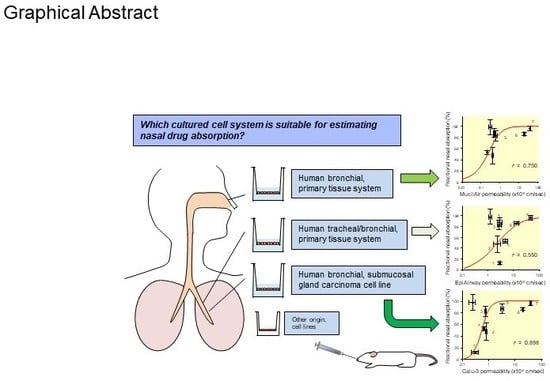
Comparison of Various Cell Lines and Three-Dimensional Mucociliary Tissue Model Systems to Estimate Drug Permeability Using an In Vitro Transport Study to Predict Nasal Drug Absorption in Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Culture of EpiAirway and MucilAir
2.3. Culture of Caco-2 and Preparation of Caco-2 Monolayers
2.4. Culture of Calu-3 and Preparation of Calu-3 Monolayers
2.5. Culture of MDCK and Preparation of MDCK Monolayers
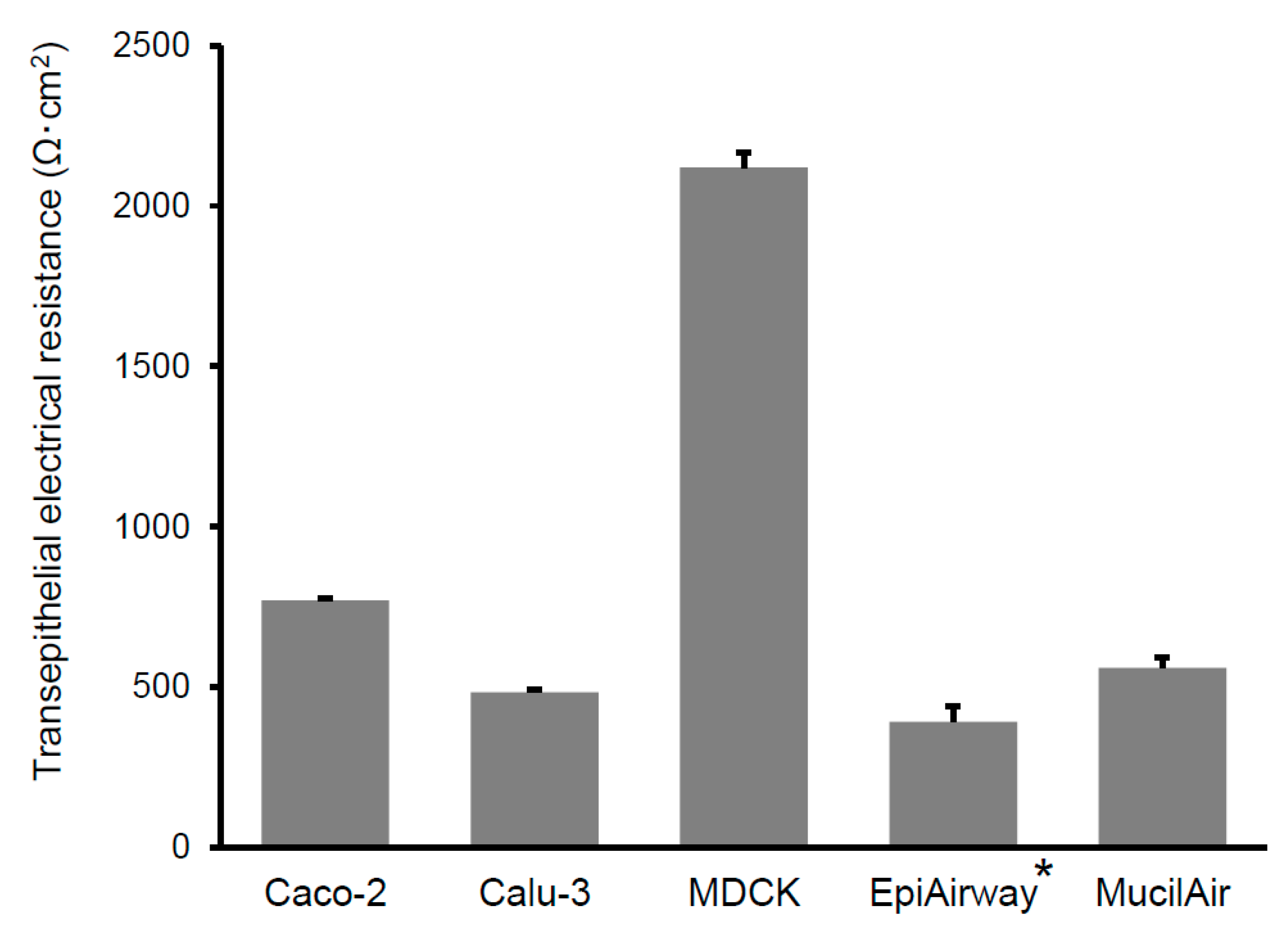
2.6. Measurement of Transepithelial Electrical Resistance
2.7. In Vitro Transepithelial Transport Study
2.8. In Vivo Study for Fractional Nasal Absorption of Atenolol, Antipyrine and Quinidine in Rats
2.8.1. Intravenous Bolus Injection
2.8.2. Nasal Administration
2.9. Sample Assay
2.9.1. Transport Study using Calu-3 and MDCK
2.9.2. Transport Study using EpiAirway
2.9.3. Transport Study using Mucilair and Animal Study
2.10. Data Fitting
3. Results
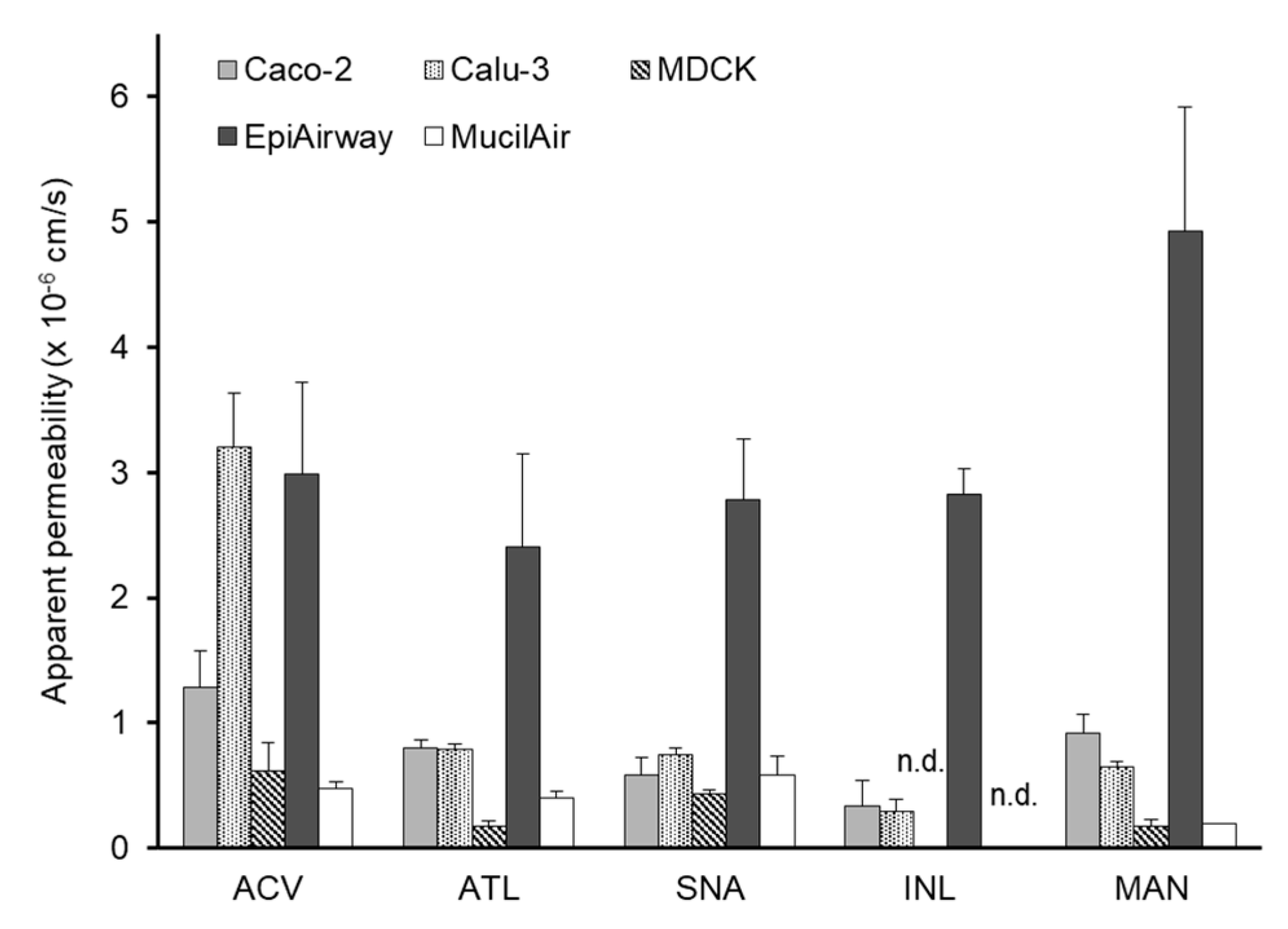
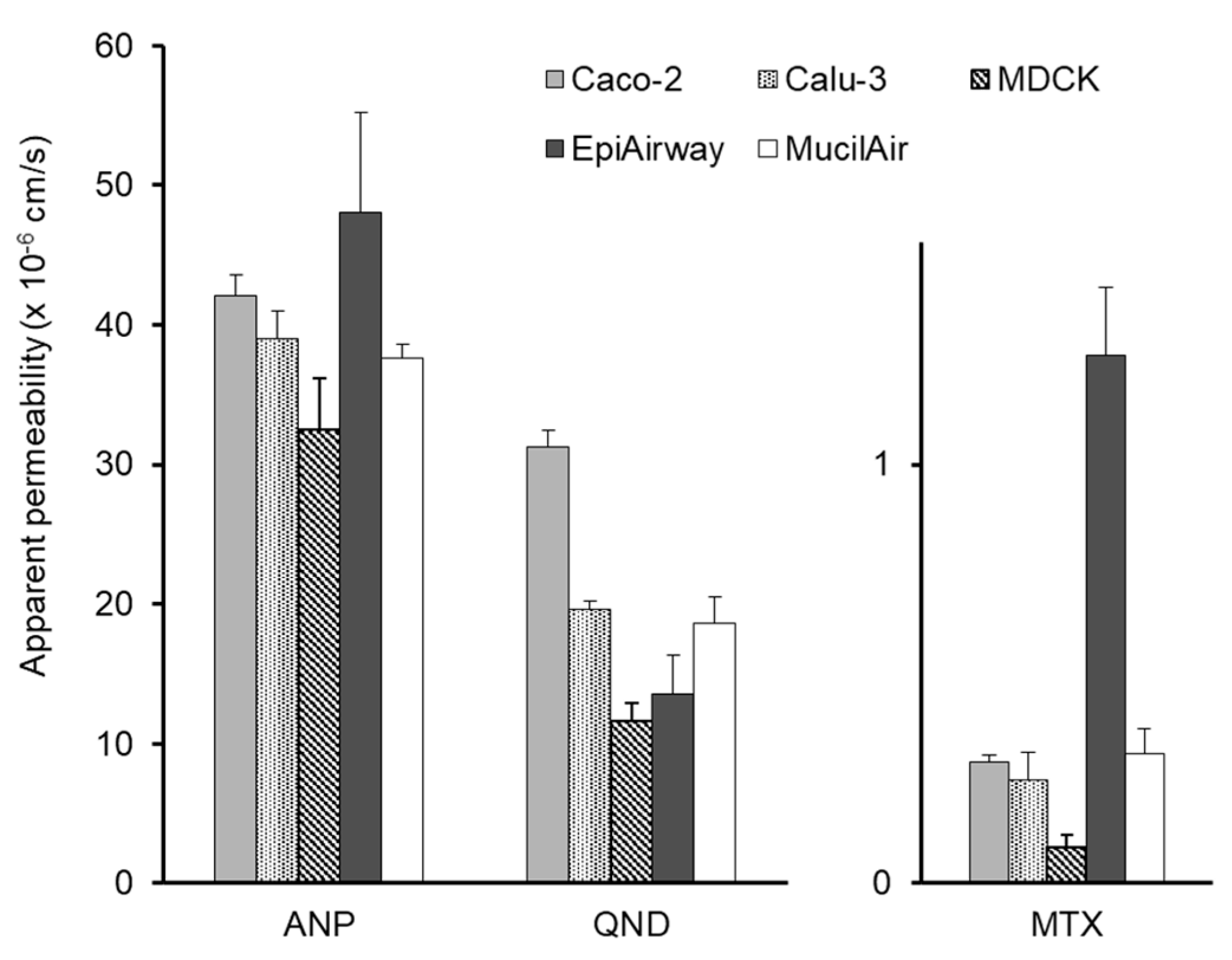
3.1. In Vitro Transepithelial Transport Study
3.2. Correlation of the Permeability with the Fraction Absorbed
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Coulet, M.; Eeckhoutte, C.; Larrieu, G.; Sutra, J.F.; Alvinerie, M.; Macé, K.; Pfeifer, A.; Zucco, F.; Stammati, A.L.; De Angelis, I.; et al. Evidence for cytochrome P4501A2-mediated protein covalent binding of thiabendazole and for its passive intestinal transport: Use of human and rabbit derived cells. Chem. Biol. Interact. 2000, 127, 109–124. [Google Scholar] [CrossRef]
- Kato, K.; Shirasaka, Y.; Kuraoka, E.; Kikuchi, A.; Iguchi, M.; Suzuki, H.; Shibasaki, S.; Kurosawa, T.; Tamai, I. Intestinal absorption mechanism of tebipenem pivoxil, a novel oral carbapenem: Involvement of human OATP family in apical membrane transport. Mol. Pharm. 2010, 7, 1747–1756. [Google Scholar] [CrossRef] [PubMed]
- Kuehn, D.; Majeed, S.; Guedj, E.; Dulize, R.; Baumer, K.; Iskandar, A.; Boue, S.; Martin, F.; Kostadinova, R.; Mathis, C.; et al. Impact assessment of repeated exposure of organotypic 3D bronchial and nasal tissue culture models to whole cigarette smoke. J. Vis. Exp. 2015, 12, 52325. [Google Scholar] [CrossRef] [PubMed]
