Targeting the Choroid Plexuses for Protein Drug Delivery
Abstract
1. Introduction
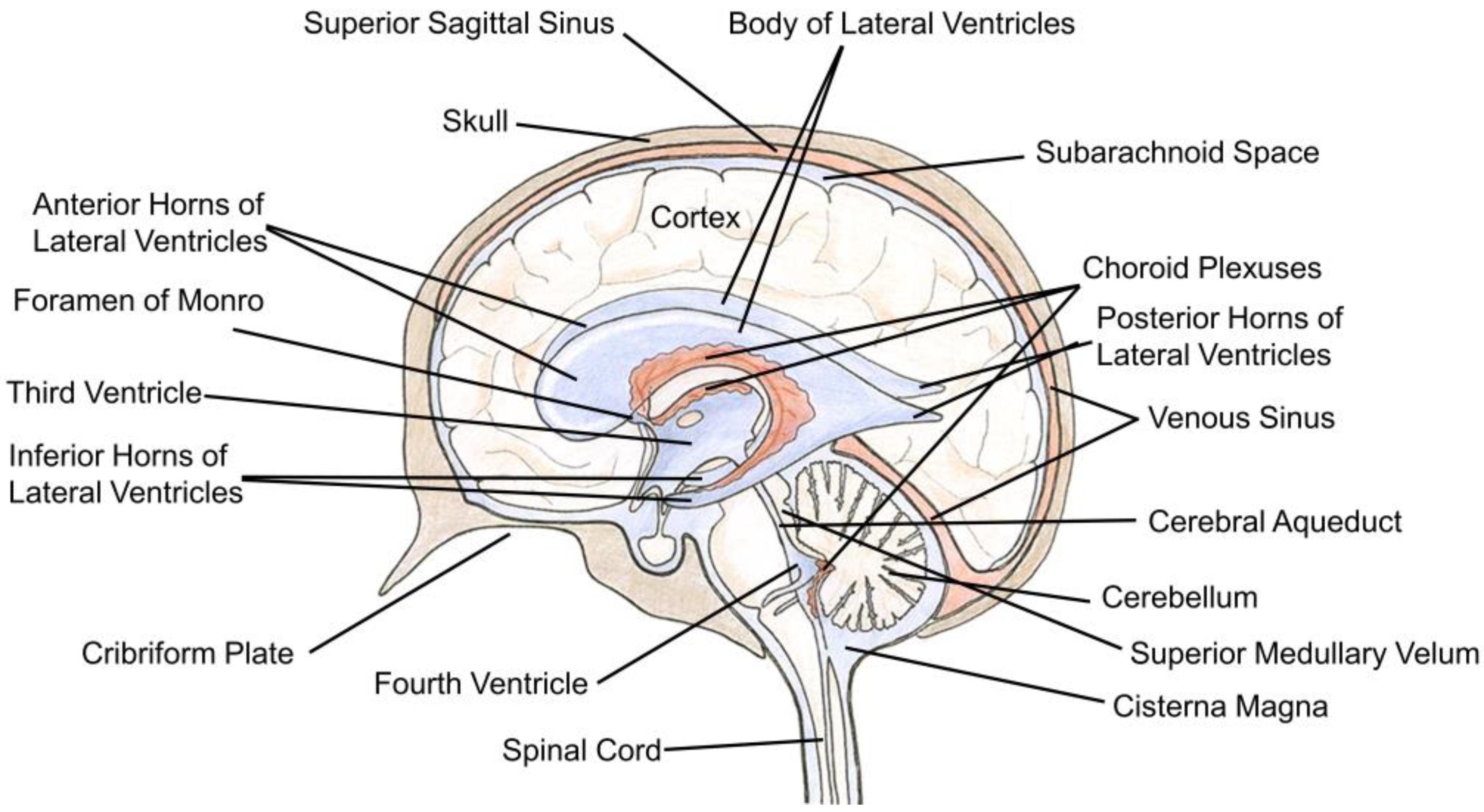
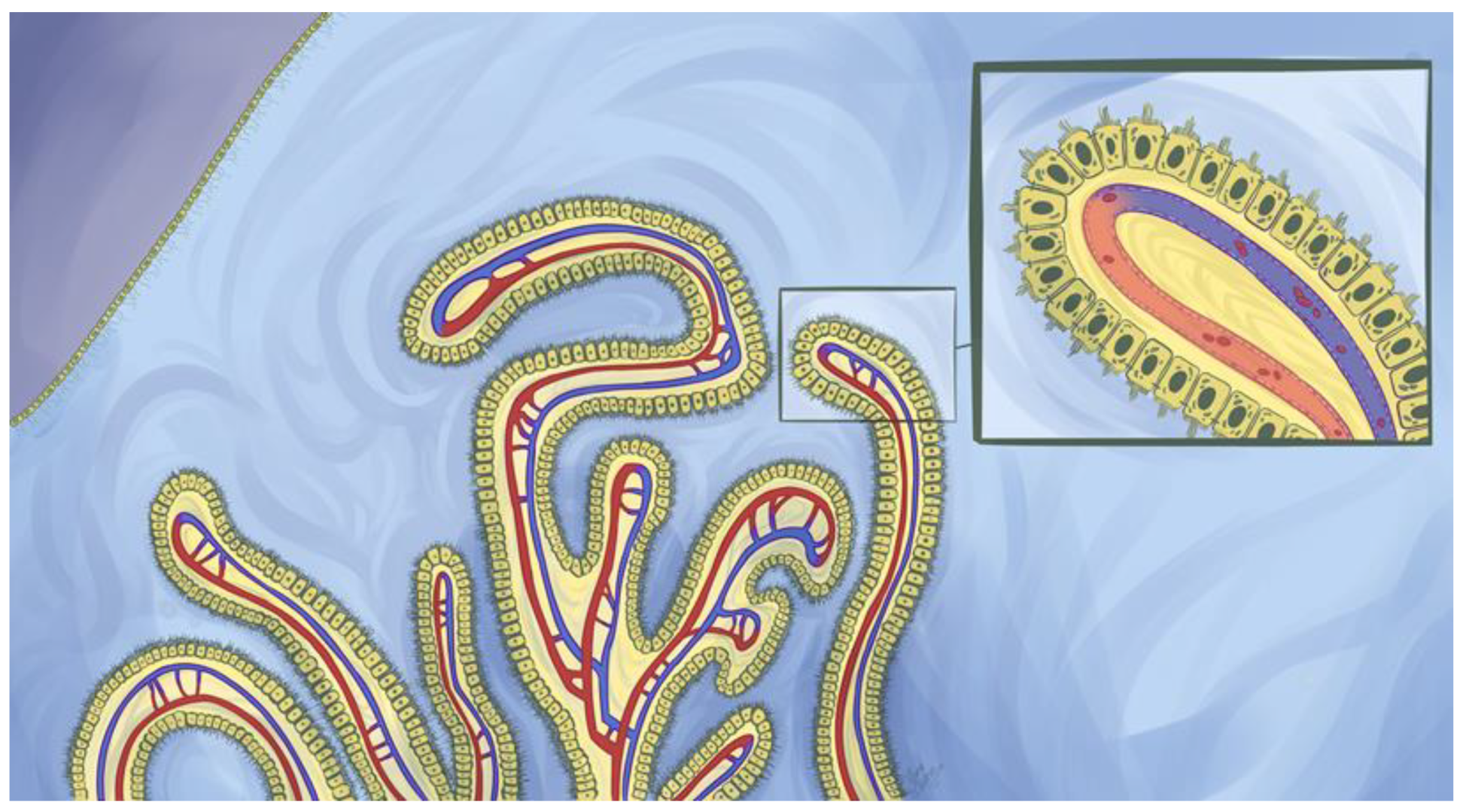
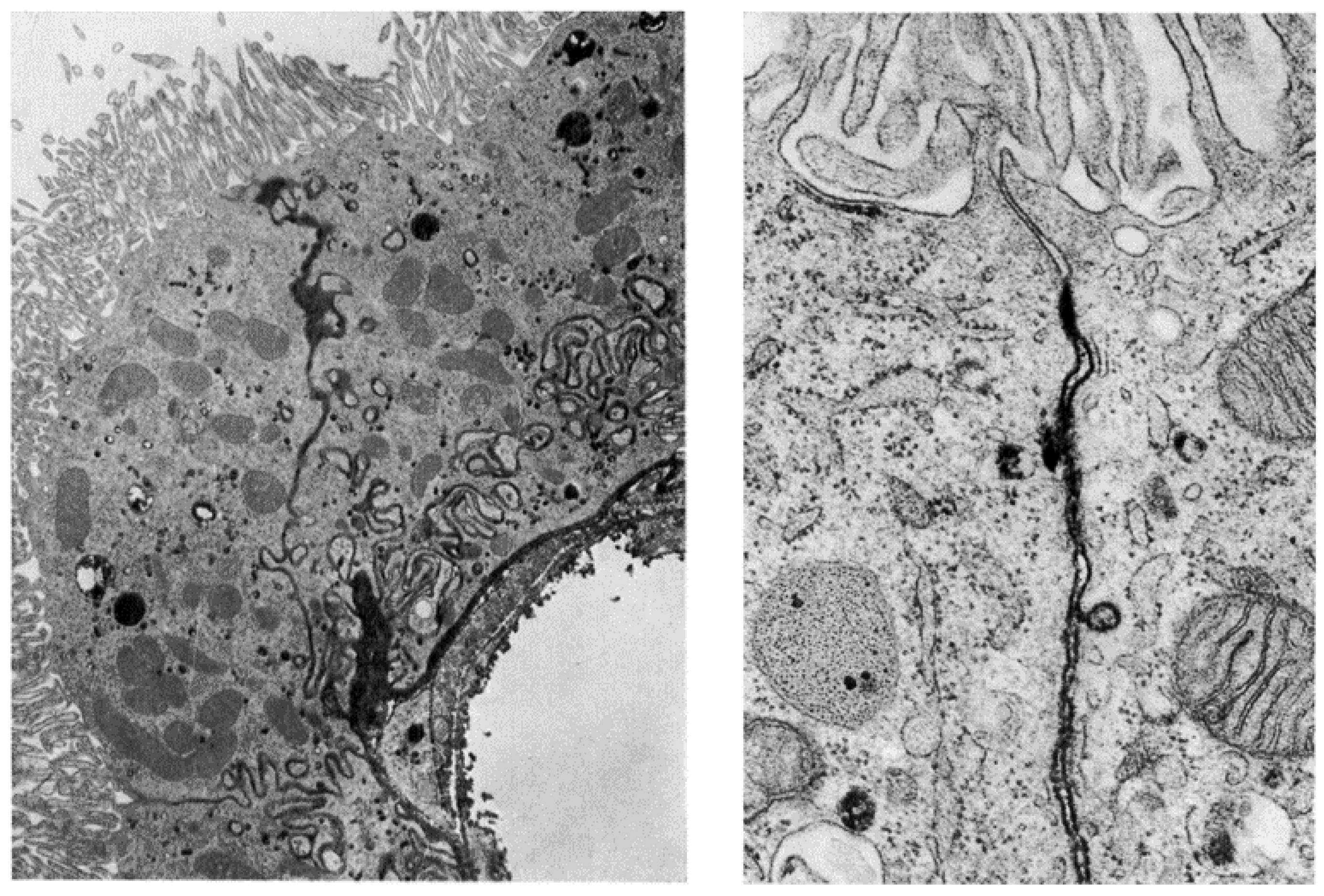
2. The Structure and Cellular Organization of the Ventricular System
3. Functions of the Choroid Plexuses
4. Peptide and Protein Transport across the Choroid Plexuses
4.1. Carrier-Mediated Transport
4.2. Receptor-Mediated Transport
4.2.1. Transferrin Receptor
4.2.2. Insulin and Insulin-Like Growth Factor Receptors
4.2.3. The Low-Density Lipoprotein Receptor Family
4.2.4. Neonatal Fc Receptor
4.2.5. SPARC
5. Preclinical Methodology to Study Protein Transport across the CPs
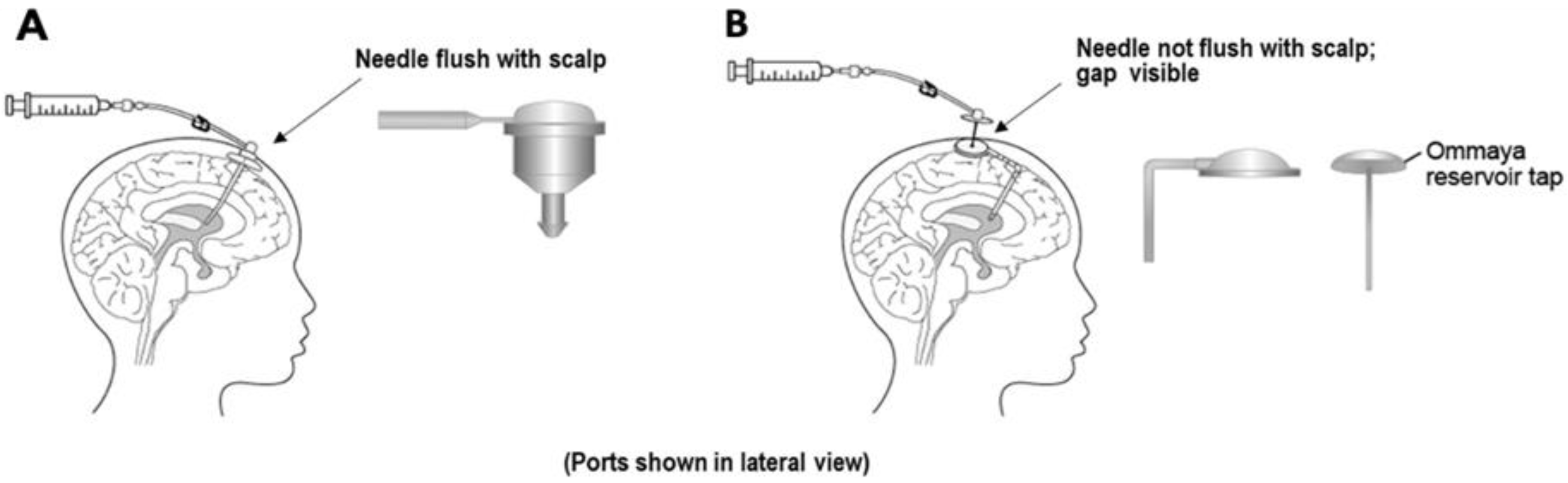
5.1. Imaging
5.2. Ventriculocisternal Perfusions
5.3. In Situ Choroid Plexus Perfusion
5.4. Choroid Plexus Epithelial Cell Culture
6. Targeting the Choroid Plexuses for Brain Delivery: Futile Strategy or Deviously Wise?
6.1. Intracranial Cerebrospinal Fluid Flow—Overview
6.2. Intracranial Barriers to Cerebrospinal Fluid-Brain Parenchyma Exchange of Protein Therapeutics
6.2.1. The Choroid Plexuses
6.2.2. The Ependymal Cells
6.2.3. The Perivascular Space
6.2.4. Why Target the Choroid Plexus for Central Nervous System Protein Drug Delivery?
7. Clinical Use of Peptide/Protein-Based Therapies for Neurological Diseases
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Drake, R.; Vogl, A.W.; Mitchell, A.W. Gray’s Anatomy for Students, 4th ed.; Elsevier Health Sciences: Philadelphia, PA, USA, 2019. [Google Scholar]
- Crossman, A.R.; Neary, D. Neuroanatomy: An Illustrated Colour Text, 6th ed.; Elsevier Health Sciences: Philadelphia, PA, USA, 2019. [Google Scholar]
- Del Bigio, M.R. Ependymal cells: Biology and pathology. Acta Neuropathol. 2010, 119, 55–73. [Google Scholar] [CrossRef] [PubMed]
- Bruni, J.E.; Del Bigio, M.R.; Clattenburg, R.E. Ependyma: Normal and pathological. A review of the literature. Brain Res. 1985, 356, 1–19. [Google Scholar] [CrossRef]
- Del Bigio, M.R. The ependyma: A protective barrier between brain and cerebrospinal fluid. Glia 1995, 14, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Gabrion, J.B.; Herbuté, S.; Bouillé, C.; Maurel, D.; Kuchler-Bopp, S.; Laabich, A.; Delaunoy, J.P. Ependymal and choroidal cells in culture: Characterization and functional differentiation. Microsc. Res. Tech. 1998, 41, 124–157. [Google Scholar] [CrossRef]
- Brightman, M.W.; Palay, S.L. The fine structure of ependyma in the brain of the rat. J. Cell Biol. 1963, 19, 415–439. [Google Scholar] [CrossRef]
- Mirzadeh, Z.; Kusne, Y.; Duran-Moreno, M.; Cabrales, E.; Gil-Perotin, S.; Ortiz, C.; Chen, B.; Garcia-Verdugo, J.M.; Sanai, N.; Alvarez-Buylla, A. Bi- and uniciliated ependymal cells define continuous floor-plate-derived tanycytic territories. Nat. Commun. 2017, 8, 13759. [Google Scholar] [CrossRef]
- Tennyson, V.M.; Pappas, G.D. An electron microscope study of ependymal cells of the fetal, early postnatal and adult rabbit. Z. Zellforsch. Mikrosk. Anat. 1962, 56, 595–618. [Google Scholar] [CrossRef]
- Bruni, J.E. Scanning and transmission electron microscopy of the ependymal lining of the third ventricle. Can. J. Neurol. Sci. 1974, 1, 59–73. [Google Scholar] [CrossRef]
- Leonhardt, H.; Desaga, U. Recent observations on ependyma and subependymal basement membranes. Acta Neurochir. (Wien.) 1975, 31, 153–159. [Google Scholar] [CrossRef]
- Cserr, H.F.; Cooper, D.N.; Milhorat, T.H. Flow of cerebral interstitial fluid as indicated by the removal of extracellular markers from rat caudate nucleus. Exp. Eye Res. 1977, 25 (Suppl. 1), 461–473. [Google Scholar] [CrossRef]
- Torack, R.M. Ultrastructural studies of subependymal extracellular spaces in adult and neonatal rat brain. Histochemistry 1980, 68, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Tsutsumi, S.; Ono, H.; Ishii, H. Subependymal hyperintense layer on CISS sequence: An MRI study. Childs Nerv. Syst. 2020. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, C. Signals that go with the flow. Trends Neurosci. 1999, 22, 143–145. [Google Scholar] [CrossRef]
- Gotow, T.; Hashimoto, P.H. Fine structure of ependymal cysts in and around the area postrema of the rat. Cell Tissue Res. 1980, 206, 303–318. [Google Scholar] [CrossRef] [PubMed]
- Brightman, M.W.; Reese, T.S. Junctions between intimately apposed cell membranes in the vertebrate brain. J. Cell Biol. 1969, 40, 648–677. [Google Scholar] [CrossRef] [PubMed]
- Whish, S.; Dziegielewska, K.M.; Møllgård, K.; Noor, N.M.; Liddelow, S.A.; Habgood, M.D.; Richardson, S.J.; Saunders, N.R. The inner CSF-brain barrier: Developmentally controlled access to the brain via intercellular junctions. Front. Neurosci. 2015, 9, 16. [Google Scholar] [CrossRef] [PubMed]
- Brightman, M.W. The intracerebral movement of proteins injected into blood and cerebrospinal fluid of mice. Prog. Brain Res. 1968, 29, 19–40. [Google Scholar] [CrossRef]
- Rall, D.P. Transport through the ependymal linings. Prog. Brain Res. 1968, 29, 159–172. [Google Scholar] [CrossRef]
- Paxinos, G. The Rat Nervous System, 4th ed.; Academic Press: London, UK, 2015. [Google Scholar]
- Duvernoy, H.M.; Risold, P.Y. The circumventricular organs: An atlas of comparative anatomy and vascularization. Brain Res. Rev. 2007, 56, 119–147. [Google Scholar] [CrossRef]
- Benarroch, E.E. Circumventricular organs: Receptive and homeostatic functions and clinical implications. Neurology 2011, 77, 1198–1204. [Google Scholar] [CrossRef]
- Mastorakos, P.; McGavern, D. The anatomy and immunology of vasculature in the central nervous system. Sci. Immunol. 2019, 4. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, E.; Guerra, M.; Peruzzo, B.; Blázquez, J.L. Tanycytes: A rich morphological history to underpin future molecular and physiological investigations. J. Neuroendocrinol. 2019, 31, e12690. [Google Scholar] [CrossRef] [PubMed]
- Langlet, F.; Mullier, A.; Bouret, S.G.; Prevot, V.; Dehouck, B. Tanycyte-like cells form a blood-cerebrospinal fluid barrier in the circumventricular organs of the mouse brain. J. Comp. Neurol. 2013, 521, 3389–3405. [Google Scholar] [CrossRef] [PubMed]
- Mullier, A.; Bouret, S.G.; Prevot, V.; Dehouck, B. Differential distribution of tight junction proteins suggests a role for tanycytes in blood-hypothalamus barrier regulation in the adult mouse brain. J. Comp. Neurol. 2010, 518, 943–962. [Google Scholar] [CrossRef] [PubMed]
- Petrov, T.; Howarth, A.G.; Krukoff, T.L.; Stevenson, B.R. Distribution of the tight junction-associated protein ZO-1 in circumventricular organs of the CNS. Brain Res. Mol. Brain Res. 1994, 21, 235–246. [Google Scholar] [CrossRef]
- Gotow, T.; Hashimoto, P.H. Fine structure of the ependyma and intercellular junctions in the area postrema of the rat. Cell Tissue Res. 1979, 201, 207–225. [Google Scholar] [CrossRef]
- Pelletier, G.; Dupont, A.; Puviani, R. Ultrastructural study of the uptake of peroxidase by the rat median eminence. Cell Tissue Res. 1975, 156, 521–532. [Google Scholar] [CrossRef]
- Müller-Fielitz, H.; Stahr, M.; Bernau, M.; Richter, M.; Abele, S.; Krajka, V.; Benzin, A.; Wenzel, J.; Kalies, K.; Mittag, J.; et al. Tanycytes control the hormonal output of the hypothalamic-pituitary-thyroid axis. Nat. Commun. 2017, 8, 484. [Google Scholar] [CrossRef]
- Balland, E.; Dam, J.; Langlet, F.; Caron, E.; Steculorum, S.; Messina, A.; Rasika, S.; Falluel-Morel, A.; Anouar, Y.; Dehouck, B.; et al. Hypothalamic tanycytes are an ERK-gated conduit for leptin into the brain. Cell Metab. 2014, 19, 293–301. [Google Scholar] [CrossRef]
- Bolborea, M.; Pollatzek, E.; Benford, H.; Sotelo-Hitschfeld, T.; Dale, N. Hypothalamic tanycytes generate acute hyperphagia through activation of the arcuate neuronal network. Proc. Natl. Acad. Sci. USA 2020, 117, 14473–14481. [Google Scholar] [CrossRef]
