Influence of Composition and Spray-Drying Process Parameters on Carrier-Free DPI Properties and Behaviors in the Lung: A review
Abstract
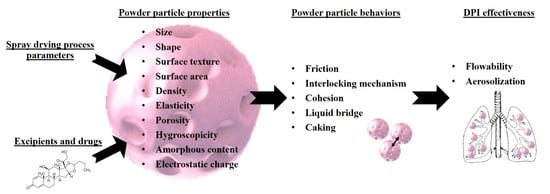
1. Introduction
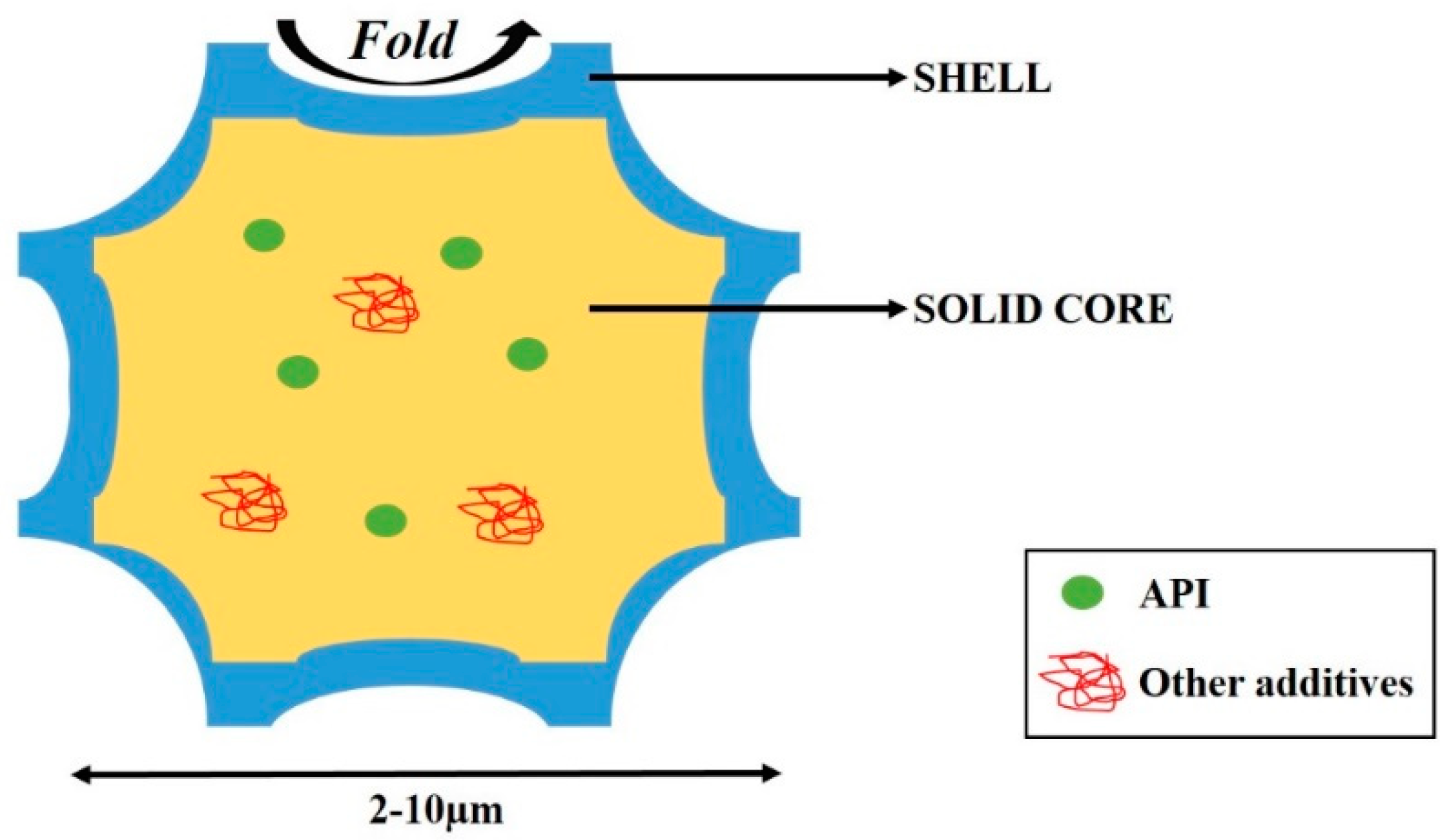
2. Excipients Forming Composite-Corrugated Particles
2.1. Excipients Forming the Core Compartment of Powder
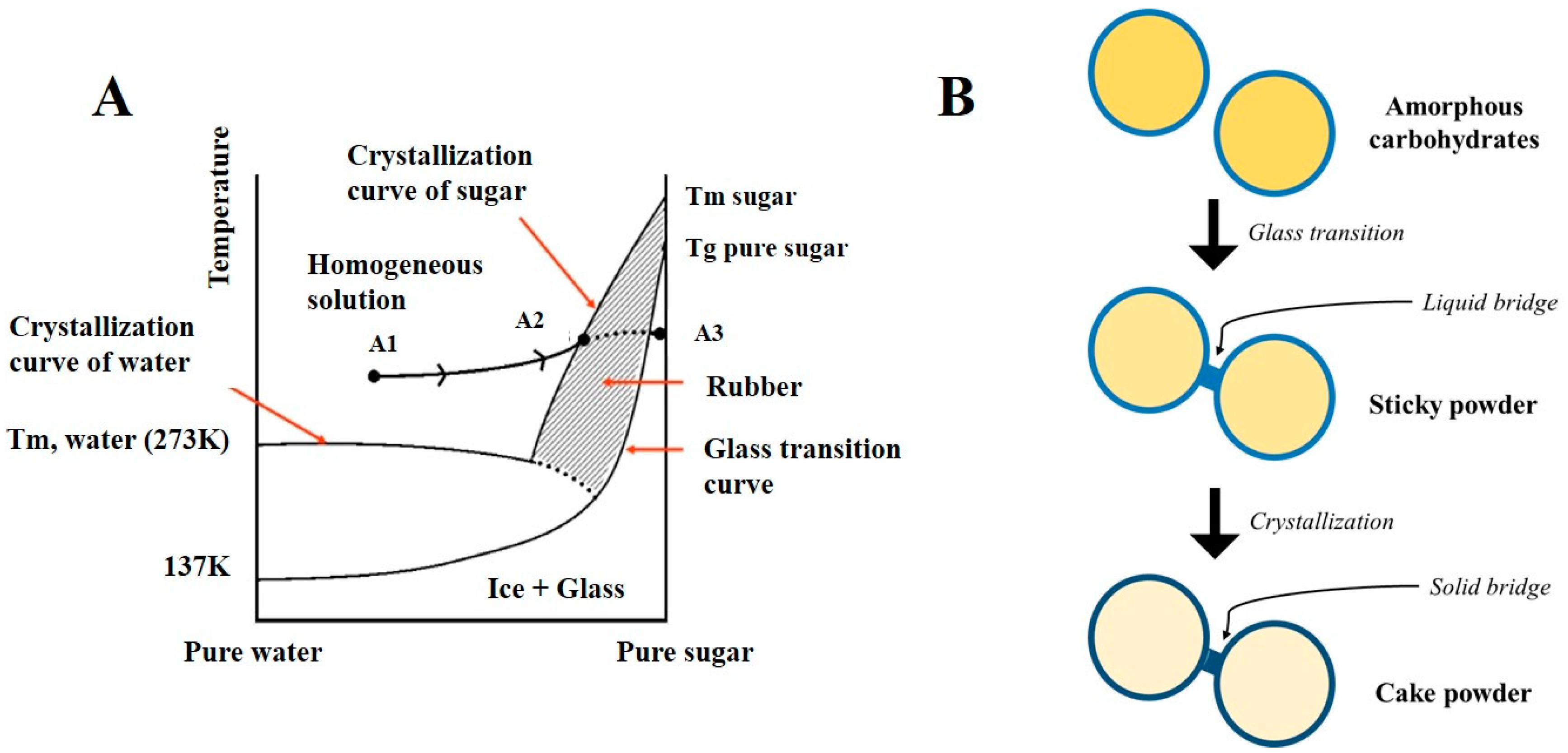
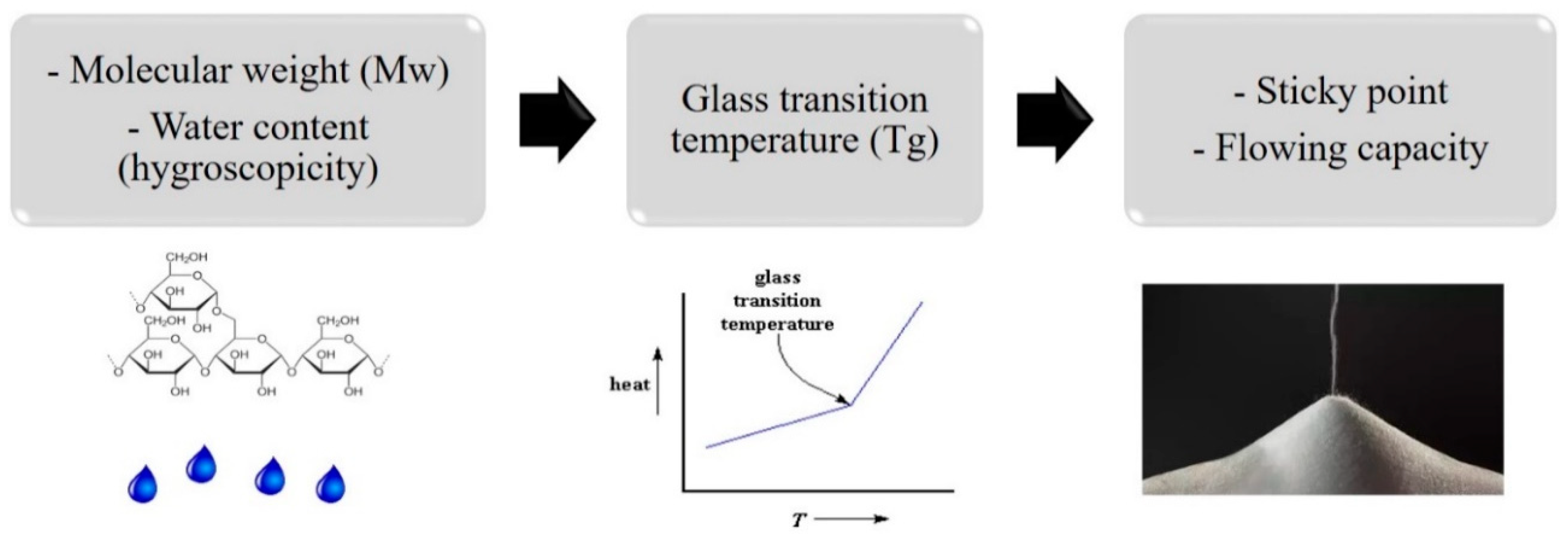
2.1.1. Influence of Molecular Weight, Hygroscopicity, and Glass Transition Temperature of Sugars on Powder Flowing Abilities
2.1.2. Importance of Sugar’s Reducing Character on Active Ingredients’ Stability
2.2. Excipients Forming the Outer Shell of the Particle Powder
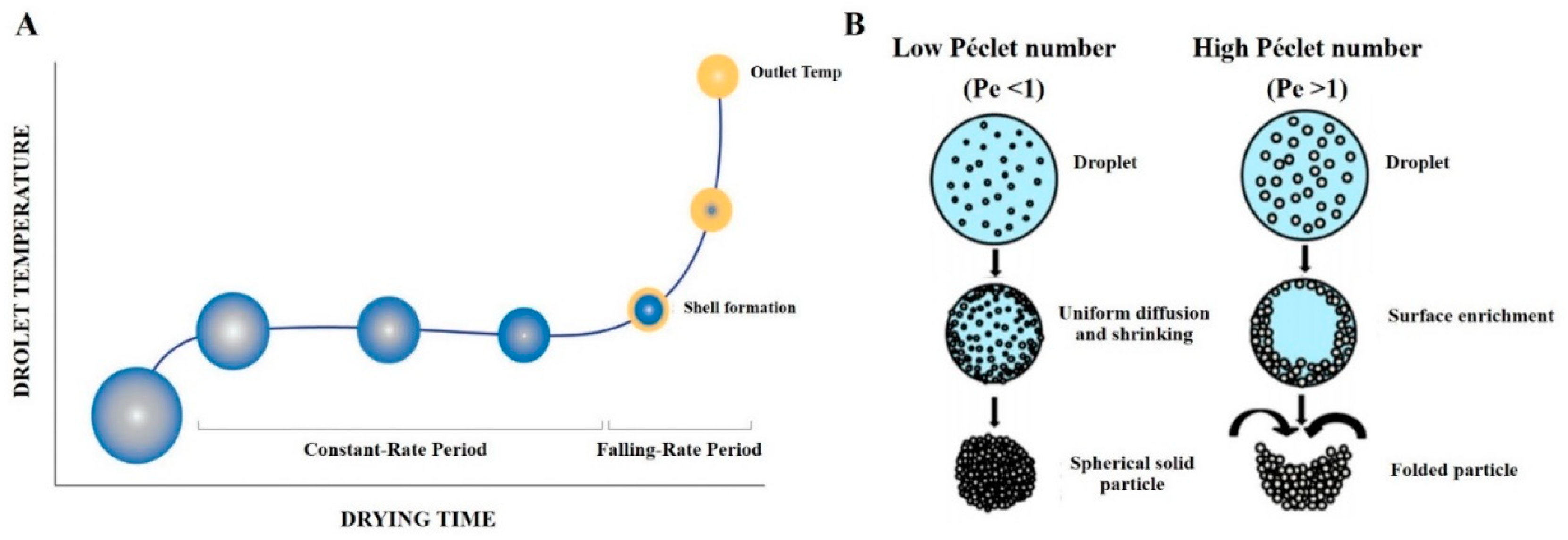
2.2.1. Drying Kinetics of a Droplet and Influence of the Péclet Number on Particle Powder Morphology
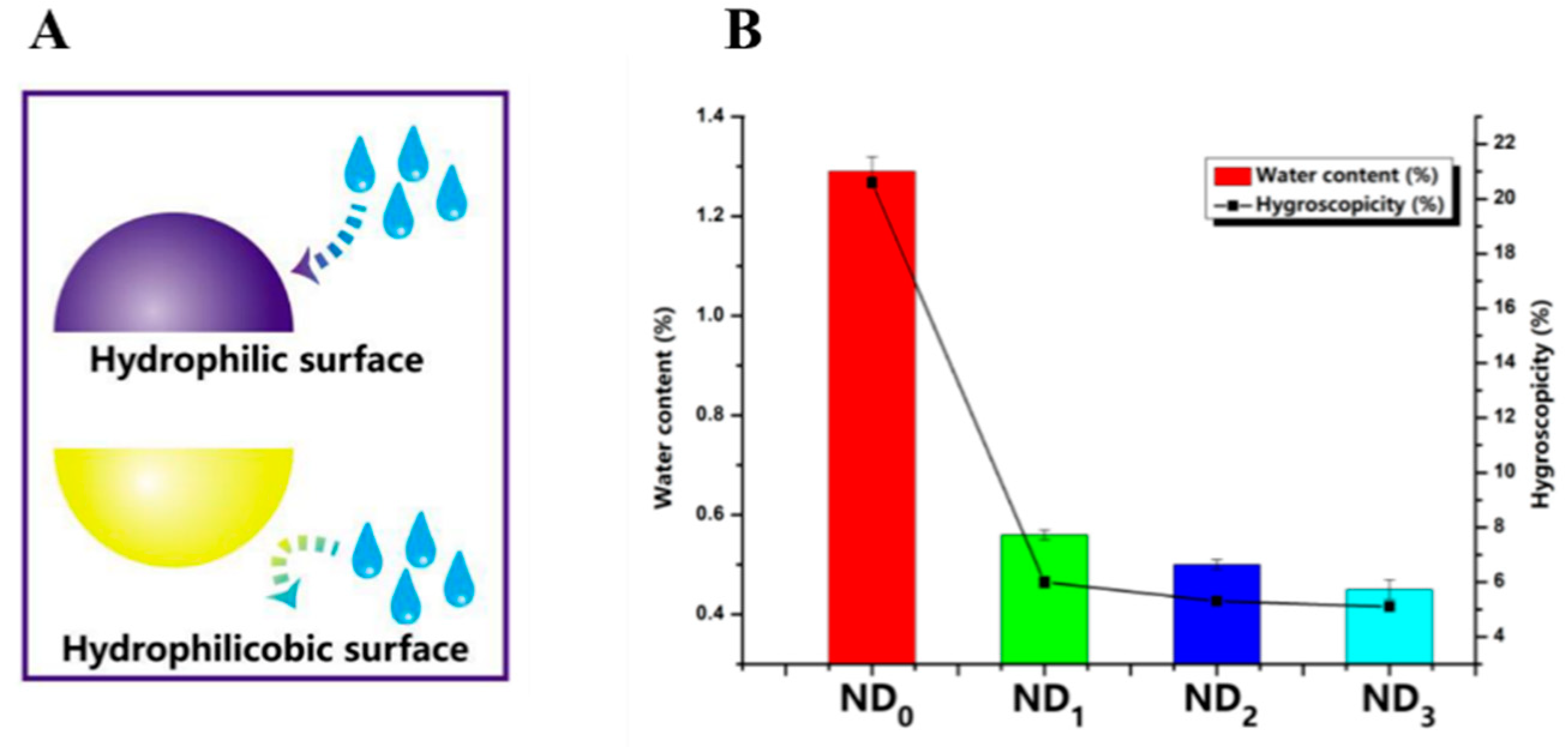
2.2.2. Amino Acids
2.3. Association of Materials to Produce Corrugated Composite Particles Produced by Spray Drying
2.3.1. Association of Oligosaccharides and Amino Acids
2.3.2. Association of Oligosaccharides
Interest of Cyclodextrins in DPI Engineering
2.4. Other Additives
2.4.1. Pore Forming Agents
2.4.2. Absorption Enhancers
3. Spray-Drying Process Parameters
3.1. Liquid Feed Concentration
3.2. Nozzle Air Pressure
3.3. Inlet Temperature
4. Aerosolization Performance Parameters
5. Conclusions and Perspectives
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Tg | Glass transition temperature |
| API | Active pharmaceutical ingredients |
| Pe | Péclet number |
| MDIs | Metered-dose inhalers |
| DPI | Dry powder inhalers |
| COPD | Chronic obstructive pulmonary disease |
| FPF | Fine particle fraction |
| NGI | Next generation inhaler |
| MMDA | Median mass aerodynamic diameter |
| T°In | Inlet temperature |
| T°Out | Outlet temperature |
References
- Pilcer, G.; Amighi, K. Formulation strategy and use of excipients in pulmonary drug delivery. Int. J. Pharm. 2010, 392, 1–19. [Google Scholar] [CrossRef]
- Healy, A.M.; Amaro, M.I.; Paluch, K.J.; Tajber, L. Dry powders for oral inhalation free of lactose carrier particles. Adv. Drug Deliv. Rev. 2014, 75, 32–52. [Google Scholar] [CrossRef] [PubMed]
- Peng, T.; Lin, S.; Niu, B.; Wang, X.; Huang, Y.; Zhang, X.; Li, G.; Pan, X.; Wu, C. Influence of physical properties of carrier on the performance of dry powder inhalers. Acta Pharm. Sin. B 2016, 6, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Ziaee, A.; Albadarin, A.B.; Padrela, L.; Femmer, T.; O’Reilly, E.; Walker, G. Spray drying of pharmaceuticals and biopharmaceuticals: Critical parameters and experimental process optimization approaches. Eur. J. Pharm. Sci. 2018, 127, 300–318. [Google Scholar] [CrossRef] [PubMed]
