 5-FU or DEX (5-FU/DEX) MSN-NH2
2.5. Characterization of 5-FU/DEX MSN-NH2
2.6. In Vitro Drug Release Study
2.7. Ex Vivo Skin Permeation Study
2.8. In Vitro Cytotoxicity Assay
2.9. Statistical Data Analysis
3. Results and Discussion
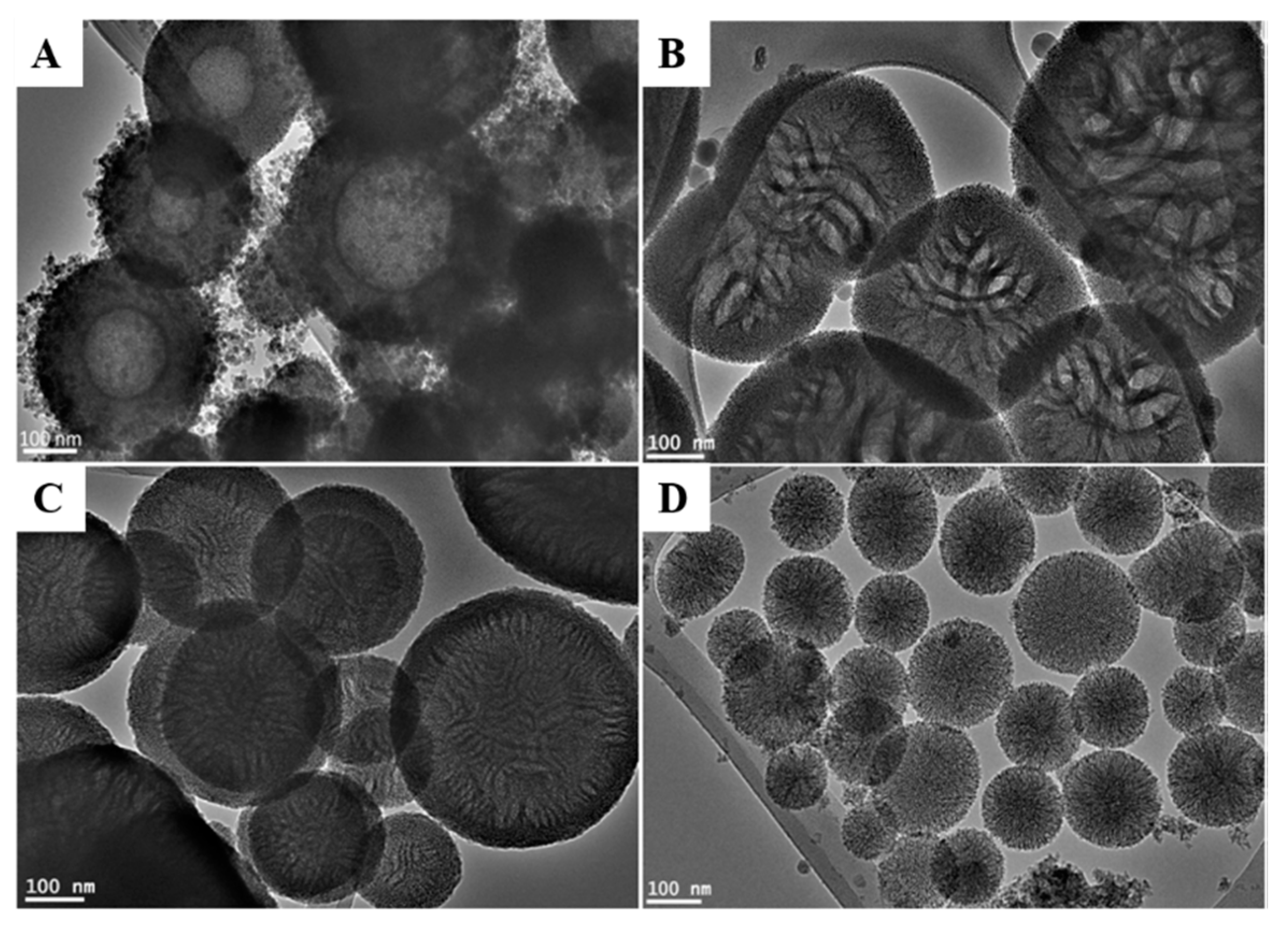
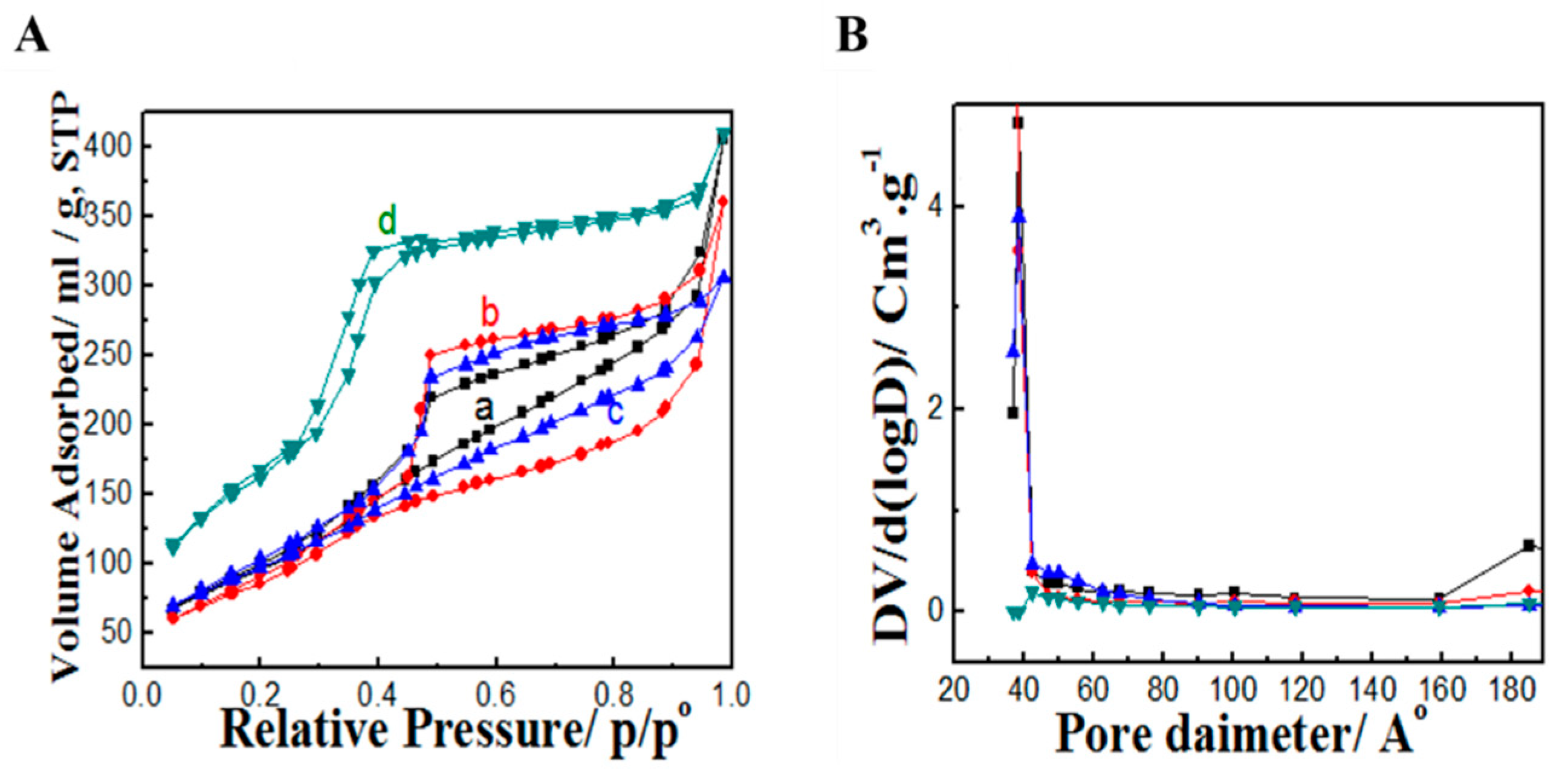
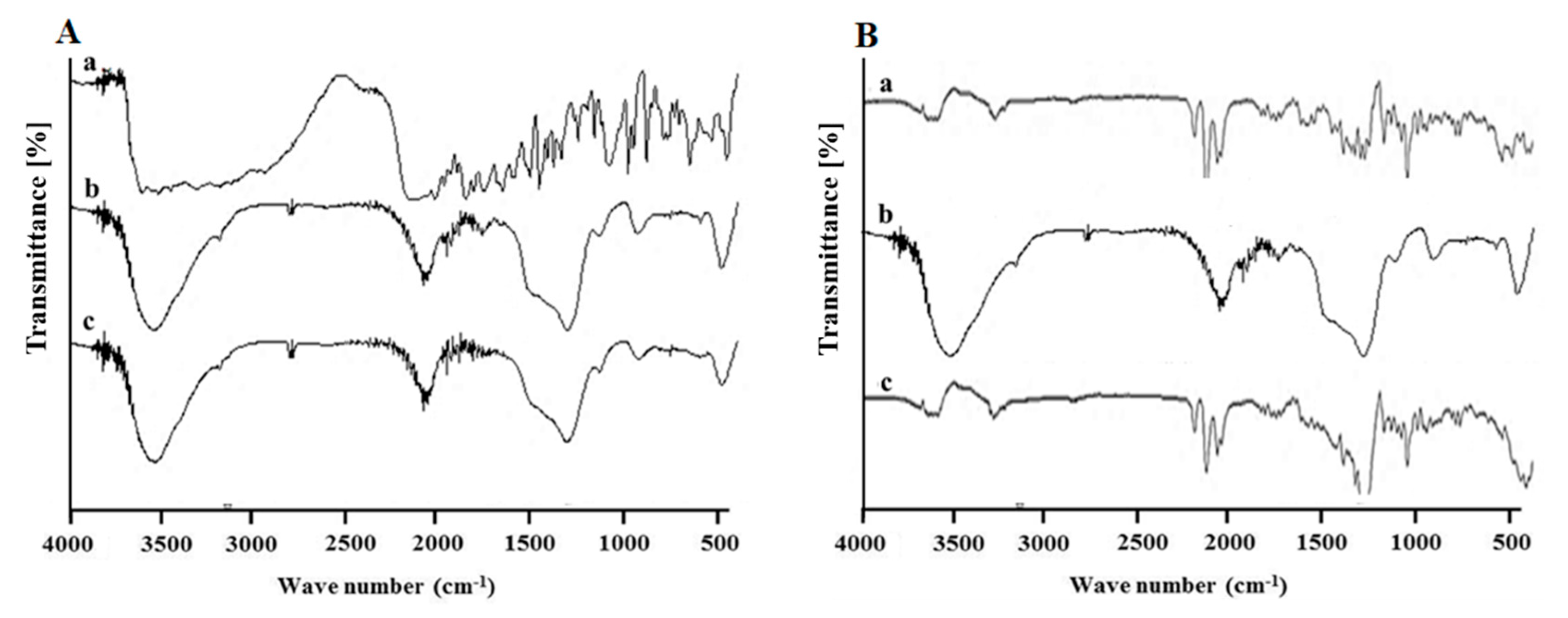
3.1. Synthesis and Characterization of MSN-NH2
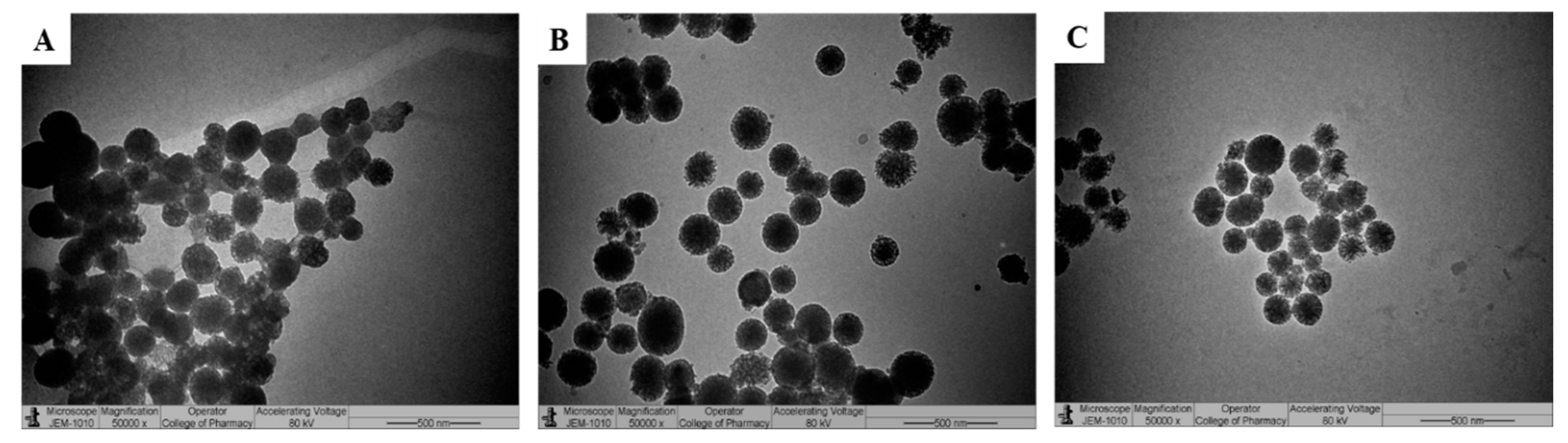
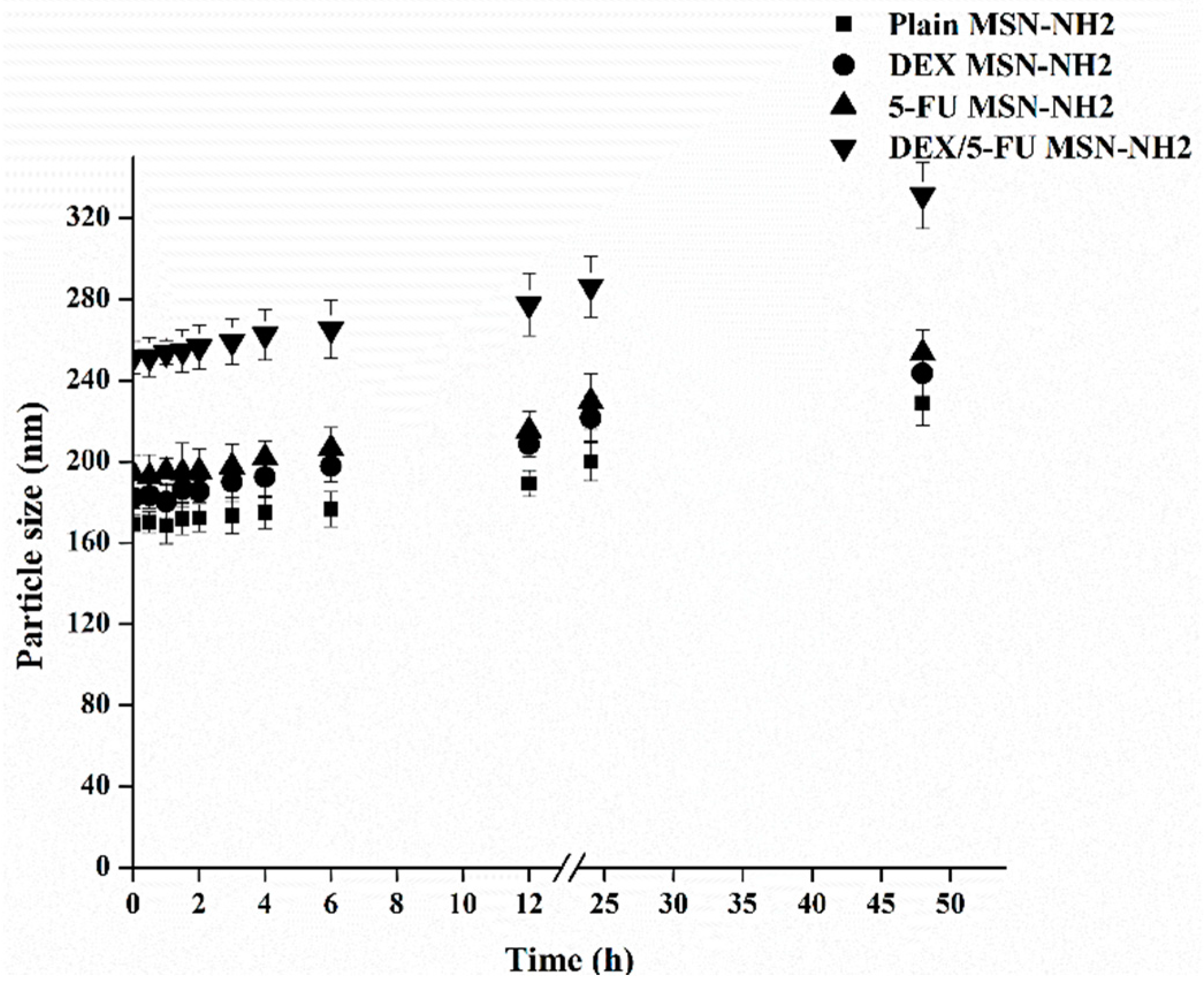
3.2. Preparation and Characterization of 5-FU/DEX Loaded MSN-NH2
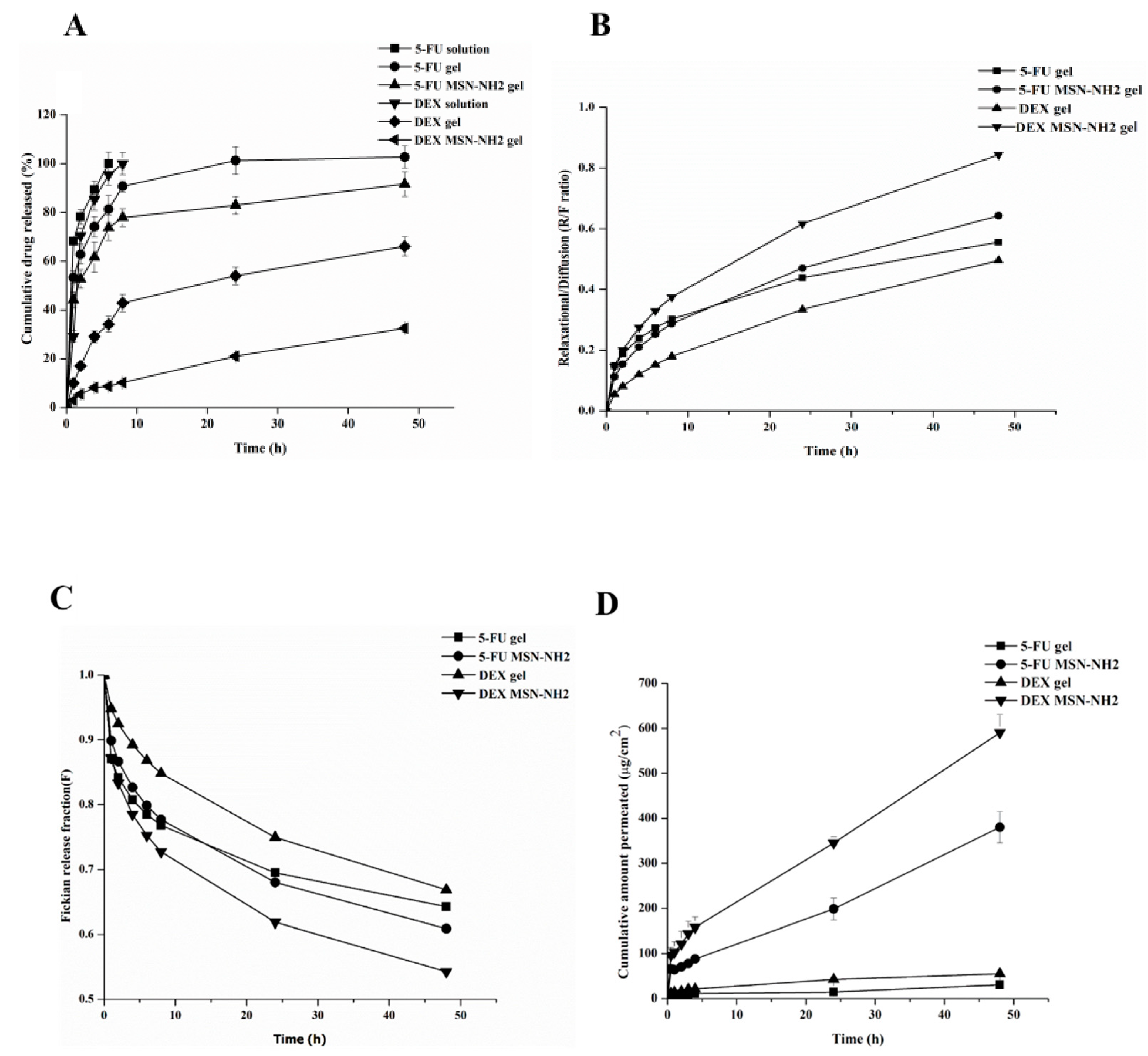
3.3. In Vitro Release Studies
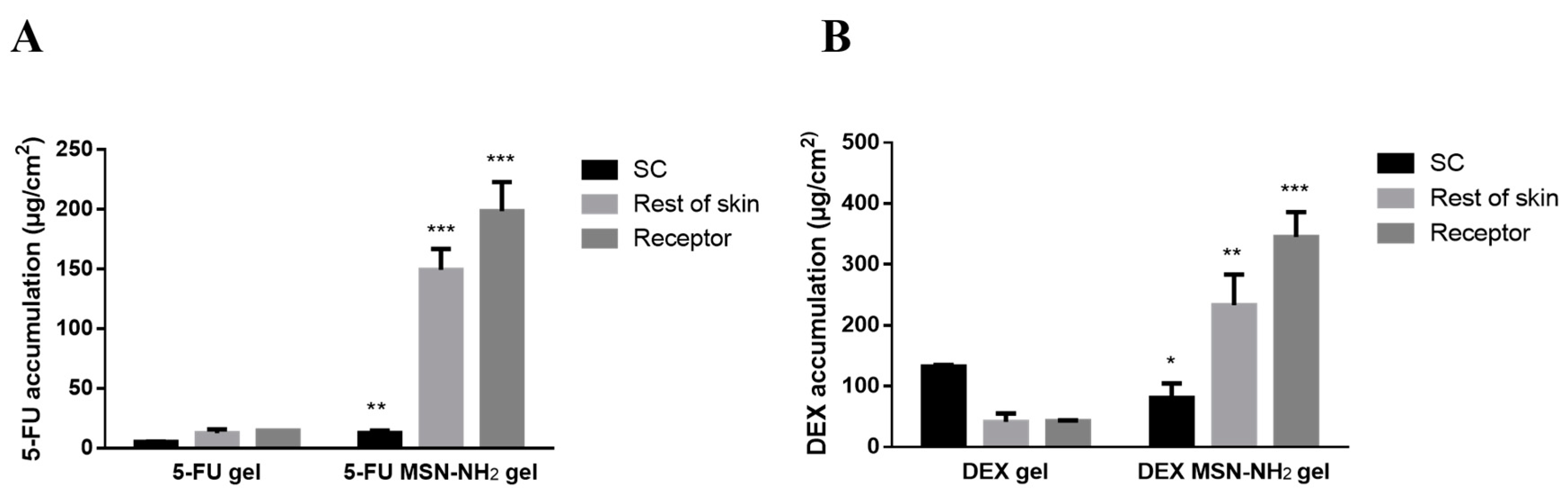
3.4. Ex Vivo Skin Permeability Studies
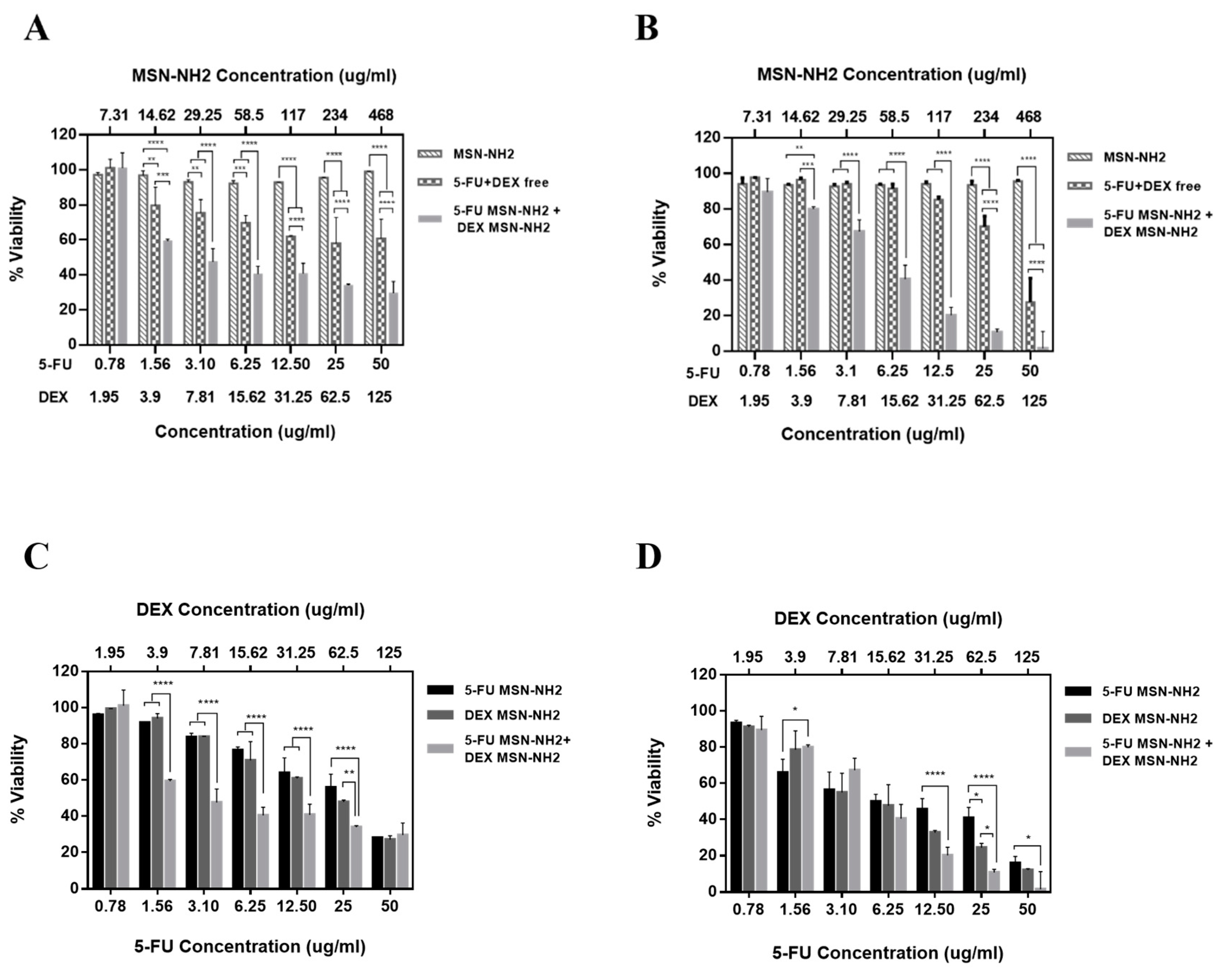
3.5. In Vitro Cytotoxicity Assay in Melanoma Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Matthews, N.H.; Li, W.Q.; Qureshi, A.A.; Weinstock, M.A.; Cho, E. Epidemiology of Melanoma. In Cutaneous Melanoma: Etiology and Therapy; Ward, W.H., Farma, J.M., Eds.; Exon Publications: Brisbane, Australia, 2017. [Google Scholar]
- Laikova, K.V.; Oberemok, V.V.; Krasnodubets, A.M.; Shumskykh, M.N.; Useinov, R.Z.; Novikov, I.A.; Temirova, Z.Z.; Gorlov, M.V.; Shved, N.A.; Kumeiko, V.V.; et al. Advances in the Understanding of Skin Cancer: Ultraviolet Radiation, Mutations, and Antisense Oligonucleotides as Anticancer Drugs. Molcules 2019, 24, 1516. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Wu, S.; Singh, R.K. Resistance to chemotherapy and molecularly targeted therapies: Rationale for combination therapy in malignant melanoma. Curr. Mol. Med. 2011, 11, 553–563. [Google Scholar] [CrossRef]
- Domingues, B.; Lopes, J.M.; Soares, P.; Populo, H. Melanoma treatment in review. ImmunoTargets Ther. 2018, 7, 35–49. [Google Scholar] [CrossRef]
- Robert, C.; Karaszewska, B.; Schachter, J.; Rutkowski, P.; Mackiewicz, A.; Stroiakovski, D.; Lichinitser, M.; Dummer, R.; Grange, F.; Mortier, L.; et al. Improved Overall Survival in Melanoma with Combined Dabrafenib and Trametinib. N. Engl. J. Med. 2015, 372, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, J.; Qin, Y.; Song, H.; Huang, P.; Song, H.; Wang, C.; Li, C.; Wang, Y.; Kong, D. Co-delivery of doxorubicin and pheophorbide A by pluronic F127 micelles for chemo-photodynamic combination therapy of melanoma. J. Mater. Chem. B 2018, 6, 3305–3314. [Google Scholar] [CrossRef] [PubMed]
