Encapsulation of a Ru(II) Polypyridyl Complex into Polylactide Nanoparticles for Antimicrobial Photodynamic Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instrumentation and Methods
2.3. Bacterial Strains and Conditions of Culture
2.4. Bacteria Photoinactivation
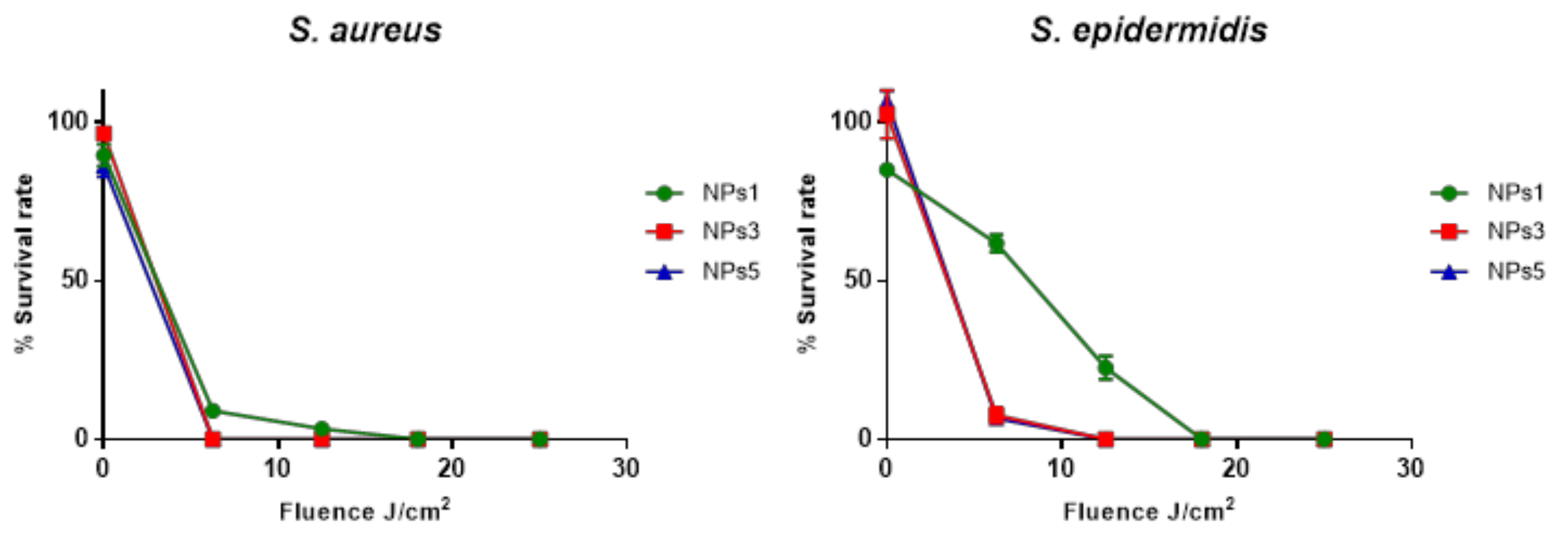
2.5. Effects of Light Fluence
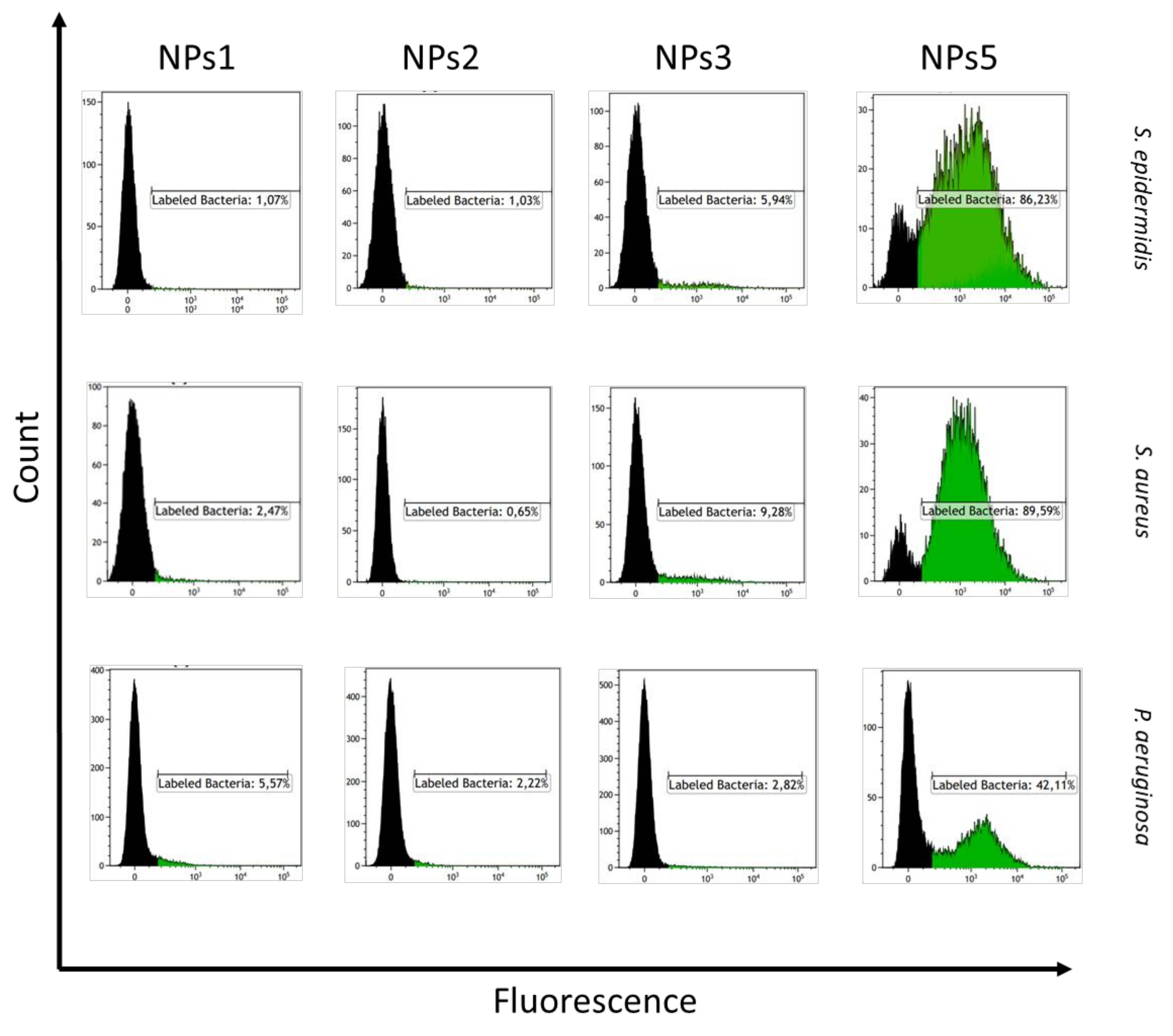
2.6. Flow Cytometry
3. Results and Discussion
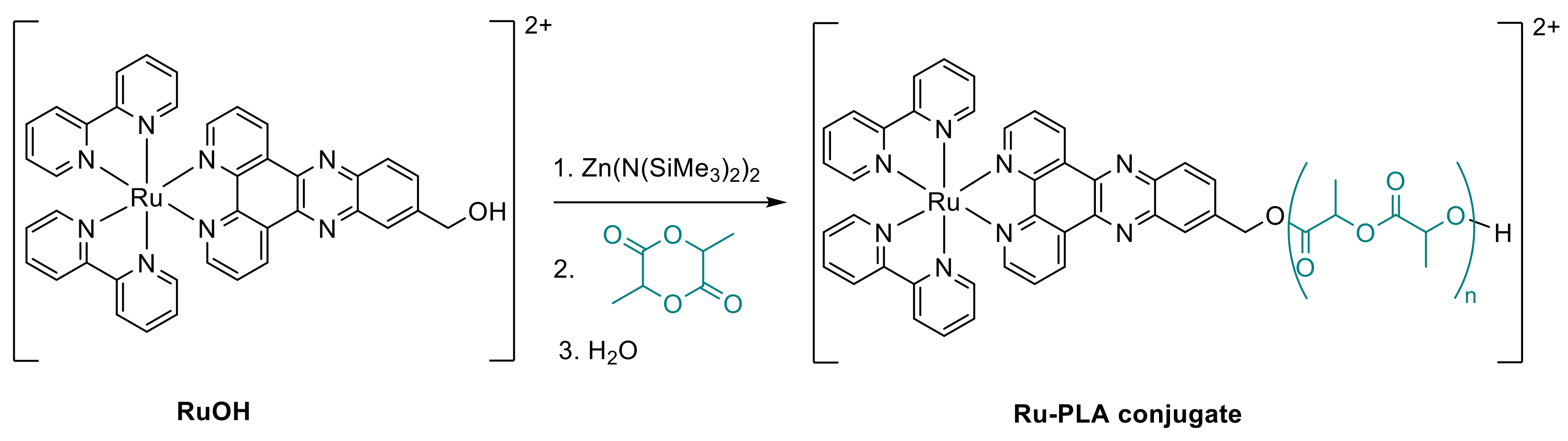
3.1. Preparation of RuPLA Nanoconjugates
3.2. Biological Evaluation of RuPLA Nanoconjugates
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Antimicrobial Resistance: Global Report on Surveillance; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Wainwright, M. Photodynamic antimicrobial chemotherapy (PACT). J. Antimicrob. Chemother. 1998, 42, 13–28. [Google Scholar] [CrossRef]
- Hamblin, M.R.; Hasan, T. Photodynamic therapy: A new antimicrobial approach to infectious disease? Photochem. Photobiol. Sci. 2004, 3, 436–450. [Google Scholar] [CrossRef]
- Hamblin, M.R. Antimicrobial photodynamic inactivation: A bright new technique to kill resistant microbes. Curr. Opin. Microbiol. 2016, 33, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Rabb, O. Uber die wirkung Fluorescirender Stoffe auf Infusorien. Z. Biol. 1900, 39, 524–546. [Google Scholar]
- Cieplik, F.; Deng, D.; Crielaard, W.; Buchalla, W.; Hellwig, E.; Al-Ahmad, A.; Maisch, T. Antimicrobial photodynamic therapy–What we know and what we don’t. Crit. Rev. Microbiol. 2018, 44, 571–589. [Google Scholar] [CrossRef] [PubMed]
- Frei, A.; Zuegg, J.; Elliott, A.G.; Baker, M.; Braese, S.; Brown, C.; Chen, F.; Dowson, C.G.; Dujardin, G.; Jung, N.; et al. Metal complexes as a promising source for new antibiotics. Chem. Sci. 2020, 11, 2627–2639. [Google Scholar] [CrossRef]
- Frei, A. Metal Complexes, an Untapped Source of Antibiotic Potential? Antibiotics 2020, 9, 90. [Google Scholar] [CrossRef]
- İşci, Ü.; Beyreis, M.; Tortik, N.; Topal, S.Z.; Glueck, M.; Ahsen, V.; Dumoulin, F.; Kiesslich, T.; Plaetzer, K. Methylsulfonyl Zn phthalocyanine: A polyvalent and powerful hydrophobic photosensitizer with a wide spectrum of photodynamic applications. Photodiagnosis Photodyn. Ther. 2016, 13, 40–47. [Google Scholar] [CrossRef]
