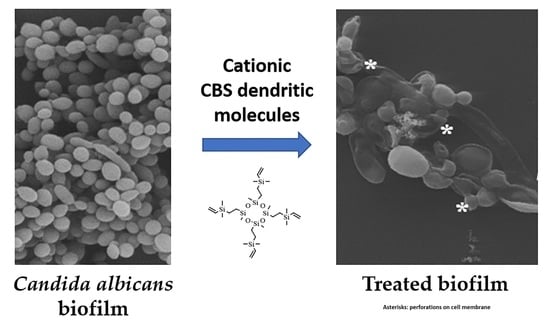
In Vitro Activity of Carbosilane Cationic Dendritic Molecules on Prevention and Treatment of Candida Albicans Biofilms
Abstract
1. Introduction
2. Materials and Methods
2.1. Candida, Growth Conditions and Stimulation for Biofilm Formation
2.2. Biofilm Formation Assay
2.3. Biofilm Quantification
2.4. Cationic CBS Dendritic Molecules
2.5. Resazurin Colorimetric Viability Assay
2.6. Drop Plate Method
2.7. Pre-Biofilm Treatment
2.8. Biofilm Treatment
2.9. Synergistic Activity of New Dendrimers and Commercial Antifungal Drugs against Candida albicans
2.10. Cytotoxicity of CBS Dendrimers and Commercial Antifungal Drugs in Hela and Human Foreskin Fibroblasts Cells
2.11. Scanning Electron Microscopy
2.12. Statistical Analysis
3. Results
3.1. Optimal Biofilm Formation
3.2. Resazurin Viability Assay
3.3. Dendrimer Activity Preventing Biofilm Formation
3.4. Dendrimer Activity Against Biofilms
3.5. ANF and CSF: Antifungal Susceptibility on Pre-Biofilms and Biofilms
3.6. Synergistic Activity of BDSQ024 and Commercial Antifungals Combinations against Candida albicans CECT 1002
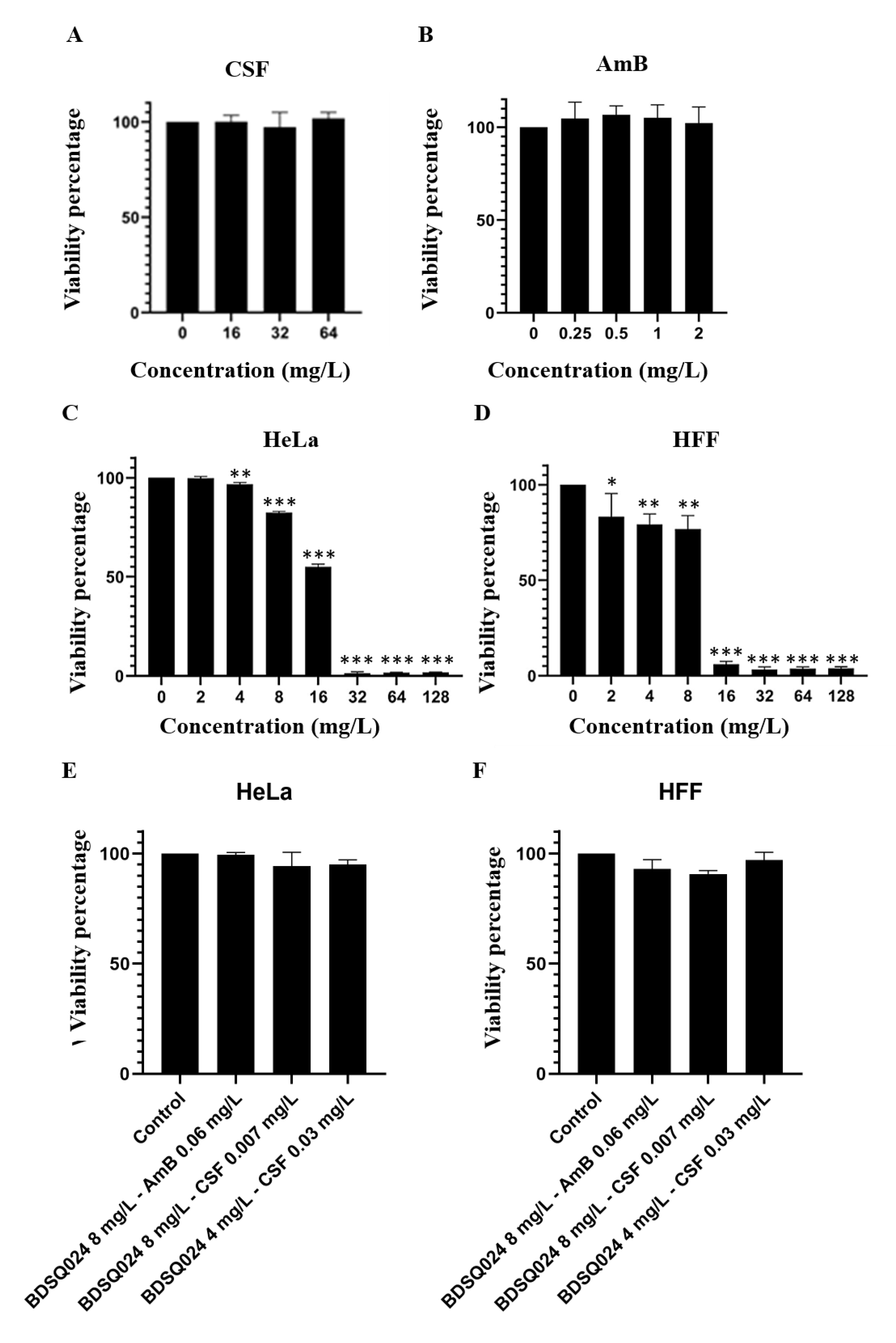
3.7. Cytotoxicity of AmB and CSF
3.8. Cytotoxicity of BDSQ024 on Hela and HFF
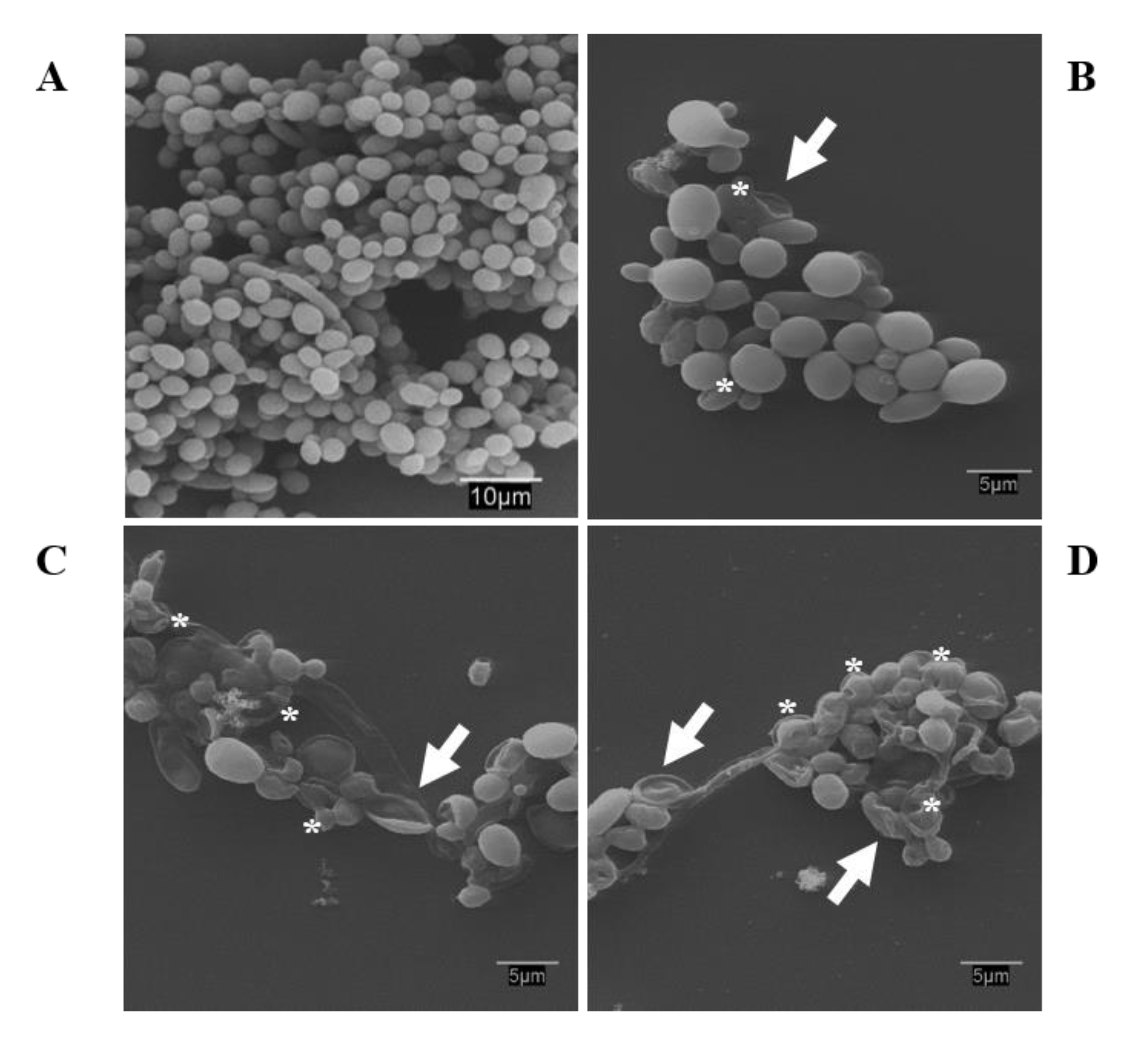
3.9. Scanning Electron Microscopy: Observations on the Biofilm Morphology
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vediyappan, G.; Rossignol, T.; d’Enfert, C. Interaction of Candida albicans biofilms with antifungals: Transcriptional response and binding of antifungals to beta-glucans. Antimicrob. Agents Chemother. 2010, 54, 2096–2111. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Casanova, N.; Bellido, A.; Espinosa-Texis, A.; Cueva, R.; Ciudad, T.; Larriba, G. Candida tropicalis Isolates from Mexican Republic Exhibit High Susceptibility to Bleomycin and Variable Susceptibility to Hydrogen Peroxide. Microb. Drug Resist. 2018, 24, 1031–1039. [Google Scholar] [CrossRef] [PubMed]
- Cortés, J.A.; Corrales, I.F. Invasive Candidiasis: Epidemiology and Risk Factors. In Fungal Infection; Loreto, É.S., Moraes Tondolo, J.S., Eds.; IntechOpen: London, UK, 2018. [Google Scholar]
- Uppuluri, P.; Pierce, C.G.; Lopez-Ribot, J.L. Candida albicans biofilm formation and its clinical consequences. Future Microbiol. 2009, 4, 1235–1237. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Tan, F.; Miao, H.; Wang, H.; Cao, Y. Effect of Shikonin Against Candida albicans Biofilms. Front. Microbiol. 2019, 10, 1085. [Google Scholar] [CrossRef] [PubMed]