- Anirudhan, T.S.; Nair, A.S.; Bino, S.J. Nanoparticle assisted solvent selective transdermal combination therapy of curcumin and 5-flurouracil for efficient cancer treatment. Carbohydr. Polym. 2017, 173, 131–142. [Google Scholar] [CrossRef]
- Sapino, S.; Oliaro-Bosso, S.; Zonari, D.; Zattoni, A.; Ugazio, E. Mesoporous silica nanoparticles as a promising skin delivery system for methotrexate. Int. J. Pharm. 2017, 530, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Young, N.P.; Townley, H.E. Characterization and Comparison of Mesoporous Silica Particles for Optimized Drug Delivery. Nanomater. Nanotechnol. 2014, 4, 2. [Google Scholar] [CrossRef]
- Slowing, I.I.; Vivero-Escoto, J.L.; Wu, C.-W.; Lin, V.S.-Y. Mesoporous silica nanoparticles as controlled release drug delivery and gene transfection carriers☆. Adv. Drug Deliv. Rev. 2008, 60, 1278–1288. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Regí, M.; Colilla, M.; Izquierdo-Barba, I.; Manzano, M. Mesoporous Silica Nanoparticles for Drug Delivery: Current Insights. Molecules 2017, 23, 47. [Google Scholar] [CrossRef] [PubMed]
- Sanjay, C.H.S.; Ghate, V.M.; Lewis, S. Mesoporous silica particles for dermal drug delivery: A review. Int. J. Appl. Pharm. 2018, 10, 23–26. [Google Scholar] [CrossRef]
- Brys, A.K.; Gowda, R.; Loriaux, D.B.; Robertson, G.P.; Mosca, P.J. Nanotechnology-based strategies for combating toxicity and resistance in melanoma therapy. Biotechnol. Adv. 2016, 34, 565–577. [Google Scholar] [CrossRef] [PubMed]
- Pearlman, D.L.; Youngberg, B.; Engelhard, C. Weekly pulse dosing schedule of fluorouracil: A new topical therapy for psoriasis. J. Am. Acad. Dermatol. 1986, 15, 1247–1252. [Google Scholar] [CrossRef]
- He, Y.; Luo, L.; Liang, S.; Long, M.; Xu, H. Amino-functionalized mesoporous silica nanoparticles as efficient carriers for anticancer drug delivery. J. Biomater. Appl. 2017, 32, 524–532. [Google Scholar] [CrossRef] [PubMed]
- She, X.; Chen, L.; Li, C.; He, C.; He, L.; Kong, L. Functionalization of Hollow Mesoporous Silica Nanoparticles for Improved 5-FU Loading. J. Nanomater. 2015, 2015, 1–9. [Google Scholar] [CrossRef]
- Manzano, M.; Aina, V.; Areán, C.; Balas, F.; Cauda, V.; Colilla, M.; Delgado, M.; Vallet-Regí, M. Studies on MCM-41 mesoporous silica for drug delivery: Effect of particle morphology and amine functionalization. Chem. Eng. J. 2008, 137, 30–37. [Google Scholar] [CrossRef]
