Development and Evaluation of Feline Tailored Amlodipine Besylate Mini-Tablets Using L-lysine as a Candidate Flavouring Agent
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Compression of Mini-Tablets
2.3. Characterisation of Mini-Tablets
2.3.1. Mass Uniformity, Thickness and Diameter
2.3.2. Tablet Strength (Hardness)
2.3.3. Content Uniformity
2.3.4. In-Vitro Dissolution Test
2.4. Electronic Taste Sensing System Measurement
2.4.1. Dose Response Curve
2.4.2. Sample Preparation
2.4.3. Sample Measurement
- Measurement of reference potential (Vr) in reference solution for 30 s;
- Measurement of electric potential (Vs) in sample (initial taste) for 30 s;
- Lightly washing of sensors in reference solution;
- Measurement of electric potential (Vr1) in reference solution again (aftertaste or CPA) for 30 s;
- Refreshing of sensors in alcohol solution to give them a complete wash before the measurement of the next sample for 30 s.
2.5. Statistical Analysis
3. Results
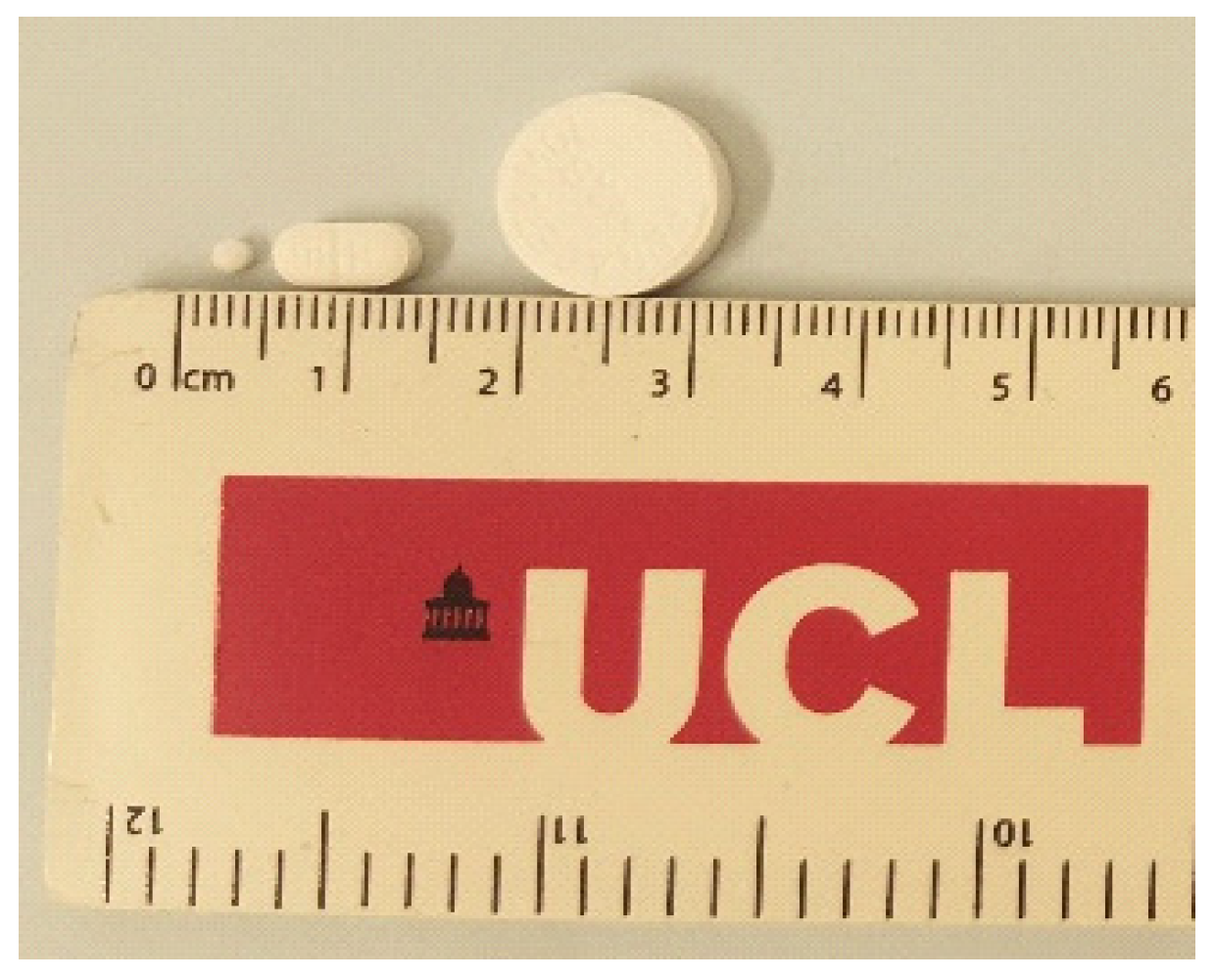
3.1. Physical Characterisation of Mini-Tablets
3.2. Electronic Taste Sensing System Measurement
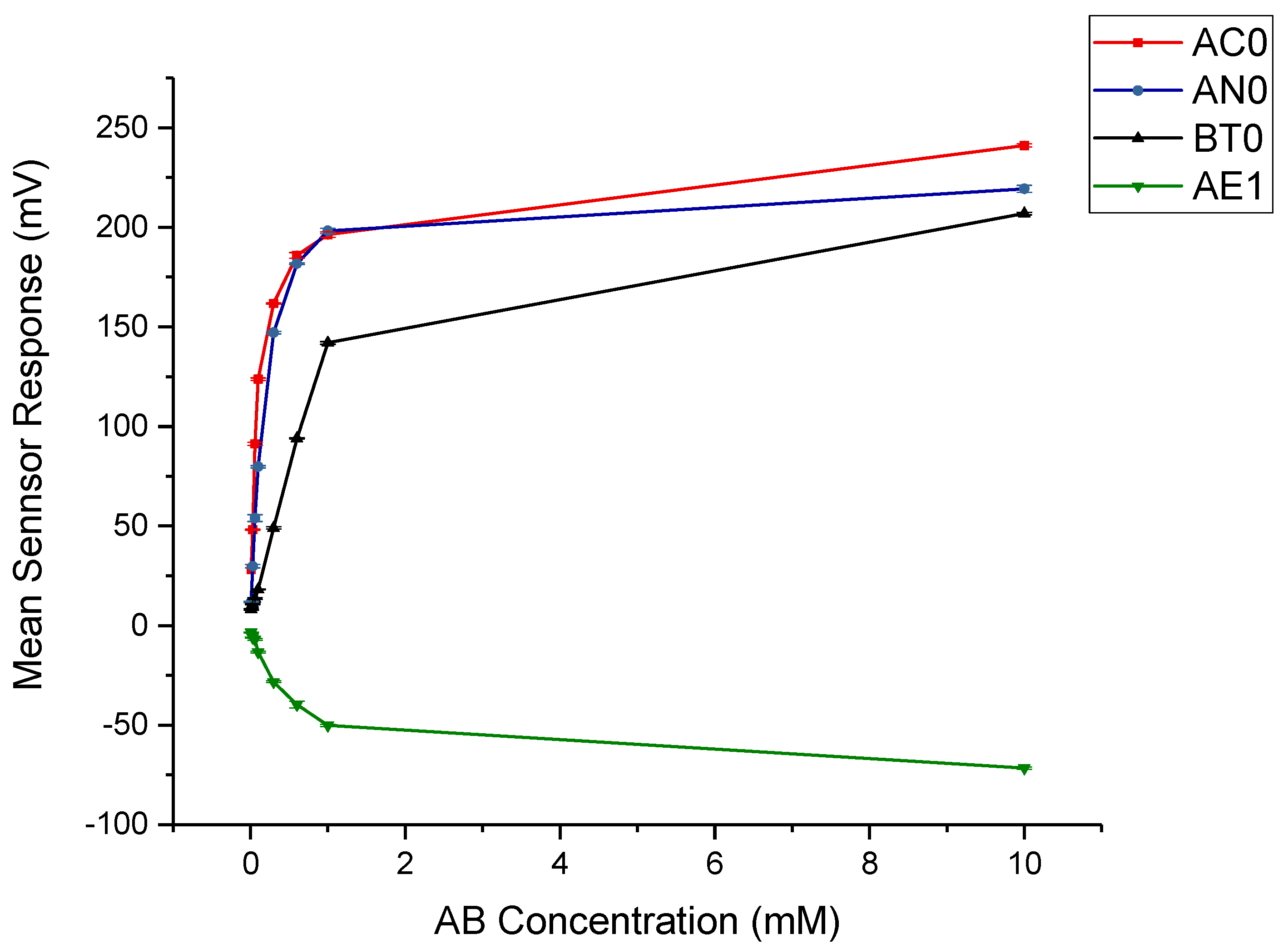
3.2.1. AB Taste Assessment
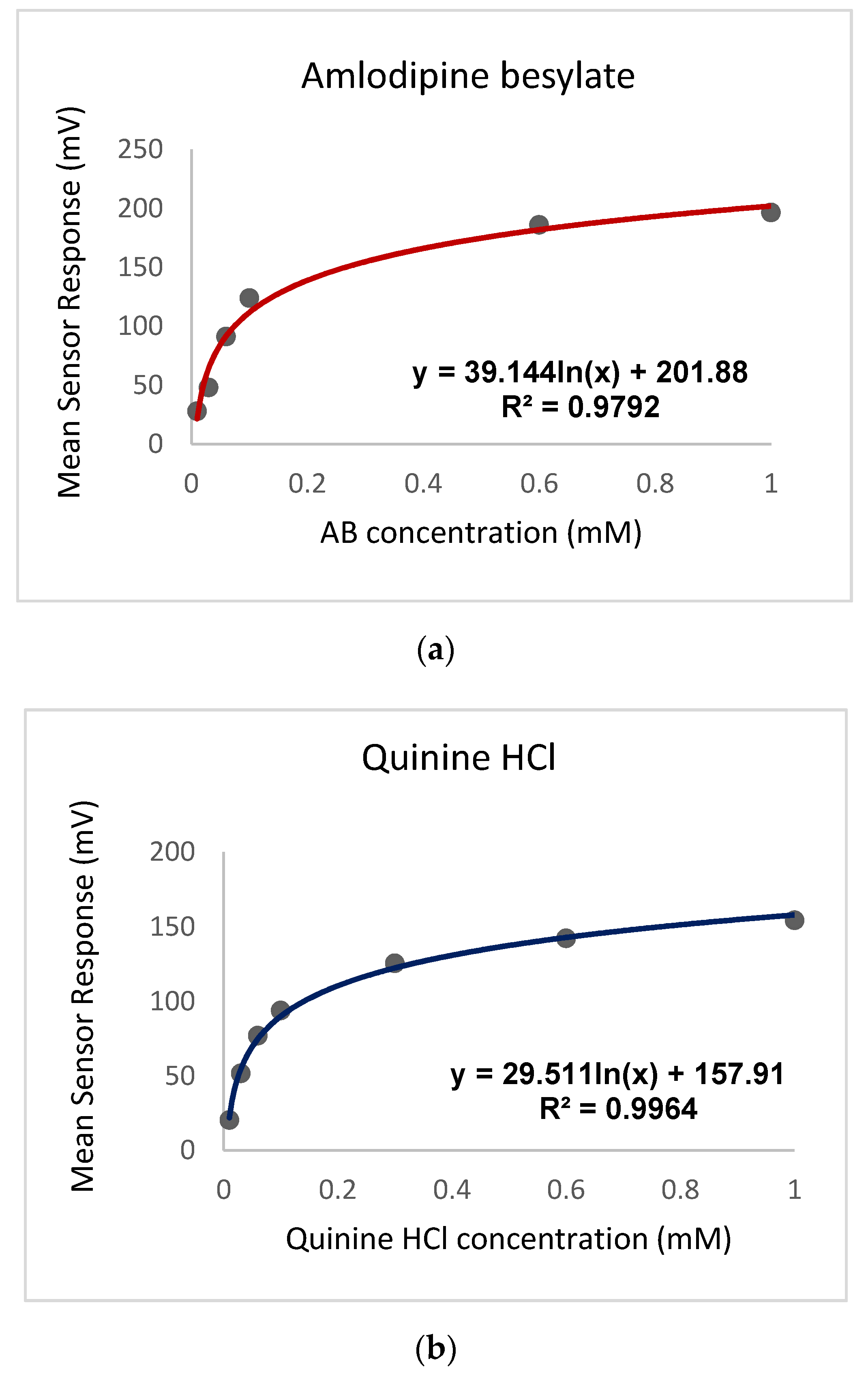
3.2.2. AB Bitterness Threshold as Compared to Quinine Hydrochloride Dihydrate
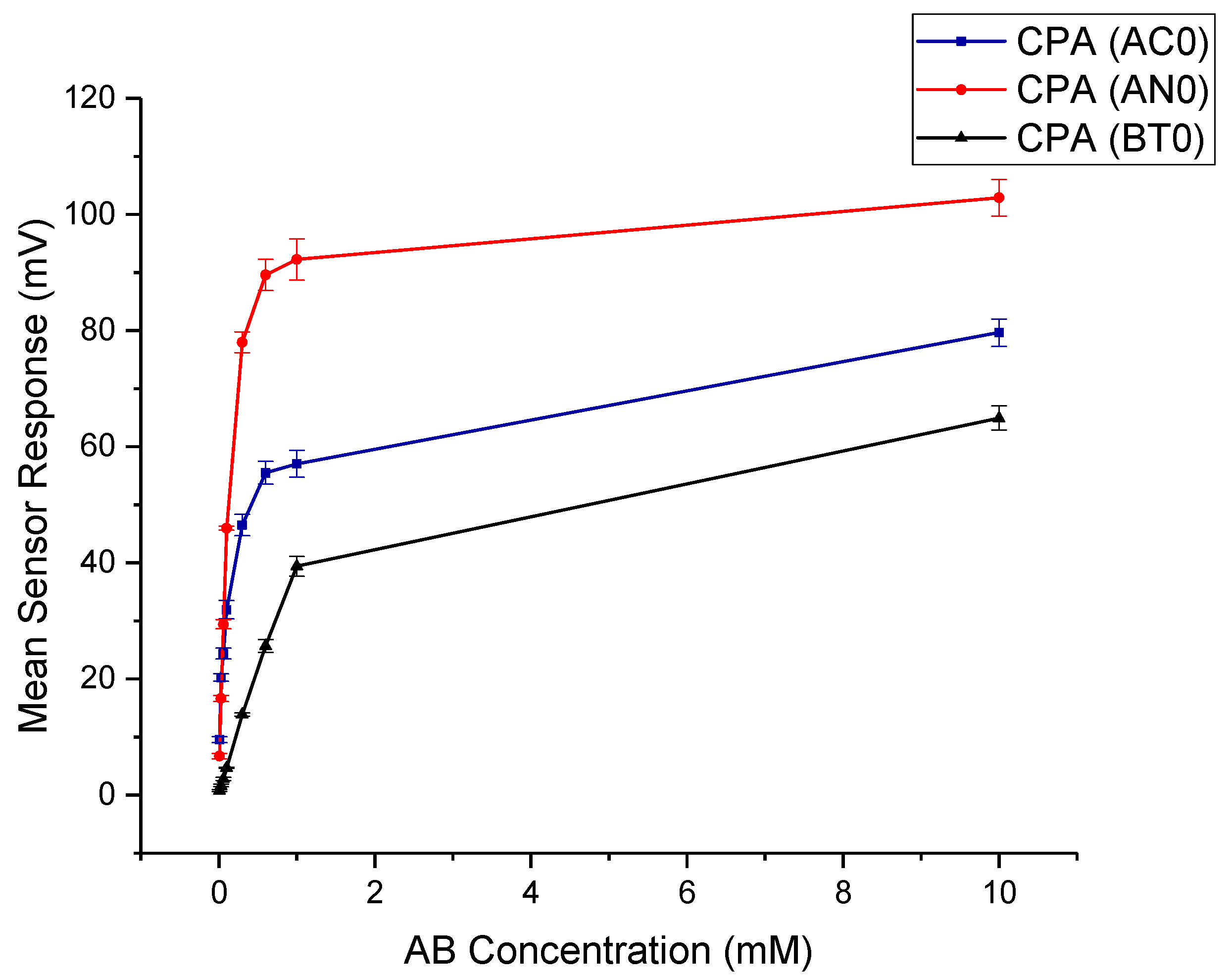
3.2.3. Aftertaste of AB
3.3. Content Uniformity and In-Vitro Dissolution Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hautala, J. Improving the Palatability of Minitablets for Feline Medication. Ph.D. Thesis, University of Helsinki, Helsinki, Finland, 31 March 2017. [Google Scholar]