- De Boer, A.H.; Hagedoorn, P.; Hoppentocht, M.; Buttini, F.; Grasmeijer, F.; Frijlink, H.W. Dry powder inhalation: Past, present and future. Expert Opin. Drug Deliv. 2017, 14, 499–512. [Google Scholar] [CrossRef] [PubMed]
- Velaga, S.P.; Djuris, J.; Cvijic, S.; Rozou, S.; Russo, P.; Colombo, G.; Rossi, A. Dry powder inhalers: An overview of the in vitro dissolution methodologies and their correlation with the biopharmaceutical aspects of the drug products. Eur. J. Pharm. Sci. 2018, 113, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Hoppentocht, M.; Hagedoorn, P.; Frijlink, H.W.; de Boer, A.H. Technological and practical challenges of dry powder inhalers and formulations. Adv. Drug Deliv. Rev. 2014, 75, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Finlay, W.H. Dry-powder inhalers. In The Mechanics of Inhaled Pharmaceutical Aerosols; Elsevier: Amsterdam, The Netherlands, 2019; pp. 213–262. [Google Scholar]
- Demoly, P.; Hagedoorn, P.; de Boer, A.H.; Frijlink, H.W. The clinical relevance of dry powder inhaler performance for drug delivery. Respir. Med. 2014, 108, 1195–1203. [Google Scholar] [CrossRef]
- Guo, C.; Ngo, D.; Ahadi, S.; Doub, W.H. Evaluation of an abbreviated impactor for fine particle fraction (FPF) determination of metered dose inhalers (MDI). AAPS PharmSciTech 2013, 14, 1004–1011. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, X.; Li, J.; Beck-Broichsitter, M.; Muenster, U.; Wang, X.; Zhao, J.; Mao, S. Optimization of budesonide-loaded large-porous microparticles for inhalation using quality by design approach. J. Drug Deliv. Sci. Technol. 2019, 53, 101140. [Google Scholar] [CrossRef]
- Cal, K.; Sollohub, K. Spray Drying Technique. I: Hardware and Process Parameters. J. Pharm. Sci. 2010, 99, 575–586. [Google Scholar] [CrossRef]
- Costa, E.; Neves, F.; Moura, C. Design of Composite Particles via Spray Drying for DPI Formulations. In ONdrugdelivery; Frederick Furness Publishing Ltd.: Loures, Portugal, 2014; pp. 12–18. [Google Scholar]
- Lebrun, P.; Krier, F.; Mantanus, J.; Grohganz, H.; Yang, M.; Rozet, E.; Boulanger, B.; Evrard, B.; Rantanen, J.; Hubert, P. Design space approach in the optimization of the spray-drying process. Eur. J. Pharm. Biopharm. 2012, 80, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Vishali, D.A.; Monisha, J.; Sivakamasundari, S.K.; Moses, J.A.; Anandharamakrishnan, C. Spray freeze drying: Emerging applications in drug delivery. J. Control. Release 2019, 300, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Padrela, L.; Rodrigues, M.A.; Duarte, A.; Dias, A.M.A.; Braga, M.E.M.; de Sousa, H.C. Supercritical carbon dioxide-based technologies for the production of drug nanoparticles/nanocrystals—A comprehensive review. Adv. Drug Deliv. Rev. 2018, 131, 22–78. [Google Scholar] [CrossRef] [PubMed]
