An Electrochemical Chip to Monitor In Vitro Glycation of Proteins and Screening of Antiglycation Potential of Drugs
Abstract
1. Introduction
2. Methods and Materials
2.1. List of Reagents
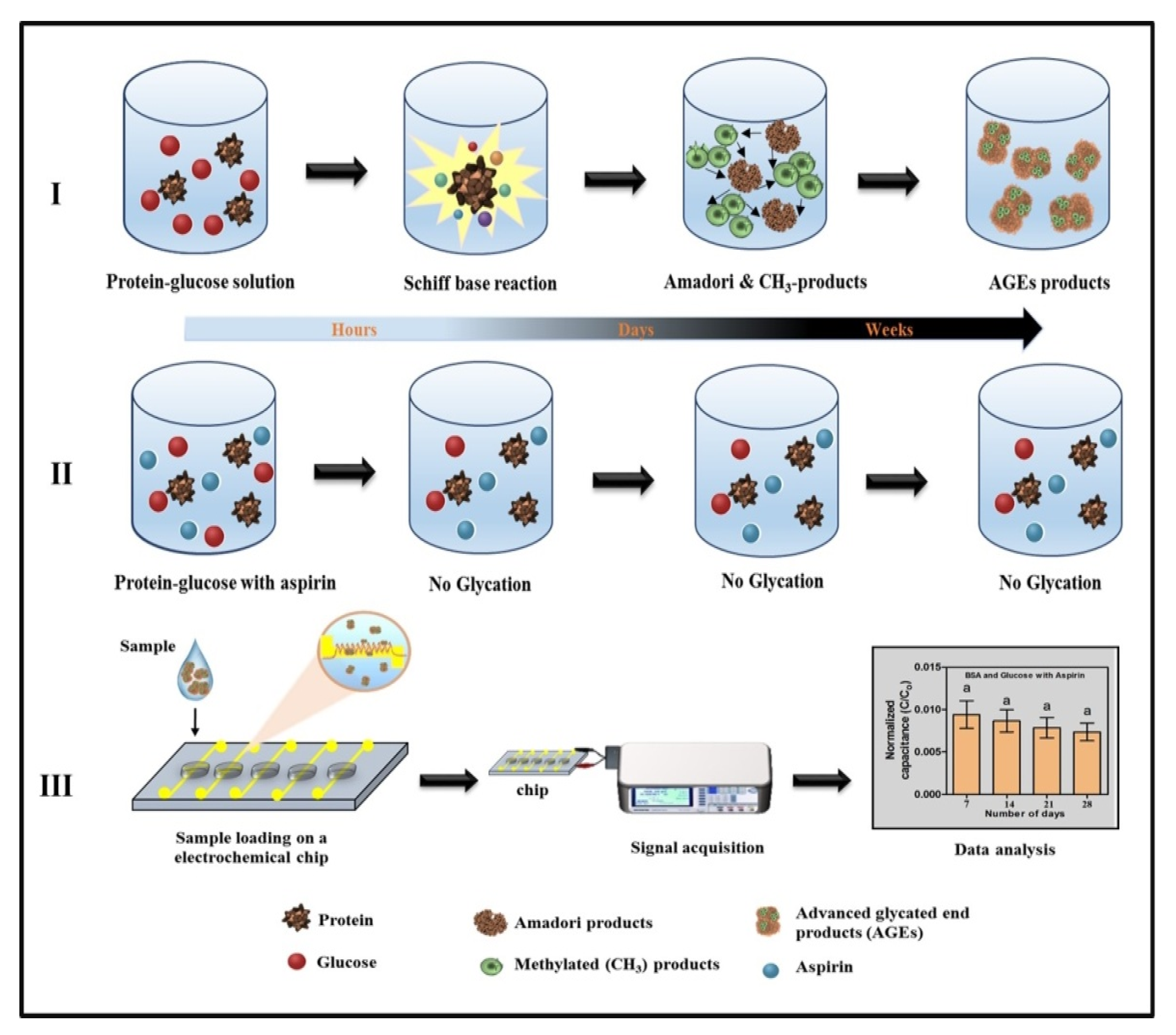
2.2. Fabrication of the Electrochemical Chip
2.3. Glycation of Proteins and Evaluation of Antiglycation Compound
2.4. Electrical Signal Measurements and Data Analysis
3. Results and Discussion
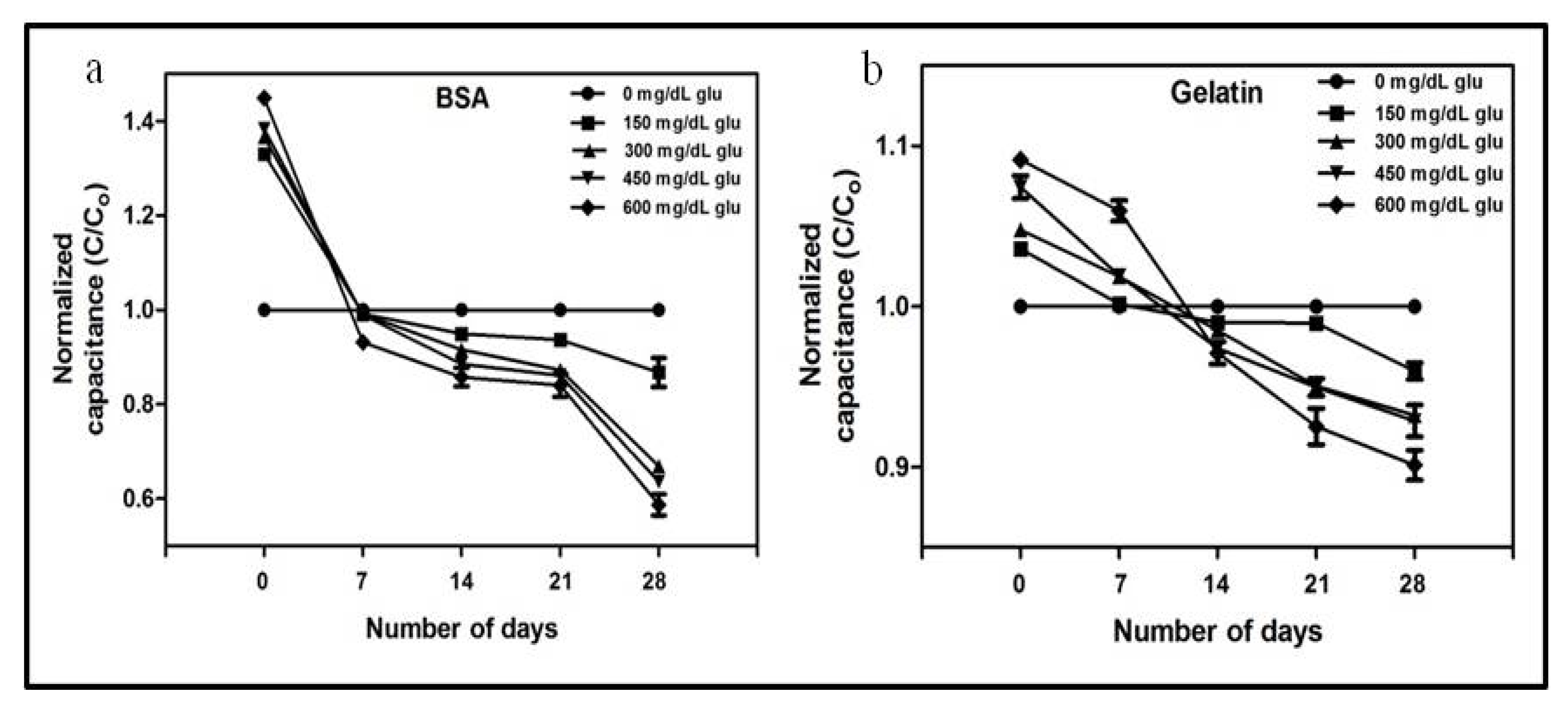
3.1. Analysis of Glycation by Capacitance Measurements
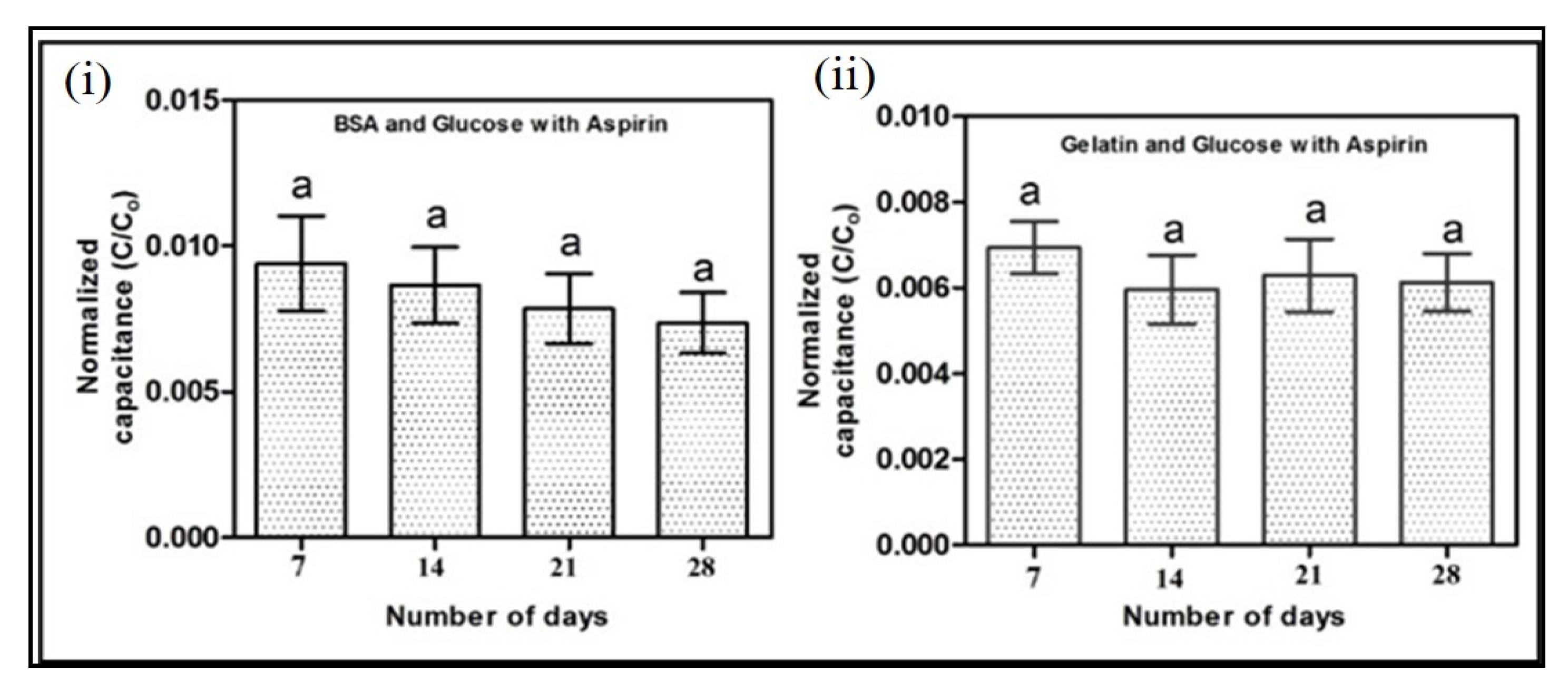
3.2. Evaluation of Antiglycation Property by Electrochemical Chip
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AGEs | advanced glycation end products |
| BSA | bovine serum albumin |
| CAD | computer-aided design |
| CML | Nε-(carboxymethyl)lysine |
| ELISA | enzyme-linked immunosorbent assay |
| GO | glyoxal |
| HPLC | high-performance liquid chromatography |
| LC-MS | liquid chromatography-mass spectrometry |
| MALDI-TOF | matrix-assisted laser desorption ionization time-of-flight |
| MG | methylglyoxal |
| NBT | nitroblue tetrazolium |
| PDMS | polydimethylsiloxane |
References
- Wild, S.; Roglic, G.; Green, A.; Sicree, R.; King, H. Global Prevalence of Diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care 2004, 27, 1047–1053. [Google Scholar] [CrossRef] [PubMed]
- Furth, A.J. The maillard reaction in aging, diabetes and nutrition. Trends Biochem. Sci. 1990, 15, 248. [Google Scholar] [CrossRef]
- Freitas, P.A.C.; Ehlert, L.R.; Camargo, J.L. Glycated albumin: A potential biomarker in diabetes. Arch. Endocrinol. Metab. 2017, 61, 296–304. [Google Scholar] [CrossRef]
- Gkogkolou, P.; Böhm, M. Advanced glycation end products. Dermato Endocrinol. 2012, 4, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Standards of Medical Care for Patients With Diabetes Mellitus. Diabetes Care 2002, 25 (Suppl. 1), s33–s49. [CrossRef]