- Vilarrasa-García, E.; Cecilia, J.A.; Maria, E.; Moya, O.; Cavalcante, C., Jr.; Cristina, D.; Azevedo, S.; Rodriguez-Castellon, E. materials “Low Cost” Pore Expanded SBA-15 Functionalized with Amine Groups Applied to CO 2 Adsorption. Materials 2015, 8, 2495–2513. [Google Scholar] [CrossRef]
- Yokoi, T.; Yoshitake, H.; Tatsumi, T. Synthesis of amino-functionalized MCM-41 via direct co-condensation and post-synthesis grafting methods using mono-, di- and tri-amino-organoalkoxysilanes. J. Mater. Chem. 2004, 14, 951–957. [Google Scholar] [CrossRef]
- Yokoi, T.; Kubota, Y.; Tatsumi, T. Amino-functionalized mesoporous silica as base catalyst and adsorbent. Appl. Catal. A Gen. 2012, 422, 14–37. [Google Scholar] [CrossRef]
- Zheng, H.; Gao, C.; Che, S. Amino and quaternary ammonium group functionalized mesoporous silica: An efficient ion-exchange method to remove anionic surfactant from AMS. Microporous Mesoporous Mater. 2008, 116, 299–307. [Google Scholar] [CrossRef]
- Kalam, M.A. The potential application of hyaluronic acid coated chitosan nanoparticles in ocular delivery of dexamethasone. Int. J. Biol. Macromol. 2016, 89, 559–568. [Google Scholar] [CrossRef]
- Alanazi, F.K.; Yassin, A.E.B.; El-Badry, M.; Mowafy, H.A.; Alsarra, I.A. Validated high-performance liquid chromatographic technique for determination of 5-fluorouracil: Applications to stability studies and simulated colonic media. J. Chromatogr. Sci. 2009, 47, 558–563. [Google Scholar] [CrossRef]
- Amin, M.K.; Boateng, J. Surface Modification of Mobile Composition of Matter (MCM)-41 Type Silica Nanoparticles for Potential Oral Mucosa Vaccine Delivery. J. Pharm. Sci. 2020, 109. [Google Scholar] [CrossRef]
- Alomrani, A.H.; Shazly, G.A.; Amara, A.A.; Badran, M.M. Itraconazole-hydroxypropyl-beta-cyclodextrin loaded deformable liposomes: In vitro skin penetration studies and antifungal efficacy using Candida albicans as model. Colloids Surf. B Biointerfaces 2014, 121, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.S.; Ali, M.S.; Zakir, F.; Alam, N.; Alam, M.I.; Ahmad, F.; Siddiqui, M.R.; Ali, M.D.; Ansari, M.S.; Ahmad, S.; et al. Enhancement of Anti-Dermatitis Potential of Clobetasol Propionate by DHA [Docosahexaenoic Acid] Rich Algal Oil Nanoemulsion Gel. Iran. J. Pharm. Res. 2016, 15, 35–52. [Google Scholar]