- Salomon, J.J.; Gausterer, J.C.; Yahara, T.; Hosoya, K.; Huwer, H.; Hittinger, M.; Schneider-Daum, N.; Lehr, C.M.; Ehrhardt, C. Organic cation transporter function in different in vitro models of human lung epithelium. Eur. J. Pharm. Sci. 2015, 80, 82–88. [Google Scholar] [CrossRef]
- Madlova, M.; Bosquillon, C.; Asker, D.; Dolezal, P.; Forbes, B. In-vitro respiratory drug absorption models possess nominal functional P-glycoprotein activity. J. Pharm. Pharm. 2009, 61, 293–301. [Google Scholar] [CrossRef]
- Russell, L.M.; Guy, R.H. Measurement and prediction of the rate and extent of drug delivery into and through the skin. Expert Opin. Drug Deliv. 2009, 6, 355–369. [Google Scholar] [CrossRef]
- Sauer, U.G.; Vogel, S.; Hess, A.; Kolle, S.N.; Ma-Hock, L.; van Ravenzwaay, B.; Landsiedel, R. In vivo-in vitro comparison of acute respiratory tract toxicity using human 3D airway epithelial models and human A549 and murine 3T3 monolayer cell systems. Toxicol. Vitr. 2013, 27, 174–190. [Google Scholar] [CrossRef]
- Deng, X.; Li, Y.; Qiu, J. Human bocavirus 1 infects commercially-available primary human airway epithelium cultures productively. J. Virol. Methods 2014, 195, 112–119. [Google Scholar] [CrossRef]
- Mohamed, H.F. Molecular analysis and anticancer properties of two identified isolates, Fusarium solani and Emericella nidulans isolated from Wady El-Natron soil in Egypt against Caco-2 (ATCC) cell line. Asian Pac. J. Trop. Biomed. 2012, 2, 863–869. [Google Scholar] [CrossRef] [Green Version]
- Konishi, Y.; Kobayashi, S. Transepithelial transport of chlorogenic acid, caffeic acid, and their colonic metabolites in intestinal Caco-2 cell monolayers. Agric. Food Chem. 2004, 52, 2518–2526. [Google Scholar] [CrossRef]