- Rohlfing, C.L.; Wiedmeyer, H.-M.; Little, R.R.; England, J.D.; Tennill, A.; Goldstein, D.E. Defining the Relationship Between Plasma Glucose and HbA1c: Analysis of glucose profiles and HbA1c in the Diabetes Control and Complications Trial. Diabetes Care 2002, 25, 275–278. [Google Scholar] [CrossRef]
- Soboleva, A.; Mavropolo-Stolyarenko, G.; Karonova, T.; Thieme, D.; Hoehenwarter, W.; Ihling, C.; Stefanov, V.E.; Grishina, T.; Frolov, A. Multiple Glycation Sites in Blood Plasma Proteins as an Integrated Biomarker of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2019, 20, 2329. [Google Scholar] [CrossRef]
- Rasheed, S.; Sánchez, S.S.; Yousuf, S.; Honoré, S.M.; Choudhary, M.I. Drug repurposing: In-vitro anti-glycation properties of 18 common drugs. PLoS ONE 2018, 13, e0190509. [Google Scholar] [CrossRef]
- Iqbal, S.; Alam, M.; Naseem, I. Vitamin D prevents glycation of proteins: Anin vitrostudy. FEBS Lett. 2016, 590, 2725–2736. [Google Scholar] [CrossRef]
- Ho, S.-C.; Chang, P.-W.; Tong, H.-T.; Yu, P.-Y. Inhibition of Fluorescent Advanced Glycation End-Products and N-Carboxymethyllysine Formation by Several Floral Herbal Infusions. Int. J. Food Prop. 2013, 17, 617–628. [Google Scholar] [CrossRef]
- Soboleva, A.; Vikhnina, M.; Grishina, T.; Frolov, A. Probing Protein Glycation by Chromatography and Mass Spectrometry: Analysis of Glycation Adducts. Int. J. Mol. Sci. 2017, 18, 2557. [Google Scholar] [CrossRef] [PubMed]
- Putta, S.; Kilari, E.K. A review on methods of estimation of advanced glycation end products. World J. Pharm. Res. 2015, 4, 689–699. [Google Scholar]
- Redman, E.A.; Ramos-Payán, M.; Mellors, J.S.; Ramsey, J.M. Analysis of Hemoglobin Glycation Using Microfluidic CE-MS: A Rapid, Mass Spectrometry Compatible Method for Assessing Diabetes Management. Anal. Chem. 2016, 88, 5324–5330. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Moon, S.W.; Jin, K.H.; Shin, J.-H. Microfluidic-based non-enzymatic glycation enhances cross-linking of human scleral tissue compared to conventional soaking. Scanning 2016, 38, 421–426. [Google Scholar] [CrossRef][Green Version]