- Savolainen, S.; Hautala, J.; Junnila, J.; Airaksinen, S.; Juppo, A.M.; Raekallio, M.; Vainio, O. Acceptability of flavoured pharmaceutically non-active mini-tablets in pet cats tested with a rapid 3-portal acceptance test with and without food. Vet. Anim. Sci. 2019, 7, 100054. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.; Kasraian, K. Pharmaceutical challenges in veterinary product development. Adv. Drug Deliv. Rev. 2002, 5454, 871–882. [Google Scholar] [CrossRef]
- Thombre, A.G. Oral delivery of medications to companion animals: Palatability considerations. Adv. Drug Deliv. Rev. 2004, 56, 1399–1413. [Google Scholar] [CrossRef]
- Moore, R.A.; Derry, S.; Wiffen, P.J.; Straube, S. Effects of food on pharmacokinetics of immediate release oral formulations of aspirin, dipyrone, paracetamol and NSAIDs—A systematic review. Br. J. Clin. Pharmacol. 2015, 80, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Y.; Jia, H.; Miyanaga, K.; Tanji, Y. Effect of milk on antibacterial activity of tetracycline against Escherichia coli and Staphylococcus aureus isolated from bovine mastitis. Appl. Microbiol. Biotechnol. 2009, 84, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Winnick, S.; Lucas, D.O.; Hartman, A.L.; Toll, D. How do you improve compliance? Pediatrics 2005, 115, e718–e724. [Google Scholar] [CrossRef] [PubMed]
- Stepien, R.L. Feline systemic hypertension. Diagnosis and management. J. Feline Med. Surg. 2011, 13, 35–43. [Google Scholar] [CrossRef]
- Forney, B. Amlodipine for Dogs and Cats. General Drug Information and Indications [Internet]. 2018. Available online: www.wedgewoodpharmacy.com (accessed on 6 September 2020).