- Elizondo-Vega, R.J.; Recabal, A.; Oyarce, K. Nutrient sensing by hypothalamic tanycytes. Front. Endocrinol. (Lausanne) 2019, 10, 244. [Google Scholar] [CrossRef] [PubMed]
- Strazielle, N.; Ghersi-Egea, J.F. Physiology of blood-brain interfaces in relation to brain disposition of small compounds and macromolecules. Mol. Pharm. 2013, 10, 1473–1491. [Google Scholar] [CrossRef] [PubMed]
- Hurley, J.V.; Anderson, R.M.; Sexton, P.T. The fate of plasma protein which escapes from blood vessels of the choroid plexus of the rat--an electron microscope study. J. Pathol. 1981, 134, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Pappas, G.D.; Tennyson, V.M. An electron microscopic study of the passage of colloidal particles from the blood vessels of the ciliary processes and choroid plexus of the rabbit. J. Cell Biol. 1962, 15, 227–239. [Google Scholar] [CrossRef]
- Wolburg, H.; Wolburg-Buchholz, K.; Liebner, S.; Engelhardt, B. Claudin-1, claudin-2 and claudin-11 are present in tight junctions of choroid plexus epithelium of the mouse. Neurosci. Lett. 2001, 307, 77–80. [Google Scholar] [CrossRef]
- Spector, R.; Keep, R.F.; Robert Snodgrass, S.; Smith, Q.R.; Johanson, C.E. A balanced view of choroid plexus structure and function: Focus on adult humans. Exp. Neurol. 2015, 267, 78–86. [Google Scholar] [CrossRef]
- Ghersi-Egea, J.F.; Strazielle, N.; Catala, M.; Silva-Vargas, V.; Doetsch, F.; Engelhardt, B. Molecular anatomy and functions of the choroidal blood-cerebrospinal fluid barrier in health and disease. Acta Neuropathol. 2018, 135, 337–361. [Google Scholar] [CrossRef]
- Spector, R.; Johanson, C.E. Vectorial ligand transport through mammalian choroid plexus. Pharm. Res. 2010, 27, 2054–2062. [Google Scholar] [CrossRef]
- Spector, R.; Johanson, C.E. The mammalian choroid plexus. Sci. Am. 1989, 261, 68–74. [Google Scholar] [CrossRef]
- Redzic, Z.B.; Preston, J.E.; Duncan, J.A.; Chodobski, A.; Szmydynger-Chodobska, J. The choroid plexus-cerebrospinal fluid system: From development to aging. Curr. Top. Dev. Biol. 2005, 71, 1–52. [Google Scholar] [CrossRef]
- Segal, M.B. The choroid plexuses and the barriers between the blood and the cerebrospinal fluid. Cell. Mol. Neurobiol. 2000, 20, 183–196. [Google Scholar] [CrossRef]
- Johansson, P.A. The choroid plexuses and their impact on developmental neurogenesis. Front. Neurosci. 2014, 8, 340. [Google Scholar] [CrossRef] [PubMed]
- Hladky, S.B.; Barrand, M.A. Fluid and ion transfer across the blood-brain and blood-cerebrospinal fluid barriers; a comparative account of mechanisms and roles. Fluids Barriers CNS 2016, 13, 19. [Google Scholar] [CrossRef] [PubMed]
- Damkier, H.H.; Brown, P.D.; Praetorius, J. Cerebrospinal fluid secretion by the choroid plexus. Physiol. Rev. 2013, 93, 1847–1892. [Google Scholar] [CrossRef]
- Cserr, H.F. Physiology of the choroid plexus. Physiol. Rev. 1971, 51, 273–311. [Google Scholar] [CrossRef] [PubMed]
- Redzic, Z.B.; Segal, M.B. The structure of the choroid plexus and the physiology of the choroid plexus epithelium. Adv. Drug Deliv. Rev. 2004, 56, 1695–1716. [Google Scholar] [CrossRef] [PubMed]
- Johanson, C.E.; Stopa, E.G.; McMillan, P.N. The blood-cerebrospinal fluid barrier: Structure and functional significance. Methods Mol. Biol. 2011, 686, 101–131. [Google Scholar] [CrossRef] [PubMed]
- Brinker, T.; Stopa, E.; Morrison, J.; Klinge, P. A new look at cerebrospinal fluid circulation. Fluids Barriers CNS 2014, 11, 10. [Google Scholar] [CrossRef]
- Sakka, L.; Coll, G.; Chazal, J. Anatomy and physiology of cerebrospinal fluid. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2011, 128, 309–316. [Google Scholar] [CrossRef]
- Spector, R.; Robert Snodgrass, S.; Johanson, C.E. A balanced view of the cerebrospinal fluid composition and functions: Focus on adult humans. Exp. Neurol. 2015, 273, 57–68. [Google Scholar] [CrossRef]
- Praetorius, J.; Damkier, H.H. Transport across the choroid plexus epithelium. Am. J. Physiol. Cell Physiol. 2017, 312, C673–C686. [Google Scholar] [CrossRef] [PubMed]
- Sotiriou, S.; Gispert, S.; Cheng, J.; Wang, Y.; Chen, A.; Hoogstraten-Miller, S.; Miller, G.F.; Kwon, O.; Levine, M.; Guttentag, S.H.; et al. Ascorbic-acid transporter Slc23a1 is essential for vitamin C transport into the brain and for perinatal survival. Nat. Med. 2002, 8, 514–517. [Google Scholar] [CrossRef] [PubMed]
- Herbert, J.; Wilcox, J.N.; Pham, K.T.; Fremeau, R.T., Jr.; Zeviani, M.; Dwork, A.; Soprano, D.R.; Makover, A.; Goodman, D.S.; Zimmerman, E.A.; et al. Transthyretin: A choroid plexus-specific transport protein in human brain. The 1986 S. Weir Mitchell award. Neurology 1986, 36, 900–911. [Google Scholar] [CrossRef]
- Richardson, S.J.; Wijayagunaratne, R.C.; D’Souza, D.G.; Darras, V.M.; Van Herck, S.L. Transport of thyroid hormones via the choroid plexus into the brain: The roles of transthyretin and thyroid hormone transmembrane transporters. Front. Neurosci. 2015, 9, 66. [Google Scholar] [CrossRef] [PubMed]
- Mazucanti, C.H.; Liu, Q.R.; Lang, D.; Huang, N.; O’Connell, J.F.; Camandola, S.; Egan, J.M. Release of insulin produced by the choroid plexis is regulated by serotonergic signaling. JCI Insight 2019, 4. [Google Scholar] [CrossRef] [PubMed]
- Falcão, A.M.; Marques, F.; Novais, A.; Sousa, N.; Palha, J.A.; Sousa, J.C. The path from the choroid plexus to the subventricular zone: Go with the flow! Front. Cell. Neurosci. 2012, 6, 34. [Google Scholar] [CrossRef] [PubMed]
- Fishman, R.A. Exchange of albumin between plasma and cerebrospinal fluid. Am. J. Physiol. 1953, 175, 96–98. [Google Scholar] [CrossRef]
- Hochwald, G.M.; Wallenstein, M. Exchange of albumin between blood, cerebrospinal fluid, and brain in the cat. Am. J. Physiol. 1967, 212, 1199–1204. [Google Scholar] [CrossRef]
- Rapoport, S.I. Passage of proteins from blood to cerebrospinal fluid. In Neurobiology of Cerebrospinal Fluid 2; Springer: New York, NY, USA, 1983; pp. 233–245. [Google Scholar]
- Morris, M.E.; Rodriguez-Cruz, V.; Felmlee, M.A. SLC and ABC transporters: Expression, localization, and species differences at the blood-brain and the blood-cerebrospinal fluid barriers. AAPS J. 2017, 19, 1317–1331. [Google Scholar] [CrossRef]
- Wang, Q.; Zuo, Z. Impact of transporters and enzymes from blood–cerebrospinal fluid barrier and brain parenchyma on CNS drug uptake. Expert Opin. Drug Metab. Toxicol. 2018, 14, 961–972. [Google Scholar] [CrossRef]
- Gomez-Zepeda, D.; Taghi, M.; Scherrmann, J.-M.; Decleves, X.; Menet, M.-C. ABC transporters at the blood–brain interfaces, their study models, and drug delivery implications in gliomas. Pharmaceutics 2020, 12, 20. [Google Scholar] [CrossRef]
- Rapoport, S.I.; Pettigrew, K.D. A heterogenous, pore-vesicle membrane model for protein transfer from blood to cerebrospinal fluid at the choroid plexus. Microvasc. Res. 1979, 18, 105–119. [Google Scholar] [CrossRef]
- Balin, B.J.; Broadwell, R.D. Transcytosis of protein through the mammalian cerebral epithelium and endothelium. I. Choroid plexus and the blood-cerebrospinal fluid barrier. J. Neurocytol. 1988, 17, 809–826. [Google Scholar] [CrossRef] [PubMed]
- Mangurian, L.P.; Walsh, R.J.; Posner, B.I. Prolactin enhancement of its own uptake at the choroid plexus. Endocrinology 1992, 131, 698–702. [Google Scholar] [CrossRef] [PubMed]
- Walsh, R.J.; Slaby, F.J.; Posner, B.I. A receptor-mediated mechanism for the transport of prolactin from blood to cerebrospinal fluid. Endocrinology 1987, 120, 1846–1850. [Google Scholar] [CrossRef]
- Di Carlo, R.; Muccioli, G.; Papotti, M.; Bussolati, G. Characterization of prolactin receptor in human brain and choroid plexus. Brain Res. 1992, 570, 341–346. [Google Scholar] [CrossRef]