- Chandra, J.; Kuhn, D.M.; Mukherjee, P.K.; Hoyer, L.L.; McCormick, T.; Ghannoum, M.A. Biofilm formation by the fungal pathogen Candida albicans: Development, architecture, and drug resistance. J. Bacteriol. 2001, 183, 5385–5394. [Google Scholar] [CrossRef]
- Nobile, C.J.; Johnson, A.D. Candida albicans Biofilms and Human Disease. Annu Rev. Microbiol. 2015, 69, 71–92. [Google Scholar] [CrossRef]
- Silva, S.; Henriques, M.; Martins, A.; Oliveira, R.; Williams, D.; Azeredo, J. Biofilms of non-Candida albicans Candida species: Quantification, structure and matrix composition. Med. Mycol. 2009, 47, 681–689. [Google Scholar] [CrossRef]
- Sardi, J.C.O.; Scorzoni, L.; Bernardi, T.; Fusco-Almeida, A.M.; Mendes Giannini, M.J.S. Candida species: Current epidemiology, pathogenicity, biofilm formation, natural antifungal products and new therapeutic options. J. Med. Microbiol. 2013, 62, 10–24. [Google Scholar] [CrossRef]
- Ramage, G.; Martinez, J.P.; Lopez-Ribot, J.L. Candida biofilms on implanted biomaterials: A clinically significant problem. FEMS Yeast Res. 2006, 6, 979–986. [Google Scholar] [CrossRef]
- Bachmann, S.P.; VandeWalle, K.; Ramage, G.; Patterson, T.F.; Wickes, B.L.; Graybill, J.R.; Lopez-Ribot, J.L. In vitro activity of caspofungin against Candida albicans biofilms. Antimicrob. Agents Chemother. 2002, 46, 3591–3596. [Google Scholar] [CrossRef]
- Kojic, E.M.; Darouiche, R.O. Candida infections of medical devices. Clin. Microbiol. Rev. 2004, 17, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Hoehamer, C.F.; Cummings, E.D.; Hilliard, G.M.; Rogers, P.D. Changes in the proteome of Candida albicans in response to azole, polyene, and echinocandin antifungal agents. Antimicrob. Agents Chemother. 2010, 54, 1655–1664. [Google Scholar] [CrossRef] [PubMed]
- Roscetto, E.; Contursi, P.; Vollaro, A.; Fusco, S.; Notomista, E.; Catania, M.R. Antifungal and anti-biofilm activity of the first cryptic antimicrobial peptide from an archaeal protein against Candida spp. clinical isolates. Sci. Rep. 2018, 8, 17570. [Google Scholar] [CrossRef] [PubMed]
- Nett, J.E.; Cain, M.T.; Crawford, K.; Andes, D.R. Optimizing a Candida biofilm microtiter plate model for measurement of antifungal susceptibility by tetrazolium salt assay. J. Clin. Microbiol. 2011, 49, 1426–1433. [Google Scholar] [CrossRef]
- Mintzer, M.A.; Dane, E.L.; O’Toole, G.A.; Grinstaff, M.W. Exploiting dendrimer multivalency to combat emerging and re-emerging infectious diseases. Mol. Pharm. 2012, 9, 342–354. [Google Scholar] [CrossRef]
- Xue, Y.; Xiao, H.; Zhang, Y. Antimicrobial polymeric materials with quaternary ammonium and phosphonium salts. Int. J. Mol. Sci. 2015, 16, 3626–3655. [Google Scholar] [CrossRef]