- White, T.D.; Boudreau, J.C. Taste preferences of the cat for neurophysiologically active compounds. Physiol. Psychol. 1975, 3, 405–410. [Google Scholar] [CrossRef]
- Verbrugghe, A.; Hesta, M. Cats and carbohydrates: The carnivore fantasy? Vet. Sci. 2017, 4, 55. [Google Scholar] [CrossRef]
- Huhtinen, M.; Derré, G.; Renoldi, H.J.; Rinkinen, M.; Adler, K.; Aspegrén, J.; Zemirline, C.; Elliott, J. Randomized Placebo-Controlled Clinical Trial of a Chewable Formulation of Amlodipine for the Treatment of Hypertension in Client-Owned Cats. J. Vet. Intern. Med. 2015, 29, 786–793. [Google Scholar] [CrossRef]
- Campbell, W.C. Use of Ivermectin in Dogs and Cats, 1st ed.; Springer: New York, NY, USA, 1989; 363p, Available online: https://link.springer.com/chapter/10.1007/978-1-4612-3626-9_18 (accessed on 6 September 2020).
- Bradshaw, J.W.S.; Goodwin, D.; Legrand-Defrétin, V.; Nott, H.M.R. Food selection by the domestic cat, an obligate carnivore. Comp. Biochem. Physiol. Part A Physiol. 1996, 114, 205–209. Available online: http://www.sciencedirect.com/science/article/pii/0300962995021337 (accessed on 6 September 2020). [CrossRef]
- Shahidah, A.A.; Farouq, A.A.; Magashi, M.A.; Sokoto, A.M. Comparative Amino Acid and Volatile Flavor Profile of Dawadawa Produced from the Seeds of P. biglobosa, G. max and H. sabdariffa. J. Adv. Microbiol. 2019, 14, 1–13. [Google Scholar] [CrossRef]
- Rizzi, G.P. The strecker degradation of amino acids: Newer avenues for flavor formation. Food Rev. Int. 2008, 24, 416–435. [Google Scholar] [CrossRef]
- Beauchamp, G.K.; Maller, O.; Rogers, J.G. Flavor preferences in cats (Felis catus and Panthera sp.). J. Comp. Physiol. Psychol. 1977, 91, 1118–1127. [Google Scholar] [CrossRef]