- Lai, Z.; Roos, P.; Olsson, Y.; Larsson, C.; Nyberg, F. Characterization of prolactin receptors in human choroid plexus. Neuroendocrinology 1992, 56, 225–233. [Google Scholar] [CrossRef]
- Rubio-Aliaga, I.; Daniel, H. Peptide transporters and their roles in physiological processes and drug disposition. Xenobiotica 2008, 38, 1022–1042. [Google Scholar] [CrossRef]
- Smith, D.E.; Clémençon, B.; Hediger, M.A. Proton-coupled oligopeptide transporter family SLC15: Physiological, pharmacological and pathological implications. Mol. Aspects Med. 2013, 34, 323–336. [Google Scholar] [CrossRef]
- Berger, U.V.; Hediger, M. Distribution of peptide transporter PEPT2 mRNA in the rat nervous system. Anat. Embryol. 1999, 199, 439–449. [Google Scholar] [CrossRef]
- Choudhuri, S.; Cherrington, N.J.; Li, N.; Klaassen, C.D. Constitutive expression of various xenobiotic and endobiotic transporter mRNAs in the choroid plexus of rats. Drug Metab. Dispos. 2003, 31, 1337–1345. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Smith, D.E.; Keep, R.F.; Brosius, F.C. Immunolocalization of the proton-coupled oligopeptide transporter PEPT2 in developing rat brain. Mol. Pharm. 2004, 1, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Shu, C.; Shen, H.; Teuscher, N.S.; Lorenzi, P.J.; Keep, R.F.; Smith, D.E. Role of PEPT2 in peptide/mimetic trafficking at the blood-cerebrospinal fluid barrier: Studies in rat choroid plexus epithelial cells in primary culture. J. Pharmacol. Exp. Ther. 2002, 301, 820–829. [Google Scholar] [CrossRef] [PubMed]
- Trambauer, J.; Fukumori, A.; Kretner, B.; Steiner, H. Chapter Six - Analyzing Amyloid-β Peptide Modulation Profiles and Binding Sites of γ-Secretase Modulators. In Methods Enzymol; Gelb, M.H., Ed.; Academic Press: Cambridge, MA, USA, 2017; Volume 584, pp. 157–183. [Google Scholar]
- Matsumoto, K.; Chiba, Y.; Fujihara, R.; Kubo, H.; Sakamoto, H.; Ueno, M. Immunohistochemical analysis of transporters related to clearance of amyloid-β peptides through blood-cerebrospinal fluid barrier in human brain. Histochem. Cell Biol. 2015, 144, 597–611. [Google Scholar] [CrossRef] [PubMed]
- Lam, F.C.; Liu, R.; Lu, P.; Shapiro, A.B.; Renoir, J.-M.; Sharom, F.J.; Reiner, P.B. β-Amyloid efflux mediated by p-glycoprotein. J. Neurochem. 2001, 76, 1121–1128. [Google Scholar] [CrossRef]
- Rao, V.V.; Dahlheimer, J.L.; Bardgett, M.E.; Snyder, A.Z.; Finch, R.A.; Sartorelli, A.C.; Piwnica-Worms, D. Choroid plexus epithelial expression of MDR1 P glycoprotein and multidrug resistance-associated protein contribute to the blood-cerebrospinal-fluid drug-permeability barrier. Proc. Natl. Acad. Sci. USA 1999, 96, 3900–3905. [Google Scholar] [CrossRef]
- Stieger, B.; Gao, B. Drug transporters in the central nervous system. Clin. Pharmacokinet. 2015, 54, 225–242. [Google Scholar] [CrossRef]
- Kuhnke, D.; Jedlitschky, G.; Grube, M.; Krohn, M.; Jucker, M.; Mosyagin, I.; Cascorbi, I.; Walker, L.C.; Kroemer, H.K.; Warzok, R.W.; et al. MDR1-P-Glycoprotein (ABCB1) mediates transport of Alzheimer’s amyloid-β peptides—Implications for the mechanisms of Aβ clearance at the Blood–Brain Barrier. Brain Pathol. 2007, 17, 347–353. [Google Scholar] [CrossRef]
- Moos, T.; Morgan, E.H. Transferrin and Transferrin Receptor Function in Brain Barrier Systems. Cell. Mol. Neurobiol. 2000, 20, 77–95. [Google Scholar] [CrossRef]
- Giometto, B.; Bozza, F.; Argentiero, V.; Gallo, P.; Pagni, S.; Piccinno, M.G.; Tavolato, B. Transferrin receptors in rat central nervous system. An immunocytochemical study. J. Neurol. Sci. 1990, 98, 81–90. [Google Scholar] [CrossRef]
- Moos, T. Immunohistochemical localization of intraneuronal transferrin receptor immunoreactivity in the adult mouse central nervous system. J. Comp. Neurol. 1996, 375, 675–692. [Google Scholar] [CrossRef]
- Aldred, A.R.; Dickson, P.W.; Marley, P.; Schreiber, G. Distribution of transferrin synthesis in brain and other tissues in the rat. J. Biol. Chem. 1987, 262, 5293–5297. [Google Scholar] [PubMed]
- Morris, C.; Candy, J.; Bloxharn, C.; Edwardson, J. Immunocytochemical localisation of transferrin in the human brain. Cells Tissues Organs 1992, 143, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Connor, J.R.; Ponnuru, P.; Wang, X.-S.; Patton, S.M.; Allen, R.P.; Earley, C.J. Profile of altered brain iron acquisition in restless legs syndrome. Brain 2011, 134, 959–968. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Miller, D.S.; Zheng, W. Intracellular localization and subsequent redistribution of metal transporters in a rat choroid plexus model following exposure to manganese or iron. Toxicol. Appl. Pharmacol. 2008, 230, 167–174. [Google Scholar] [CrossRef][Green Version]
- Deane, R.; Zheng, W.; Zlokovic, B.V. Brain capillary endothelium and choroid plexus epithelium regulate transport of transferrin-bound and free iron into the rat brain. J. Neurochem. 2004, 88, 813–820. [Google Scholar] [CrossRef]
- Werner, H.; Roberts, C.; Raizada, M.; Bondy, C.; Adamo, M.; LeRoith, D. Developmental regulation of the insulin and insulin-like growth factor receptors in the central nervous system. In Receptors in the Developing Nervous System; Zagon, I.S., McLaughlin, P.J., Eds.; Chapman & Hall: London, UK, 1993; pp. 109–127. [Google Scholar]
- MARKS, J.L.; PORTE JR, D.; STAHL, W.L.; BASKIN, D.G. Localization of insulin receptor mRNA in rat brain by in situ hybridization. Endocrinology 1990, 127, 3234–3236. [Google Scholar] [CrossRef]
- Baskin, D.G.; Brewitt, B.; Davidson, D.A.; Corp, E.; Paquette, T.; Figlewicz, D.P.; Lewellen, T.K.; Graham, M.K.; Woods, S.G.; Dorsa, D.M. Quantitative autoradiographic evidence for insulin receptors in the choroid plexus of the rat brain. Diabetes 1986, 35, 246–249. [Google Scholar] [CrossRef][Green Version]
- Werther, G.A.; Hogg, A.; Oldfield, B.J.; McKinley, M.J.; Figdor, R.; Mendelsohn, F.A. Localization and characterization of insulin-like growth factor-I receptors in rat brain and pituitary gland using in vitro autoradiography and computerized densitometry* a distinct distribution from insulin receptors. J. Neuroendocrinol. 1989, 1, 369–377. [Google Scholar] [CrossRef]
- Schulingkamp, R.; Pagano, T.; Hung, D.; Raffa, R. Insulin receptors and insulin action in the brain: Review and clinical implications. Neurosci. Biobehav. Rev. 2000, 24, 855–872. [Google Scholar] [CrossRef]
- Marks, J.L.; Porte, D., Jr.; Baskin, D.G. Localization of type I insulin-like growth factor receptor messenger RNA in the adult rat brain by in situ hybridization. Mol. Endocrinol. 1991, 5, 1158–1168. [Google Scholar] [CrossRef] [PubMed]
- Ayer-le Lievre, C.; Stahlbom, P.A.; Sara, V.R. Expression of IGF-I and -II mRNA in the brain and craniofacial region of the rat fetus. Development 1991, 111, 105–115. [Google Scholar] [PubMed]
- Lee, W.H.; Michels, K.M.; Bondy, C.A. Localization of insulin-like growth factor binding protein-2 messenger RNA during postnatal brain development: Correlation with insulin-like growth factors I and II. Neuroscience 1993, 53, 251–265. [Google Scholar] [CrossRef]
- Couce, M.E.; Weatherington, A.J.; McGinty, J.F. Expression of insulin-like growth factor-II (IGF-II) and IGF-II/mannose-6-phosphate receptor in the rat hippocampus: An in situ hybridization and immunocytochemical study. Endocrinology 1992, 131, 1636–1642. [Google Scholar] [CrossRef]
- De Keyser, J.; Wilczak, N.; De Backer, J.P.; Herroelen, L.; Vauquelin, G. Insulin-like growth factor-I receptors in human brain and pituitary gland: An autoradiographic study. Synapse 1994, 17, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, C.; Blay, P.; Nielsen, F.C.; Gammeltoft, S. Gene expression and receptor binding of insulin-like growth factor-II in pig choroid plexus epithelial cells. J. Neurochem. 1992, 58, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Ocrant, I.; Parmelee, J.T. Immunofluorescent cytometry and electron microscopic immunolocalization of insulin-like growth factor (IGF)-II receptors in infant rat choroid plexus. Mol. Cell. Neurosci. 1992, 3, 354–359. [Google Scholar] [CrossRef]