- Garcia-Gallego, S.; Franci, G.; Falanga, A.; Gomez, R.; Folliero, V.; Galdiero, S.; de la Mata, F.J.; Galdiero, M. Function Oriented Molecular Design: Dendrimers as Novel Antimicrobials. Molecules 2017, 22, 1581. [Google Scholar] [CrossRef]
- Chen, C.Z.; Beck-Tan, N.C.; Dhurjati, P.; van Dyk, T.K.; LaRossa, R.A.; Cooper, S.L. Quaternary ammonium functionalized poly (propylene imine) dendrimers as effective antimicrobials: Structure-activity studies. Biomacromolecules 2000, 1, 473–480. [Google Scholar] [CrossRef]
- Fuentes-Paniagua, E.; Sánchez-Nieves, J.; Hernández-Ros, J.M.; Fernández-Ezequiel, A.; Soliveri, J.; Copa-Patiño, J.L.; Gómez, R.; Javier de la Mata, F. Structure–activity relationship study of cationic carbosilane dendritic systems as antibacterial agents. RSC Adv. 2016, 6, 7022–7033. [Google Scholar] [CrossRef]
- Heredero-Bermejo, I.; Hernández-Ros, J.M.; Sánchez-García, L.; Maly, M.; Verdú-Expósito, C.; Soliveri, J.; Javier de la Mata, F.; Copa-Patiño, J.L.; Pérez-Serrano, J.; Sánchez-Nieves, J.; et al. Ammonium and guanidine carbosilane dendrimers and dendrons as microbicides. Eur. Polym. J. 2018, 101, 159–168. [Google Scholar] [CrossRef]
- Wronska, N.; Majoral, J.P.; Appelhans, D.; Bryszewska, M.; Lisowska, K. Synergistic Effects of Anionic/Cationic Dendrimers and Levofloxacin on Antibacterial Activities. Molecules 2019, 24, 2894. [Google Scholar] [CrossRef] [PubMed]
- Martin-Perez, T.; Lozano-Cruz, T.; Criado-Fornelio, A.; Ortega, P.; Gomez, R.; de la Mata, F.J.; Perez-Serrano, J. Synthesis and in vitro activity of new biguanide-containing dendrimers on pathogenic isolates of Acanthamoeba polyphaga and Acanthamoeba griffini. Parasitol. Res. 2019, 118, 1953–1961. [Google Scholar] [CrossRef] [PubMed]
- Ortega, P.; Sánchez-Nieves, J.; Cano, J.; Gómez, R.; de la Mata, F.J. Poly (carbosilane) dendrimers and other silicon-containing dendrimers. In Dendrimer Chemistry: Synthetic Approaches Towards Complex. Architectures; Malkoch, M., García Gallego, S., Eds.; The Royal Society of Chemistry: London, UK, 2020. [Google Scholar]
- Janaszewska, A.; Lazniewska, J.; Trzepiński, P.; Marcinkowska, M.; Klajnert-Maculewicz, B. Cytotoxicity of Dendrimers. Biomolecules 2019, 9, 330. [Google Scholar] [CrossRef] [PubMed]
- Heredero-Bermejo, I.; Sanchez-Nieves, J.; Soliveri, J.; Gomez, R.; de la Mata, F.J.; Copa-Patino, J.L.; Perez-Serrano, J. In vitro anti-Acanthamoeba synergistic effect of chlorhexidine and cationic carbosilane dendrimers against both trophozoite and cyst forms. Int. J. Pharm. 2016, 509, 1–7. [Google Scholar] [CrossRef]
- Zielinska, P.; Staniszewska, M.; Bondaryk, M.; Koronkiewicz, M.; Urbanczyk-Lipkowska, Z. Design and studies of multiple mechanism of anti-Candida activity of a new potent Trp-rich peptide dendrimers. Eur. J. Med. Chem. 2015, 105, 106–119. [Google Scholar] [CrossRef]