- AlMomen, A.; Cho, S.; Yang, C.-H.; Li, Z.; Jarboe, E.A.; Peterson, C.M.; Huh, K.M.; Janát-Amsbury, M.M. Thermosensitive progesterone hydrogel: A safe and effective new formulation for vaginal application. Pharm. Res. 2015, 32, 2266–2279. [Google Scholar] [CrossRef]
- Che, S.; Garcia-Bennett, A.E.; Yokoi, T.; Sakamoto, K.; Kunieda, H.; Terasaki, O.; Tatsumi, T. A novel anionic surfactant templating route for synthesizing mesoporous silica with unique structure. Nat. Mater. 2003, 2, 801–805. [Google Scholar] [CrossRef]
- Bao, Y.; Wang, T.; Kang, Q.; Shi, C.; Ma, J. Micelle-template synthesis of hollow silica spheres for improving water vapor permeability of waterborne polyurethane membrane. Sci. Rep. 2017, 7, 46638. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.; Yu, S. Fourier Transform Infrared Spectroscopic Analysis of Protein Secondary Structures. Acta Biochim. Biophys. Sin. 2007, 39, 549–559. [Google Scholar] [CrossRef] [PubMed]
- White, L.; Tripp, C. Reaction of (3-Aminopropyl)dimethylethoxysilane with Amine Catalysts on Silica Surfaces. J. Colloid Interface Sci. 2000, 232, 400–407. [Google Scholar] [CrossRef]
- Gross, K.; Kircik, L.; Kricorian, G. 5% 5-Fluorouracil Cream for the Treatment of Small Superficial Basal Cell Carcinoma: Efficacy, Tolerability, Cosmetic Outcome, and Patient Satisfaction. Dermatol. Surg. 2007, 33, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Bargman, H.; Hochman, J. Topical treatment of Bowen’s disease with 5-Fluorouracil. J. Cutan. Med. Surg. 2003, 7, 101–105. [Google Scholar] [CrossRef]
- Tawfik, A.A.; Fathy, M.; Badawi, A.M.; Abdallah, N.; Shokeir, H. Topical 5 fluorouracil cream vs combined 5 fluorouracil and fractional erbium YAG laser for treatment of severe hypertrophic scars. Clin. Cosmet. Investig. Dermatol. 2019, 12, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Werschler, W.P. Considerations for Use of Fluorouracil Cream 0.5% for the Treatment of Actinic Keratosis in Elderly Patients. J. Clin. Aesthetic Dermatol. 2008, 1, 22–27. [Google Scholar]
- Dhiman, M.K.; Dhiman, A.; Sawant, K.K. Transbuccal Delivery of 5-Fluorouracil: Permeation Enhancement and Pharmacokinetic Study. AAPS PharmSciTech 2009, 10, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Dobos, J.; Kenessey, I.; Tímár, J.; Ladányi, A. Glucocorticoid Receptor Expression and Antiproliferative Effect of Dexamethasone on Human Melanoma Cells. Pathol. Oncol. Res. 2011, 17, 729–734. [Google Scholar] [CrossRef]