- Bolos, M.; Fernandez, S.; Torres-Aleman, I. Oral administration of a GSK3 inhibitor increases brain insulin-like growth factor I levels. J. Biol. Chem. 2010, 285, 17693–17700. [Google Scholar] [CrossRef]
- Dieckmann, M.; Dietrich, M.F.; Herz, J. Lipoprotein receptors–an evolutionarily ancient multifunctional receptor family. Biol. Chem. 2010, 391, 1341–1363. [Google Scholar] [CrossRef]
- Pohlkamp, T.; Wasser, C.R.; Herz, J. Functional roles of the interaction of APP and lipoprotein receptors. Front. Mol. Neurosci. 2017, 10, 54. [Google Scholar] [CrossRef]
- Strazielle, N.; Ghersi-Egea, J.-F. Potential pathways for CNS drug delivery across the blood-cerebrospinal fluid barrier. Curr. Pharm. Des. 2016, 22, 5463–5476. [Google Scholar] [CrossRef]
- Ruzali, W.A.W.; Kehoe, P.G.; Love, S. LRP1 expression in cerebral cortex, choroid plexus and meningeal blood vessels: Relationship to cerebral amyloid angiopathy and APOE status. Neurosci. Lett. 2012, 525, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Pascale, C.L.; Miller, M.C.; Chiu, C.; Boylan, M.; Caralopoulos, I.N.; Gonzalez, L.; Johanson, C.E.; Silverberg, G.D. Amyloid-beta transporter expression at the blood-CSF barrier is age-dependent. Fluids Barriers CNS 2011, 8, 21. [Google Scholar] [CrossRef] [PubMed]
- Page, K.J.; Hollister, R.D.; Hyman, B.T. Dissociation of apolipoprotein and apolipoprotein receptor response to lesion in the rat brain: An in situ hybridization study. Neuroscience 1998, 85, 1161–1171. [Google Scholar] [CrossRef]
- Fujiyoshi, M.; Tachikawa, M.; Ohtsuki, S.; Ito, S.; Uchida, Y.; Akanuma, S.i.; Kamiie, J.; Hashimoto, T.; Hosoya, K.i.; Iwatsubo, T. Amyloid-β peptide (1–40) elimination from cerebrospinal fluid involves low-density lipoprotein receptor-related protein 1 at the blood-cerebrospinal fluid barrier. J. Neurochem. 2011, 118, 407–415. [Google Scholar] [CrossRef]
- Behl, M.; Zhang, Y.; Monnot, A.D.; Jiang, W.; Zheng, W. Increased beta-amyloid levels in the choroid plexus following lead exposure and the involvement of low-density lipoprotein receptor protein-1. Toxicol. Appl. Pharmacol. 2009, 240, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Bachinsky, D.R.; Stamenkovic, I.; Strickland, D.K.; Brown, D.; Andres, G.; McCluskey, R.T. Organ distribution in rats of two members of the low-density lipoprotein receptor gene family, gp330 and LRP/alpha 2MR, and the receptor-associated protein (RAP). J. Histochem. Cytochem. 1994, 42, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Wolf, B.; Lopes, M.; VandenBerg, S.; Gonias, S. Characterization and immunohistochemical localization of alpha 2-macroglobulin receptor (low-density lipoprotein receptor-related protein) in human brain. Am. J. Clin. Pathol. 1992, 141, 37. [Google Scholar]
- Shibata, M.; Yamada, S.; Kumar, S.R.; Calero, M.; Bading, J.; Frangione, B.; Holtzman, D.M.; Miller, C.A.; Strickland, D.K.; Ghiso, J. Clearance of Alzheimer’s amyloid-β 1–40 peptide from brain by LDL receptor–related protein-1 at the blood-brain barrier. J. Clin. Investig. 2000, 106, 1489–1499. [Google Scholar] [CrossRef]
- Bento-Abreu, A.; Velasco, A.; Polo-Hernández, E.; Pérez-Reyes, P.L.; Tabernero, A.; Medina, J.M. Megalin is a receptor for albumin in astrocytes and is required for the synthesis of the neurotrophic factor oleic acid. J. Neurochem. 2008, 106, 1149–1159. [Google Scholar] [CrossRef]
- Chun, J.T.; Wang, L.; Pasinetti, G.M.; Finch, C.E.; Zlokovic, B.V. Glycoprotein 330/megalin (LRP-2) has low prevalence as mRNA and protein in brain microvessels and choroid plexus. Exp. Neurol. 1999, 157, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Spuch, C.; Antequera, D.; Pascual, C.; Abilleira, S.; Blanco, M.; Moreno-Carretero, M.J.; Romero-López, J.; Ishida, T.; Molina, J.A.; Villarejo, A.; et al. Soluble megalin is reduced in cerebrospinal fluid samples of Alzheimer’s disease patients. Front. Cell. Neurosci. 2015, 9, 134. [Google Scholar] [CrossRef]
- Hammad, S.M.; Ranganathan, S.; Loukinova, E.; Twal, W.O.; Argraves, W.S. Interaction of apolipoprotein J-amyloid beta-peptide complex with low density lipoprotein receptor-related protein-2/megalin. A mechanism to prevent pathological accumulation of amyloid beta-peptide. J. Biol. Chem. 1997, 272, 18644–18649. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, M.O.; Spuch, C.; Antequera, D.; Rodal, I.; de Yébenes, J.G.; Molina, J.A.; Bermejo, F.; Carro, E. Megalin mediates the transport of leptin across the blood-CSF barrier. Neurobiol. Aging 2008, 29, 902–912. [Google Scholar] [CrossRef]
- Carro, E.; Spuch, C.; Trejo, J.L.; Antequera, D.; Torres-Aleman, I. Choroid plexus megalin is involved in neuroprotection by serum insulin-like growth factor I. J. Neurosci. Res. 2005, 25, 10884–10893. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Iijima, H.; Goto, K.; Sakai, J.; Ishii, H.; Kim, H.J.; Suzuki, H.; Kondo, H.; Saeki, S.; Yamamoto, T. Human apolipoprotein E receptor 2. A novel lipoprotein receptor of the low density lipoprotein receptor family predominantly expressed in brain. J. Biol. Chem. 1996, 271, 8373–8380. [Google Scholar] [CrossRef] [PubMed]
- Burk, R.F.; Hill, K.E.; Motley, A.K.; Winfrey, V.P.; Kurokawa, S.; Mitchell, S.L.; Zhang, W. Selenoprotein P and apolipoprotein E receptor-2 interact at the blood-brain barrier and also within the brain to maintain an essential selenium pool that protects against neurodegeneration. FASEB J. 2014, 28, 3579–3588. [Google Scholar] [CrossRef]
- Boyles, J.K.; Pitas, R.E.; Wilson, E.; Mahley, R.W.; Taylor, J.M. Apolipoprotein E associated with astrocytic glia of the central nervous system and with nonmyelinating glia of the peripheral nervous system. J. Clin. Investig. 1985, 76, 1501–1513. [Google Scholar] [CrossRef]
- Pyzik, M.; Sand, K.M.K.; Hubbard, J.J.; Andersen, J.T.; Sandlie, I.; Blumberg, R.S. The Neonatal Fc Receptor (FcRn): A Misnomer? Front. Immunol. 2019, 10. [Google Scholar] [CrossRef]
- Schlachetzki, F.; Zhu, C.; Pardridge, W.M. Expression of the neonatal Fc receptor (FcRn) at the blood–brain barrier. J. Neurochem. 2002, 81, 203–206. [Google Scholar] [CrossRef]
- Farrell, C.L.; Yang, J.; Pardridge, W.M. GLUT-1 glucose transporter is present within apical and basolateral membranes of brain epithelial interfaces and in microvascular endothelia with and without tight junctions. J. Histochem. Cytochem. 1992, 40, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Latvala, S.; Jacobsen, B.; Otteneder, M.B.; Herrmann, A.; Kronenberg, S. Distribution of FcRn across species and tissues. J. Histochem. Cytochem. 2017, 65, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Liddelow, S.A.; Dziegielewska, K.M.; Møllgård, K.; Phoenix, T.N.; Temple, S.; VandeBerg, J.L.; Saunders, N.R. SPARC/osteonectin, an endogenous mechanism for targeting albumin to the blood–cerebrospinal fluid interface during brain development. Eur. J. Neurosci. 2011, 34, 1062–1073. [Google Scholar] [CrossRef] [PubMed]
- Liddelow, S.A.; Dzięgielewska, K.M.; Møllgård, K.; Whish, S.C.; Noor, N.M.; Wheaton, B.J.; Gehwolf, R.; Wagner, A.; Traweger, A.; Bauer, H.; et al. Cellular specificity of the blood-CSF barrier for albumin transfer across the choroid plexus epithelium. PLoS ONE 2014, 9, e106592. [Google Scholar] [CrossRef] [PubMed]
- Klatzo, I.; Miquel, J.; Ferris, P.J.; Prokop, J.D.; Smith, D.E. Observations on the passage of the fluorescein labeled serum proteins (FLSP) from the cerebrospinal fluid. J. Neuropathol. Exp. Neurol. 1964, 23, 18–35. [Google Scholar] [CrossRef] [PubMed]
