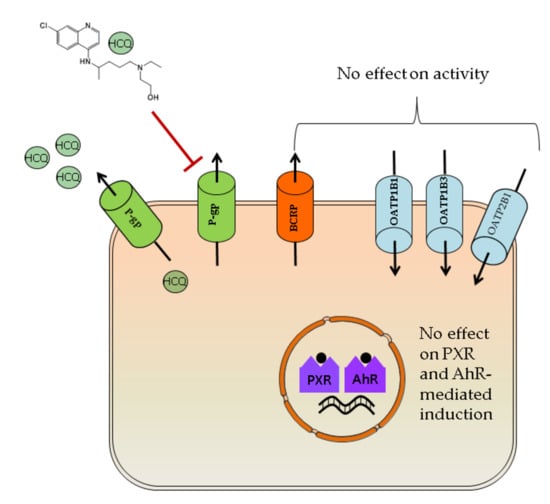
Interaction of Hydroxychloroquine with Pharmacokinetically Important Drug Transporters
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cytotoxicity Assays
2.3. P-gp Inhibition Assay (Calcein-AM Uptake Assay)
2.4. BCRP Inhibition Assay (Flow Cytometric Pheophorbide A Efflux Assay)
2.5. Flow Cytometric OATP1B1 and OATP1B3 Inhibition Assay
2.6. Flow Cytometric OATP2B1 Inhibition Assay
2.7. Growth Inhibition Assays with Resistant Cell Lines for Assessing P-gp and BCRP Substrate Characteristics
2.8. Induction Assay
2.9. Statistical Analyses
3. Results
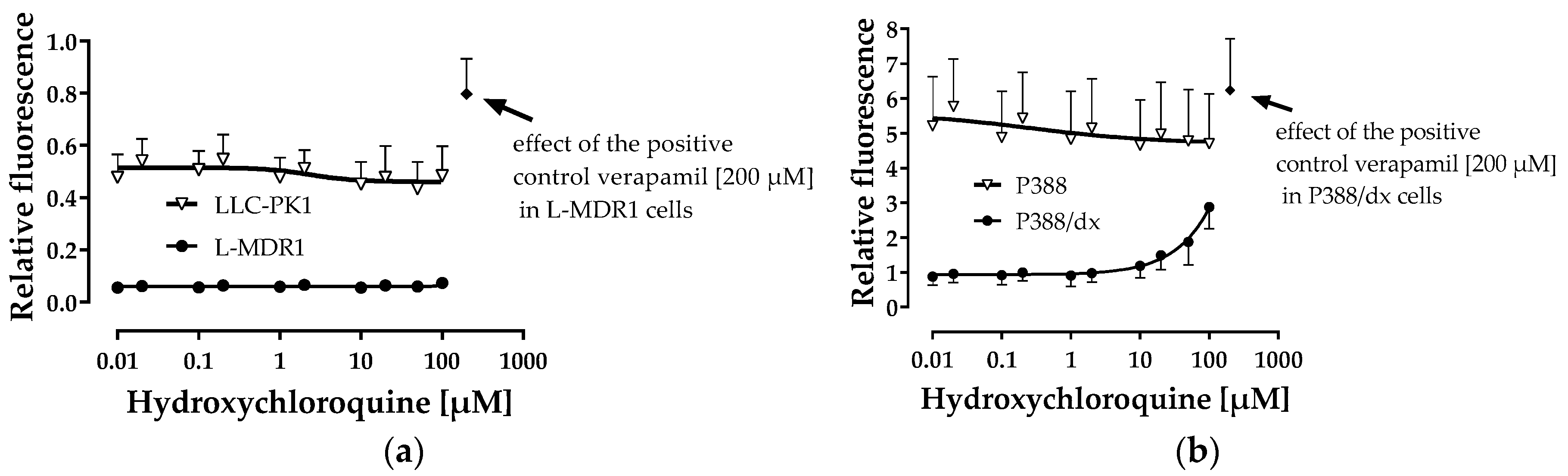
3.1. Inhibition of Drug Transporters by Hydroxychloroquine
3.2. P-gp and BCRP Substrate Characteristics of Hydroxychloroquine
3.3. Induction of PXR and AhR Regulated Genes by Hydroxychloroquine
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Easterbrook, M. Detection and prevention of maculopathy associated with antimalarial agents. Int. Ophthalmol. Clin. 1999, 39, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Pancă, A.G.; Popa-Cherecheanu, A.; Marinescu, B.; Geamănu, C.D.; Voinea, L.M. Retinal toxicity associated with chronic exposure to hydroxychloroquine and its ocular screening. Review. J. Med. Life 2014, 7, 322–326. [Google Scholar]
- Olsen, N.J.; Schleich, M.A.; Karp, D.R. Multifaceted effects of hydroxychloroquine in human disease. Semin. Arthritis Rheum. 2013, 43, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Schrezenmeier, E.; Dörner, T. Mechanisms of action of hydroxychloroquine and chloroquine: Implications for rheumatology. Nat. Rev. Rheumatol. 2020, 16, 155–166. [Google Scholar] [PubMed]
- Rolain, J.M.; Colson, P.; Raoult, D. Recycling of chloroquine and its hydroxyl analogue to face bacterial, fungal and viral infections in the 21st century. Int. J. Antimicrob. Agents 2007, 30, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Plantone, D.; Koudriavtseva, T. Current and future use of chloroquine and hydroxychloroquine in infectious, immune, neoplastic, and neurological diseases: A Mini-Review. Clin. Drug Investig. 2018, 38, 653–671. [Google Scholar] [PubMed]
- Hashem, A.M.; Alghamdi, B.S.; Algaissi, A.A.; Alshehri, F.S.; Bukhari, A.; Alfaleh, M.A.; Memish, Z.A. Therapeutic use of chloroquine and hydroxychloroquine in COVID-19 and other viral infections: A narrative review. Travel Med. Infect. Dis. 2020, 35, 101735. [Google Scholar] [CrossRef]
- Wahie, S.; Daly, A.K.; Cordell, H.J.; Goodfield, M.J.; Jones, S.K.; Lovell, C.R.; Carmichael, A.J.; Carr, M.M.; Drummond, A.; Natarajan, S.; et al. Clinical and pharmacogenetic influences on response to hydroxychloroquine in discoid lupus erythematosus: A retrospective cohort study. J. Investig. Dermatol. 2011, 131, 1981–1986. [Google Scholar] [CrossRef]