- Mittal, R.; Sharma, A.; Arora, S. Ufasomes Mediated Cutaneous Delivery of Dexamethasone: Formulation and Evaluation of Anti-Inflammatory Activity by Carrageenin-Induced Rat Paw Edema Model. J. Pharm. 2012, 2013, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-N.; Ko, S.A.; Lee, S.H.; Huh, B.K.; Bin Choy, Y. Amine-grafted SBA-15 for ophthalmic delivery of dexamethasone. J. Solid State Chem. 2018, 268, 102–107. [Google Scholar] [CrossRef]
- Merino, V.; López, A.; Kalia, Y.N.; Guy, R.H. Electrorepulsion Versus Electroosmosis: Effect of pH on the lontophoretic Flux of 5-Fluorouracil. Pharm. Res. 1999, 16, 758–761. [Google Scholar] [CrossRef]
- Moodley, T.; Singh, M. Polymeric Mesoporous Silica Nanoparticles for Enhanced Delivery of 5-Fluorouracil in Vitro. Pharmaceutics 2019, 11, 288. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wang, Y.; Peng, Z.; She, M.; Kong, L. Physichemical Property and Morphology of 5-Fluorouracil Loaded Chitosan Nanoparticles; IEEE: New York City, USA, 2010. [Google Scholar]
- Puwang, L.; Yichao, W.; Zheng, P.; Fenghua, S.; Lingxue, K. Development of chitosan nanoparticles as drug delivery systems for 5-fluorouracil and leucovorin blends. Carbohydr. Polym. 2011, 85, 698–704. [Google Scholar] [CrossRef]
- Alami-Milani, M.; Zakeri-Milani, P.; Valizadeh, H.; Salehi, R.; Jelvehgari, M. Preparation and evaluation of PCL-PEG-PCL micelles as potential nanocarriers for ocular delivery of dexamethasone. Iran J. Basic Med. Sci. 2018, 21, 153–164. [Google Scholar]
- Mayba, J.N.; Gooderham, M.J. A Guide to Topical Vehicle Formulations. J. Cutan. Med. Surg. 2017, 22, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Gaikwad, D.V.; Yadav, V.; Dhavale, R.; Choudhari, P.; Jadhav, S. Effect of Carbopol 934 and 940 on Fluconazole Release from Topical Gel Formulation: A Factorial Approach. Curr. Pharma Res. 2012, 2, 487–493. [Google Scholar]
- Li, J.Q.; Yang, J.C.; Liang, J.X.; Wang, S.L. Pharmacokinetic study and clinical evaluation of a slow-release 5-fluorouracil implant in pancreatic cancer patients. Anti-Cancer Drugs 2016, 27, 60–65. [Google Scholar] [CrossRef]
- Fanciullino, R.; Giacometti, S.; Mercier, C.; Aubert, C.; Blanquicett, C.; Piccerelle, P.; Ciccolini, J. In vitro and in vivo reversal of resistance to 5-fluorouracil in colorectal cancer cells with a novel stealth double-liposomal formulation. Br. J. Cancer 2007, 97, 919–926. [Google Scholar] [CrossRef]
