TMS-Induced Controlled BBB Opening: Preclinical Characterization and Implications for Treatment of Brain Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Open-Window Craniotomy
2.3. Noncovalent Conjugation of Succinimidyl Ester (NHS) to IGF-Trap
2.4. Fluorescent Angiography for Assessing Vascular Permeability
2.5. Repetitive Transcranial Magnetic Stimulation
2.6. Electrocorticography Recording and Analysis
2.7. Evaluation of rTMS-Induced Brain Injury
2.8. Statistical Analysis
3. Results
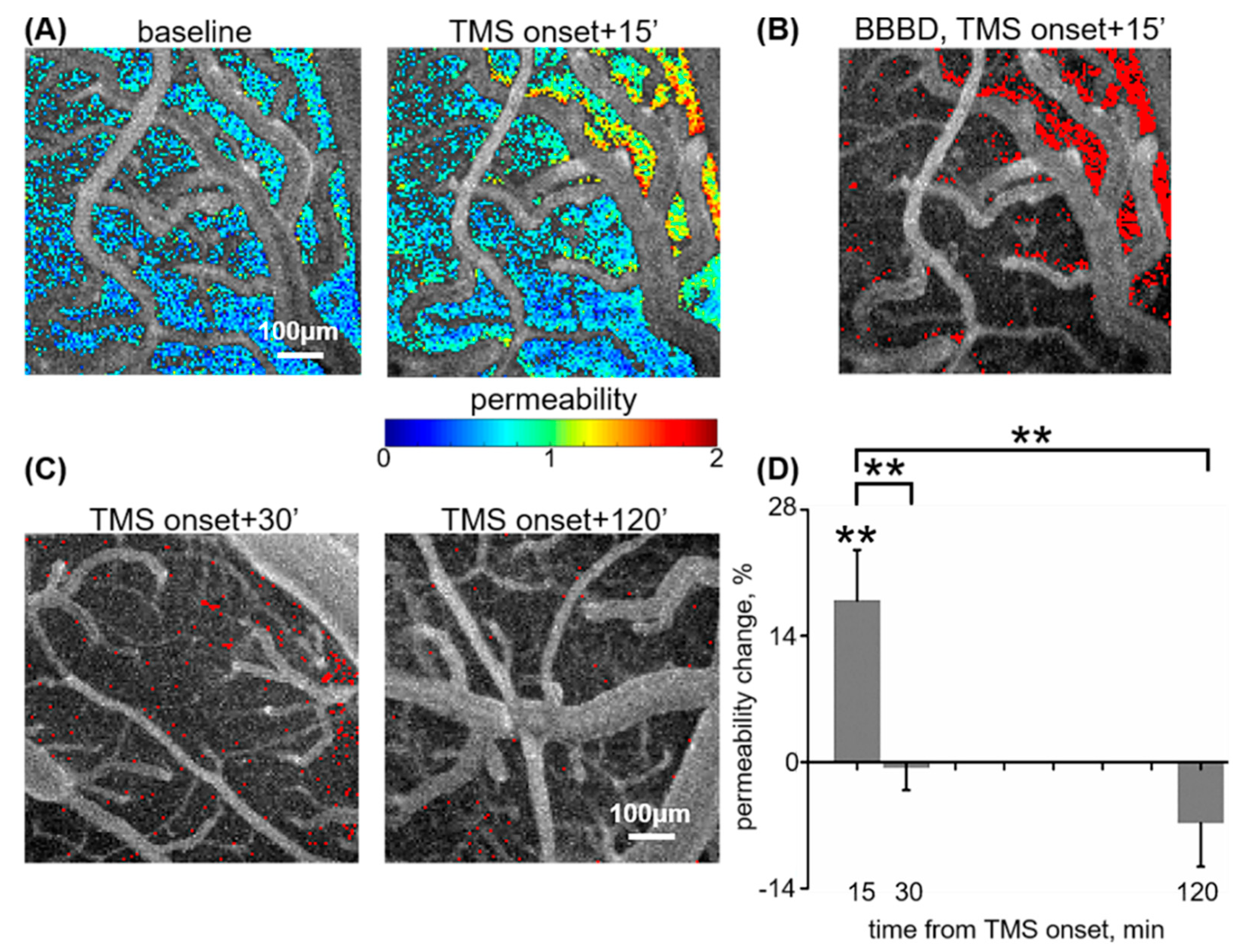
3.1. rTMS-Induced BBB Opening Is Transient
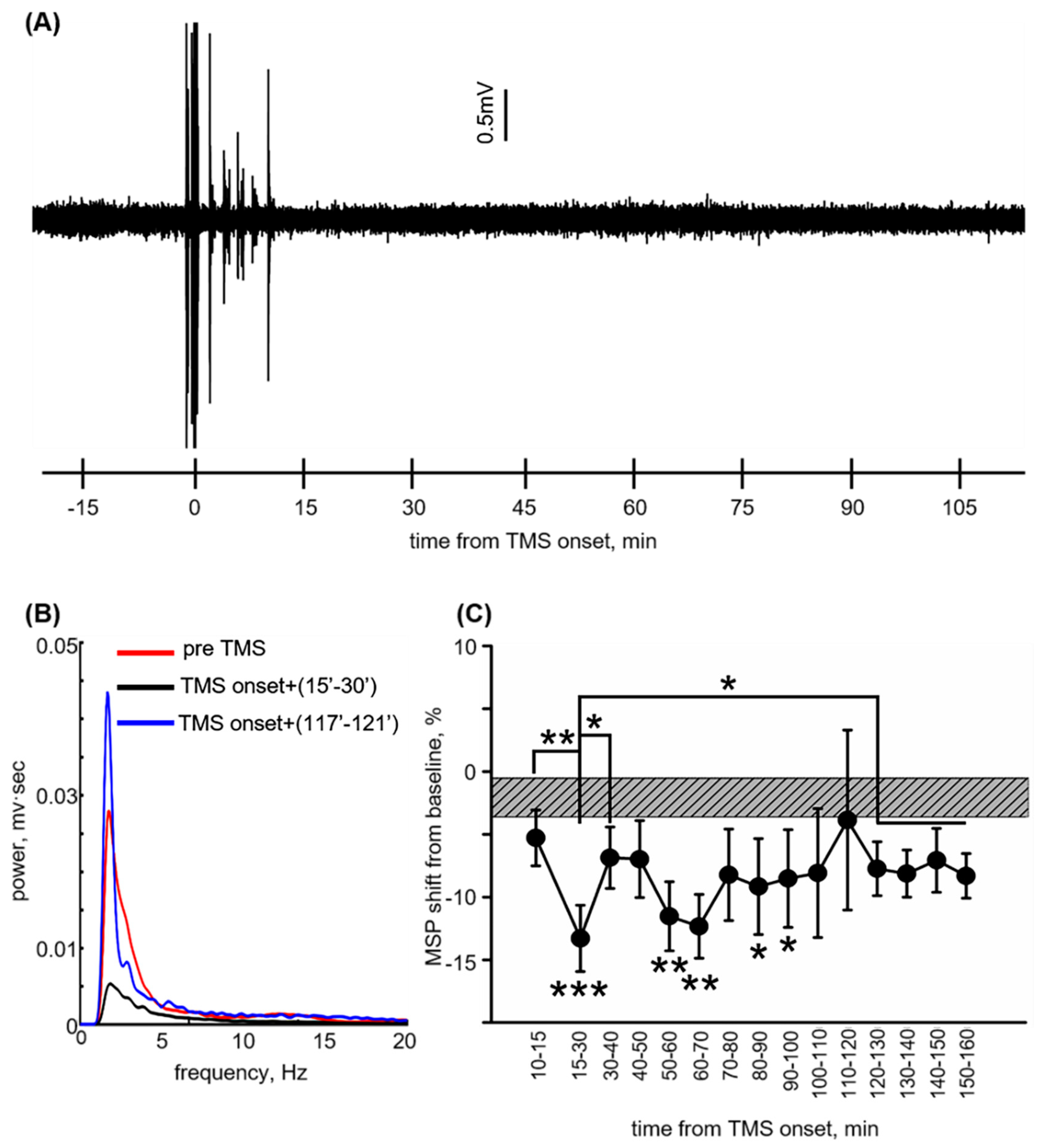
3.2. Reversible ECoG Depression Coincides with BBB Normalization
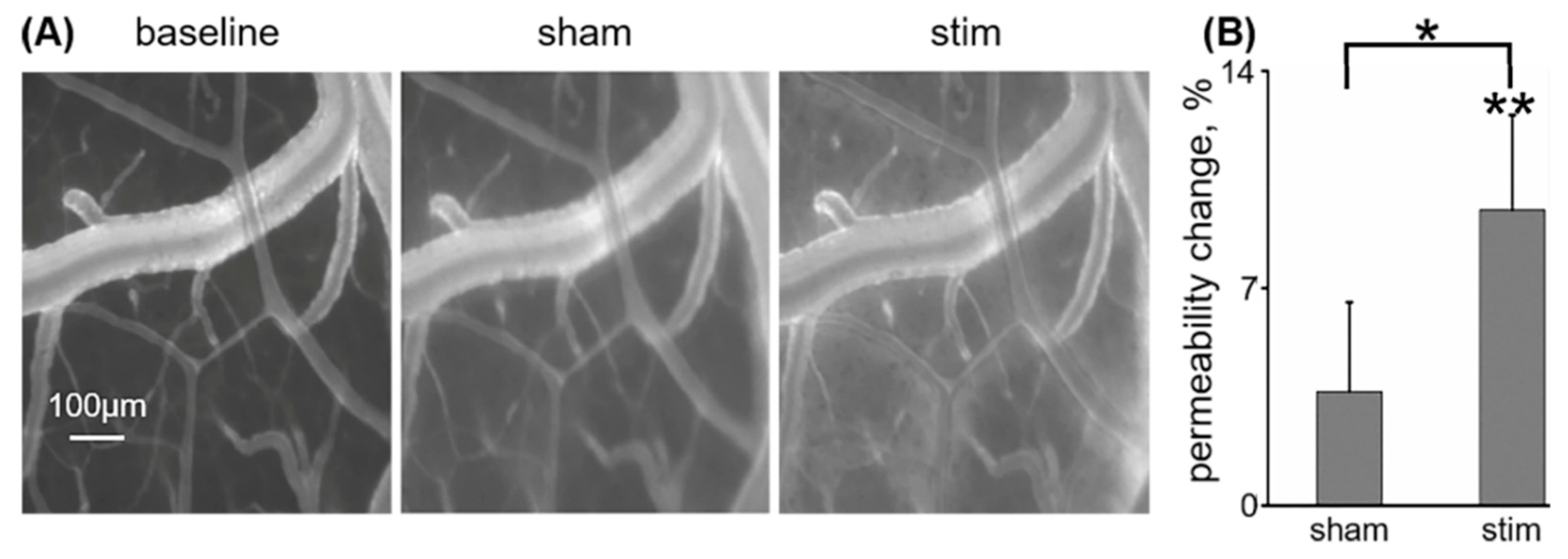
3.3. Low-Frequency rTMS Increases Cortical Vascular Permeability to IGF-Trap
3.4. Repeated Low-Frequency rTMS Does Not Induce Brain Injury
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Availability of Data and Materials
Abbreviations
| ACSF: | Artificial cerebrospinal fluid |
| BBB: | Blood–brain barrier |
| BBBD: | BBB dysfunction |
| CNS: | Central nervous system |
| DCE: | Dynamic contrast-enhanced |
| DF: | Dominant frequency |
| ECoG: | Electrocorticography |
| EV: | Extravascular |
| IGF: | Insulin-like growth factor |
| IT: | Intensity vs. time |
| MRI: | Magnetic resonance imaging |
| MSP: | Mean spectral power |
| MWU: | Mann–Whitney U |
| NaFlu: | Sodium fluorescein |
| NHS: | Succinimidyl ester |
| PBS: | Phosphate buffered saline |
| PI: | Permeability index |
| RB: | Rose Bengal |
| rMT: | Resting motor threshold |
| rTMS: | Repetitive transcranial magnetic stimulation |
| SEM: | Squared error of the mean |
References
- Abbott, N.J.; Patabendige, A.A.K.; Dolman, D.E.M.; Yusof, S.R.; Begley, D.J. Structure and function of the blood-brain barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Arvanitis, C.D.; Ferraro, G.B.; Jain, R.K. The blood–brain barrier and blood–tumour barrier in brain tumours and metastases. Nat. Rev. Cancer 2020, 20, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Groothuis, D.R. The blood-brain and blood-tumor barriers: A review of strategies for increasing drug delivery. Neuro-Oncology 2000, 2, 45–59. [Google Scholar] [CrossRef]