- Focaroli, S.; Mah, P.T.; Hastedt, J.E.; Gitlin, I.; Oscarson, S.; Fahy, J.V.; Healy, A.M. A Design of Experiment (DoE) approach to optimise spray drying process conditions for the production of trehalose/leucine formulations with application in pulmonary delivery. Int. J. Pharm. 2019, 562, 228–240. [Google Scholar] [CrossRef]
- BeMiller, J.N. Oligosaccharides. In Carbohydrate Chemistry for Food Scientists; Elsevier: Amsterdam, The Netherlands, 2019; pp. 49–74. [Google Scholar]
- Littringer, E.M.; Mescher, A.; Eckhard, S.; Schröttner, H.; Langes, C.; Fries, M.; Grieser, U.; Walzel, P.; Urbanetz, N.A. Spray Drying of Mannitol as a Drug Carrier—The Impact of Process Parameters on Product Properties. Dry. Technol. 2012, 30, 114–124. [Google Scholar] [CrossRef]
- Karner, S.; Littringer, E.M.; Urbanetz, N.A. Triboelectrics: The influence of particle surface roughness and shape on charge acquisition during aerosolization and the DPI performance. Powder Technol. 2014, 262, 22–29. [Google Scholar] [CrossRef]
- Mönckedieck, M.; Kamplade, J.; Fakner, P.; Urbanetz, N.A.; Walzel, P.; Steckel, H.A.; Scherließ, R. Spray drying of mannitol carrier particles with defined morphology and flow characteristics for dry powder inhalation. Dry. Technol. 2017, 35, 1843–1857. [Google Scholar] [CrossRef]
- Roe, K.D.; Labuza, T.P. Glass Transition and Crystallization of Amorphous Trehalose-sucrose Mixtures. Int. J. Food Prop. 2005, 8, 559–574. [Google Scholar] [CrossRef]
- Schebor, C.; Mazzobre, M.F.; del Pilar Buera, M. Glass transition and time-dependent crystallization behavior of dehydration bioprotectant sugars. Carbohydr. Res. 2010, 345, 303–308. [Google Scholar] [CrossRef]
- Ogain, O.N.; Li, J.; Tajber, L.; Corrigan, O.I.; Healy, A.M. Particle engineering of materials for oral inhalation by dry powder inhalers. I-Particles of sugar excipients (trehalose and raffinose) for protein delivery. Int. J. Pharm. 2011, 405, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Ramezani, V.; Vatanara, A.; Seyedabadi, M.; Nabi Meibodi, M.; Fanaei, H. Application of cyclodextrins in antibody microparticles: Potentials for antibody protection in spray drying. Drug Dev. Ind. Pharm. 2017, 43, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Huang, Z.; Zhang, X.; Huang, Y.; Cui, Y.; Ma, C.; Wang, G.; Freeman, T.; Lu, X.Y.; Pan, X.; et al. Low density, good flowability cyclodextrin-raffinose binary carrier for dry powder inhaler: Anti-hygroscopicity and aerosolization performance enhancement. Expert Opin. Drug Deliv. 2018, 15, 443–457. [Google Scholar] [CrossRef] [PubMed]