- Jose, J.; Charyulu, R.N. Prolonged drug delivery system of an antifungal drug by association with polyamidoamine dendrimers. Int. J. Pharm. Investig. 2016, 6, 123–127. [Google Scholar] [CrossRef]
- Winnicka, K.; Sosnowska, K.; Wieczorek, P.; Sacha, P.T.; Tryniszewska, E. Poly (amidoamine) dendrimers increase antifungal activity of clotrimazole. Biol. Pharm. Bull. 2011, 34, 1129–1133. [Google Scholar] [CrossRef]
- Winnicka, K.; Wroblewska, M.; Wieczorek, P.; Sacha, P.T.; Tryniszewska, E. Hydrogel of ketoconazole and PAMAM dendrimers: Formulation and antifungal activity. Molecules 2012, 17, 4612–4624. [Google Scholar] [CrossRef]
- Khairnar, G.A.; Chavan-Patil, A.B.; Palve, P.R.; Bhise, S.B.; Mourya, V.K.; Kulkarni, C.G. Dendrimers: Potential tool for enhancement of antifungal activity. Int. J. PharmTech Res. 2010, 2, 736–739. [Google Scholar]
- Ramage, G.; Bachmann, S.; Patterson, T.F.; Wickes, B.L.; Lopez-Ribot, J.L. Investigation of multidrug efflux pumps in relation to fluconazole resistance in Candida albicans biofilms. J. Antimicrob. Chemother. 2002, 49, 973–980. [Google Scholar] [CrossRef]
- Ramage, G.; Vande Walle, K.; Wickes, B.L.; Lopez-Ribot, J.L. Standardized method for in vitro antifungal susceptibility testing of Candida albicans biofilms. Antimicrob. Agents Chemother. 2001, 45, 2475–2479. [Google Scholar] [CrossRef] [PubMed]
- Negri, M.; Goncalves, V.; Silva, S.; Henriques, M.; Azeredo, J.; Oliveira, R. Crystal violet staining to quantify Candida adhesion to epithelial cells. Br. J. Biomed. Sci. 2010, 67, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Juarez-Perez, E.J.; Viñas, C.; Teixidor, F.; Núñez, R. Polyanionic Carbosilane and Carbosiloxane Metallodendrimers Based on Cobaltabisdicarbollide Derivatives. Organometallics 2009, 28, 5550–5559. [Google Scholar] [CrossRef]
- Andrea, G.D. Antifungal Effect of Synthetic Dendritic Compounds on Selected Clinical Strains of Candida albicans and C. glabrata. Master’s Thesis, University of Alcala, Madrid, Spain, 2018. [Google Scholar]
- Barrios-Gumiel, A.; Sanchez-Nieves, J.; Perez-Serrano, J.; Gomez, R.; de la Mata, F.J. PEGylated AgNP covered with cationic carbosilane dendrons to enhance antibacterial and inhibition of biofilm properties. Int. J. Pharm. 2019, 569, 118591. [Google Scholar] [CrossRef]
- Sanz del Olmo, N.; Carloni, R.; Bajo, A.M.; Ortega, P.; Fattori, A.; Gomez, R.; Ottaviani, M.F.; Garcia-Gallego, S.; Cangiotti, M.; de la Mata, F.J. Insight into the antitumor activity of carbosilane Cu(ii)-metallodendrimers through their interaction with biological membrane models. Nanoscale 2019, 11, 13330–13342. [Google Scholar] [CrossRef] [PubMed]