- Boudreau, J.C.; Alev, N. Classification of chemoresponsive tongue units of the cat geniculate ganglion. Brain Res. 1973, 54, 157–175. Available online: http://www.sciencedirect.com/science/article/pii/0006899373900425 (accessed on 6 September 2020). [CrossRef]
- Sivén, M.; Savolainen, S.; Räntilä, S.; Männikkö, S.; Vainionpää, M.; Airaksinen, S.; Raekallio, M.; Vainio, O.; Juppo, A.M. Difficulties in administration of oral medication formulations to pet cats: An e-survey of cat owners. Vet. Rec. 2017, 180, 250. Available online: http://veterinaryrecord.bmj.com/content/180/10/250.abstract (accessed on 6 September 2020). [CrossRef] [PubMed]
- Thomson, S.A.; Tuleu, C.; Wong, I.C.K.; Keady, S.; Pitt, K.G.; Sutcliffe, A.G. Minitablets: New modality to deliver medicines to preschool-aged children. Pediatrics 2009, 123, e235–e238. [Google Scholar] [CrossRef]
- Spomer, N.; Klingmann, V.; Stoltenberg, I.; Lerch, C.; Meissner, T.; Breitkreutz, J. Acceptance of uncoated mini-tablets in young children: Results from a prospective exploratory cross-over study. Arch. Dis. Child. 2012, 97, 283–286. [Google Scholar] [CrossRef]
- Anand, V.; Kataria, M.; Kukkar, V.; Saharan, V.; Choudhury, P.K. The latest trends in the taste assessment of pharmaceuticals. Drug Discov. Today 2007, 12, 257–265. [Google Scholar] [CrossRef]
- Gupta, H.; Sharma, A.; Kumar, S.; Roy, S. E-Tongue: A Tool for Taste Evaluation. Recent Pat. Drug Deliv. Formul. 2009, 4, 82–89. [Google Scholar] [CrossRef]
- Li, X.; Li, W.; Wang, H.; Cao, J.; Maehashi, K.; Huang, L.; Bachmanov, A.A.; Reed, D.R.; Legrand-Defretin, V.; Beauchamp, G.K.; et al. Pseudogenization of a sweet-receptor gene accounts for cats’ indifference toward sugar. PLoS Genet 2005, 1, e3. [Google Scholar] [CrossRef] [PubMed]