- Chuang, Y.-C.; Lan, K.-C.; Hsieh, K.-M.; Jang, L.-S.; Chen, M.-K. Detection of glycated hemoglobin (HbA1c) based on impedance measurement with parallel electrodes integrated into a microfluidic device. Sensors Actuators B Chem. 2012, 171, 1222–1230. [Google Scholar] [CrossRef]
- Wu, C.-C.; Lin, H.-I.; Chang, K.-W.; Mai, J.D.; Lin, H.-I.; Lee, G.-B. Measurement of glycated hemoglobin levels using an integrated microfluidic system. Microfluid. Nanofluidics 2014, 18, 613–621. [Google Scholar] [CrossRef]
- Thornalley, P.J.; Battah, S.; Ahmed, N.; Karachalias, N.; Agalou, S.; Babaei-Jadidi, R.; Dawnay, A. Quantitative screening of advanced glycation endproducts in cellular and extracellular proteins by tandem mass spectrometry. Biochem. J. 2003, 375, 581–592. [Google Scholar] [CrossRef]
- Smuda, M.; Henning, C.; Raghavan, C.T.; Johar, K.; Vasavada, A.R.; Nagaraj, R.H.; Glomb, M.A. Comprehensive Analysis of Maillard Protein Modifications in Human Lenses: Effect of Age and Cataract. Biochemistry 2015, 54, 2500–2507. [Google Scholar] [CrossRef]
- Matsui, T.; Joo, H.D.; Lee, J.M.; Ju, S.M.; Tao, W.H.; Higashimoto, Y.; Fukami, K.; Yamagishi, S.-I. Development of a monoclonal antibody-based ELISA system for glyceraldehyde-derived advanced glycation end products. Immunol. Lett. 2015, 167, 141–146. [Google Scholar] [CrossRef]
- Walker, S.; Howie, A.; Smith, A. The measurement of glycosylated albumin by reduction of alkaline nitro-blue tetrazolium. Clin. Chim. Acta 1986, 156, 197–206. [Google Scholar] [CrossRef]
- Sahu, A.; Sarkar, P.D. Comparative study of NBT reduction method for estimation of glycated protein (serum fructoseamine) with glycated HbA1c estimated on DCA 2000+Analyzer (immunoagglutination inhibition). Indian J. Physiol. Pharmacol. 2009, 52, 408–412. [Google Scholar]