- Pardridge, W.M. The blood-brain barrier: Bottleneck in brain drug development. NeuroRx 2005, 2, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Rayes, R.F.; Elahi, S.M.; Lu, Y.; Hancock, M.A.; Massie, B.; Rowe, G.E.; Aomari, H.; Hossain, S.; Durocher, Y.; et al. The IGF-Trap: Novel Inhibitor of Carcinoma Growth and Metastasis. Mol. Cancer Ther. 2015, 14, 982–993. [Google Scholar] [CrossRef] [PubMed]
- Vaniotis, G.; Moffett, S.; Sulea, T.; Wang, N.; Elahi, S.M.; Lessard, E.; Baardsnes, J.; Perrino, S.; Durocher, Y.; Frystyk, J.; et al. Enhanced anti-metastatic bioactivity of an IGF-TRAP re-engineered to improve physicochemical properties. Sci. Rep. 2018, 8, 17361. [Google Scholar] [CrossRef] [PubMed]
- Rapoport, S.I. Osmotic opening of the blood-brain barrier: Principles, mechanism, and therapeutic applications. Cell. Mol. Neurobiol. 2000, 20, 217–230. [Google Scholar] [CrossRef]
- Brown, R.C.; Egleton, R.D.; Davis, T.P. Mannitol opening of the blood-brain barrier: Regional variation in the permeability of sucrose, but not 86Rb+ or albumin. Brain Res. 2004, 1014, 221–227. [Google Scholar] [CrossRef]
- Kiviniemi, V.; Korhonen, V.; Kortelainen, J.; Rytky, S.; Keinänen, T.; Tuovinen, T.; Isokangas, M.; Sonkajärvi, E.; Siniluoto, T.; Nikkinen, J.; et al. Real-time monitoring of human blood-brain barrier disruption. PLoS ONE 2017, 12, e0174072. [Google Scholar] [CrossRef]
- Van Vliet, E.; Da Costa, A.; Redeker, S.; Van Schaik, R.; Aronica, E.; Gorter, J. Blood-brain barrier leakage may lead to progression of temporal lobe epilepsy. Brain 2007, 130, 521–534. [Google Scholar] [CrossRef]
- McDannold, N.; Vykhodtseva, N.; Hynynen, K. Targeted disruption of the blood-brain barrier with focused ultrasound: Association with cavitation activity. Phys. Med. Biol. 2006, 51, 793–807. [Google Scholar] [CrossRef] [PubMed]
- Mesiwala, A.H.; Farrell, L.; Wenzel, H.J.; Silbergeld, D.L.; Crum, L.A.; Winn, H.R.; Mourad, P.D. High-intensity focused ultrasound selectively disrupts the blood-brain barrier in vivo. Ultrasound Med. Biol. 2002, 28, 389–400. [Google Scholar] [CrossRef]
- Abrahao, A.; Meng, Y.; Llinas, M.; Huang, Y.; Hamani, C.; Mainprize, T.; Aubert, I.; Heyn, C.; Black, S.E.; Hynynen, K.; et al. First-in-human trial of blood–brain barrier opening in amyotrophic lateral sclerosis using MR-guided focused ultrasound. Nat. Commun. 2019, 10, 4373. [Google Scholar] [CrossRef]