- Tabary, N.; Mahieu, A.; Willart, J.F.; Dudognon, E.; Danède, F.; Descamps, M.; Bacquet, M.; Martel, B. Characterization of the hidden glass transition of amorphous cyclomaltoheptaose. Carbohydr. Res. 2011, 346, 2193–2199. [Google Scholar] [CrossRef]
- Zheng, Y.; Chow, A.H. Production and characterization of a spray-dried hydroxypropyl-beta-cyclodextrin/quercetin complex. Drug Dev. Ind. Pharm. 2009, 35, 727–734. [Google Scholar] [CrossRef]
- Dufour, G.; Bigazzi, W.; Wong, N.; Boschini, F.; de Tullio, P.; Piel, G.; Cataldo, D.; Evrard, B. Interest of cyclodextrins in spray-dried microparticles formulation for sustained pulmonary delivery of budesonide. Int. J. Pharm. 2015, 495, 869–878. [Google Scholar] [CrossRef]
- Srichana, T.; Suedee, R.; Reanmongkol, W. Cyclodextrin as a potential drug carrier in salbutamol dry powder aerosols: The in-vitro deposition and toxicity studies of the complexes. Respir. Med. 2001, 95, 513–519. [Google Scholar] [CrossRef]
- Cabral-Marques, H.; Almeida, R. Optimisation of spray-drying process variables for dry powder inhalation (DPI) formulations of corticosteroid/cyclodextrin inclusion complexes. Eur. J. Pharm. Biopharm. 2009, 73, 121–129. [Google Scholar] [CrossRef]
- Simon, A.; Amaro, M.I.; Cabral, L.M.; Healy, A.M.; de Sousa, V.P. Development of a novel dry powder inhalation formulation for the delivery of rivastigmine hydrogen tartrate. Int. J. Pharm. 2016, 501, 124–138. [Google Scholar] [CrossRef]
- Mensink, M.A.; Frijlink, H.W.; van der Voort Maarschalk, K.; Hinrichs, W.L. Inulin, a flexible oligosaccharide. II: Review of its pharmaceutical applications. Carbohydr. Polym. 2015, 134, 418–428. [Google Scholar] [CrossRef]
- Carrigy, N.B.; Ordoubadi, M.; Liu, Y.; Melhem, O.; Barona, D.; Wang, H.; Milburn, L.; Ruzycki, C.A.; Finlay, W.H.; Vehring, R. Amorphous pullulan trehalose microparticle platform for respiratory delivery. Int. J. Pharm. 2019, 563, 156–168. [Google Scholar] [CrossRef] [PubMed]
- Hartel, R.W.; Ergun, R.; Vogel, S. Phase/State Transitions of Confectionery Sweeteners: Thermodynamic and Kinetic Aspects. Compr. Rev. Food Sci. F 2011, 10, 17–32. [Google Scholar] [CrossRef]
- Yu, L.; Mishra, D.; Rigsbee, R. Determination of the Glass Properties of D-Mannitol Using Sorbitol as an Impurity. J. Pharm. Sci. 1998, 87, 774–777. [Google Scholar] [CrossRef] [PubMed]