- Ciosek, P.; Wróblewski, W. Potentiometric electronic tongues for foodstuff and biosample recognition-an overview. Sensors 2011, 11, 4688–4701. [Google Scholar] [CrossRef]
- Podrazka, M.; Báczyńska, E.; Kundys, M.; Jeleń, P.S.; Nery, E.W. Electronic tongue—A tool for all tastes? Biosensors 2018, 8, 3. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, Y.; Usui, R.; Ikezaki, H.; Tahara, K.; Takeuchi, H. An advanced technique using an electronic taste-sensing system to evaluate the bitterness of orally disintegrating films and the evaluation of model films. Int. J. Pharm. 2017, 531, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Choonara, Y.E.; Pillay, V.; Carmichael, T.; Danckwerts, M.P. An in vitro study of the design and development of a novel doughnut-shaped minitablet for intraocular implantation. Int. J. Pharm. 2006, 310, 15–24. [Google Scholar] [CrossRef]
- Maheswari, K.M.; Devineni, P.K.; Deekonda, S.; Shaik, S.; Uppala, N.P.; Nalluri, B.N. Development and Evaluation of Mouth Dissolving Films of Amlodipine Besylate for Enhanced Therapeutic Efficacy. J. Pharm. 2014, 2014, 20949. [Google Scholar] [CrossRef]
- Conti, B.; Genta, I.; Giunchedi, P.; Modena, T. Testing of “in vitro” dissolution behaviour of microparticulate drug delivery systems. Drug Dev. Ind. Pharm. 1995, 21, 1223–1233. [Google Scholar] [CrossRef]
- Tolbert, M.K.; Olin, S.; MacLane, S.; Gould, E.; Steiner, J.M.; Vaden, S.; Price, J. Evaluation of Gastric pH and Serum Gastrin Concentrations in Cats with Chronic Kidney Disease. J. Vet. Intern. Med. 2017, 31, 1414–1419. [Google Scholar] [CrossRef]