- Furubayashi, T.; Kamaguchi, A.; Kawaharada, K.; Masaoka, Y.; Kataoka, M.; Yamashita, S.; Higashi, Y.; Sakane, T. Evaluation of the contribution of the nasal cavity and gastrointestinal tract to drug absorption following nasal application to rats. Biol. Pharm. Bull. 2007, 30, 608–611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furubayashi, T.; Kamaguchi, A.; Kawaharada, K.; Masaoka, Y.; Kataoka, M.; Yamashita, S.; Higashi, Y.; Sakane, T. Kinetic model to predict the absorption of nasally applied drugs from in vitro transcellular permeability of drugs. Biol. Pharm. Bull. 2007, 30, 1007–1010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taub, M.E.; Kristensen, L.; Frokjaer, S. Optimized conditions for MDCK permeability and turbidimetric solubility studies using compounds representative of BCS classes I–IV. Eur. J. Pharm. Sci. 2002, 15, 331–340. [Google Scholar] [CrossRef]
- Amasheh, S.; Meiri, N.; Gitter, A.H.; Schöneberg, T.; Mankertz, J.; Schulzke, J.D.; Fromm, M. Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells. J. Cell Sci. 2002, 115, 4969–4976. [Google Scholar] [CrossRef] [Green Version]
- Ye, L.; Yang, X.; Yang, Z.; Gao, S.; Yin, T.; Liu, W.; Wang, F.; Hu, M.; Liu, Z. The role of efflux transporters on the transport of highly toxic aconitine, mesaconitine, hypaconitine, and their hydrolysates, as determined in cultured Caco-2 and transfected MDCKII cells. Toxicol. Lett. 2013, 216, 86–99. [Google Scholar] [CrossRef]
- Haws, C.; Finkbeiner, W.E.; Widdicombe, J.H.; Wine, J.J. CFTR in Calu-3 human airway cells: Channel properties and role in cAMP-activated Cl− conductance. Am. J. Physiol. 1994, 266, 502–512. [Google Scholar] [CrossRef]
- Forbes, I.I. Human airway epithelial cell lines for in vitro drug transport and metabolism studies. Pharm. Sci. Technol. Today 2000, 3, 18–27. [Google Scholar] [CrossRef]
- Shen, B.Q.; Finkbeiner, W.E.; Wine, J.J.; Mrsny, R.J.; Widdicombe, J.H. Calu-3: A human airway epithelial cell line that shows cAMP-dependent Cl− secretion. Am. J. Physiol. 1994, 266, 493–501. [Google Scholar] [CrossRef]
- Mathia, N.R.; Timoszyk, J.; Stetsko, P.I.; Megill, J.R.; Smith, R.L.; Wall, D.A. Permeability characteristics of calu-3 human bronchial epithelial cells: In vitro-in vivo correlation to predict lung absorption in rats. J. Drug Target. 2002, 10, 3–40. [Google Scholar] [CrossRef]