- Patil, L.D.; Verma, U.; Patil, U.D.; Naik, J.; Narkhede, J.S. Inclusion of Aceclofenac in Mesoporous Silica Nanoparticles: Drug Release Study and Statistical Optimization of Encapsulation Efficiency by Response Surface Methodology. Mater. Technol. 2019, 34, 751–763. [Google Scholar] [CrossRef]
- Berlier, G.; Gastaldi, L.; Sapino, S.; Miletto, I.; Bottinelli, E.; Chirio, D.; Ugazio, E. MCM-41 as a useful vector for rutin topical formulations: Synthesis, characterization and testing. Int. J. Pharm. 2013, 457, 177–186. [Google Scholar] [CrossRef]
- Petrilli, R.; Eloy, J.O.; Lopez, R.F.V.; Lee, R.J. Cetuximab Immunoliposomes Enhance Delivery of 5-FU to Skin Squamous Carcinoma Cells. Anti-Cancer Agents Med. Chem. 2017, 17, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Vicentini, F.T.M.C.; Simi, T.R.M.; Del Ciampo, J.O.; Wolga, N.O.; Pitol, D.L.; Iyomasa, M.M.; Bentley, M.V.L.B.; Fonseca, M.J.V. Quercetin in w/o microemulsion: In vitro and in vivo skin penetration and efficacy against UVB-induced skin damages evaluated in vivo. Eur. J. Pharm. Biopharm. 2008, 69, 948–957. [Google Scholar] [CrossRef]
- Chinembiri, T.N.; Gerber, M.; Du Plessis, L.; Du Preez, J.; Du Plessis, J. Topical Delivery of 5-Fluorouracil from Pheroid™ Formulations and the in Vitro Efficacy Against Human Melanoma. AAPS PharmSciTech 2015, 16, 1390–1399. [Google Scholar] [CrossRef] [PubMed]
- Naguib, Y.W.; Kumar, A.; Cui, Z. The effect of microneedles on the skin permeability and antitumor activity of topical 5-fluorouracil. Acta Pharm. Sin. B 2014, 4, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Trotta, M.; Ugazio, E.; Peira, E.; Pulitano, C. Influence of ion pairing on topical delivery of retinoic acid from microemulsions. J. Control. Release 2003, 86, 315–321. [Google Scholar] [CrossRef]
- Asiri, A.M.; Song, H.; Ong, W.Y.; Han, M.Y.; Huang, D. Positively charged and pH self-buffering quantum dots for efficient cellular uptake by charge mediation and monitoring cell membrane permeability. Nanotechnology 2009, 20. [Google Scholar] [CrossRef]
- Sapino, S.; Ugazio, E.; Gastaldi, L.; Miletto, I.; Berlier, G.; Zonari, D.; Oliaro-Bosso, S. Mesoporous silica as topical nanocarriers for quercetin: Characterization and in vitro studies. Eur. J. Pharm. Biopharm. 2015, 89, 116–125. [Google Scholar] [CrossRef]