- Ashraf, J.M.; Ahmad, S.; Choi, I.; Ahmad, S.; Farhan, M.; Tatyana, G.; Shahab, U. Recent advances in detection of AGEs: Immunochemical, bioanalytical and biochemical approaches. IUBMB Life 2015, 67, 897–913. [Google Scholar] [CrossRef]
- Zhernovaya, O.S.; Tuchin, V.V.; Meglinski, I.V. Monitoring of blood proteins glycation by refractive index and spectral measurements. Laser Phys. Lett. 2008, 5, 460–464. [Google Scholar] [CrossRef]
- Vigneshwaran, N.; Bijukumar, G.; Karmakar, N.; Anand, S.; Misra, A. Autofluorescence characterization of advanced glycation end products of hemoglobin. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2005, 61, 163–170. [Google Scholar] [CrossRef]
- Perrone, A.; Giovino, A.; Benny, J.; Martinelli, F. Advanced Glycation End Products (AGEs): Biochemistry, Signaling, Analytical Methods, and Epigenetic Effects. Oxidative Med. Cell. Longev. 2020, 2020, 1–18. [Google Scholar] [CrossRef]
- Żurawska-Płaksej, E.; Rorbach-Dolata, A.; Wiglusz, K.; Piwowar, A. The effect of glycation on bovine serum albumin conformation and ligand binding properties with regard to gliclazide. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 189, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Sadowska-Bartosz, I.; Stefaniuk, I.; Galiniak, S.; Bartosz, G. Glycation of bovine serum albumin by ascorbate in vitro: Possible contribution of the ascorbyl radical? Redox Biol. 2015, 6, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, S.; Saraswathi, N. Vanillin restrains non-enzymatic glycation and aggregation of albumin by chemical chaperone like function. Int. J. Biol. Macromol. 2016, 87, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Jing, H. Glycation of bovine serum albumin with monosaccharides inhibits heat-induced protein aggregation. RSC Adv. 2016, 6, 115183–115188. [Google Scholar] [CrossRef]