- De la Mata de la Mata, F.J.; Gómez, R.; Sánchez-Nieves, J.; Mencia-Berlinches, G.; Cano-Sierra, J.; Copa-Patiño, J.L.; Soliveri, J.; Pérez-Serrano, J.; Valiente-Martínez, M.; Gutiérrez-Ulloa, C.E.; et al. Carbosilane Dendrons Functionalized with Fatty Acids: Formation of Micelles and Uses. Oficina Española de Patentes y Marcas Patent No. ES2657282A1, 2 March 2018. Oficina Española de Patentes y Marcas Patent No. ES2657282B1, 17 December 2018. [Google Scholar]
- Fernandez, J.; Acosta, G.; Pulido, D.; Maly, M.; Copa-Patino, J.L.; Soliveri, J.; Royo, M.; Gomez, R.; Albericio, F.; Ortega, P.; et al. Carbosilane Dendron-Peptide Nanoconjugates as Antimicrobial Agents. Mol. Pharm. 2019, 16, 2661–2674. [Google Scholar] [CrossRef]
- National Committee for Clinical Laboratory Standards. Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts: Approved Standard M27-A; NCCLS: Wayne, PA, USA, 1997. [Google Scholar]
- Zarei Mahmoudabadi, A.; Zarrin, M.; Kiasat, N. Biofilm Formation and Susceptibility to Amphotericin B and Fluconazole in Candida albicans. Jundishapur J. Microbiol. 2014, 7, e17105. [Google Scholar] [CrossRef] [PubMed]
- Kerekes, E.B.; Deak, E.; Tako, M.; Tserennadmid, R.; Petkovits, T.; Vagvolgyi, C.; Krisch, J. Anti-biofilm forming and anti-quorum sensing activity of selected essential oils and their main components on food-related micro-organisms. J. Appl. Microbiol. 2013, 115, 933–942. [Google Scholar] [CrossRef]
- Thomas, P.; Sekhar, A.C.; Upreti, R.; Mujawar, M.M.; Pasha, S.S. Optimization of single plate-serial dilution spotting (SP-SDS) with sample anchoring as an assured method for bacterial and yeast cfu enumeration and single colony isolation from diverse samples. Biotechnol. Rep. 2015, 8, 45–55. [Google Scholar] [CrossRef]
- Ravi, N.S.; Aslam, R.F.; Veeraraghavan, B. A New Method for Determination of Minimum Biofilm Eradication Concentration for Accurate Antimicrobial Therapy. Methods Mol. Biol. 2019, 1946, 61–67. [Google Scholar] [CrossRef]
- Serra, E.; Hidalgo-Bastida, L.A.; Verran, J.; Williams, D.; Malic, S. Antifungal Activity of Commercial Essential Oils and Biocides against Candida albicans. Pathogens 2018, 7, 15. [Google Scholar] [CrossRef] [PubMed]
- Kohno, Y.; Ohno, H.; Miyazaki, Y.; Higashiyama, Y.; Yanagihara, K.; Hirakata, Y.; Fukushima, K.; Kohno, S. In vitro and in vivo activities of novel fluoroquinolones alone and in combination with clarithromycin against clinically isolated Mycobacterium avium complex strains in Japan. Antimicrob. Agents Chemother. 2007, 51, 4071–4076. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sims, C.R.; Ostrosky-Zeichner, L.; Rex, J.H. Invasive candidiasis in immunocompromised hospitalized patients. Arch. Med. Res. 2005, 36, 660–671. [Google Scholar] [CrossRef] [PubMed]