- Li, F.; Collins, J.G.; Keene, F.R. Ruthenium complexes as antimicrobial agents. Chem. Soc. Rev. 2015, 44, 2529–2542. [Google Scholar] [CrossRef]
- Southam, H.M.; Butler, J.A.; Chapman, J.A.; Poole, R.K. The Microbiology of Ruthenium Complexes. Adv. Microb. Physiol. 2017, 71, 1–96. [Google Scholar]
- Golbaghi, G.; Groleau, M.-C.; de los Santos, Y.L.; Doucet, N.; Déziel, E.; Castonguay, A. Cationic RuII Cyclopentadienyl Complexes with Antifungal Activity against Several Candida Species. ChemBioChem 2020. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, F.P.; Gyarfas, E.C.; Rogers, W.P.; Koch, J.H. Biological Activity of Complex Ions. Nature 1952, 170, 190–191. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, F.P.; Reid, I.K.; Shulman, A.; Laycock, G.M.; Dixson, S. The biological actions of 1,10-phenanthroline and 2,2'-bipyridine hydrochlorides, quaternary salts and metal chelates and related compounds. 1. Bacteriostatic action on selected gram-positive, gram-negative and acid-fast bacteria. Aust. J. Exp. Biol. Med. Sci. 1969, 47, 203–218. [Google Scholar] [CrossRef] [PubMed]
- Bolhuis, A.; Hand, L.; Marshall, J.E.; Richards, A.D.; Rodger, A.; Aldrich-Wright, J. Antimicrobial activity of ruthenium-based intercalators. Eur. J. Pharm. Sci. 2011, 42, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Mulyana, Y.; Feterl, M.; Warner, J.M.; Collins, J.G.; Keene, F.R. The antimicrobial activity of inert oligonuclear polypyridylruthenium(II) complexes against pathogenic bacteria, including MRSA. Dalton Trans. 2011, 40, 5032–5038. [Google Scholar] [CrossRef] [PubMed]
- Weber, D.K.; Sani, M.-A.; Downton, M.T.; Separovic, F.; Keene, F.R.; Collins, J.G. Membrane Insertion of a Dinuclear Polypyridylruthenium(II) Complex Revealed by Solid-State NMR and Molecular Dynamics Simulation: Implications for Selective Antibacterial Activity. J. Am. Chem. Soc. 2016, 138, 15267–15277. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Harry, E.J.; Bottomley, A.L.; Edstein, M.D.; Birrell, G.W.; Woodward, C.E.; Keene, F.R.; Collins, J.G. Dinuclear ruthenium(II) antimicrobial agents that selectively target polysomes in vivo. Chem. Sci. 2013, 5, 685–693. [Google Scholar] [CrossRef]