- Xia, S.; Li, Y.; Zhao, Q.; Li, J.; Xia, Q.; Zhang, X.; Huang, Q. Probing Conformational Change of Bovine Serum Albumin–Dextran Conjugates under Controlled Dry Heating. J. Agric. Food Chem. 2015, 63, 4080–4086. [Google Scholar] [CrossRef]
- Cooper, M.E.; Thallas, V.; Forbes, J.M.; Scalbert, E.; Sastra, S.; Darby, I.A.; Soulis, T. The cross-link breaker, N-phenacylthiazolium bromide prevents vascular advanced glycation end-product accumulation. Diabetologia 2000, 43, 660–664. [Google Scholar] [CrossRef] [PubMed]
- Chinchansure, A.A.; Korwar, A.M.; Kulkarni, M.J.; Joshi, S.P. Recent development of plant products with anti-glycation activity: A review. RSC Adv. 2015, 5, 31113–31138. [Google Scholar] [CrossRef]
- Meenatchi, P.; Purushothaman, A.; Maneemegalai, S. Antioxidant, antiglycation and insulinotrophic properties of Coccinia grandis (L.) in vitro: Possible role in prevention of diabetic complications. J. Tradit. Complement. Med. 2016, 7, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Pringle, N.A.; Van De Venter, M.; Koekemoer, T.C. Gelatin as a convenient surrogate protein to model the in vitro effects of advanced glycation end-product formation. Exp. Dermatol. 2018, 27, 1309–1311. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Zhang, Y.; Wang, T.-H.; Yang, S. Continuous dielectrophoretic bacterial separation and concentration from physiological media of high conductivity. Lab Chip 2011, 11, 2893–2900. [Google Scholar] [CrossRef]
- Cohen, M.P.; Hud, E. Production and characterization of monoclonal antibodies against human glycoalbumin. J. Immunol. Methods 1989, 117, 121–129. [Google Scholar] [CrossRef]
- Fu, M.-X.; Thorpe, S.; Baynes, J.W. Effects of Aspirin on Glycation, Glycoxidation, and Crosslinking of Collagen. In Maillard Reactions in Chemistry, Food and Health; Labuza, T.P., Monnier, V., Baynes, J., O’Brien, J., Eds.; Woodhead Publishing: Sawston, UK, 2005; pp. 95–100. [Google Scholar]