- Buitink, J.; van den, D.I.J.; Hoekstra, F.; Alberda, M.; Hemminga, M.A. High Critical Temperature above Tg May Contribute to the Stability of Biological Systems. Biophys. J. 2000, 79, 1119–1128. [Google Scholar] [CrossRef]
- Zhang, L.; Zeng, X.; Fu, N.; Tang, X.; Sun, Y.; Lin, L. Maltodextrin: A consummate carrier for spray-drying of xylooligosaccharides. Food Res. Int. 2018, 106, 383–393. [Google Scholar] [CrossRef]
- Amorij, J.P.; Huckriede, A.; Wilschut, J.; Frijlink, H.W.; Hinrichs, W.L. Development of stable influenza vaccine powder formulations: Challenges and possibilities. Pharm. Res. 2008, 25, 1256–1273. [Google Scholar] [CrossRef]
- Hashemi, N.; Milani, E.; Mortezavi, S.A.; Yazdi, F.T. Sticky point temperature as a suitable method in evaluation of shelf life of food powders. Bull. de la Soc. R. des Sci. de Liège 2017, 86, 7–12. [Google Scholar]
- De Melo Ramos, F.; Ubbink, J.; Júnior, V.S.; Prata, A.S. Drying of Maltodextrin solution in a vacuum spray dryer. Chem. Eng. Res. Des. 2019, 146, 78–86. [Google Scholar] [CrossRef]
- Steckel, H.; Bolzen, N. Alternative sugars as potential carriers for dry powder inhalations. Int. J. Pharm. 2004, 270, 297–306. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, X.; Wang, W.; Huang, Z.; Zhao, Z.; Wang, G.; Cai, S.; Jing, H.; Huang, Y.; Pan, X.; et al. Moisture-Resistant Co-Spray-Dried Netilmicin with l-Leucine as Dry Powder Inhalation for the Treatment of Respiratory Infections. Pharmaceutics 2018, 10, 252. [Google Scholar] [CrossRef]
- Mangal, S.; Meiser, F.; Tan, G.; Gengenbach, T.; Denman, J.; Rowles, M.R.; Larson, T.; Morton, D.A.V. Relationship between surface concentration of L-leucine and bulk powder properties in spray dried formulations. Eur. J. Pharm. Biopharm. 2015, 94, 160–169. [Google Scholar] [CrossRef]
- Osman, A.; Goehring, L.; Patti, A.; Stitt, H.; Shokri, N. Fundamental Investigation of the Drying of Solid Suspensions. Ind. Eng. Chem. Res. 2017, 56, 10506–10513. [Google Scholar] [CrossRef]
- Mezhericher, M.; Levy, A.; Borde, I. Heat and mass transfer of single droplet/wet particle drying. Chem. Eng. Sci. 2008, 63, 12–23. [Google Scholar] [CrossRef]
- Vehring, R. Pharmaceutical particle engineering via spray drying. Pharm. Res. 2008, 25, 999–1022. [Google Scholar] [CrossRef]
- Chen, X.D.; Sidhu, H.; Nelson, M. Theoretical probing of the phenomenon of the formation of the outermost surface layer of a multi-component particle, and the surface chemical composition after the rapid removal of water in spray drying. Chem. Eng. Sci. 2011, 66, 6375–6384. [Google Scholar] [CrossRef]
- Fluid Air. Experts in Solid Dosage Technology. Available online: http://www.fluidairinc.com/encapsulation.html#undefined2 (accessed on 24 August 2019).