- Lam, P.-L.; Lu, G.-L.; Hon, K.-M.; Lee, K.-W.; Ho, C.-L.; Wang, X.; Tang, J.C.-O.; Lam, K.-H.; Wong, R.S.-M.; Kok, S.H.-L.; et al. Development of ruthenium(II) complexes as topical antibiotics against methicillin resistant Staphylococcus aureus. Dalton Trans. 2014, 43, 3949–3957. [Google Scholar] [CrossRef]
- Gorle, A.K.; Feterl, M.; Warner, J.M.; Wallace, L.; Keene, F.R.; Collins, J.G. Tri- and tetra-nuclear polypyridyl ruthenium(ii) complexes as antimicrobial agents. Dalton Trans. 2014, 43, 16713–16725. [Google Scholar] [CrossRef]
- Gorle, A.K.; Feterl, M.; Warner, J.M.; Primrose, S.; Constantinoiu, C.C.; Keene, F.R.; Collins, J.G. Mononuclear Polypyridylruthenium(II) Complexes with High Membrane Permeability in Gram-Negative Bacteria—in particular Pseudomonas aeruginosa. Chem. Eur. J. 2015, 21, 10472–10481. [Google Scholar] [CrossRef]
- Kumar, S.V.; Scottwell, S.Ø.; Waugh, E.; McAdam, C.J.; Hanton, L.R.; Brooks, H.J.L.; Crowley, J.D. Antimicrobial Properties of Tris(homoleptic) Ruthenium(II) 2-Pyridyl-1,2,3-triazole “Click” Complexes against Pathogenic Bacteria, Including Methicillin-Resistant Staphylococcus aureus (MRSA). Inorg. Chem. 2016, 55, 9767–9777. [Google Scholar] [CrossRef] [PubMed]
- Mårtensson, A.K.F.; Bergentall, M.; Tremaroli, V.; Lincoln, P. Diastereomeric bactericidal effect of Ru(phenanthroline)2dipyridophenazine. Chirality 2016, 28, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Smitten, K.L.; Southam, H.M.; de la Serna, J.B.; Gill, M.R.; Jarman, P.J.; Smythe, C.G.W.; Poole, R.K.; Thomas, J.A. Using Nanoscopy To Probe the Biological Activity of Antimicrobial Leads That Display Potent Activity against Pathogenic, Multidrug Resistant, Gram-Negative Bacteria. ACS Nano 2019, 13, 5133–5146. [Google Scholar] [CrossRef] [PubMed]
- Smitten, K.L.; Fairbanks, S.D.; Robertson, C.C.; de la Serna, J.B.; Foster, S.J.; Thomas, J.A. Ruthenium based antimicrobial theranostics – using nanoscopy to identify therapeutic targets and resistance mechanisms in Staphylococcus aureus. Chem. Sci. 2020, 11, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, R.F.; Fletcher, N.C.; McCague, P.J.; Donnelly, J.; McCarron, P.A.; Tunney, M.M. Design, Synthesis and Photodynamic Antimicrobial Activity of Ruthenium Trischelate Diimine Complexes. Lett. Drug Des. Discov. 2007, 4, 175–179. [Google Scholar] [CrossRef]