- Bowsher, D. Pathways of absorption of protein from the cerebrospinal fluid: An autoradiographic study in the cat. Anat. Rec. 1957, 128, 23–39. [Google Scholar] [CrossRef]
- Evans, P.G.; Sokolska, M.; Alves, A.; Harrison, I.F.; Ohene, Y.; Nahavandi, P.; Ismail, O.; Miranda, E.; Lythgoe, M.F.; Thomas, D.L.; et al. Non-invasive MRI of blood-cerebrospinal fluid barrier function. Nat. Commun 2020, 11, 2081. [Google Scholar] [CrossRef]
- Iliff, J.J.; Wang, M.; Liao, Y.; Plogg, B.A.; Peng, W.; Gundersen, G.A.; Benveniste, H.; Vates, G.E.; Deane, R.; Goldman, S.A.; et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci. Transl. Med. 2012, 4, 147ra111. [Google Scholar] [CrossRef]
- Chodobski, A.; Ghersi-Egea, J.F.; Nicholson, C.; Nagaraja, T.N.; Szmydynger-Chodobska, J. The quest for a better insight into physiology of fluids and barriers of the brain: The exemplary career of Joseph D. Fenstermacher. Fluids Barriers CNS 2015, 12, 1. [Google Scholar] [CrossRef]
- Segal, M.B. Transport of nutrients across the choroid plexus. Microsc. Res. Tech. 2001, 52, 38–48. [Google Scholar] [CrossRef]
- Fenstermacher, J.D. Ventriculocisternal perfusion as a technique for studying transport and metabolism within the brain. In Research Methods in Neurochemistry; Springer: New York, NY, USA, 1972; pp. 165–178. [Google Scholar]
- Pollay, M.; Stevens, A.; Estrada, E.; Kaplan, R. Extracorporeal perfusion of choroid plexus. J. Appl. Physiol. 1972, 32, 612–617. [Google Scholar] [CrossRef] [PubMed]
- Preston, J.E.; Segal, M.B. The steady-state amino acid fluxes across the perfused choroid plexus of the sheep. Brain Res. 1990, 525, 275–279. [Google Scholar] [CrossRef]
- Deane, R.; Segal, M.B. The transport of sugars across the perfused choroid plexus of the sheep. J. Physiol. 1985, 362, 245–260. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.A.; Preston, J.E.; Wilson, M.R.; Farrell, C.L.; Segal, M.B. Leptin transport at the blood--cerebrospinal fluid barrier using the perfused sheep choroid plexus model. Brain Res. 2001, 895, 283–290. [Google Scholar] [CrossRef]
- Erb, U.; Schwerk, C.; Schroten, H.; Karremann, M. Review of functional in vitro models of the blood-cerebrospinal fluid barrier in leukaemia research. J. Neurosci. Methods 2020, 329, 108478. [Google Scholar] [CrossRef] [PubMed]
- Redzic, Z.B. Studies on the human choroid plexus in vitro. Fluids Barriers CNS 2013, 10, 10. [Google Scholar] [CrossRef]
- Strazielle, N.; Ghersi-Egea, J.F. Demonstration of a coupled metabolism-efflux process at the choroid plexus as a mechanism of brain protection toward xenobiotics. J. Neurosci. 1999, 19, 6275–6289. [Google Scholar] [CrossRef]
- Johnson, B.A.; Coutts, M.; Vo, H.M.; Hao, X.; Fatima, N.; Rivera, M.J.; Sims, R.J.; Neel, M.J.; Kang, Y.J.; Monuki, E.S. Accurate, strong, and stable reporting of choroid plexus epithelial cells in transgenic mice using a human transthyretin BAC. Fluids Barriers CNS 2018, 15, 22. [Google Scholar] [CrossRef]
- Ishiwata, I.; Ishiwata, C.; Ishiwata, E.; Sato, Y.; Kiguchi, K.; Tachibana, T.; Hashimoto, H.; Ishikawa, H. Establishment and characterization of a human malignant choroids plexus papilloma cell line (HIBCPP). Hum. Cell 2005, 18, 67–72. [Google Scholar] [CrossRef]
- Schwerk, C.; Papandreou, T.; Schuhmann, D.; Nickol, L.; Borkowski, J.; Steinmann, U.; Quednau, N.; Stump, C.; Weiss, C.; Berger, J.; et al. Polar invasion and translocation of Neisseria meningitidis and Streptococcus suis in a novel human model of the blood-cerebrospinal fluid barrier. PLoS ONE 2012, 7, e30069. [Google Scholar] [CrossRef]
- Shi, L.Z.; Li, G.J.; Wang, S.; Zheng, W. Use of Z310 cells as an in vitro blood-cerebrospinal fluid barrier model: Tight junction proteins and transport properties. Toxicol. In Vitro 2008, 22, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Zhao, Q. Establishment and characterization of an immortalized Z310 choroidal epithelial cell line from murine choroid plexus. Brain Res. 2002, 958, 371–380. [Google Scholar] [CrossRef]
- Kläs, J.; Wolburg, H.; Terasaki, T.; Fricker, G.; Reichel, V. Characterization of immortalized choroid plexus epithelial cell lines for studies of transport processes across the blood-cerebrospinal fluid barrier. Cerebrospinal Fluid Res. 2010, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Stukas, S.; Robert, J.; Lee, M.; Kulic, I.; Carr, M.; Tourigny, K.; Fan, J.; Namjoshi, D.; Lemke, K.; DeValle, N.; et al. Intravenously injected human apolipoprotein A-I rapidly enters the central nervous system via the choroid plexus. J. Am. Heart Assoc. 2014, 3, e001156. [Google Scholar] [CrossRef]
- Lun, M.P.; Johnson, M.B.; Broadbelt, K.G.; Watanabe, M.; Kang, Y.J.; Chau, K.F.; Springel, M.W.; Malesz, A.; Sousa, A.M.; Pletikos, M.; et al. Spatially heterogeneous choroid plexus transcriptomes encode positional identity and contribute to regional CSF production. J. Neurosci. 2015, 35, 4903–4916. [Google Scholar] [CrossRef]
- Keep, R.F.; Jones, H.C. A morphometric study on the development of the lateral ventricle choroid plexus, choroid plexus capillaries and ventricular ependyma in the rat. Brain Res. Dev. Brain Res. 1990, 56, 47–53. [Google Scholar] [CrossRef]
- Saunders, N.R.; Dziegielewska, K.M.; Møllgård, K.; Habgood, M.D. Physiology and molecular biology of barrier mechanisms in the fetal and neonatal brain. J. Physiol. 2018, 596, 5723–5756. [Google Scholar] [CrossRef]
- Kratzer, I.; Liddelow, S.A.; Saunders, N.R.; Dziegielewska, K.M.; Strazielle, N.; Ghersi-Egea, J.F. Developmental changes in the transcriptome of the rat choroid plexus in relation to neuroprotection. Fluids Barriers CNS 2013, 10, 25. [Google Scholar] [CrossRef]
- Reiber, H. Proteins in cerebrospinal fluid and blood: Barriers, CSF flow rate and source-related dynamics. Restor. Neurol. Neurosci. 2003, 21, 79–96. [Google Scholar]
- Gribkoff, V.K.; Kaczmarek, L.K. The need for new approaches in CNS drug discovery: Why drugs have failed, and what can be done to improve outcomes. Neuropharmacology 2017, 120, 11–19. [Google Scholar] [CrossRef]
- Pardridge, W.M. CSF, blood-brain barrier, and brain drug delivery. Expert Opin. Drug Deliv. 2016, 13, 963–975. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. Blood-brain barrier and delivery of protein and gene therapeutics to brain. Front. Aging Neurosci. 2019, 11, 373. [Google Scholar] [CrossRef] [PubMed]
- Hladky, S.B.; Barrand, M.A. Mechanisms of fluid movement into, through and out of the brain: Evaluation of the evidence. Fluids Barriers CNS 2014, 11, 26. [Google Scholar] [CrossRef] [PubMed]
- Bothwell, S.W.; Janigro, D.; Patabendige, A. Cerebrospinal fluid dynamics and intracranial pressure elevation in neurological diseases. Fluids Barriers CNS 2019, 16, 9. [Google Scholar] [CrossRef] [PubMed]
- Linninger, A.A.; Tangen, K.; Hsu, C.-Y.; Frim, D. Cerebrospinal fluid mechanics and its coupling to cerebrovascular dynamics. Annu. Rev. Fluid Mech. 2016, 48, 219–257. [Google Scholar] [CrossRef]
- Mortensen, K.N.; Sanggaard, S.; Mestre, H.; Lee, H.; Kostrikov, S.; Xavier, A.L.R.; Gjedde, A.; Benveniste, H.; Nedergaard, M. Impaired glymphatic transport in spontaneously hypertensive rats. J. Neurosci. 2019, 39, 6365–6377. [Google Scholar] [CrossRef] [PubMed]
- Strecker, E.P.; Scheffel, U.; Kelley, J.E.; James, A.E., Jr. Cerebrospinal fluid absorption in communicating hydrocephalus. Evaluation of transfer of radioactive albumin from subarachnoid space to plasma. Neurology 1973, 23, 854–864. [Google Scholar] [CrossRef] [PubMed]
- James, A.E., Jr.; Strecker, E.P.; Sperber, E.; Flor, W.J.; Merz, T.; Burns, B. An alternative pathway of cerebrospinal fluid absorption in communicating hydrocephalus. Transependymal movement. Radiology 1974, 111, 143–146. [Google Scholar] [CrossRef]