- Lee, J.Y.; Vinayagamoorthy, N.; Han, K.; Kwok, S.K.; Ju, J.H.; Park, K.S.; Jung, S.H.; Park, S.W.; Chung, Y.J.; Park, S.H. Association of polymorphisms of cytochrome P450 2D6 with blood hydroxychloroquine levels in patients with systemic lupus erythematosus. Arthritis Rheumatol. 2016, 68, 184–190. [Google Scholar] [CrossRef]
- Somer, M.; Kallio, J.; Pesonen, U.; Pyykkö, K.; Huupponen, R.; Scheinin, M. Influence of hydroxychloroquine on the bioavailability of oral metoprolol. Br. J. Clin. Pharmacol. 2000, 49, 549–554. [Google Scholar]
- Nakanishi, T.; Tamai, I. Interaction of drug or food with drug transporters in intestine and liver. Curr. Drug Metab. 2015, 16, 753–764. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.X.; Yi, X.L.; Fan, H.R.; Wu, W.D.; Zhang, X.; Xiao, X.F.; He, X. Effects of drug transporters on pharmacological responses and safety. Curr. Drug Metab. 2015, 16, 732–752. [Google Scholar] [CrossRef] [PubMed]
- König, J.; Müller, F.; Fromm, M.F. Transporters and drug-drug interactions: Important determinants of drug disposition and effects. Pharmacol. Rev. 2013, 65, 944–966. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J.; Haefeli, W.E. Impact of ATP-binding cassette transporters on human immunodeficiency virus therapy. Int. Rev. Cell. Mol. Biol. 2010, 280, 219–279. [Google Scholar]
- McFeely, S.J.; Wu, L.; Ritchie, T.K.; Unadkat, J. Organic anion transporting polypeptide 2B1—More than a glass-full of drug interactions. Pharmacol. Ther. 2019, 196, 204–215. [Google Scholar] [CrossRef]
- Leden, I. Digoxin-hydroxychloroquine interaction? Acta Med. Scand. 1982, 211, 411–412. [Google Scholar] [CrossRef]
- Weiss, J.; Dormann, S.M.; Martin-Facklam, M.; Kerpen, C.J.; Ketabi-Kiyanvash, N.; Haefeli, W.E. Inhibition of P-glycoprotein by newer antidepressants. J. Pharmacol. Exp. Ther. 2003, 305, 197–204. [Google Scholar] [CrossRef]
- Fröhlich, M.; Albermann, N.; Sauer, A.; Walter-Sack, I.; Haefeli, W.E.; Weiss, J. In vitro and ex vivo evidence for modulation of P-glycoprotein activity by progestins. Biochem. Pharmacol. 2004, 68, 2409–2416. [Google Scholar] [CrossRef]
- Schinkel, A.H.; Wagenaar, E.; Mol, C.A.; van Deemter, L. P-glycoprotein in the bloodbrain barrier of mice influences the brain penetration and pharmacological activity of many drugs. J. Clin. Investig. 1996, 97, 2517–2524. [Google Scholar] [CrossRef]
- Boesch, D.; Gavériaux, C.; Jachez, B.; Pourtier-Manzanedo, A.; Bollinger, P.; Loor, F. In vivo circumvention of P-glycoprotein-mediated multidrug resistance of tumor cells with SDZ PSC 833. Cancer Res. 1991, 51, 4226–4233. [Google Scholar]