- Mason, K.L.; Erb Downward, J.R.; Mason, K.D.; Falkowski, N.R.; Eaton, K.A.; Kao, J.Y.; Young, V.B.; Huffnagle, G.B. Candida albicans and bacterial microbiota interactions in the cecum during recolonization following broad-spectrum antibiotic therapy. Infect. Immun. 2012, 80, 3371–3380. [Google Scholar] [CrossRef]
- Ghrenassia, E.; Mokart, D.; Mayaux, J.; Demoule, A.; Rezine, I.; Kerhuel, L.; Calvet, L.; De Jong, A.; Azoulay, E.; Darmon, M. Candidemia in critically ill immunocompromised patients: Report of a retrospective multicenter cohort study. Ann. Intensive Care 2019, 9, 62. [Google Scholar] [CrossRef]
- Taff, H.T.; Mitchell, K.F.; Edward, J.A.; Andes, D.R. Mechanisms of Candida biofilm drug resistance. Future Microbiol. 2013, 8, 1325–1337. [Google Scholar] [CrossRef]
- Rasines, B.; Hernandez-Ros, J.M.; de las Cuevas, N.; Copa-Patino, J.L.; Soliveri, J.; Munoz-Fernandez, M.A.; Gomez, R.; de la Mata, F.J. Water-stable ammonium-terminated carbosilane dendrimers as efficient antibacterial agents. Dalton Trans. 2009, 8704–8713. [Google Scholar] [CrossRef]
- Jin, Y.; Yip, H.K.; Samaranayake, Y.H.; Yau, J.Y.; Samaranayake, L.P. Biofilm-forming ability of Candida albicans is unlikely to contribute to high levels of oral yeast carriage in cases of human immunodeficiency virus infection. J. Clin. Microbiol. 2003, 41, 2961–2967. [Google Scholar] [CrossRef]
- Melo, A.S.; Bizerra, F.C.; Freymuller, E.; Arthington-Skaggs, B.A.; Colombo, A.L. Biofilm production and evaluation of antifungal susceptibility amongst clinical Candida spp. isolates, including strains of the Candida parapsilosis complex. Med. Mycol. 2011, 49, 253–262. [Google Scholar] [CrossRef]
- De la Cruz-Claure, M.L.; Cèspedes-Llave, A.A.; Ulloa, M.T.; Benito-Lama, M.; Domínguez-Álvarez, E.; Bastida, A. Inhibition–Disruption of Candida glabrata Biofilms: Symmetrical Selenoesters as Potential Anti-Biofilm Agents. Microorganisms 2019, 7, 664. [Google Scholar] [CrossRef]
- Kuhn, D.M.; Balkis, M.; Chandra, J.; Mukherjee, P.K.; Ghannoum, M.A. Uses and limitations of the XTT assay in studies of Candida growth and metabolism. J. Clin. Microbiol. 2003, 41, 506–508. [Google Scholar] [CrossRef] [PubMed]
- Waters, E.M.; Rowe, S.E.; O’Gara, J.P.; Conlon, B.P. Convergence of Staphylococcus aureus Persister and Biofilm Research: Can Biofilms Be Defined as Communities of Adherent Persister Cells? PLoS Pathog. 2016, 12, e1006012. [Google Scholar] [CrossRef] [PubMed]