- Abdelhakim, H.E.; Coupe, A.; Tuleu, C.; Edirisinghe, M.; Craig, D.Q.M. Electrospinning Optimization of Eudragit e PO with and without Chlorpheniramine Maleate Using a Design of Experiment Approach. Mol. Pharm. 2019, 16, 2557–2568. [Google Scholar] [CrossRef]
- Westberg, A. Characterization of Mini-Tablets: Evaluation of Disintegration and Dissolution Methods. Master’s Thesis, Umea University, Umea, Sweden, 2019. [Google Scholar]
- Chay, S.K.; Keating, A.V.; James, C.; Aliev, A.E.; Haider, S.; Craig, D.Q.M. Evaluation of the taste-masking effects of (2-hydroxypropyl)-β-cyclodextrin on ranitidine hydrochloride; A combined biosensor, spectroscopic and molecular modelling assessment. RSC Adv. 2018, 8, 3564–3573. [Google Scholar] [CrossRef]
- Soto, J.; Keeley, A.; Keating, A.V.; Mohamed-Ahmed, A.H.A.; Sheng, Y.; Winzenburg, G.; Turner, R.; Desset-Brèthes, S.; Orlu, M.; Tuleu, C. Rats can predict aversiveness of Active Pharmaceutical Ingredients. Eur. J. Pharm. Biopharm. 2018, 133, 77–84. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Guideline on the Demonstration of Palatability of Veterinary Medicinal Products; European Medicines Agency: Amsterdam, The Netherlands, 2014; Volume 44, pp. 1–7. [Google Scholar]


| Mini-Tablet Composition (% w/w) | |||||
|---|---|---|---|---|---|
| Ingredients | Functions | Formulation A (AB Unmasked) | Formulation B (AB Masked) | Formulation C (Placebo Unmasked) | Formulation D (Placebo Masked) |
| AB | API | 15.4 | 15.4 | 0 | 0 |
| l-lysine | Taste-masking agent | 0 | 10 | 0 | 10 |
| Starch-1500 | Disintegrant | 10 | 10 | 10 | 10 |
| Mg Stearate | Lubricant | 2 | 2 | 2 | 2 |
| Disintequik | Bulking agent | 72.6 | 62.6 | 88 | 78 |