- Hidalgo, I.J.; Raub, T.J.; Borchardt, R.T. Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology 1989, 96, 736–749. [Google Scholar] [CrossRef]
- Cavet, M.E.; West, M.; Simmons, N.L. Transepithelial transport of the fluoroquinolone ciprofloxacin by human airway epithelial Calu-3 cells. Antimicrob. Agents Chemother. 1997, 41, 2693–2698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donalisio, M.; Rusnati, M.; Cagno, V.; Civra, A.; Bugatti, A.; Giuliani, A.; Pirri, G.; Volante, M.; Papotti, M.; Landolfo, S.; et al. Inhibition of human respiratory syncytial virus infectivity by a dendrimeric heparan sulfate-binding peptide. Antimicrob. Agents Chemother. 2012, 56, 5278–5288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reus, A.A.; Mass, W.J.; Jansen, H.T.; Constant, S.; Staal, Y.C.; van Triel, J.J.; Kuper, C.F. Feasibility of a 3D human airway epitherial model to study respiratory absorption. Toxicol. Vitr. 2014, 28, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Duff, T.; Carter, S.; Feldman, G.; McEwan, G.; Pfaller, W.; Rhodes, P.; Ryan, M.; Hawksworth, G. Transepithelial Resistance and Inulin Permeability as Endpoints in in Vitro Nephrotoxicity Testing. Altern. Lab. Anim. 2002, 30, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Hirohashi, T.; Suzuki, H.; Sugiyama, Y. Characterization of the transport properties of cloned rat multidrug resistance-associated protein 3 (MRP3). J. Biol. Chem. 1999, 274, 15181–15185. [Google Scholar] [CrossRef] [Green Version]
- Kitamura, Y.; Hirouchi, M.; Kusuhara, H.; Schuetz, J.D.; Sugiyama, Y. Increasing systemic exposure of methotrexate by active efflux mediated by multidrug resistance-associated protein 3 (mrp3/abcc3). J. Pharm. Exp. 2008, 327, 465–473. [Google Scholar] [CrossRef] [Green Version]
- Thiebaud, N.; Menetrier, F.; Belloir, C.; Minn, A.L.; Neiers, F.; Artur, Y.; Le Bon, A.M.; Heydel, J.M. Expression and differential localization of xenobiotic transporters in the rat olfactory neuro-epithelium. Neurosci. Lett. 2011, 505, 180–185. [Google Scholar] [CrossRef]