- Rezai, A.R.; Ranjan, M.; D’Haese, P.-F.; Haut, M.W.; Carpenter, J.; Najib, U.; Mehta, R.I.; Chazen, J.L.; Zibly, Z.; Yates, J.R.; et al. Noninvasive hippocampal blood−brain barrier opening in Alzheimer’s disease with focused ultrasound. Proc. Natl. Acad. Sci. USA 2020, 117, 9180–9182. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.E.; Drazic, J.; Ganguly, M.; Stefanovic, B.; Hynynen, K. Two-photon fluorescence microscopy study of cerebrovascular dynamics in ultrasound-induced blood-brain barrier opening. J. Cereb. Blood Flow Metab. 2011, 31, 1852–1862. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, Z.I.; Kim, S.; Jikaria, N.; Qureshi, F.; Milo, B.; Lewis, B.K.; Bresler, M.; Burks, S.R.; Frank, J.A. Disrupting the blood–brain barrier by focused ultrasound induces sterile inflammation. Proc. Natl. Acad. Sci. USA 2017, 114, E75–E84. [Google Scholar] [CrossRef] [PubMed]
- Lippmann, K.; Kamintsky, L.; Kim, S.Y.; Lublinsky, S.; Prager, O.; Nichtweiss, J.F.; Salar, S.; Kaufer, D.; Heinemann, U.; Friedman, A. Epileptiform activity and spreading depolarization in the blood–brain barrier-disrupted peri-infarct hippocampus are associated with impaired GABAergic inhibition and synaptic plasticity. J. Cereb. Blood Flow Metab. 2017, 37, 1803–1819. [Google Scholar] [CrossRef]
- Swissa, E.; Serlin, Y.; Vazana, U.; Prager, O.; Friedman, A. Blood-brain barrier dysfunction in status epileptics: Mechanisms and role in epileptogenesis. Epilepsy Behav. 2019, 101, 106285. [Google Scholar] [CrossRef]
- Van Vliet, E.; Aronica, E.; Gorter, J. Blood-brain barrier dysfunction, seizures and epilepsy. Semin. Cell Dev. Biol. 2015, 38, 26–34. [Google Scholar] [CrossRef]
- Bar-Klein, G.; Lublinsky, S.; Kamintsky, L.; Noyman, I.; Veksler, R.; Dalipaj, H.; Senatorov, V.; Swissa, E.; Rosenbach, D.; Elazary, N.; et al. Imaging blood–brain barrier dysfunction as a biomarker for epileptogenesis. Brain 2017, 140, 1692–1705. [Google Scholar] [CrossRef]
- Tomkins, O.; Shelef, I.; Kaizerman, I.; Eliushin, A.; Afawi, Z.; Misk, A.; Gidon, M.; Cohen, A.; Zumsteg, D.; Friedman, A. Blood-brain barrier disruption in post-traumatic epilepsy. J. Neurol. Neurosurg. Psychiatry 2008, 79, 774–777. [Google Scholar] [CrossRef] [PubMed]