- Urios, P.; Grigorova-Borsos, A.-M.; Sternberg, M. Aspirin inhibits the formation of pentosidine, a cross-linking advanced glycation end product, in collagen. Diabetes Res. Clin. Prac. 2007, 77, 337–340. [Google Scholar] [CrossRef]
- Parker, R.; Doyle, F.J.; Peppas, N. The intravenous route to blood glucose control. IEEE Eng. Med. Boil. Mag. 2001, 20, 65–73. [Google Scholar] [CrossRef]
- Pethig, R.; Kell, D.B. The passive electrical properties of biological systems: Their significance in physiology, biophysics and biotechnology. Phys. Med. Biol. 1987, 32, 933–970. [Google Scholar] [CrossRef]
- Johnson, R.N.; Metcalf, P.A.; Baker, J.R. Fructosamine: A new approach to the estimation of serum glycosylprotein. An index of diabetic control. Clin. Chim. Acta 1983, 127, 87–95. [Google Scholar] [CrossRef]
- Wei, B.; Berning, K.; Quan, C.; Zhang, Y.T. Glycation of antibodies: Modification, methods and potential effects on biological functions. mAbs 2017, 9, 586–594. [Google Scholar]
- Mashiba, S.; Uchida, K.; Okuda, S.; Tomita, S. Measurement of glycated albumin by the nitroblue tetrazolium colorimetric method. Clin. Chim. Acta 1992, 212, 3–15. [Google Scholar] [PubMed]
- Ahmad, S.; Moinuddin; Ali, A. Immunological studies on glycated human IgG. Life Sci. 2012, 90, 980–987. [Google Scholar]
- Wani, A.; Mushtag, S.; Ahsan, H.; Ahmad, R. Biochemical studies of in vitro glycation of human DNA. Asian J. Biomed. Pharm. Sci. 2012, 9, 23–27. [Google Scholar]
- Bohinc, K.; Kralj-Iglič, V.; Iglič, A. Thickness of electrical double layer. Effect of ion size. Electrochim. Acta 2001, 46, 3033–3040. [Google Scholar]
- Sajithlal, G.; Chithra, P.; Chandrakasan, G. Advanced glycation end products induce crosslinking of collagen in vitro. Biochim. Biophys. Acta Bioenerg. 1998, 1407, 215–224. [Google Scholar]