- Abbott, N.J.; Pizzo, M.E.; Preston, J.E.; Janigro, D.; Thorne, R.G. The role of brain barriers in fluid movement in the CNS: Is there a ‘glymphatic’ system? Acta Neuropathol. 2018, 135, 387–407. [Google Scholar] [CrossRef]
- Pizzo, M.E.; Wolak, D.J.; Kumar, N.N.; Brunette, E.; Brunnquell, C.L.; Hannocks, M.J.; Abbott, N.J.; Meyerand, M.E.; Sorokin, L.; Stanimirovic, D.B.; et al. Intrathecal antibody distribution in the rat brain: Surface diffusion, perivascular transport and osmotic enhancement of delivery. J. Physiol. 2018, 596, 445–475. [Google Scholar] [CrossRef]
- Ichimura, T.; Fraser, P.A.; Cserr, H.F. Distribution of extracellular tracers in perivascular spaces of the rat brain. Brain Res. 1991, 545, 103–113. [Google Scholar] [CrossRef]
- Nabeshima, S.; Reese, T.S.; Landis, D.M.; Brightman, M.W. Junctions in the meninges and marginal glia. J. Comp. Neurol. 1975, 164, 127–169. [Google Scholar] [CrossRef] [PubMed]
- Castro Dias, M.; Mapunda, J.A.; Vladymyrov, M.; Engelhardt, B. Structure and Junctional Complexes of Endothelial, Epithelial and Glial Brain Barriers. Int. J. Mol. Sci. 2019, 20, 5372. [Google Scholar] [CrossRef] [PubMed]
- Rennels, M.L.; Gregory, T.F.; Blaumanis, O.R.; Fujimoto, K.; Grady, P.A. Evidence for a ‘paravascular’ fluid circulation in the mammalian central nervous system, provided by the rapid distribution of tracer protein throughout the brain from the subarachnoid space. Brain Res. 1985, 326, 47–63. [Google Scholar] [CrossRef]
- Rennels, M.L.; Blaumanis, O.R.; Grady, P.A. Rapid solute transport throughout the brain via paravascular fluid pathways. Adv. Neurol. 1990, 52, 431–439. [Google Scholar]
- Iliff, J.J.; Wang, M.; Zeppenfeld, D.M.; Venkataraman, A.; Plog, B.A.; Liao, Y.; Deane, R.; Nedergaard, M. Cerebral arterial pulsation drives paravascular CSF-interstitial fluid exchange in the murine brain. J. Neurosci. 2013, 33, 18190–18199. [Google Scholar] [CrossRef]
- Mestre, H.; Tithof, J.; Du, T.; Song, W.; Peng, W.; Sweeney, A.M.; Olveda, G.; Thomas, J.H.; Nedergaard, M.; Kelley, D.H. Flow of cerebrospinal fluid is driven by arterial pulsations and is reduced in hypertension. Nat. Commun 2018, 9, 4878. [Google Scholar] [CrossRef]
- Mestre, H.; Mori, Y.; Nedergaard, M. The brain’s glymphatic system: Current controversies. Trends Neurosci. 2020, 43, 458–466. [Google Scholar] [CrossRef]
- Wardlaw, J.M.; Benveniste, H.; Nedergaard, M.; Zlokovic, B.V.; Mestre, H.; Lee, H.; Doubal, F.N.; Brown, R.; Ramirez, J.; MacIntosh, B.J.; et al. Perivascular spaces in the brain: Anatomy, physiology and pathology. Nat. Rev. Neurol. 2020, 16, 137–153. [Google Scholar] [CrossRef]
- Hannocks, M.J.; Pizzo, M.E.; Huppert, J.; Deshpande, T.; Abbott, N.J.; Thorne, R.G.; Sorokin, L. Molecular characterization of perivascular drainage pathways in the murine brain. J. Cereb. Blood Flow Metab. 2018, 38, 669–686. [Google Scholar] [CrossRef]
- Wagner, H.J.; Pilgrim, C.; Brandl, J. Penetration and removal of horseradish peroxidase injected into the cerebrospinal fluid: Role of cerebral perivascular spaces, endothelium and microglia. Acta Neuropathol. 1974, 27, 299–315. [Google Scholar] [CrossRef] [PubMed]
- Wolak, D.J.; Thorne, R.G. Diffusion of macromolecules in the brain: Implications for drug delivery. Mol. Pharm. 2013, 10, 1492–1504. [Google Scholar] [CrossRef] [PubMed]
- Ray, L.A.; Heys, J.J. Fluid flow and mass transport in brain tissue. Fluids 2019, 4, 196. [Google Scholar] [CrossRef]
- Weller, R.O.; Sharp, M.M.; Christodoulides, M.; Carare, R.O.; Møllgård, K. The meninges as barriers and facilitators for the movement of fluid, cells and pathogens related to the rodent and human CNS. Acta Neuropathol. 2018, 135, 363–385. [Google Scholar] [CrossRef] [PubMed]
- Louveau, A.; Plog, B.A.; Antila, S.; Alitalo, K.; Nedergaard, M.; Kipnis, J. Understanding the functions and relationships of the glymphatic system and meningeal lymphatics. J. Clin InvestIG. 2017, 127, 3210–3219. [Google Scholar] [CrossRef] [PubMed]
- Bakker, E.; Naessens, D.M.P.; VanBavel, E. Paravascular spaces: Entry to or exit from the brain? Exp. Physiol. 2019, 104, 1013–1017. [Google Scholar] [CrossRef]
- Plog, B.A.; Nedergaard, M. The glymphatic system in central nervous system health and disease: Past, present, and future. Annu. Rev. Pathol. 2018, 13, 379–394. [Google Scholar] [CrossRef]
- Bakker, E.N.; Bacskai, B.J.; Arbel-Ornath, M.; Aldea, R.; Bedussi, B.; Morris, A.W.; Weller, R.O.; Carare, R.O. Lymphatic clearance of the brain: Perivascular, paravascular and significance for neurodegenerative diseases. Cell. Mol. Neurobiol. 2016, 36, 181–194. [Google Scholar] [CrossRef]
- Rosenberg, G.A.; Kyner, W.T.; Estrada, E. Bulk flow of brain interstitial fluid under normal and hyperosmolar conditions. Am. J. Physiol. 1980, 238, F42–F49. [Google Scholar] [CrossRef]
- Pollay, M. The function and structure of the cerebrospinal fluid outflow system. Cerebrospinal Fluid Res. 2010, 7, 9. [Google Scholar] [CrossRef]
- Chen, L.; Elias, G.; Yostos, M.P.; Stimec, B.; Fasel, J.; Murphy, K. Pathways of cerebrospinal fluid outflow: A deeper understanding of resorption. Neuroradiology 2015, 57, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Sandrone, S.; Moreno-Zambrano, D.; Kipnis, J.; van Gijn, J. A (delayed) history of the brain lymphatic system. Nat. Med. 2019, 25, 538–540. [Google Scholar] [CrossRef] [PubMed]
- Johnston, M.; Papaiconomou, C. Cerebrospinal fluid transport: A lymphatic perspective. News Physiol. Sci. 2002, 17, 227–230. [Google Scholar] [CrossRef]
- Da Mesquita, S.; Fu, Z.; Kipnis, J. The meningeal lymphatic system: A new player in neurophysiology. Neuron 2018, 100, 375–388. [Google Scholar] [CrossRef]
- Bradbury, M.W.; Westrop, R.J. Factors influencing exit of substances from cerebrospinal fluid into deep cervical lymph of the rabbit. J. Physiol. 1983, 339, 519–534. [Google Scholar] [CrossRef] [PubMed]
- Boulton, M.; Young, A.; Hay, J.; Armstrong, D.; Flessner, M.; Schwartz, M.; Johnston, M. Drainage of CSF through lymphatic pathways and arachnoid villi in sheep: Measurement of 125I-albumin clearance. Neuropathol. Appl. Neurobiol. 1996, 22, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Ineichen, B.V.; Detmar, M.; Proulx, S.T. Outflow of cerebrospinal fluid is predominantly through lymphatic vessels and is reduced in aged mice. Nat. Commun. 2017, 8, 1434. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.H.; Cho, H.; Kim, J.H.; Kim, S.H.; Ham, J.S.; Park, I.; Suh, S.H.; Hong, S.P.; Song, J.H.; Hong, Y.K.; et al. Meningeal lymphatic vessels at the skull base drain cerebrospinal fluid. Nature 2019, 572, 62–66. [Google Scholar] [CrossRef]
- Papisov, M.I.; Belov, V.V.; Gannon, K.S. Physiology of the intrathecal bolus: The leptomeningeal route for macromolecule and particle delivery to CNS. Mol. Pharm. 2013, 10, 1522–1532. [Google Scholar] [CrossRef]
- Ma, Q.; Ries, M.; Decker, Y.; Müller, A.; Riner, C.; Bücker, A.; Fassbender, K.; Detmar, M.; Proulx, S.T. Rapid lymphatic efflux limits cerebrospinal fluid flow to the brain. Acta Neuropathol. 2019, 137, 151–165. [Google Scholar] [CrossRef]
- Thorne, R.G.; Frey, W.H., 2nd. Delivery of neurotrophic factors to the central nervous system: Pharmacokinetic considerations. Clin. Pharmacokinet 2001, 40, 907–946. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.E.; Streicher, E.; Milkovic, K.; Klatzo, I. Observations on the transport of proteins by the isolated choroid plexus. Acta Neuropathol. 1964, 3, 372–386. [Google Scholar] [CrossRef]
- Aleshire, S.L.; Hajdu, I.; Bradley, C.A.; Parl, F.F. Choroid plexus as a barrier to immunoglobulin delivery into cerebrospinal fluid. J. Neurosurg. 1985, 63, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Zlokovic, B.V.; Martel, C.L.; Matsubara, E.; McComb, J.G.; Zheng, G.; McCluskey, R.T.; Frangione, B.; Ghiso, J. Glycoprotein 330/megalin: Probable role in receptor-mediated transport of apolipoprotein J alone and in a complex with Alzheimer disease amyloid beta at the blood-brain and blood-cerebrospinal fluid barriers. Proc. Natl. Acad. Sci. USA 1996, 93, 4229–4234. [Google Scholar] [CrossRef] [PubMed]