- Breedveld, P.; Zelcer, N.; Pluim, D.; Sönmezer, O.; Tibben, M.M.; Beijnen, J.H.; Schinkel, A.H.; van Tellingen, O.; Borst, P.; Schellens, J.H. Mechanism of the pharmacokinetic interaction between methotrexate and benzimidazoles: Potential role for breast cancer resistance protein in clinical drug-drug interactions. Cancer Res. 2004, 64, 5804–5811. [Google Scholar] [CrossRef] [Green Version]
- Endter, S.; Francombe, D.; Ehrhardt, C.; Gumbleton, M. RT-PCR analysis of ABC, SLC and SLCO drug transporters in human lung epithelial cell models. J. Pharm. Pharm. 2009, 61, 583–591. [Google Scholar] [CrossRef]
- Toda, R.; Kawazu, K.; Oyabu, M.; Miyazaki, T.; Kiuchi, Y. Comparison of drug permeabilities across the blood-retinal barrier, blood-aqueous humor barrier, and blood-brain barrier. J. Pharm. Sci. 2011, 100, 3904–3911. [Google Scholar] [CrossRef]
- Knowles, M.; Murray, G.; Shallal, J.; Askin, F.; Ranga, V.; Gatzy, J.; Boucher, R. Bioelectric properties and ion flow across excised human bronchi. J. Appl. Physiol. Respir. Env. Exerc. Physiol. 1984, 56, 868–877. [Google Scholar]
- Ballard, S.T.; Taylor, A.E. Bioelectric properties of proximal bronchiolar epithelium. Am. J. Physiol. 1994, 267, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Joris, L.; Quinton, P.M. Components of electrogenic transport in unstimulated equine tracheal epithelium. Am. J. Physiol. 1991, 260, 510–515. [Google Scholar] [CrossRef] [PubMed]
- Chemuturi, N.V.; Hayden, P.; Klausner, M.; Donovan, M.D. Comparison of human tracheal/bronchial epithelial cell culture and bovine nasal respiratory explants for nasal drug transport studies. J. Pharm. Sci. 2005, 94, 1976–1985. [Google Scholar] [CrossRef] [PubMed]
- Hellinger, E.; Veszelka, S.; Tóth, A.E.; Walter, F.; Kittel, A.; Bakk, M.L.; Tihanyi, K.; Háda, V.; Nakagawa, S.; Duy, T.D.; et al. Comparison of brain capillary endothelial cell-based and epithelial (MDCK-MDR1, Caco-2, and VB-Caco-2) cell-based surrogate blood-brain barrier penetration models. Eur. J. Pharm. Biopharm. 2012, 82, 340–351. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.H.; Bian, Y.C.; Liu, Y.; Sheng, R.; Jiang, H.D.; Yu, L.S.; Hu, Y.Z.; Zeng, S. Evaluation of blood-brain barrier and blood-cerebrospinal fluid barrier permeability of 2-phenoxy-indan-1-one derivatives using in vitro cell models. Int. J. Pharm. 2014, 460, 101–107. [Google Scholar] [CrossRef]
- Yoneda, K. Mucous blanket of rat bronchus, an ultrastructural study. Am. Rev. Respir. Dis. 1976, 114, 837–842. [Google Scholar]
- Matsui, H.; Grubb, B.R.; Tarran, R.; Randell, S.H.; Gatzy, J.T.; Davis, C.W.; Boucher, R.C. Evidence for periciliary liquid layer depletion, not abnormal ion composition, in the pathogenesis of cystic fibrosis airways disease. Cell 1998, 95, 1005–1015. [Google Scholar]
- Sigurdsson, H.H.; Kirch, J.; Lehr, C.M. Mucus as a barrier to lipophilic drugs. Int. J. Pharm. 2013, 453, 56–64. [Google Scholar] [CrossRef]
- Haghi, M.; Young, P.M.; Traini, D.; Jaiswal, R.; Gong, J.; Bebawy, M. Time-and passage-dependent characteristics of a Calu-3 respiratory epithelial cell model. Drug Dev. Ind. Pharm. 2010, 36, 1207–1214. [Google Scholar] [CrossRef]
- Lavelle, J.P.; Negrete, H.O.; Poland, P.A.; Kinlough, C.L.; Meyers, S.D.; Hughey, R.P.; Zeidel, M.L. Low permeabilities of MDCK cell monolayers: A model barrier epithelium. Am. J. Physiol. 1997, 273, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Torky, A.R.; Stehfest, E.; Viehweger, K.; Taege, C.; Foth, H. Immuno-histochemical detection of MRPs in human lung cells in culture. Toxicology 2005, 207, 437–450. [Google Scholar] [CrossRef] [PubMed]
- van de Donk, H.J.; Merkus, F.W. Decreases in ciliary beat frequency due to intranasal administration of propranolol. J. Pharm. Sci. 1982, 71, 595–596. [Google Scholar] [CrossRef] [PubMed]
- Murakami, T.; Takano, M. Intestinal efflux transporters and drug absorption. Expert Opin. Drug Metab. Toxicol. 2008, 4, 923–939. [Google Scholar] [CrossRef] [PubMed]
- Verstraelen, J.; Reichl, S. Expression analysis of MDR1, BCRP and MRP3 transporter proteins in different in vitro and ex vivo cornea models for drug absorption studies. Int. J. Pharm. 2013, 441, 765–775. [Google Scholar] [CrossRef] [PubMed]