- Conlon, B.P.; Rowe, S.E.; Gandt, A.B.; Nuxoll, A.S.; Donegan, N.P.; Zalis, E.A.; Clair, G.; Adkins, J.N.; Cheung, A.L.; Lewis, K. Persister formation in Staphylococcus aureus is associated with ATP depletion. Nat. Microbiol. 2016, 1. [Google Scholar] [CrossRef] [PubMed]
- LaFleur, M.D.; Kumamoto, C.A.; Lewis, K. Candida albicans biofilms produce antifungal-tolerant persister cells. Antimicrob. Agents Chemother. 2006, 50, 3839–3846. [Google Scholar] [CrossRef]
- Mathe, L.; Van Dijck, P. Recent insights into Candida albicans biofilm resistance mechanisms. Curr Genet. 2013, 59, 251–264. [Google Scholar] [CrossRef]
- Wuyts, J.; Van Dijck, P.; Holtappels, M. Fungal persister cells: The basis for recalcitrant infections? PLoS Pathog. 2018, 14, e1007301. [Google Scholar] [CrossRef]
- Grela, E.; Zdybicka-Barabas, A.; Pawlikowska-Pawlega, B.; Cytrynska, M.; Wlodarczyk, M.; Grudzinski, W.; Luchowski, R.; Gruszecki, W.I. Modes of the antibiotic activity of amphotericin B against Candida albicans. Sci. Rep. 2019, 9, 17029. [Google Scholar] [CrossRef]
- Pesee, S.; Angkananuwat, C.; Tancharoensukjit, S.; Muanmai, S.; Sirivan, P.; Bubphawas, M.; Tanarerkchai, N. In vitro activity of Caspofungin combined with Fluconazole on mixed Candida albicans and Candida glabrata biofilm. Med. Mycol. 2016, 54, 384–393. [Google Scholar] [CrossRef]
- Staniszewska, M.; Bondaryk, M.; Swoboda-Kopec, E.; Siennicka, K.; Sygitowicz, G.; Kurzatkowski, W. Candida albicans morphologies revealed by scanning electron microscopy analysis. Br. J. Microbiol. 2013, 44, 813–821. [Google Scholar] [CrossRef]
- Campitelli, M.; Zeineddine, N.; Samaha, G.; Maslak, S. Combination Antifungal Therapy: A Review of Current Data. J. Clin. Med. Res. 2017, 9, 451–456. [Google Scholar] [CrossRef]

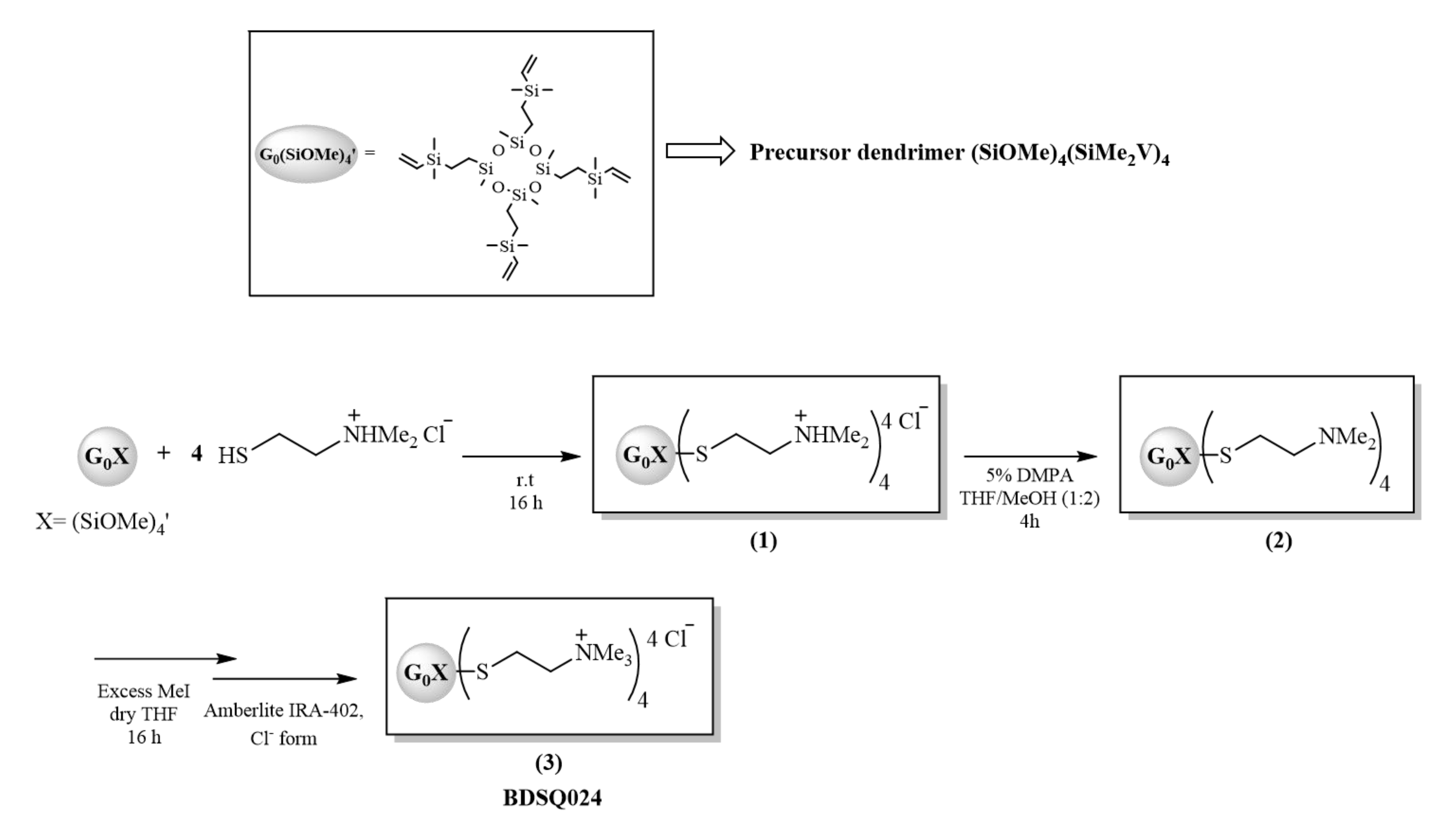
| Lab Name | Compound | Core | Functional Groups | R |
|---|---|---|---|---|
| BDSQ024 | C48H120 Cl4N4O4S4Si8 | (SiOMe)4 | 4, NMe3+ | - |
| AG3 | C64H160Cl8N8O4S8Si8 | (SiOMe)4 | 8 NMe2H+ | [36] |
| BDAB24 | HSG1(SNMe3+)2:HSHPEG, ratio 1:1 | AgNP | -NMe3+, -PEG | [37] |
| BDAB27 | HSG2(SNMe3+)4:HSHPEG, ratio 1:1 | AgNP | -NMe3+, -PEG | [37] |
| BDAB28 | HSG3(SNMe3+)4:HSHPEG, ratio 3:1 | AgNP | -NMe3+, -PEG | [37] |
| BDAT2 | C56H100Cu4N16O28Si5 | Si | 4 pyridine-imine-Cu | [38] |
| BDLS001 | C51H114Cl6N18O3S6Si3 | C6H3O3 | (NHC(NH)NH2)6 (6 Guanidine) | [21] |
| BDEF134 | C47H102I4N4O2S4Si3 | Dendron | F.P.: HOC6H4O; P: 4 NMe3+ | [20] |
| BDEF135 | C91H206I8N8O2S8Si7 | Dendron | F.P.: HOC6H4O; P: 8 NMe3+ | [20] |
| ChG2 | C69H145I4N5O2S4Si3 | Dendron | F.P: Cholesterol; P: 4 NMe3+ | [39] |
| ChG3 | C121H265I8N9O2S8Si7 | Dendron | F.P: Cholesterol; P: 8 NMe3+ | [39] |