- Pavek, P.; Merino, G.; Wagenaar, E.; Bolscher, E.; Novotna, M.; Jonker, J.W.; Schinkel, A.H. Human breast cancer resistance protein: Interactions with steroid drugs, hormones, the dietary carcinogen 2-amino-1-methyl-6-phenylimidazo(4,5-b)pyridine, and transport of cimetidine. J. Pharmacol. Exp. Ther. 2005, 312, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J.; Rose, J.; Storch, C.H.; Ketabi-Kiyanvash, N.; Sauer, A.; Haefeli, W.E.; Efferth, T. Modulation of human BCRP (ABCG2) activity by anti-HIV drugs. J. Antimicrob. Chemother. 2007, 59, 238–245. [Google Scholar] [PubMed]
- König, J.; Cui, Y.; Nies, A.T.; Keppler, D. Localization and genomic organization of a new hepatocellular organic anion transporting polypeptide. J. Biol. Chem. 2000, 275, 23161–23168. [Google Scholar] [CrossRef] [PubMed]
- König, J.; Cui, Y.; Nies, A.T.; Keppler, D. A novel human organic anion transporting polypeptide localized to the basolateral hepatocyte membrane. Am. J. Physiol. Gastrointest. Liver Physiol. 2000, 278, G156–G164. [Google Scholar]
- Weiss, J.; Theile, D.; Spalwisz, A.; Burhenne, J.; Riedel, K.D.; Haefeli, W.E. Influence of sildenafil and tadalafil on the enzyme- and transporter-inducing effects of bosentan and ambrisentan in LS180 cells. Biochem. Pharmacol. 2013, 85, 265–273. [Google Scholar]
- Grosser, G.; Döring, B.; Uegele, B.; Geyer, J.; Kulling, S.E.; Soukup, S.T. Transport of the soy isoflavone diadzein and its conjugative metabolites by the carriers SOAT, NTCP, OAT4, and OATP2B1. Arch. Toxicol. 2015, 89, 2253–2263. [Google Scholar]
- Peters, T.; Lindenmaier, H.; Haefeli, W.E.; Weiss, J. Interaction of the mitotic kinesin Eg5 inhibitor monastrol with P-glycoprotein. Naunyn Schmiedebergs Arch. Pharmacol. 2006, 372, 291–299. [Google Scholar]
- Weiss, J.; Theile, D.; Dvorak, Z.; Haefeli, W.E. Interaction potential of the multitargeted receptor tyrosine kinase inhibitor dovitinib with drug transporters and drug metabolising enzymes assessed in vitro. Pharmaceutics 2014, 6, 632–650. [Google Scholar]
- Albermann, N.; Schmitz-Winnenthal, F.H.; Z’graggen, K.; Volk, C.; Hoffmann, M.M.; Haefeli, W.E.; Weiss, J. Expression of the drug transporters MDR1/ABCB1, MRP1/ABCC1, MRP2/ABCC2, BCRP/ABCG2, and PXR in peripheral blood mononuclear cells and their relationship with the expression in intestine and liver. Biochem. Pharmacol. 2005, 70, 949–958. [Google Scholar]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, research0034.1. [Google Scholar]
- Dvorak, Z.; Vrzal, R.; Henklova, P.; Jancova, P.; Anzenbacherova, E.; Maurel, P.; Svecova, L.; Pavek, P.; Ehrmann, J.; Havlik, R.; et al. JNK inhibitor SP600125 is a partial agonist of human aryl hydrocarbon receptor and induces CYP1A1 and CYP1A2 genes in primary human hepatocytes. Biochem. Pharmacol. 2008, 5, 580–588. [Google Scholar]