- Mercier, C.; Jacqueroux, E.; He, Z.; Hodin, S.; Constant, S.; Perek, N.; Boudard, D.; Delavenne, X. Pharmacological characterization of the 3D MucilAir™ nasal model. Eur. J. Pharm. Biopharm. 2019, 139, 186–196. [Google Scholar] [CrossRef] [PubMed]
- König, J.; Rost, D.; Cui, Y.; Keppler, D. Characterization of the human multidrug resistance protein isoform MRP3 localized to the basolateral hepatocyte membrane. Hepatology 1999, 29, 1156–1163. [Google Scholar] [CrossRef]
- Sakamoto, A.; Matsumaru, T.; Yamamura, N.; Suzuki, S.; Uchida, Y.; Tachikawa, M.; Terasaki, T. Drug transporter protein quantification of immortalized human lung cell lines derived from tracheobronchial epithelial cells (Calu-3 and BEAS2-B), bronchiolar-alveolar cells (NCI-H292 and NCI-H441), and alveolar type II-like cells (A549) by liquid chromatography-tandem mass spectrometry. J. Pharm. Sci. 2015, 104, 3029–3038. [Google Scholar]
- Feng, B.; West, M.; Patel, N.C.; Wager, T.; Hou, X.; Johnson, J.; Tremaine, L.; Liras, J. Validation of Human MDR1-MDCK and BCRP-MDCK Cell Lines to Improve the Prediction of Brain Penetration. J. Pharm. Sci. 2019, 108, 2476–2483. [Google Scholar] [CrossRef]
- Nakanishi, T.; Haruta, T.; Shirasaka, Y.; Tamai, I. Organic cation transporter-mediated renal secretion of ipratropium and tiotropium in rats and humans. Drug. Metab. Dispos. 2011, 39, 117–122. [Google Scholar] [CrossRef]
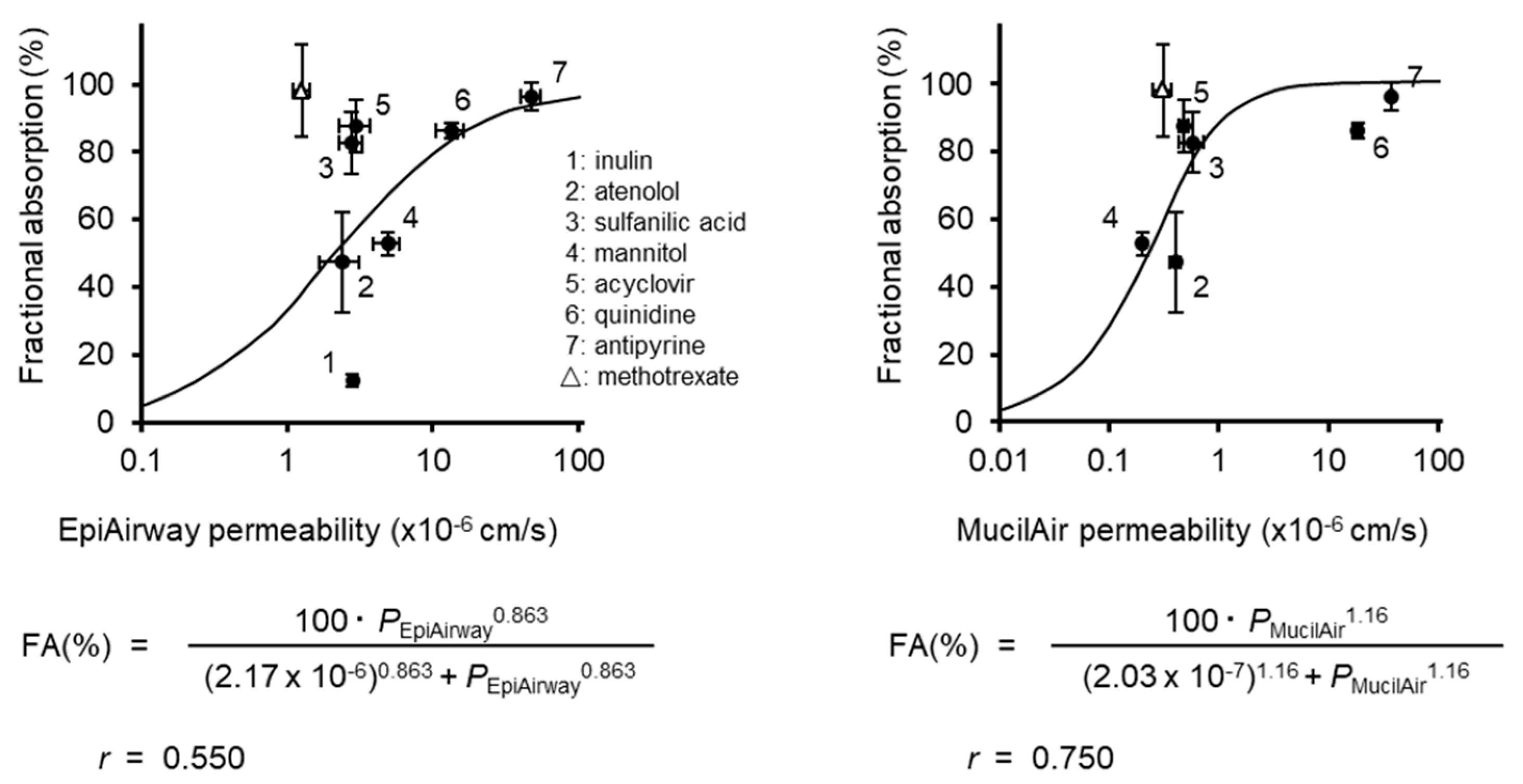
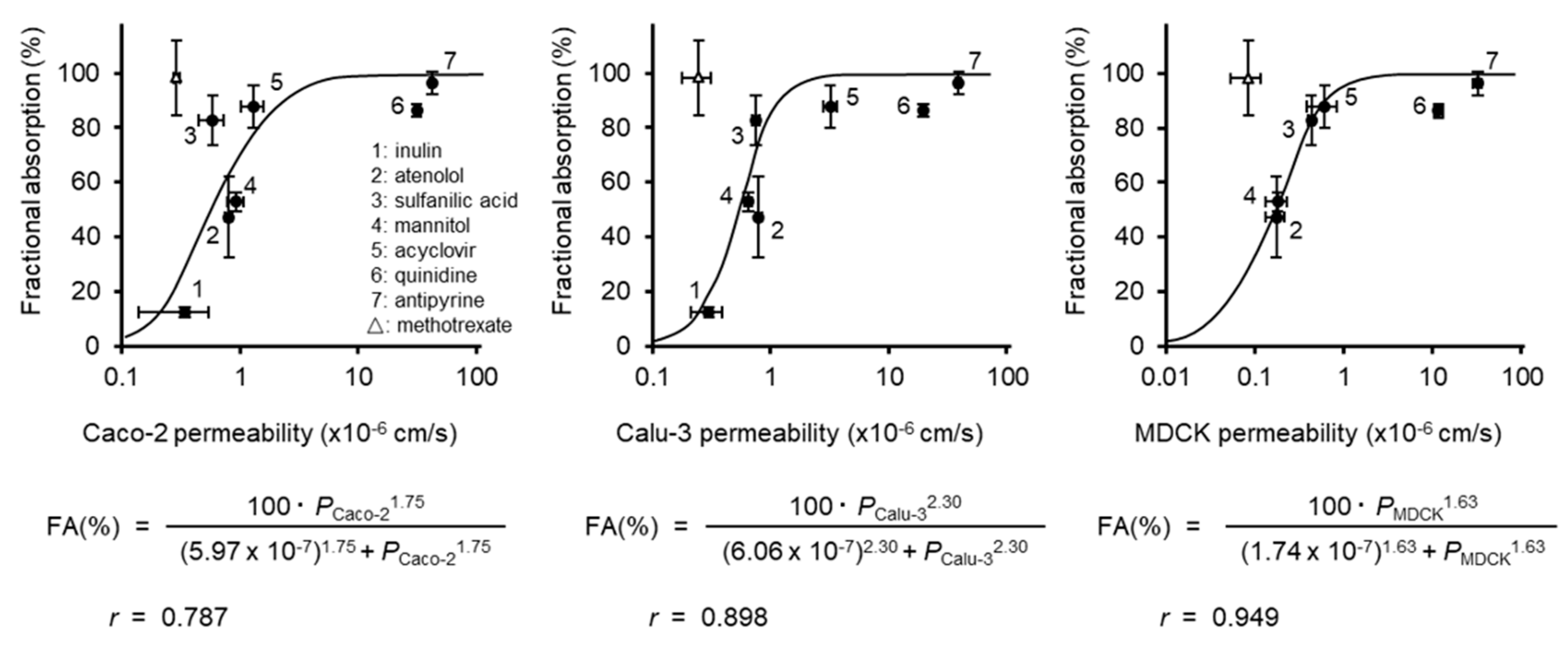
| Compounds | Permeability (×10−6cm/s ± S.E.) | Fractional Absorption*a (% ± S.E.) | ||||
|---|---|---|---|---|---|---|
| Caco-2 | Calu-3 | MDCK | EpiAirway | MucilAir | ||
| Acyclovir | 1.29 ± 0.28 | 3.20 ± 0.43 | 0.61 ± 0.23 | 2.99 ± 0.73 | 0.48 ± 0.05 | 87.7 ± 7.9 |
| Atenolol | 0.80 ± 0.06 | 0.79 ± 0.05 | 0.17 ± 0.04 | 2.40 ± 0.74 | 0.40 ± 0.05 | 47.3 ± 14.9 |
| Sulfanilic acid | 0.58 ± 0.14 | 0.75 ± 0.05 | 0.43 ± 0.04 | 2.78 ± 0.40 | 0.58 ± 0.15 | 82.7 ± 9.0 |
| Inulin | 0.34 ± 0.20 *1 | 0.30 ± 0.09 *1 | - | 2.83 ± 0.20 *2 | - | 12.4 ± 2.0 *1 |