- Christensen, E.I.; Birn, H.; Verroust, P.; Moestrup, S.K. Membrane receptors for endocytosis in the renal proximal tubule. Int. Rev. Cytol. 1998, 180, 237–284. [Google Scholar] [CrossRef]
- Dickson, L.E.; Wagner, M.C.; Sandoval, R.M.; Molitoris, B.A. The proximal tubule and albuminuria: Really! J. Am. Soc. Nephrol. 2014, 25, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Olstad, E.W.; Ringers, C.; Hansen, J.N.; Wens, A.; Brandt, C.; Wachten, D.; Yaksi, E.; Jurisch-Yaksi, N. Ciliary beating compartmentalizes cerebrospinal fluid flow in the brain and regulates ventricular development. Curr. Biol. 2019, 29, 229–241.e226. [Google Scholar] [CrossRef] [PubMed]
- Faubel, R.; Westendorf, C.; Bodenschatz, E.; Eichele, G. Cilia-based flow network in the brain ventricles. Science 2016, 353, 176–178. [Google Scholar] [CrossRef]
- Siyahhan, B.; Knobloch, V.; de Zélicourt, D.; Asgari, M.; Schmid Daners, M.; Poulikakos, D.; Kurtcuoglu, V. Flow induced by ependymal cilia dominates near-wall cerebrospinal fluid dynamics in the lateral ventricles. J. R Soc. Interface 2014, 11, 20131189. [Google Scholar] [CrossRef]
- Dreha-Kulaczewski, S.; Joseph, A.A.; Merboldt, K.D.; Ludwig, H.C.; Gärtner, J.; Frahm, J. Inspiration is the major regulator of human CSF flow. J. Neurosci. 2015, 35, 2485–2491. [Google Scholar] [CrossRef]
- Yan, Q.; Matheson, C.; Sun, J.; Radeke, M.J.; Feinstein, S.C.; Miller, J.A. Distribution of intracerebral ventricularly administered neurotrophins in rat brain and its correlation with trk receptor expression. Exp. Neurol. 1994, 127, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Raheel, H.; Ghaffari, S.; Khosraviani, N.; Mintsopoulos, V.; Auyeung, D.; Wang, C.; Kim, Y.H.; Mullen, B.; Sung, H.K.; Ho, M.; et al. CD36 mediates albumin transcytosis by dermal but not lung microvascular endothelial cells: Role in fatty acid delivery. Am. J. Physiol. Lung Cell Mol. Physiol. 2019, 316, L740–L750. [Google Scholar] [CrossRef] [PubMed]
- Plog, B.A.; Mestre, H.; Olveda, G.E.; Sweeney, A.M.; Kenney, H.M.; Cove, A.; Dholakia, K.Y.; Tithof, J.; Nevins, T.D.; Lundgaard, I.; et al. Transcranial optical imaging reveals a pathway for optimizing the delivery of immunotherapeutics to the brain. JCI Insight 2018, 3. [Google Scholar] [CrossRef] [PubMed]
- Brøchner, C.B.; Holst, C.B.; Møllgård, K. Outer brain barriers in rat and human development. Front. Neurosci. 2015, 9. [Google Scholar] [CrossRef] [PubMed]
- Balslev, Y.; Saunders, N.R.; MØllgard, K. Ontogenetic development of diffusional restriction to protein at the pial surface of the rat brain: An electron microscopical study. J. Neurocytol. 1997, 26, 133–148. [Google Scholar] [CrossRef] [PubMed]
- Kida, S.; Steart, P.V.; Zhang, E.-T.; Weller, R.O. Perivascular cells act as scavengers in the cerebral perivascular spaces and remain distinct from pericytes, microglia and macrophages. Acta Neuropathol. 1993, 85, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, C.; Strazielle, N.; Ghersi-Egea, J.-F. Brain leukocyte infiltration initiated by peripheral inflammation or experimental autoimmune encephalomyelitis occurs through pathways connected to the CSF-filled compartments of the forebrain and midbrain. J. Neuroinflammation 2012, 9, 187. [Google Scholar] [CrossRef]
- Huang, J.; Khademi, M.; Fugger, L.; Lindhe, Ö.; Novakova, L.; Axelsson, M.; Malmeström, C.; Constantinescu, C.; Lycke, J.; Piehl, F.; et al. Inflammation-related plasma and CSF biomarkers for multiple sclerosis. Proc. Natl. Acad. Sci. USA 2020, 117, 12952–12960. [Google Scholar] [CrossRef]
- Maschke, M.; Kastrup, O.; Diener, H.-C. CNS Manifestations of Cytomegalovirus Infections. CNS Drugs 2002, 16, 303–315. [Google Scholar] [CrossRef]
- Koh, L.; Zakharov, A.; Johnston, M. Integration of the subarachnoid space and lymphatics: Is it time to embrace a new concept of cerebrospinal fluid absorption? Cereb. Fluid Res. 2005, 2, 6. [Google Scholar] [CrossRef]
- Goodman, T.; Hajihosseini, M.K. Hypothalamic tanycytes—Masters and servants of metabolic, neuroendocrine, and neurogenic functions. Front. Neurosci. 2015, 9. [Google Scholar] [CrossRef] [PubMed]
- Jalalvand, E.; Robertson, B.; Tostivint, H.; Löw, P.; Wallén, P.; Grillner, S. Cerebrospinal fluid-contacting neurons sense pH changes and motion in the hypothalamus. J. Neurosci. Res. 2018, 38, 7713–7724. [Google Scholar] [CrossRef] [PubMed]
- Kakkis, E.; McEntee, M.; Vogler, C.; Le, S.; Levy, B.; Belichenko, P.; Mobley, W.; Dickson, P.; Hanson, S.; Passage, M. Intrathecal enzyme replacement therapy reduces lysosomal storage in the brain and meninges of the canine model of MPS I. Mol. Genet. Metab. 2004, 83, 163–174. [Google Scholar] [CrossRef]
- Calias, P.; Papisov, M.; Pan, J.; Savioli, N.; Belov, V.; Huang, Y.; Lotterhand, J.; Alessandrini, M.; Liu, N.; Fischman, A.J.; et al. CNS penetration of intrathecal-lumbar idursulfase in the monkey, dog and mouse: Implications for neurological outcomes of lysosomal storage disorder. PLoS ONE 2012, 7, e30341. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Manickam, D.S.; Brynskikh, A.; Kabanov, A.V. Agile delivery of protein therapeutics to CNS. J. Control. Release 2014, 190, 637–663. [Google Scholar] [CrossRef] [PubMed]
- Schulz, A.; Ajayi, T.; Specchio, N.; de Los Reyes, E.; Gissen, P.; Ballon, D.; Dyke, J.P.; Cahan, H.; Slasor, P.; Jacoby, D.; et al. Study of intraventricular cerliponase alfa for CLN2 disease. N. Engl. J. Med. 2018, 378, 1898–1907. [Google Scholar] [CrossRef]
- Mole, S.E.; Schulz, A.; Haltia, M. Chapter 4—The neuronal ceroid-lipofuscinoses (Batten disease). In Rosenberg’s Molecular and Genetic Basis of Neurological and Psychiatric Disease, 6th ed.; Rosenberg, R.N., Pascual, J.M., Eds.; Academic Press: London, UK, 2020. [Google Scholar] [CrossRef]
- de Los Reyes, E.; Lehwald, L.; Augustine, E.F.; Berry-Kravis, E.; Butler, K.; Cormier, N.; Demarest, S.; Lu, S.; Madden, J.; Olaya, J. Intracerebroventricular cerliponase alfa for neuronal ceroid lipofuscinosis type 2 disease: Clinical practice considerations from US clinics. Pediatr. Neurol. 2020. [Google Scholar] [CrossRef]
- Markham, A. Cerliponase alfa: First global approval. Drugs 2017, 77, 1247–1249. [Google Scholar] [CrossRef]
- Casacó, A.; López, G.; García, I.; Rodríguez, J.A.; Fernández, R.; Figueredo, J.; Torres, L.; Perera, A.; Batista, J.; Leyva, R.; et al. Phase I single-dose study of intracavitary-administered Nimotuzumab labeled with 188 Re in adult recurrent high-grade glioma. Cancer Biol. Ther. 2008, 7, 333–339. [Google Scholar] [CrossRef]
- Zalutsky, M.R.; Reardon, D.A.; Akabani, G.; Coleman, R.E.; Friedman, A.H.; Friedman, H.S.; McLendon, R.E.; Wong, T.Z.; Bigner, D.D. Clinical experience with alpha-particle emitting 211At: Treatment of recurrent brain tumor patients with 211At-labeled chimeric antitenascin monoclonal antibody 81C6. J. Nucl. Med. 2008, 49, 30–38. [Google Scholar] [CrossRef]
- Pandit-Taskar, N.; Zanzonico, P.B.; Kramer, K.; Grkovski, M.; Fung, E.K.; Shi, W.; Zhang, Z.; Lyashchenko, S.K.; Fung, A.M.; Pentlow, K.S.; et al. Biodistribution and dosimetry of intraventricularly administered (124)I-omburtamab in patients with metastatic leptomeningeal tumors. J. Nucl. Med. 2019, 60, 1794–1801. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
| Transporter/Receptors | Expression | Localization | Endogenous Substrate | Direction | Function | |
|---|---|---|---|---|---|---|
| Peptide transporter (PTR) family | PEPT2/SLC15A2 | mRNA (r) [74,75] | A of CPECs (r) [76] | Di-/tripeptide [73] | Efflux, CSF→luminal [76] | Removal of neuropeptides, peptide fragments, and peptide-like drugs from CSF [76,77] |