- Taha, I.E.; Ak-Suwayeh, A.S.; Tayel, M.M.; Badran, M.M. Fast ultra-fine self-nanoemulsifying drug delivery system for improving in vitro gastric dissolution of poor water soluble drug. Acta Pol. Pharm. Drug Res. 2015, 72. [Google Scholar]
- Moghaddam, S.P.H.; Mohammadpour, R.; Ghandehari, H. In vitro and in vivo evaluation of degradation, toxicity, biodistribution, and clearance of silica nanoparticles as a function of size, porosity, density, and composition. J. Control. Release 2019, 312, 1–15. [Google Scholar] [CrossRef]
- Morris, A.S.; Adamcakova-Dodd, A.; Lehman, S.E.; Wongrakpanich, A.; Thorne, P.S.; Larsen, S.C.; Salem, A.K. Amine modification of nonporous silica nanoparticles reduces inflammatory response following intratracheal instillation in murine lungs. Toxicol. Lett. 2016, 241, 207–215. [Google Scholar] [CrossRef]
- Bowman, C.R.; Bailey, F.C.; Elrod-Erickson, M.; Neigh, A.M.; Otter, R.R. Effects of silver nanoparticles on zebrafish (Danio rerio) and Escherichia coli (ATCC 25922): A comparison of toxicity based on total surface area versus mass concentration of particles in a model eukaryotic and prokaryotic system. Environ. Toxicol. Chem. 2012, 31, 1793–1800. [Google Scholar] [CrossRef]
- Zhang, C.; Beckermann, B.; Kallifatidis, G.; Liu, Z.; Rittgen, W.; Edler, L.; Büchler, P.; Debatin, K.-M.; Büchler, M.W.; Friess, H.; et al. Corticosteroids induce chemotherapy resistance in the majority of tumour cells from bone, brain, breast, cervix, melanoma and neuroblastoma. Int. J. Oncol. 2006, 29, 1295–1301. [Google Scholar] [CrossRef] [PubMed]
- Kong, B.; Seog, J.H.; Graham, L.M.; Lee, S.B. Experimental considerations on the cytotoxicity of nanoparticles. Nanomedicine 2011, 6, 929–941. [Google Scholar] [CrossRef] [PubMed]


| Ratio Drug:MSN-NH2 | (Drug:MSN-NH2) (mg in 25 mL) | EE% | LC% | LC mg (Drug)/mg (MSN-NH2) |
|---|---|---|---|---|
| DEX | ||||
| 1:5 | 5:25 | 47.4 ± 3.15 | 8.66 ± 0.63 | 0.087 ± 0.006 mg/1 mg |
| 1:1 | 25:25 | 18.89 ± 0.89 | 15.89 ± 0.88 | 0.159 ± 0.009 mg/1 mg |
| 5:1 | 125:25 | 15.92 ± 0.79 | 44.72 ± 4.21 | 0.447 ± 0.042 mg/1 mg |
| 5-FU | ||||
| 1:1 | 25:25 | 18.01 ± 3.72 | 15.26 ± 3.13 | 0.153 ± 0.031 mg/1 mg. |
| 5:1 | 125:25 | 3.6 ± 1.4 | 12.6 ± 5.50 | 0.126 ± 0.055 mg/1 mg. |
| Codes | Zero Order | First Order | Higuchi (Diffusion) | Korsmey-er Peppas | “n” Value | K |