- Lei, W.; Zhou, Q.; Jiang, G.; Zhang, B.; Wang, X. Photodynamic inactivation of Escherichia coli by Ru(II) complexes. Photochem. Photobiol. Sci. 2011, 10, 887–890. [Google Scholar] [CrossRef]
- Arenas, Y.; Monro, S.; Shi, G.; Mandel, A.; McFarland, S.; Lilge, L. Photodynamic inactivation of Staphylococcus aureus and methicillin-resistant Staphylococcus aureus with Ru(II)-based type I/type II photosensitizers. Photodiagn. Photodyn. Therapy 2013, 10, 615–625. [Google Scholar] [CrossRef]
- Frei, A.; Rubbiani, R.; Tubafard, S.; Blacque, O.; Anstaett, P.; Felgenträger, A.; Maisch, T.; Spiccia, L.; Gasser, G. Synthesis, Characterization, and Biological Evaluation of New Ru(II) Polypyridyl Photosensitizers for Photodynamic Therapy. J. Med. Chem. 2014, 57, 7280–7292. [Google Scholar] [CrossRef]
- Le Gall, T.; Lemercier, G.; Chevreux, S.; Tücking, K.-S.; Ravel, J.; Thétiot, F.; Jonas, U.; Schönherr, H.; Montier, T. Ruthenium(II) Polypyridyl Complexes as Photosensitizers for Antibacterial Photodynamic Therapy: A Structure–Activity Study on Clinical Bacterial Strains. ChemMedChem 2018, 13, 2229–2239. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, C.; Rees, T.W.; Ke, L.; Ji, L.; Chao, H. Harnessing ruthenium(II) as photodynamic agents: Encouraging advances in cancer therapy. Coord. Chem. Rev. 2018, 363, 17–28. [Google Scholar] [CrossRef]
- Yin, R.; Agrawal, T.; Khan, U.; Gupta, G.K.; Rai, V.; Huang, Y.-Y.; Hamblin, M.R. Antimicrobial photodynamic inactivation in nanomedicine: Small light strides against bad bugs. Nanomedicine 2015, 10, 2379–2404. [Google Scholar] [CrossRef] [PubMed]
- Perni, S.; Prokopovich, P.; Pratten, J.; Parkin, I.P.; Wilson, M. Nanoparticles: Their potential use in antibacterial photodynamic therapy. Photochem. Photobiol. Sci. 2011, 10, 712–720. [Google Scholar] [CrossRef] [PubMed]
- Maldonado-Carmona, N.; Ouk, T.-S.; Calvete, M.J.F.; Pereira, M.M.; Villandier, N.; Leroy-Lhez, S. Conjugating biomaterials with photosensitizers: Advances and perspectives for photodynamic antimicrobial chemotherapy. Photochem. Photobiol. Sci. 2020, 19, 445–461. [Google Scholar] [CrossRef] [PubMed]