- Singh, V.P.; Bali, A.; Singh, N.; Jaggi, A.S. Advanced Glycation End Products and Diabetic Complications. Korean J. Physiol. Pharmacol. 2014, 18, 1–14. [Google Scholar]
- Naumowicz, M.; Figaszewski, Z.A. The effect of pH on the electrical capacitance of phosphatidylcholine-phosphatidylserine system in bilayer lipid membrane. J. Membr. Biol. 2014, 247, 361–369. [Google Scholar]
- Brey, W.S.; A Heeb, M.; Ward, T.M. Dielectric measurements of water sorbed on ovalbumin and lysozyme. J. Colloid Interface Sci. 1969, 30, 13–20. [Google Scholar]
- Gudiksen, K.L.; Gitlin, I.; Whitesides, G.M. Differentiation of proteins based on characteristic patterns of association and denaturation in solutions of SDS. Proc. Natl. Acad. Sci. USA 2006, 103, 7968–7972. [Google Scholar]
- Tatham, M.H.; Cole, C.; Scullion, P.; Wilkie, R.; Westwood, N.J.; Stark, L.A.; Hay, R.T. A Proteomic Approach to Analyze the Aspirin-mediated Lysine Acetylome. Mol. Cell. Proteom. 2016, 16, 310–326. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, Z.A.; Park, S. An Electrochemical Chip to Monitor In Vitro Glycation of Proteins and Screening of Antiglycation Potential of Drugs. Pharmaceutics 2020, 12, 1011. https://doi.org/10.3390/pharmaceutics12111011
Khan ZA, Park S. An Electrochemical Chip to Monitor In Vitro Glycation of Proteins and Screening of Antiglycation Potential of Drugs. Pharmaceutics. 2020; 12(11):1011. https://doi.org/10.3390/pharmaceutics12111011
Chicago/Turabian StyleKhan, Zeeshan A., and Seungkyung Park. 2020. "An Electrochemical Chip to Monitor In Vitro Glycation of Proteins and Screening of Antiglycation Potential of Drugs" Pharmaceutics 12, no. 11: 1011. https://doi.org/10.3390/pharmaceutics12111011
APA StyleKhan, Z. A., & Park, S. (2020). An Electrochemical Chip to Monitor In Vitro Glycation of Proteins and Screening of Antiglycation Potential of Drugs. Pharmaceutics, 12(11), 1011. https://doi.org/10.3390/pharmaceutics12111011