- Friedman, A. Blood–brain barrier dysfunction, status epilepticus, seizures, and epilepsy: A puzzle of a chicken and egg? Epilepsia 2011, 52, 19–20. [Google Scholar] [CrossRef] [PubMed]
- Rüber, T.; David, B.; Lüchters, G.; Nass, R.D.; Friedman, A.; Surges, R.; Stöcker, T.; Weber, B.; Deichmann, R.; Schlaug, G.; et al. Evidence for peri-ictal blood–brain barrier dysfunction in patients with epilepsy. Brain 2018, 141, 2952–2965. [Google Scholar] [CrossRef] [PubMed]
- Schoknecht, K.; Kikhia, M.; Lemale, C.L.; Liotta, A.; Lublinsky, S.; Mueller, S.; Boehm-Sturm, P.; Friedman, A.; Dreier, J.P. The role of spreading depolarizations and electrographic seizures in early injury progression of the rat photothrombosis stroke model. J. Cereb. Blood Flow Metab. 2020, 0271678X20915801. [Google Scholar] [CrossRef]
- Sharp, C.; Hines, I.; Houghton, J.; Warren, A.; Jackson, T.; Jawahar, A.; Nanda, A.; Elrod, J.; Long, A.; Chi, A.; et al. Glutamate causes a loss in human cerebral endothelial barrier integrity through activation of NMDA receptor. Am. J. Physiol. Heart Circ. Physiol. 2003, 285, H2592–H2598. [Google Scholar] [CrossRef]
- Sharp, C.D.; Houghton, J.; Elrod, J.W.; Warren, A.; Iv, T.H.J.; Jawahar, A.; Nanda, A.; Minagar, A.; Alexander, J.S.; Christopher, D. N-methyl-D-aspartate receptor activation in human cerebral endothelium promotes intracellular oxidant stress. Am. J. Physiol. Heart Circ. Physiol. 2005, 288, H1893–H1899. [Google Scholar] [CrossRef]
- Vazana, U.; Veksler, R.; Pell, G.S.; Prager, O.; Fassler, M.; Chassidim, Y.; Roth, Y.; Shahar, H.; Zangen, A.; Raccah, R.; et al. Glutamate-Mediated Blood–Brain Barrier Opening: Implications for Neuroprotection and Drug Delivery. J. Neurosci. 2016, 36, 7727–7739. [Google Scholar] [CrossRef]
- Maccabee, P.J.; Eberle, L.; Amassian, V.E.; Cracco, R.Q.; Rudell, A. Spatial distribution of the electric field induced in volume by round and figure ‘8’ magnetic coils: Relevance to activation of sensory nerve fibers. Electroencephalogr. Clin. Neurophysiol. 1990, 76, 131–141. [Google Scholar] [CrossRef]
- Zangen, A.; Roth, Y.; Voller, B.; Hallett, M. Transcranial magnetic stimulation of deep brain regions: Evidence for efficacy of the H-coil. Clin. Neurophysiol. 2005, 116, 775–779. [Google Scholar] [CrossRef]