- Mari, C.; Pierroz, V.; Rubbiani, R.; Patra, M.; Hess, J.; Spingler, B.; Oehninger, L.; Schur, J.; Ott, I.; Salassa, L.; et al. DNA intercalating Ru(II) polypyridyl complexes as effective photosensitizers in photodynamic therapy. Chem. Eur. J. 2014, 20, 14421–14436. [Google Scholar] [CrossRef]
- Soliman, N.; McKenzie, L.K.; Karges, J.; Bertrand, E.; Tharaud, M.; Jakubaszek, M.; Guérineau, V.; Goud, B.; Hollenstein, M.; Gasser, G.; et al. Ruthenium-initiated polymerization of lactide: A route to remarkable cellular uptake for photodynamic therapy of cancer. Chem. Sci. 2020, 11, 2657–2663. [Google Scholar] [CrossRef]
- Ikada, Y.; Jamshidi, K.; Tsuji, H.; Hyon, S.H. Stereocomplex formation between enantiomeric poly(lactides). Macromolecules 1987, 20, 904–906. [Google Scholar] [CrossRef]
- Tsuji, H. Poly(lactide) Stereocomplexes: Formation, Structure, Properties, Degradation, and Applications. Macromol. Biosci. 2005, 5, 569–597. [Google Scholar] [CrossRef]
- Marin, P.; Tschan, M.J.-L.; Isnard, F.; Robert, C.; Haquette, P.; Trivelli, X.; Chamoreau, L.-M.; Guérineau, V.; del Rosal, I.; Maron, L.; et al. Polymerization of rac-Lactide Using Achiral Iron Complexes: Access to Thermally Stable Stereocomplexes. Angew. Chem. Int. Ed. 2019, 58, 12585–12589. [Google Scholar] [CrossRef]
- Pereira, M.A.; Faustino, M.A.F.; Tome, J.P.C.; Neves, M.G.P.M.S.; Tomé, A.C.; Cavaleiro, J.A.S.; Cunha, A.; Almeida, A. Influence of external bacterial structures on the efficiency of photodynamic inactivation by a cationic porphyrin. Photochem. Photobiol. Sci. 2014, 13, 680–690. [Google Scholar] [CrossRef]
- Malik, Z.; Hanania, J.; Nitzan, Y. New trends in photobiology bactericidal effects of photoactivated porphyrins—An alternative approach to antimicrobial drugs. J. Photochem. Photobiol. B 1990, 5, 281–293. [Google Scholar] [CrossRef]
- Minnock, A.; Vernon, D.I.; Schofield, J.; Griffiths, J.; Parish, J.H.; Brown, S.T. Photoinactivation of bacteria. Use of a cationic water-soluble zinc phthalocyanine to photoinactivate both Gram-negative and Gram-positive bacteria. J. Photochem. Photobiol. B 1996, 32, 159–164. [Google Scholar] [CrossRef]
- Salton, M.R.J.; Kim, K.-S. Chapter 2 Structure. In Medical Microbiology, 4th ed.; Baron, S., Ed.; University of Texas Medical Branch at Galveston: Galveston, TX, USA, 1996. [Google Scholar]