|---|---|---|---|---|---|---|
| 5-FU gel | 0.647 | 0.604 | 0.821 | 0.989 | 0.159 | 59.119 |
| 5-FU MSN-NH2-gel | 0.652 | 0.557 | 0.833 | 0.987 | 0.167 | 49.671 |
| DEX gel | 0.701 | 0.502 | 0.964 | 0.982 | 0.368 | 16.602 |
| DEX MSN-NH2 gel | 0.922 | 0.701 | 0.994 | 0.998 | 0.602 | 3.139 |
| Codes | Peppas Sahlin | K1 | K2 | “m” Value |
|---|---|---|---|---|
| 5-FU gel | 0.999 | 61.692 | 9.170 | 0.341 |
| 5-FU MSN-NH2-gel | 0.991 | 42.468 | 4.783 | 0.450 |
| DEX gel | 0.992 | 14.249 | 0.781 | 0.569 |
| DEX MSN-NH2 gel | 0.999 | 3.070 | 0.452 | 0.452 |
| Formula Code | Flux, J (µg/cm2/h) | Permeability Coefficient Kp × 10−3 (cm h−1) | Lag Time (h) | Enhancement Ratio (ER) |
|---|---|---|---|---|
| 5-FU gel | 1.401 ± 0.018 | 1.75 ± 0.291 | 0.5 | - |
| 5-FU MSN-NH2 gel | 6.540 ± 0.207 | 8.22 ± 0.122 | 0.5 | 4.668 |
| DEX gel | 3.403 ± 0.008 | 1.70 ± 0.130 | 0.5 | - |
| DEX MSN-NH2 gel | 19.330 ± 0.117 | 38.71 ± 0.297 | 0.5 | 5.68 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almomen, A.; El-Toni, A.M.; Badran, M.; Alhowyan, A.; Abul Kalam, M.; Alshamsan, A.; Alkholief, M. The Design of Anionic Surfactant-Based Amino-Functionalized Mesoporous Silica Nanoparticles and their Application in Transdermal Drug Delivery. Pharmaceutics 2020, 12, 1035. https://doi.org/10.3390/pharmaceutics12111035
Almomen A, El-Toni AM, Badran M, Alhowyan A, Abul Kalam M, Alshamsan A, Alkholief M. The Design of Anionic Surfactant-Based Amino-Functionalized Mesoporous Silica Nanoparticles and their Application in Transdermal Drug Delivery. Pharmaceutics. 2020; 12(11):1035. https://doi.org/10.3390/pharmaceutics12111035
Chicago/Turabian StyleAlmomen, Aliyah, Ahmed M. El-Toni, Mohammed Badran, Adel Alhowyan, Mohd Abul Kalam, Aws Alshamsan, and Musaed Alkholief. 2020. "The Design of Anionic Surfactant-Based Amino-Functionalized Mesoporous Silica Nanoparticles and their Application in Transdermal Drug Delivery" Pharmaceutics 12, no. 11: 1035. https://doi.org/10.3390/pharmaceutics12111035
APA StyleAlmomen, A., El-Toni, A. M., Badran, M., Alhowyan, A., Abul Kalam, M., Alshamsan, A., & Alkholief, M. (2020). The Design of Anionic Surfactant-Based Amino-Functionalized Mesoporous Silica Nanoparticles and their Application in Transdermal Drug Delivery. Pharmaceutics, 12(11), 1035. https://doi.org/10.3390/pharmaceutics12111035