- Levkovitz, Y.; Isserles, M.; Padberg, F.; Lisanby, S.H.; Bystritsky, A.; Xia, G.; Tendler, A.; Daskalakis, Z.J.; Winston, J.L.; Dannon, P.; et al. Efficacy and safety of deep transcranial magnetic stimulation for major depression: A prospective multicenter randomized controlled trial. World Psychiatry 2015, 14, 64–73. [Google Scholar] [CrossRef]
- Carmi, L.; Tendler, A.; Bystritsky, A.; Hollander, E.; Blumberger, D.M.; Daskalakis, J.; Ward, H.; Lapidus, K.; Goodman, W.; Casuto, L.; et al. Efficacy and Safety of Deep Transcranial Magnetic Stimulation for Obsessive-Compulsive Disorder: A Prospective Multicenter Randomized Double-Blind Placebo-Controlled Trial. Am. J. Psychiatry 2019, 176, 931–938. [Google Scholar] [CrossRef] [PubMed]
- Zangen, A.; Hyodo, K. Transcranial magnetic stimulation induces increases in extracellular levels of dopamine and glutamate in the nucleus accumbens. Neuroreport 2002, 13, 2401–2405. [Google Scholar] [CrossRef] [PubMed]
- Chassidim, Y.; Veksler, R.; Lublinsky, S.; Pell, G.; Friedman, A.; Shelef, I. Quantitative imaging assessment of blood-brain barrier permeability in humans. Fluids Barriers CNS 2013, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Chassidim, Y.; Vazana, U.; Prager, O.; Veksler, R.; Bar-Klein, G.; Schoknecht, K.; Fassler, M.; Lublinsky, S.; Shelef, I. Analyzing the blood-brain barrier: The benefits of medical imaging in research and clinical practice. Semin. Cell Dev. Biol. 2015, 38, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Veksler, R.; Shelef, I.; Friedman, A. Blood-Brain Barrier Imaging in Human Neuropathologies. Arch. Med. Res. 2014, 45, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Prager, O.; Chassidim, Y.; Klein, C.; Levi, H.; Shelef, I.; Friedman, A. Dynamic in vivo imaging of cerebral blood flow and blood-brain barrier permeability. Neuroimage 2010, 49, 337–344. [Google Scholar] [CrossRef]
- Chen, Y.; Qi, S.; Perrino, S.; Hashimoto, M.; Brodt, P. Targeting the IGF-Axis for Cancer Therapy: Development and Validation of an IGF-Trap as a Potential Drug. Cells 2020, 9, 1098. [Google Scholar] [CrossRef]
- Schoknecht, K.; Prager, O.; Vazana, U.; Kamintsky, L.; Harhausen, D.; Zille, M.; Figge, L.; Chassidim, Y.; Schellenberger, E.; Kovács, R.; et al. Monitoring stroke progression: In vivo imaging of cortical perfusion, blood-brain barrier permeability and cellular damage in the rat photothrombosis model. J. Cereb. Blood Flow Metab. 2014, 34, 1791–1801. [Google Scholar] [CrossRef]