| Entry | Tacticity | Mn,NMR [a] (g/mol) | %RuOH [b] wt% | NPs | Dz ± SD [c] (nm) | PdI ± SD [c] | ζ (mV) [c] |
|---|---|---|---|---|---|---|---|
| P1 | Atactic | 7000 | 15 | NPs1 | 149.7 ± 0.451 | 0.117 ± 0.005 | 33.4 ± 2.14 |
| P2 | Atactic | 4000 | 25 | NPs2 | 128.1 ± 0.737 | 0.171 ± 0.011 | 31.2 ± 2.22 |
| P3 | Isotactic | 4000 | 25 | NPs3 | 116.2 ± 0.551 | 0.167 ± 0.004 | 4.26 ± 0.401 |
| P4 | Isotactic | 4000 | 25 | ||||
| P5 | Atactic | 2000 | 53 | NPs4 | 286.2 ± 3.10 | 0.173 ± 0.011 | N.D. |
| P6 | Isotactic | 7000 | 15 | NPs5 | 177.3 ± 1.55 | 0.183 ± 0.011 | 10.9 ± 0.231 |
| P7 | Isotactic | 7000 | 15 |
| NPs | MIC (µM) | MBC (µM) | ||||||
|---|---|---|---|---|---|---|---|---|
| S. aureus | S. epidermidis | S. aureus | S. epidermidis | |||||
| Light | Dark | Light | Dark | Light | Dark | Light | Dark | |
| RuOH | >50 | >50 | >50 | >50 | >50 | >50 | >50 | >50 |
| NPs1 | 25 | >50 | 25 | >50 | >50 | >50 | 25 | >50 |
| NPs2 | 50 | >50 | 50 | >50 | >50 | >50 | 50 | >50 |
| NPs3 | 25 | >50 | 12.5 | >50 | 50 | >50 | 12.5 | >50 |
| NPs4 | >50 | >50 | N.D. | N.D. | >50 | >50 | N.D. | N.D. |
| NPs5 | 12.5 | >50 | 12.5 | >50 | 25 | >50 | 12.5 | >50 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soliman, N.; Sol, V.; Ouk, T.-S.; Thomas, C.M.; Gasser, G. Encapsulation of a Ru(II) Polypyridyl Complex into Polylactide Nanoparticles for Antimicrobial Photodynamic Therapy. Pharmaceutics 2020, 12, 961. https://doi.org/10.3390/pharmaceutics12100961
Soliman N, Sol V, Ouk T-S, Thomas CM, Gasser G. Encapsulation of a Ru(II) Polypyridyl Complex into Polylactide Nanoparticles for Antimicrobial Photodynamic Therapy. Pharmaceutics. 2020; 12(10):961. https://doi.org/10.3390/pharmaceutics12100961
Chicago/Turabian StyleSoliman, Nancy, Vincent Sol, Tan-Sothea Ouk, Christophe M. Thomas, and Gilles Gasser. 2020. "Encapsulation of a Ru(II) Polypyridyl Complex into Polylactide Nanoparticles for Antimicrobial Photodynamic Therapy" Pharmaceutics 12, no. 10: 961. https://doi.org/10.3390/pharmaceutics12100961
APA StyleSoliman, N., Sol, V., Ouk, T.-S., Thomas, C. M., & Gasser, G. (2020). Encapsulation of a Ru(II) Polypyridyl Complex into Polylactide Nanoparticles for Antimicrobial Photodynamic Therapy. Pharmaceutics, 12(10), 961. https://doi.org/10.3390/pharmaceutics12100961