| Mannitol | 0.92 ± 0.15 *3 | 0.64 ± 0.04 *3 | 0.18 ± 0.05 *3,b | 4.93 ± 0.99 *3 | 0.2 ± 0.0 *4,c | 52.9 ± 3.4 *3 |
| Antipyrine | 42.1 ± 1.47 | 39.0 ± 2.02 | 32.5 ± 3.71 | 48.1 ± 7.15 | 37.6 ± 0.99 | 96.3 ± 4.2 |
| Quinidine | 31.2 ± 1.25 | 19.6 ± 0.60 | 11.6 ± 1.31 | 13.5 ± 2.86 | 18.6 ± 1.93 | 86.3 ± 2.4 |
| Methotrexate | 0.29 ± 0.02 | 0.25 ± 0.07 | 0.09 ± 0.03 | 1.26 ± 0.16 | 0.31 ± 0.06 | 98.2 ± 13.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Furubayashi, T.; Inoue, D.; Nishiyama, N.; Tanaka, A.; Yutani, R.; Kimura, S.; Katsumi, H.; Yamamoto, A.; Sakane, T. Comparison of Various Cell Lines and Three-Dimensional Mucociliary Tissue Model Systems to Estimate Drug Permeability Using an In Vitro Transport Study to Predict Nasal Drug Absorption in Rats. Pharmaceutics 2020, 12, 79. https://doi.org/10.3390/pharmaceutics12010079
Furubayashi T, Inoue D, Nishiyama N, Tanaka A, Yutani R, Kimura S, Katsumi H, Yamamoto A, Sakane T. Comparison of Various Cell Lines and Three-Dimensional Mucociliary Tissue Model Systems to Estimate Drug Permeability Using an In Vitro Transport Study to Predict Nasal Drug Absorption in Rats. Pharmaceutics. 2020; 12(1):79. https://doi.org/10.3390/pharmaceutics12010079
Chicago/Turabian StyleFurubayashi, Tomoyuki, Daisuke Inoue, Noriko Nishiyama, Akiko Tanaka, Reiko Yutani, Shunsuke Kimura, Hidemasa Katsumi, Akira Yamamoto, and Toshiyasu Sakane. 2020. "Comparison of Various Cell Lines and Three-Dimensional Mucociliary Tissue Model Systems to Estimate Drug Permeability Using an In Vitro Transport Study to Predict Nasal Drug Absorption in Rats" Pharmaceutics 12, no. 1: 79. https://doi.org/10.3390/pharmaceutics12010079
APA StyleFurubayashi, T., Inoue, D., Nishiyama, N., Tanaka, A., Yutani, R., Kimura, S., Katsumi, H., Yamamoto, A., & Sakane, T. (2020). Comparison of Various Cell Lines and Three-Dimensional Mucociliary Tissue Model Systems to Estimate Drug Permeability Using an In Vitro Transport Study to Predict Nasal Drug Absorption in Rats. Pharmaceutics, 12(1), 79. https://doi.org/10.3390/pharmaceutics12010079