- Guizar-Sicairos, M.; Thurman, S.T.; Fienup, J.R. Efficient subpixel image registration algorithms. Opt. Lett. 2008, 33, 156–158. [Google Scholar] [CrossRef]
- Nachar, N. The Mann-Whitney U: A Test for Assessing Whether Two Independent Samples Come from the Same Distribution. Tutor. Quant. Methods Psychol. 2008, 4, 13–20. [Google Scholar] [CrossRef]
- Seiffert, E.; Dreier, J.; Ivens, S.; Bechmann, I.; Tomkins, O.; Heinemann, U.; Friedman, A. Lasting blood-brain barrier disruption induces epileptic focus in the rat somatosensory cortex. J. Neurosci. 2004, 24, 7829–7836. [Google Scholar] [CrossRef] [PubMed]
- Gersner, R.; Kravetz, E.; Feil, J.; Pell, G.; Zangen, A. Long-term effects of repetitive transcranial magnetic stimulation on markers for neuroplasticity: Differential outcomes in anesthetized and awake animals. J. Neurosci. 2011, 31, 7521–7526. [Google Scholar] [CrossRef]
- Li, B.; Virtanen, J.P.; Oeltermann, A.; Schwarz, C.; Giese, M.A.; Ziemann, U.; Benali, A.; Neurophysiology, S.; Reichardt, W.; Neuroscience, I. Lifting the veil on the dynamics of neuronal activities evoked by transcranial magnetic stimulation. eLife 2017, 6, e30552. [Google Scholar] [CrossRef] [PubMed]
- Prager, O.; Kamintsky, L.; Hasam-henderson, L.; Schoknecht, K.; Wuntke, V.; Papageorgiou, I.; Swolinsky, J.; Muoio, V.; Bar-klein, G.; Vazana, U.; et al. Seizure-induced microvascular injury is associated with impaired neurovascular coupling and blood–brain barrier dysfunction. Epilepsia 2019, 60, 322–336. [Google Scholar] [CrossRef] [PubMed]
- Delorme, A.; Sejnowski, T.; Makeig, S. Enhanced detection of artifacts in EEG data using higher-order statistics and independent component analysis. Neuroimage 2007, 34, 1443–1449. [Google Scholar] [CrossRef] [PubMed]
- Tadel, F.; Baillet, S.; Mosher, J.; Pantazis, D.; Leahy, R. Brainstorm: A User-Friendly Application for MEG/EEG Analysis. Comput. Intell. Neurosci. 2011, 2011, 879716. [Google Scholar] [CrossRef]