- Boraey, M.A.; Hoe, S.; Sharif, H.; Miller, D.P.; Lechuga-Ballesteros, D.; Vehring, R. Improvement of the dispersibility of spray-dried budesonide powders using leucine in an ethanol–water cosolvent system. Powder Technol. 2013, 236, 171–178. [Google Scholar] [CrossRef]
- Carr, A.G.; Mammucari, R.; Foster, N.R. Particle formation of budesonide from alcohol-modified subcritical water solutions. Int. J. Pharm. 2011, 405, 169–180. [Google Scholar] [CrossRef]
- Rattanupatam, T.; Srichana, T. Budesonide dry powder for inhalation: Effects of leucine and mannitol on the efficiency of delivery. Drug Deliv. 2014, 21, 397–405. [Google Scholar] [CrossRef]
- Yang, X.F.; Xu, Y.; Qu, D.S.; Li, H.Y. The influence of amino acids on aztreonam spray-dried powders for inhalation. Asian J. Pharm. Sci. 2015, 10, 541–548. [Google Scholar] [CrossRef]
- Shetty, N.; Park, H.; Zemlyanov, D.; Mangal, S.; Bhujbal, S.; Zhou, Q. Influence of excipients on physical and aerosolization stability of spray dried high-dose powder formulations for inhalation. Int. J. Pharm. 2018, 544, 222–234. [Google Scholar] [CrossRef]
- Lechuga-Ballesteros, D.; Charan, C.; Stults, C.L.M.; Stevenson, C.L.; Miller, D.P.; Vehring, R.; Tep, V.; Kuo, M.C. Trileucine improves aerosol performance and stability of spray-dried powders for inhalation. J. Pharm. Sci. 2008, 97, 287–302. [Google Scholar] [CrossRef]
- Lu, W.D.; Rades, T.; Rantanen, J.; Yang, M.S. Inhalable co-amorphous budesonide-arginine dry powders prepared by spray drying. Int. J. Pharm. 2019, 365, 1–8. [Google Scholar] [CrossRef]
- Chang, Y.X.; Yang, J.J.; Pan, R.L.; Chang, Q.; Liao, Y.H. Anti-hygroscopic effect of leucine on spray-dried herbal extract powders. Powder Technol. 2014, 266, 388–395. [Google Scholar] [CrossRef]
- Seville, P.C.; Learoyd, T.P.; Li, Y.H.; Williamson, I.J.; Birchall, J.C. Amino acid-modified spray-dried powders with enhanced aerosolisation properties for pulmonary drug delivery. Powder Technol. 2007, 178, 40–50. [Google Scholar] [CrossRef]
- Vozone, C.; Cabral-Marques, H. Complexation of Budesonide in Cyclodextrins and Particle Aerodynamic Characterization of the Complex Solid Form for Dry Powder Inhalation. J. Incl. Phenom. Macrocycl. 2002, 44, 111–115. [Google Scholar] [CrossRef]
- Jansook, P.; Ogawa, N.; Loftsson, T. Cyclodextrins: Structure, physicochemical properties and pharmaceutical applications. Int. J. Pharm. 2018, 535, 272–284. [Google Scholar] [CrossRef]
- Jacob, S.; Nair, A.B. Cyclodextrin complexes: Perspective from drug delivery and formulation. Drug Dev. Res. 2018, 79, 201–217. [Google Scholar] [CrossRef]
- Lechanteur, A.; das Neves, J.; Sarmento, B. The role of mucus in cell-based models used to screen mucosal drug delivery. Adv. Drug Deliv. Rev. 2017, 124, 50–63. [Google Scholar] [CrossRef]
- Hu, X.; Wang, C.F.; Wang, L.B.; Liu, Z.X.; Wu, L.; Zhang, G.Q.; Yu, L.; Ren, X.H.; York, P.; Sun, L.X.; et al. Nanoporous CD-MOF particles with uniform and inhalable size for pulmonary delivery of budesonide. Int. J. Pharm. 2019, 564, 153–161. [Google Scholar] [CrossRef]