| Formulation | Weight Variation (mg) ± SD | Thickness (mm) ± SD | Diameter (mm) ± SD | Hardness (N) ± SD | Content Uniformity (%) ± SD |
|---|---|---|---|---|---|
| A | 6.26 ± 0.20 | 1.59 ± 0.08 | 1.98 ± 0.02 | 10.80 ± 2.20 | 103 ± 5.00 |
| B | 6.29 ± 0.30 | 1.66 ± 0.05 | 1.98 ± 0.02 | 8.83 ± 0.40 | 105 ± 1.40 |
| C | 6.39 ± 0.20 | 1.61 ± 0.04 | 1.98 ± 0.02 | 11.60 ± 1.70 | Placebo |
| D | 6.46 ± 0.30 | 1.88 ± 0.05 | 1.96 ± 0.03 | 5.75 ± 2.20 | Placebo |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ekweremadu, C.S.; Abdelhakim, H.E.; Craig, D.Q.M.; Barker, S.A. Development and Evaluation of Feline Tailored Amlodipine Besylate Mini-Tablets Using L-lysine as a Candidate Flavouring Agent. Pharmaceutics 2020, 12, 917. https://doi.org/10.3390/pharmaceutics12100917
Ekweremadu CS, Abdelhakim HE, Craig DQM, Barker SA. Development and Evaluation of Feline Tailored Amlodipine Besylate Mini-Tablets Using L-lysine as a Candidate Flavouring Agent. Pharmaceutics. 2020; 12(10):917. https://doi.org/10.3390/pharmaceutics12100917
Chicago/Turabian StyleEkweremadu, Chinedu S., Hend E. Abdelhakim, Duncan Q. M. Craig, and Susan A. Barker. 2020. "Development and Evaluation of Feline Tailored Amlodipine Besylate Mini-Tablets Using L-lysine as a Candidate Flavouring Agent" Pharmaceutics 12, no. 10: 917. https://doi.org/10.3390/pharmaceutics12100917
APA StyleEkweremadu, C. S., Abdelhakim, H. E., Craig, D. Q. M., & Barker, S. A. (2020). Development and Evaluation of Feline Tailored Amlodipine Besylate Mini-Tablets Using L-lysine as a Candidate Flavouring Agent. Pharmaceutics, 12(10), 917. https://doi.org/10.3390/pharmaceutics12100917