- Watson, B.; Dietrich, W.; Prado, R.; Ginsberg, M. Argon laser-induced arterial photothrombosis Characterization and possible application to therapy of arteriovenous malformations. J. Neurosurg. 1987, 66, 748–754. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.-Y.; Lin, Y.-S.; Kang, K.-H.; Chao, T.-K. Reversible blood–brain barrier disruption by repeated transcranial focused ultrasound allows enhanced extravasation. J. Control. Release 2011, 150, 111–116. [Google Scholar] [CrossRef]
- Bellavance, M.-A.; Blanchette, M.; Fortin, D. Recent Advances in Blood–Brain Barrier Disruption as a CNS Delivery Strategy. AAPS J. 2008, 10, 166–177. [Google Scholar] [CrossRef]
- Casula, E.P.; Tarantino, V.; Basso, D.; Arcara, G.; Marino, G.; Toffolo, G.M.; Rothwell, J.C.; Bisiacchi, P.S. Low-frequency rTMS inhibitory effects in the primary motor cortex: Insights from TMS-evoked potentials. Neuroimage 2014, 98, 225–232. [Google Scholar] [CrossRef]
- Siebner, H.R.; Mentschel, C.; Auer, C.; Lehner, C.; Conrad, B. Repetitive transcranial magnetic stimulation causes a short-term increase in the duration of the cortical silent period in patients with Parkinson’s disease. Neurosci. Lett. 2000, 284, 147–150. [Google Scholar] [CrossRef]
- Murphy, S.C.; Palmer, L.M.; Nyffeler, T.; Müri, R.M.; Larkum, M.E. Transcranial magnetic stimulation (TMS) inhibits cortical dendrites. eLife 2016, 5, e13598. [Google Scholar] [CrossRef] [PubMed]
- Premoli, I.; Castellanos, N.; Rivolta, D.; Belardinelli, P.; Bajo, R.; Zipser, C.; Espenhahn, S.; Heidegger, T.; Müller-Dahlhaus, F.; Ziemann, U. TMS-EEG Signatures of GABAergic Neurotransmission in the Human Cortex. J. Neurosci. 2014, 34, 5603–5612. [Google Scholar] [CrossRef] [PubMed]
- Fierro, B.; Brighina, F.; Vitello, G.; Piazza, A.; Scalia, S.; Giglia, G.; Daniele, O.; Pascual-Leone, A. Modulatory effects of low- and high-frequency repetitive transcranial magnetic stimulation on visual cortex of healthy subjects undergoing light deprivation. J. Physiol. 2005, 565, 659–665. [Google Scholar] [CrossRef]
- Tovar, V.; Alsinet, C.; Villanueva, A.; Hoshida, Y.; Chiang, D.Y.; Solé, M.; Thung, S.; Moyano, S.; Toffanin, S.; Mínguez, B.; et al. IGF activation in a molecular subclass of hepatocellular carcinoma and pre-clinical efficacy of IGF-1R blockage. J. Hepatol. 2010, 52, 550–559. [Google Scholar] [CrossRef]
- Scharf, J.; Dombrowski, F.; Ramadori, G. The IGF axis and hepatocarcinogenesis. J. Clin. Pathol. Mol. Pathol. 2001, 54, 138–144. [Google Scholar] [CrossRef]