| EG2 | C70H146I4N4O2S4Si3 | Dendron | F.P: E vitamin; P: 4 NMe3+ | [39] |
| EG3 | C122H266I8N8O2S8Si7 | Dendron | F.P: E vitamin; P: 8 NMe3+ | [39] |
| YF017 | C41H91Cl4N5O2S4Si3 | Dendron | F.P: Maleimide; P: 4 NMe2H+ | [40] |
| Compounds | Biofilm Formation (mg/L) Range: 2–512 mg/L | |
|---|---|---|
| MBIC | MFC | |
| BDSQ024 * | 16–32 | 32 |
| AG3 | 128 | 128 |
| BDAB24 | >512 | >512 |
| BDAB27 | 128 | 512 |
| BDAB28 | 256 | >512 |
| BDAT2 | 128 | 128 |
| BDLS001 | 32 | 64 |
| BDEF134 | 32–64 | >512 |
| BDEF135 | 128 | >512 |
| ChG2 | >512 | >512 |
| ChG3 | 512 | >512 |
| EG2 | 512 | >512 |
| EG3 | >512 | >512 |
| YF017* | 32 | 32 |
| MBICs (mg/L) | FICI | Relationship | |||
|---|---|---|---|---|---|
| BDSQ024 Individual | AmB Individual | BDSQ024 in Combination with AmB | AmB in Combination with BDSQ024 | ||
| 16–32 | 0.25 | 8 | 0.06 | 0.49 | Low synergy |
| 16–32 | 0.25 | 4 | 0.125 | 0.62 | Non-synergy |
| BDSQ024 individual | CSF individual | BDSQ024 in combination with CSF | CSF in combination with BDSQ024 | FICI | Relationship |
| 16–32 | 0.25–0.5 | 8 | 0.007 | 0.26 | Synergy |
| 16–32 | 0.25–0.5 | 4 | 0.03 | 0.18 | Synergy |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heredero-Bermejo, I.; Gómez-Casanova, N.; Quintana, S.; Soliveri, J.; de la Mata, F.J.; Pérez-Serrano, J.; Sánchez-Nieves, J.; Copa-Patiño, J.L. In Vitro Activity of Carbosilane Cationic Dendritic Molecules on Prevention and Treatment of Candida Albicans Biofilms. Pharmaceutics 2020, 12, 918. https://doi.org/10.3390/pharmaceutics12100918
Heredero-Bermejo I, Gómez-Casanova N, Quintana S, Soliveri J, de la Mata FJ, Pérez-Serrano J, Sánchez-Nieves J, Copa-Patiño JL. In Vitro Activity of Carbosilane Cationic Dendritic Molecules on Prevention and Treatment of Candida Albicans Biofilms. Pharmaceutics. 2020; 12(10):918. https://doi.org/10.3390/pharmaceutics12100918
Chicago/Turabian StyleHeredero-Bermejo, Irene, Natalia Gómez-Casanova, Sara Quintana, Juan Soliveri, Francisco Javier de la Mata, Jorge Pérez-Serrano, Javier Sánchez-Nieves, and José Luis Copa-Patiño. 2020. "In Vitro Activity of Carbosilane Cationic Dendritic Molecules on Prevention and Treatment of Candida Albicans Biofilms" Pharmaceutics 12, no. 10: 918. https://doi.org/10.3390/pharmaceutics12100918
APA StyleHeredero-Bermejo, I., Gómez-Casanova, N., Quintana, S., Soliveri, J., de la Mata, F. J., Pérez-Serrano, J., Sánchez-Nieves, J., & Copa-Patiño, J. L. (2020). In Vitro Activity of Carbosilane Cationic Dendritic Molecules on Prevention and Treatment of Candida Albicans Biofilms. Pharmaceutics, 12(10), 918. https://doi.org/10.3390/pharmaceutics12100918