- Nolan, L.M.; Tajber, L.; McDonald, B.F.; Barham, A.S.; Corrigan, O.I.; Healy, A.M. Excipient-free nanoporous microparticles of budesonide for pulmonary delivery. Eur. J. Pharm. Sci. 2009, 37, 593–602. [Google Scholar] [CrossRef]
- Li, H.Y.; Seville, P.C.; Williamson, I.J.; Birchall, J.C. The use of absorption enhancers to enhance the dispersibility of spray-dried powders for pulmonary gene therapy. J. Gene Med. 2005, 7, 1035–1043. [Google Scholar] [CrossRef]
- Sosnik, A.; Seremeta, K.P. Advantages and challenges of the spray-drying technology for the production of pure drug particles and drug-loaded polymeric carriers. Adv. Colloid Interface Sci. 2015, 223, 40–54. [Google Scholar] [CrossRef]
- Tonon, R.V.; Brabet, C.; Hubinger, M.D. Influence of process conditions on the physicochemical properties of açai (Euterpe oleraceae Mart.) powder produced by spray drying. J. Food Eng. 2008, 88, 411–418. [Google Scholar] [CrossRef]
- Singh, A.; Van den Mooter, G. Spray drying formulation of amorphous solid dispersions. Adv. Drug Deliv. Rev. 2016, 100, 27–50. [Google Scholar] [CrossRef]
- Stahl, K.; Claesson, M.; Lilliehorn, P.; Lindén, H.; Bäckström, K. The effect of process variables on the degradation and physical properties of spray dried insulin intended for inhalation. Int. J. Pharm. 2002, 233, 227–237. [Google Scholar] [CrossRef]
- Wang, H.; Nobes, D.S.; Vehring, R. Particle Surface Roughness Improves Colloidal Stability of Pressurized Pharmaceutical Suspensions. Pharm. Res. 2019, 36, 43. [Google Scholar] [CrossRef]
- Adi, S.; Adi, H.; Tang, P.; Traini, D.; Chan, H.K.; Young, P.M. Micro-particle corrugation, adhesion and inhalation aerosol efficiency. Eur. J. Pharm. Sci. 2008, 35, 12–18. [Google Scholar] [CrossRef]
- Elsayed, M.M.A. Microstructural characterization of carrier-based dry powder inhalation excipients: Insights and guidance. Int. J. Pharm. 2019, 568, 118482. [Google Scholar] [CrossRef]
- Karner, S.; Anne Urbanetz, N. The impact of electrostatic charge in pharmaceutical powders with specific focus on inhalation-powders. J. Aerosol. Sci. 2011, 42, 428–445. [Google Scholar] [CrossRef]

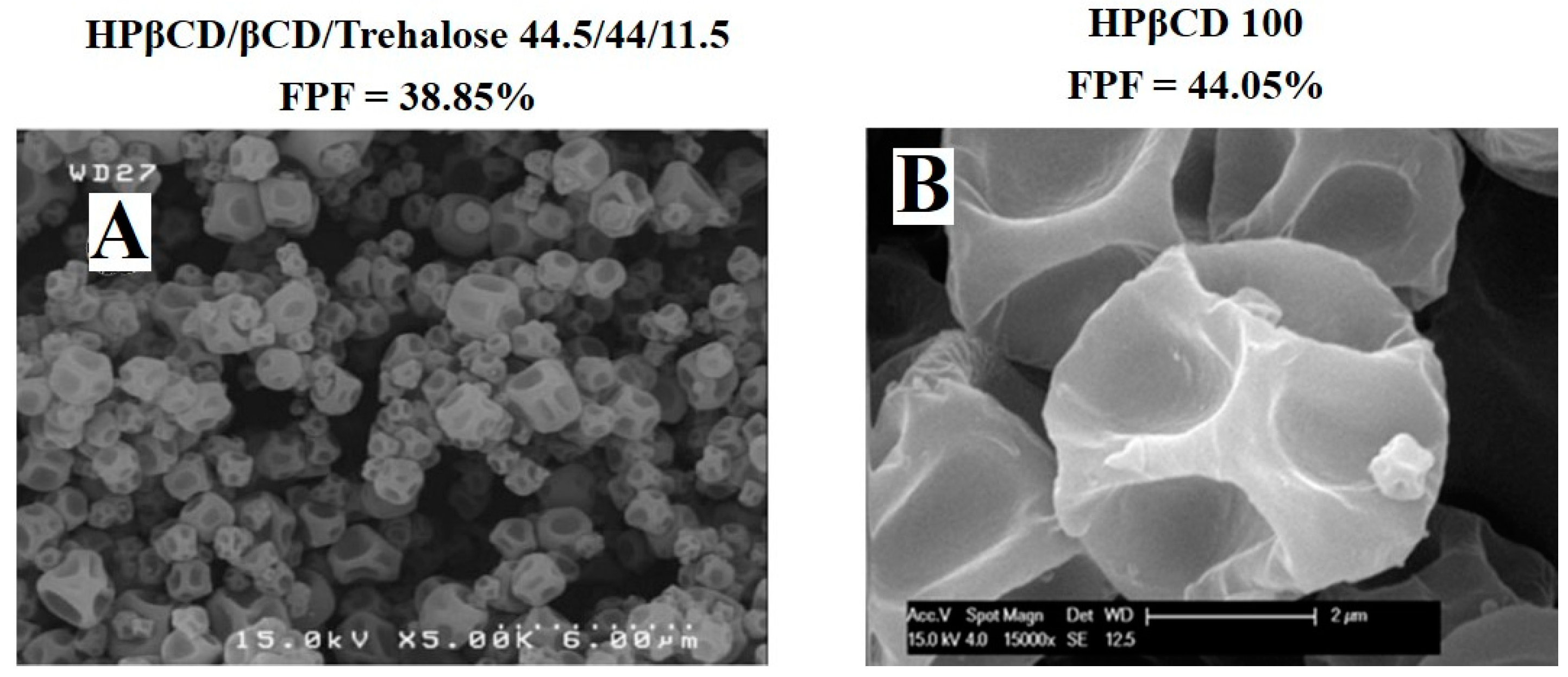
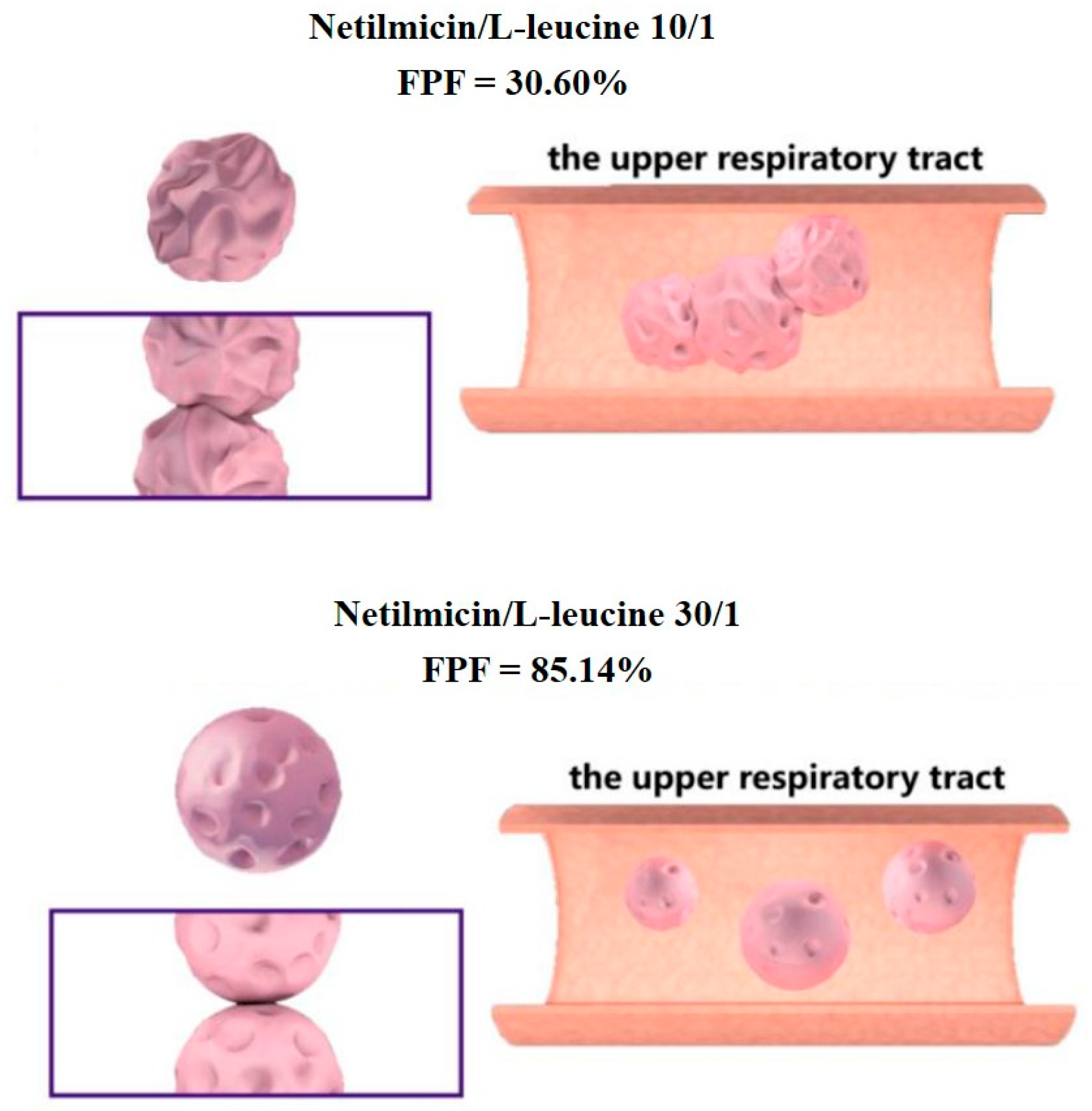
| Oligosaccharide | Type (Number of Monosaccharide) | Molecular Weight (g/mol) | Conformation | Tg (°C) | Reducing Sugar | Ref |
| Mannitol | Polyol | 182.2 | Linear | / | No | [14,19,20] |
| Trehalose | Glucose (2) | 342.3 | Linear | 97–106 [22,23] | No | [17,24,25] |
| Raffinose | Galactose (1), glucose (1), fructose (1) | 504.4 | Linear | 80 [23] | No | [24,26] |
| β-CD | Glucopyranose (7) | 1135.0 | Cyclic | 292 [27] | No | [25] |
| HP-β-CD | Glucopyranose (7) | 1541.5 | Cyclic | 220 [28] | No | [25,26,29] |
| DM-β-CD | Glucopyranose (7) | 1303.3 | Cyclic | / | No | [30] |
| γ-CD | Glucopyranose (8) | 1297.1 | Cyclic | / | No | [30,31] |
| Polysaccharide | Type (Number of Monosaccharide) | Molecular Weight (g/mol) | Conformation | Tg (°C) | Reducing Sugar | Ref |