- Ayed-Boussema, I.; Pascussi, J.M.; Maurel, P.; Bacha, H.; Hassen, W. Zearalenone activates pregnane X receptor; constitutive androstane receptor and aryl hydrocarbon receptor and corresponding phase I target genes mRNA in primary cultures of human hepatocytes. Environ. Toxicol. Pharmacol. 2011, 31, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Cerveny, L.; Svecova, L.; Anzenbacherova, E.; Vrzal, R.; Staud, F.; Dvorak, Z.; Ulrichova, J.; Anzenbacher, P.; Pavek, P. Valproic acid induces CYP3A4 and MDR1 gene expression by activation of constitutive androstane receptor and pregnane X receptor pathways. Drug Metab. Dispos. 2007, 35, 1032–1041. [Google Scholar] [PubMed]
- Zisowsky, J.; Koegel, S.; Leyers, S.; Devarakonda, K.; Kassack, M.U.; Osmak, M.; Jaehde, U. Relevance of drug uptake and efflux for cisplatin sensitivity of tumor cells. Biochem. Pharmacol. 2007, 73, 298–307. [Google Scholar] [PubMed]
- Meo, S.A.; Klonoff, D.C.; Akram, J. Efficacy of chloroquine and hydroxychloroquine in the treatment of COVID-19. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 4539–4547. [Google Scholar] [PubMed]
- Tett, S.E.; Cutler, D.J.; Day, R.O.; Brown, K.F. A dose-ranging study of the pharmacokinetics of hydroxy-chloroquine following intravenous administration to healthy volunteers. Br. J. Clin. Pharmacol. 1988, 26, 303–313. [Google Scholar] [PubMed]
- Rainsford, K.D.; Parke, A.L.; Clifford-Rashotte, M.; Kean, W.F. Therapy and pharmacological properties of hydroxychloroquine and chloroquine in treatment of systemic lupus erythematosus, rheumatoid arthritis and related diseases. Inflammopharmacology 2015, 23, 231–269. [Google Scholar] [CrossRef]
- Francès, C.; Cosnes, A.; Duhaut, P.; Zahr, N.; Soutou, B.; Ingen-Housz-Oro, S.; Bessis, D.; Chevrant-Breton, J.; Cordel, N.; Lipsker, D.; et al. Low blood concentration of hydroxychloroquine in patients with refractory cutaneous lupus erythematosus: A French multicenter prospective study. Arch. Dermatol. 2012, 148, 479–484. [Google Scholar]
- Costedoat-Chalumeau, N.; Amoura, Z.; Hulot, J.S.; Hammoud, H.A.; Aymard, G.; Cacoub, P.; Francès, C.; Wechsler, B.; Huong du, L.T.; Ghillani, P.; et al. Low blood concentration of hydroxychloroquine is a marker for and predictor of disease exacerbations in patients with systemic lupus erythematosus. Arthritis Rheum. 2006, 54, 3284–3290. [Google Scholar]
- Cutler, D.J. Possible mechanisms of action of antimalarials in rheumatic disease. Agents Actions Suppl. 1993, 44, 139–143. [Google Scholar]
- Yamagishi, T.; Sahni, S.; Sharp, D.M.; Arvind, A.; Jansson, P.J.; Richardson, D.R. P-glycoprotein mediates drug resistance via a novel mechanism involving lysosomal sequestration. J. Biol. Chem. 2013, 288, 31761–33171. [Google Scholar] [CrossRef]
- Kansal, A.; Tripathi, D.; Rai, M.K.; Agarwal, V. Persistent expression and function of P-glycoprotein on peripheral blood lymphocytes identifies corticosteroid resistance in patients with systemic lupus erythematosus. Clin. Rheumatol. 2016, 35, 341–3439. [Google Scholar] [CrossRef]