- Andrews, D.W.; Resnicoff, M.; Flanders, A.E.; Kenyon, L.; Curtis, M.; Merli, G.; Baserga, R.; Iliakis, G.; Aiken, R.D. Results of a Pilot Study Involving the Use of an Antisense Oligodeoxynucleotide Directed Against the Insulin-Like Growth Factor Type I Receptor in Malignant Astrocytomas. J. Clin. Oncol. 2001, 19, 2189–2200. [Google Scholar] [CrossRef]
- Seccareccia, E.; Brodt, P. The role of the insulin-like growth factor-I receptor in malignancy: An update. Growth Horm. IGF Res. 2012, 22, 193–199. [Google Scholar] [CrossRef]
- Kaufmann, A.M.; Cardoso, E.R. Aggravation of vasogenic cerebral edema by multiple-dose mannitol. J. Neurosurg. 1992, 77, 584–589. [Google Scholar] [CrossRef]
- Rossi, S.; Rossini, P.M. TMS in cognitive plasticity and the potential for rehabilitation. Trends Cogn. Sci. 2004, 8, 273–279. [Google Scholar] [CrossRef]
- Rossi, S.; Hallett, M.; Rossini, P.M.; Pascual-Leone, A. Safety of TMS Consensus Group. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin. Neurophysiol. 2009, 120, 2008–2039. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vazana, U.; Schori, L.; Monsonego, U.; Swissa, E.; Pell, G.S.; Roth, Y.; Brodt, P.; Friedman, A.; Prager, O. TMS-Induced Controlled BBB Opening: Preclinical Characterization and Implications for Treatment of Brain Cancer. Pharmaceutics 2020, 12, 946. https://doi.org/10.3390/pharmaceutics12100946
Vazana U, Schori L, Monsonego U, Swissa E, Pell GS, Roth Y, Brodt P, Friedman A, Prager O. TMS-Induced Controlled BBB Opening: Preclinical Characterization and Implications for Treatment of Brain Cancer. Pharmaceutics. 2020; 12(10):946. https://doi.org/10.3390/pharmaceutics12100946
Chicago/Turabian StyleVazana, Udi, Lior Schori, Uri Monsonego, Evyatar Swissa, Gabriel S. Pell, Yiftach Roth, Pnina Brodt, Alon Friedman, and Ofer Prager. 2020. "TMS-Induced Controlled BBB Opening: Preclinical Characterization and Implications for Treatment of Brain Cancer" Pharmaceutics 12, no. 10: 946. https://doi.org/10.3390/pharmaceutics12100946
APA StyleVazana, U., Schori, L., Monsonego, U., Swissa, E., Pell, G. S., Roth, Y., Brodt, P., Friedman, A., & Prager, O. (2020). TMS-Induced Controlled BBB Opening: Preclinical Characterization and Implications for Treatment of Brain Cancer. Pharmaceutics, 12(10), 946. https://doi.org/10.3390/pharmaceutics12100946