| Inulin | Fructose (23) | 4 143.7 | Linear | 156 | No | [32,33] |
| Pullulan | Maltotriose (n) | 986.8 | Linear | 261 | No | [34] |
| Oligosaccharide(s) | Amino Acid | Optimal Ratio (%w/w) Oligosaccharides/Amino Acid (or If No Oligosaccharide, API/Amino acid) | API | Solvant (v/v) | FPF (%) | Solid Content (w/v %) | Ref |
|---|---|---|---|---|---|---|---|
| / | L-leucine | 82.5/17.5 | Budesonide | Ethanol/Water 75/25 | 66 | 0.7 | [50] |
| / | L-leucine | 1/50 | Budesonide | Ethanol/water 20/80 | 49.4 | 1 | [52] |
| / | L-leucine | 97/3 | Netilmicin | Water | 85.1 | 3.3 | [43] |
| / | Arginine | 71/29 | Budesonide | Water | 61.6 | 1 | [56] |
| / | Trileucine | 85/15 | -Gentamicine -Netilmicin | Ethanol/water 55/45 | 49.3 62.4 | 1 1 | [55] |
| / | L-leucine | 40/60 | Aztreonam | Water | 61.7 | 5 | [53] |
| / | Histidine | 40/60 | Aztreonam | Water | 51.4 | 5 | [53] |
| / | Glycine | 40/60 | Aztreonam | Water | 0.29 | 5 | [53] |
| / | L-leucine | 50/50 | Ciprofloxacin | Water | 1.6 | [54] | |
| Trehalose | L-leucine | 90/10 | / | Water | 68.5 | 5 | [17] |
| Inulin | L-leucine | 80/20 | Rivastigmine | Ethanol/water 30/70 | 64.2 | 1 | [32] |
| Raffinose | Trileucine | 84/16 | Albuterol | Water | 65 | 1 | [55] |
| Lactose | L-leucine | 80/20 | Salbutamol | Water | 78 | 2 | [58] |
| HPβCD | / | / | Budesonide | Water | 44 | 10 | [29] |
| DM-β-CD | / | / | Budesonide | Water | 67 | - | [59] |
| Trehalose | / | / | Model protein (Lysozyme) | Ethanol/water 80/20% | 62.3 | 1 | [24] |
| Raffinose | / | / | Model protein (Lysozyme) | Ethanol/water 80/20% | 50.1 | 1 | [24] |
| Pullulan/Trehalose | / | 10/90 | / | Water | 40 | 0.2 | [34] |
| HPβCD/βCD/Trehalose | / | 44.5/44/11.5 | Antibody | Water | 39 | 0.1 | [25] |
| HPβCD/Raffinose | / | 60/40 | Budesonide | Water | 70 | 2 | [26] |
| Process Parameters | Particle Powder Properties |
|---|---|
| Liquid feed concentration |
|
| Feed rate |
|
| Nozzle air pressure |
|
| Inlet temperature |
|
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lechanteur, A.; Evrard, B. Influence of Composition and Spray-Drying Process Parameters on Carrier-Free DPI Properties and Behaviors in the Lung: A review. Pharmaceutics 2020, 12, 55. https://doi.org/10.3390/pharmaceutics12010055
Lechanteur A, Evrard B. Influence of Composition and Spray-Drying Process Parameters on Carrier-Free DPI Properties and Behaviors in the Lung: A review. Pharmaceutics. 2020; 12(1):55. https://doi.org/10.3390/pharmaceutics12010055
Chicago/Turabian StyleLechanteur, Anna, and Brigitte Evrard. 2020. "Influence of Composition and Spray-Drying Process Parameters on Carrier-Free DPI Properties and Behaviors in the Lung: A review" Pharmaceutics 12, no. 1: 55. https://doi.org/10.3390/pharmaceutics12010055
APA StyleLechanteur, A., & Evrard, B. (2020). Influence of Composition and Spray-Drying Process Parameters on Carrier-Free DPI Properties and Behaviors in the Lung: A review. Pharmaceutics, 12(1), 55. https://doi.org/10.3390/pharmaceutics12010055