- Li, Y.; Cao, F.; Li, M.; Li, P.; Yu, Y.; Xiang, L.; Xu, T.; Lei, J.; Tai, Y.Y.; Zhu, J.; et al. Hydroxychloroquine induced lung cancer suppression by enhancing chemo-sensitization and promoting the transition of M2-TAMs to M1-like macrophages. J. Exp. Clin. Cancer Res. 2018, 37, 259. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, Y.D.; Strong, J.M.; Reynolds, K.S.; Huang, S.M. A regulatory viewpoint on transporter-based drug interactions. Xenobiotica 2008, 38, 709–724. [Google Scholar] [CrossRef] [PubMed]
- Liu, X. Transporter-mediated drug-drug interactions and their significance. Adv. Exp. Med. Biol. 2019, 1141, 241–291. [Google Scholar] [PubMed]
- Xu, C.; Zhu, L.; Chan, T.; Lu, X.; Shen, W.; Madigan, M.C.; Gillies, M.C.; Zhou, F. Chloroquine and hydroxychloroquine are novel inhibitors of human organic anion transporting polypeptide 1A2. J. Pharm. Sci. 2016, 105, 884–890. [Google Scholar] [CrossRef]
- Ramadoss, P.; Marcus, C.; Perdew, G.H. Role of the aryl hydrocarbon receptor in drug metabolism. Expert Opin. Drug Metab. Toxicol. 2005, 1, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.P.; Wang, B.; Yang, M.; Boutros, P.C.; Macaulay, J.; Xu, H.; Chuang, A.I.; Kosuge, K.; Yamamoto, M.; Takahashi, S.; et al. Aryl hydrocarbon receptor is a transcriptional activator of the human breast cancer resistance protein (BCRP/ABCG2). Mol. Pharmacol. 2010, 78, 175–185. [Google Scholar] [CrossRef]



| Gene | Forward Primer 5′-3′ | Reverse Primer 5′-3′ | A [°C] | Ref. |
|---|---|---|---|---|
| ABCB1 | CCCATCATTGCAATAGCAGG | TGTTCAAACTTCTGCTCCTG | 60 | [29] |
| ABCG2 | AGATGGGTTTCCAAGCGTTCAT | CCAGTCCCAGTACGACTGTGACA | 57 | [29] |
| CYP1A1 | TCCGGGACATCACAGACAGC | ACCCTGGGGTTCATCACCAA | 65 | [31] |
| CYP1A2 | CATCCCCACAGCACAACAA | TCCCACTTGGCCAGGACTTC | 63 | [32] |
| CYP3A4 | TTCAGCAAGAAGAACAA | GGTTGAAGAAGTCTCTAAGC | 57 | [33] |
| GU | TTCACCAGGATCCACCTCTG | AGCACTCTCGTCGGTGACTG | 61 | [34] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weiss, J.; Bajraktari-Sylejmani, G.; Haefeli, W.E. Interaction of Hydroxychloroquine with Pharmacokinetically Important Drug Transporters. Pharmaceutics 2020, 12, 919. https://doi.org/10.3390/pharmaceutics12100919
Weiss J, Bajraktari-Sylejmani G, Haefeli WE. Interaction of Hydroxychloroquine with Pharmacokinetically Important Drug Transporters. Pharmaceutics. 2020; 12(10):919. https://doi.org/10.3390/pharmaceutics12100919
Chicago/Turabian StyleWeiss, Johanna, Gzona Bajraktari-Sylejmani, and Walter E. Haefeli. 2020. "Interaction of Hydroxychloroquine with Pharmacokinetically Important Drug Transporters" Pharmaceutics 12, no. 10: 919. https://doi.org/10.3390/pharmaceutics12100919
APA StyleWeiss, J., Bajraktari-Sylejmani, G., & Haefeli, W. E. (2020). Interaction of Hydroxychloroquine with Pharmacokinetically Important Drug Transporters. Pharmaceutics, 12(10), 919. https://doi.org/10.3390/pharmaceutics12100919