| P-glycoprotein | Pgp/MDR1 | mRNA and protein (r, m h) [82] | A of CPECs (h) [81] | Aβ [80,83] | Efflux, CSF→luminal [80,83] | - |
| Transferrin (Tf) receptor | TfR | mRNA and protein (r) [90] | CPECs (h) [89], (r) [85], (m) [86], vesicles around nuclei of CPECs (r) [90] | Tf [86,91] | Unidirectional uptake (luminal→CP epithelium) [91] | Uptake of Tf bound iron into CPECs and slow release of iron to CSF [91] |
| Insulin receptor | mRNA (r) [93] | CPECs (r) [94,95] | Insulin [94] | Luminal→CSF [94] | May transport insulin from the blood into the CSF with intermediate compartment and saturable process [94] | |
| Insulin-like growth factor receptors | IGF1R | mRNA (r) [97], (r fetus) [98] | CPECs (r) [95], (h) [101], on surface of CPECs (p) [102] | IGF-I, IGF-II, insulin [96] | Luminal→CSF [104] | Mediates effects of IGF-I and IGF-II [102] |
| IGF2R | mRNA (r) [99,100] | Intracellular of CPECs (p) [102], epithelium and endothelium CP (infant r) [103] | IGF-I, IGF-II [96] | - | - | |
| Low density lipoprotein (LDL) receptor | LDLR | - | A of CPECs (h) [79] | LDL [107] | - | - |
| LDL receptor-related proteins (LRPs) | LRP1 (LRP/α2-macroglobulin receptor) | mRNA and protein (h) [108,112], (r) [109], mRNA (r) [110], protein (r) [111] | Diffuse cellular of CPECs (r) [113], CPECs (h) [79] | α2-macroglobulin [35], Aβ [35,111,112] | Efflux (CSF→luminal) [109] | May involve in the clearance of protease/α2-macroglobulin complexes from the CSF [35] Maintains brain homeostasis of Aβand partly mediates the elimination of Aβ from CSF [111,112] May associate with apolipoprotein E (apoE) to influence the severity of cerebral amyloid angiopathy and Alzheimer’s disease [108] |
| LRP2 (megalin/glycoprotein 330) | mRNA and protein (r) [109,117] | A of CPECs (r) [109,116], CPECs and ventricular ependyma (h) [79] | Leptin [120], IGF-I [104,121], ApoJ [119] | Bidirectional transport [35] | Mediates entry of leptin into CSF across CP [120] Mediates penetration of peripheral IGF-I in the CSF and mediates IGF-I-induced clearance of Aβ [121] Bind with ApoJ and mediates clearance of Aβ1-40-apoJ from CSF [119] | |
| LRP8 (ApoE receptor 2) | mRNA (r) [122] | CPECs (r) [122], (m) [123] | ApoE [122], selenoprotein P (Sepp1) [123] | - | May involve in the uptake of ApoE phospholipid discoidal particles or ApoE-enriched high-density lipoprotein in brain [122] Facilitates uptake of Sepp1 [123] | |
| Neonatal Fc receptor | FcRn | mRNA (r) [35] | Diffuse cellular of CPECs (r) [35,126], CPECs (monkey, r, m) [128] | IgG [126] | - | May mediate transcytosis of IgG [126] |
| Name | Brand Name | Description | Condition/Disease | Route of Administration | Initial Approval Year |
|---|---|---|---|---|---|
| Cerliponase alfa | Brineura™ | Recombinant human tripeptidyl peptidase-1 | Neuronal ceroid lipofuscinosis type 2 disease | i.c.v. | 2017 |
| Glatiramer acetate | Copaxone® | Acetate salts of synthetic polypeptides of L-glutamic acid, L-alanine, L-tyrosine, and L-lysine | Relapsing forms of multiple sclerosis | s.c. | 1996 |
| Peginterferon beta-1a | Plegridy™ | Interferon beta-1a | Relapsing forms of multiple sclerosis | s.c. | 2014 |
| Natalizumab | TysabriI® | Humanized IgG4k monoclonal antibody | Relapsing forms of multiple sclerosis | i.v. | 2004 |
| Ocrelizumab | Ocrevus™ | Humanized anti-CD20 monoclonal antibody | Relapsing or primary progressive forms of multiple sclerosis | i.v. | 2017 |
| Ofatumumab | Kesimpta® | Anti-CD20 monoclonal antibody | Relapsing forms of multiple sclerosis | s.c. | 2020 |
| Eptinezumab | Vyepti™ | Humanized IgG1 antibody antagonizing CGRPR | Adult migraine | i.v. | 2020 |
| Erenumab | Aimovig™ | Human monoclonal antibody antagonizing CGRPR | Adult migraine | s.c. | 2018 |
| Fremanezumab | Ajovy™ | Humanized IgG2 antagonizing CGRPR | Adult migraine | s.c. | 2018 |
| Galcanezumab | Emgality™ | Humanized IgG4 antagonizing CGRPR | Adult migraine | s.c. | 2018 |
| Dinutuximab | Unituxin™ | GD2-binding monoclonal antibody | Pediatric patients with high-risk neuroblastoma | i.v. | 2015 |
| Name | Description | Condition/Disease | Route of Administration | Status | Reference/Clinical Trial Identifier |
|---|---|---|---|---|---|
| Aducanumab | Human monoclonal antibody targeting Aβ | Alzheimer’s disease | i.v. | Phase III (under review) | NCT02477800; NCT02484547 |
| Gantenerumab | Human IgG1 antibody targeting Aβ | Alzheimer’s disease | s.c. | Phase III | NCT01224106; NCT01760005; NCT02051608; NCT03443973; NCT03444870 |
| ABBV-8E12 | Humanized IgG4 anti-tau antibody | Alzheimer’s disease | i.v. | Phase II | NCT02880956 |
| AL002 | Anti-human TREM2 antibody | Alzheimer’s disease | i.v. | Phase I | NCT03635047 |
| AL003 | Anti-human SIGLEC 3 antibody | Alzheimer’s disease | i.v. | Phase I | NCT03822208 |
| Crenezumab | Humanized IgG4 monoclonal antibody targeting Aβ | Alzheimer’s disease | i.v. | Phase II | NCT01397578; NCT01343966; NCT01723826; NCT01998841; NCT02670083 |
| Donanemab | Humanized IgG1 monoclonal antibody targeting N3pG- Aβ | Alzheimer’s disease | i.v. | Phase II | NCT03367403 |
| JNJ-63733657 | Monoclonal antibody targeting the mid-region of tau | Alzheimer’s disease | i.v. | Phase I | NCT03375697 |
| Semorinemab | Anti-tau IgG4 antibody | Alzheimer’s disease | i.v. | Phase II | NCT02820896; NCT03828747; NCT03289143 |
| Solanezumab | Humanized monoclonal IgG1 antibody | Alzheimer’s disease | i.v. | Phase III | NCT00329082; NCT00749216; NCT00904683; NCT00905372; NCT01148498; NCT01127633; NCT01760005; NCT01900665; NCT02008357; NCT02760602 |
| Zagotenemab | Humanized anti-tau antibody | Alzheimer’s disease | i.v. | Phase II | NCT03518073 |
| Opicinumab | Monoclonal antibody targeting LINGO1 | Multiple sclerosis | i.v. | Phase II | NCT02833142; NCT03222973; NCT01721161 |
| Rindopepimut | EGFRvIII peptide vaccine | Glioblastoma | i.d.l | Phase II | NCT01480479; NCT01498328; NCT00458601 |
| Durvalumab | Human IgG1κ monoclonal antibody | Glioblastoma | i.v. | Phase II | NCT02336165 |
| 125I-MAB-425 | Anti-epidermal growth factor receptor-425 monoclonal antibody | Glioblastoma | i.v. or i.a. | Phase II | NCT01317888 |
| 131I-chTNT-1/B MAb | Monoclonal antibody targeting DNA-histone H1 complex | Glioblastoma | s.c. | Phase II | NCT00677716; NCT00509301; NCT00128635; NCT00004017 |
| 188Re-labeled Nimotuzumab | Humanized monoclonal antibody targeting epidermal growth factor receptors | Glioblastoma and astrocytoma | Intracavity | Phase I | [227] |
| 211At-labeled 81C6 mAb | Chimeric antitenascin monoclonal antibody | Brain tumor | Intracavity | Phase I | [228]; NCT00003461 |
| 131I-Omburtamab | Murine monoclonal antibody targeting 4Ig-B7-H3 | Neuroblastoma and leptomeningeal metastases | i.c.v. | Phase II/III | [229]; NCT03275402 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bryniarski, M.A.; Ren, T.; Rizvi, A.R.; Snyder, A.M.; Morris, M.E. Targeting the Choroid Plexuses for Protein Drug Delivery. Pharmaceutics 2020, 12, 963. https://doi.org/10.3390/pharmaceutics12100963
Bryniarski MA, Ren T, Rizvi AR, Snyder AM, Morris ME. Targeting the Choroid Plexuses for Protein Drug Delivery. Pharmaceutics. 2020; 12(10):963. https://doi.org/10.3390/pharmaceutics12100963
Chicago/Turabian StyleBryniarski, Mark A., Tianjing Ren, Abbas R. Rizvi, Anthony M. Snyder, and Marilyn E. Morris. 2020. "Targeting the Choroid Plexuses for Protein Drug Delivery" Pharmaceutics 12, no. 10: 963. https://doi.org/10.3390/pharmaceutics12100963
APA StyleBryniarski, M. A., Ren, T., Rizvi, A. R., Snyder, A. M., & Morris, M. E. (2020). Targeting the Choroid Plexuses for Protein Drug Delivery. Pharmaceutics, 12(10), 963. https://doi.org/10.3390/pharmaceutics12100963






