Both IgM and IgG Antibodies against Polyethylene Glycol Can Alter the Biological Activity of Methoxy Polyethylene Glycol-Epoetin Beta in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Ethical Statement
2.3. Plasma Sample Collection
2.4. Human Anti-PEG Antibody Assays
2.5. Clinical Relevance of Anemia Patients
2.6. Mouse Monoclonal Anti-PEG Antibody Assay
2.7. Immunoblotting
2.8. Measurement of Anti-PEG Antibodies in Mice Serum
2.9. Red Blood Cell Measurement
2.10. 125I- PEG-EPO Labeling
2.11. Biodistribution of 125I- PEG-EPO
2.12. Pharmacokinetics of 125I- PEG-EPO
2.13. Preparation of 6.3 F(ab’)2 Antibody
2.14. Statistical Analysis
3. Results
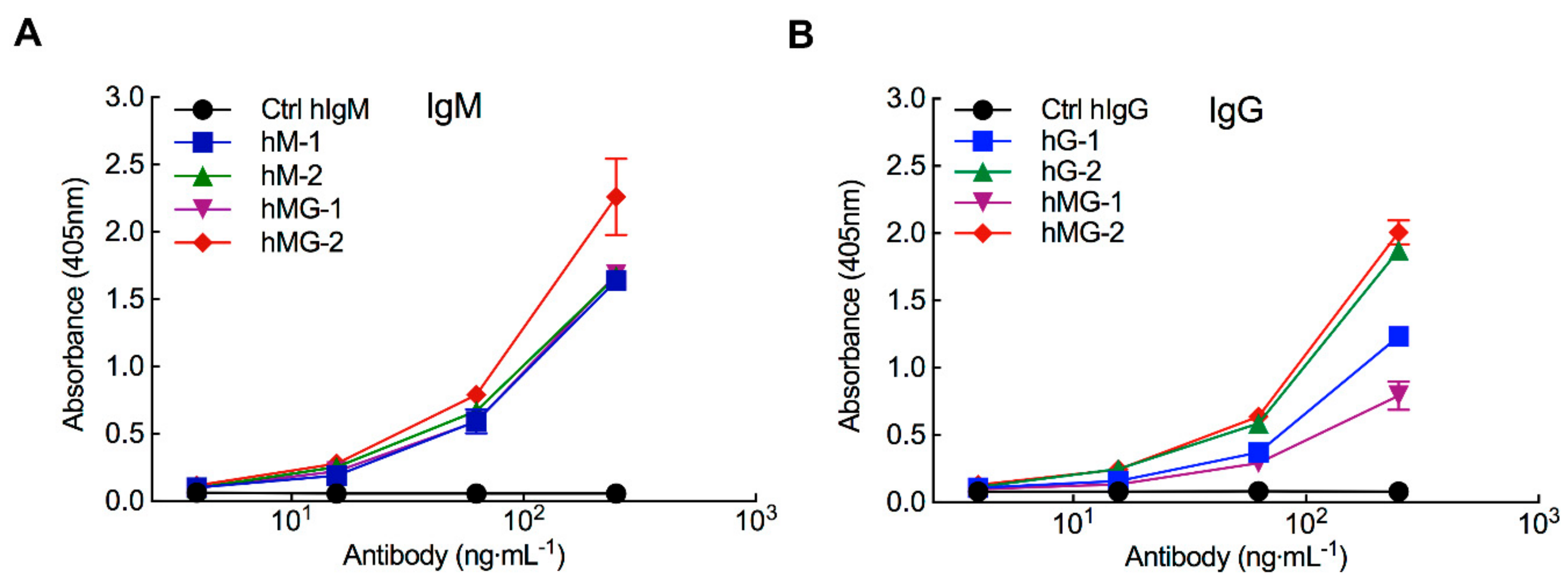
3.1. Pre-Existing Anti-PEG Antibodies in Healthy Donors Bind to PEG-EPO
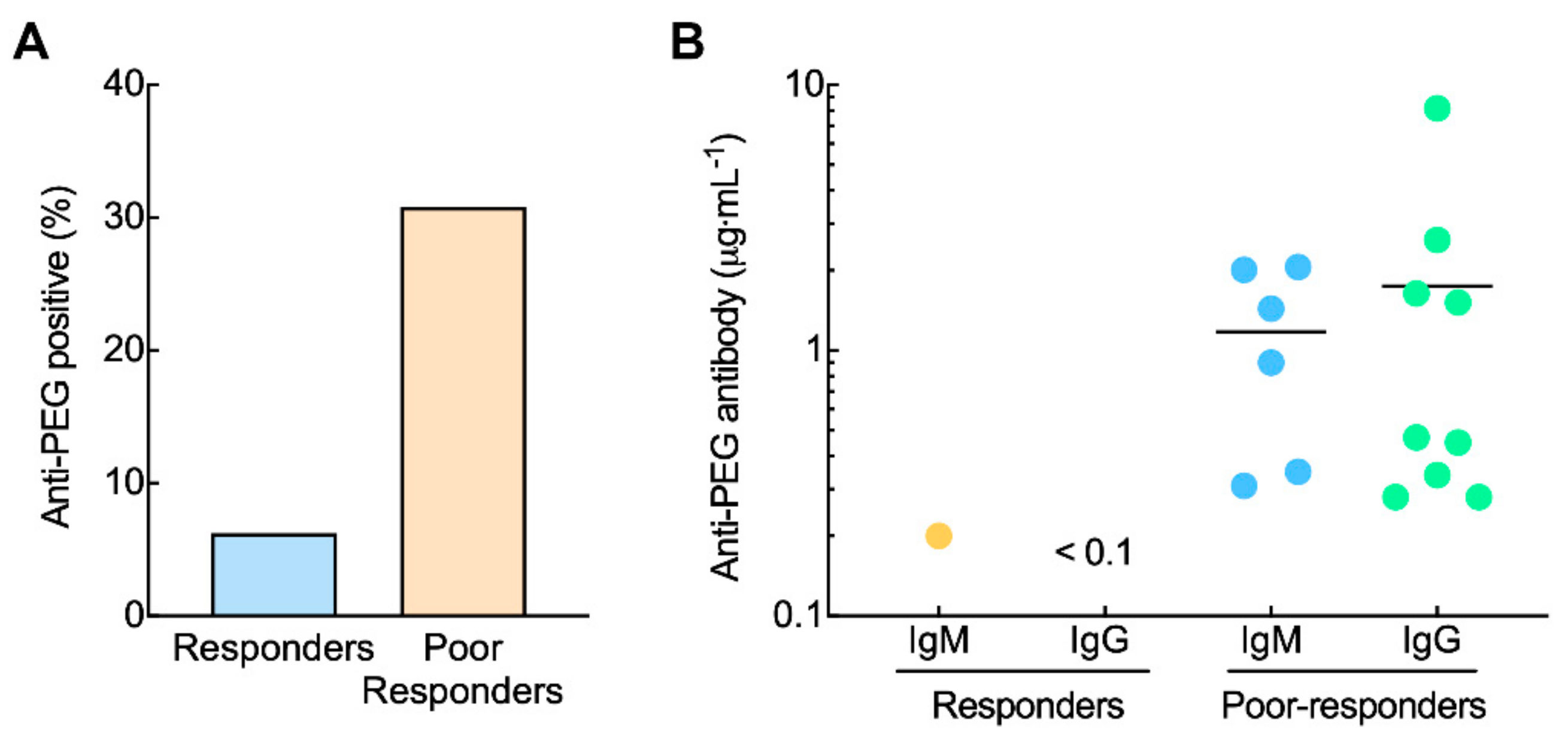
3.2. Pre-Existing Anti-PEG Antibodies in Anemia Patients Receiving PEG-EPO
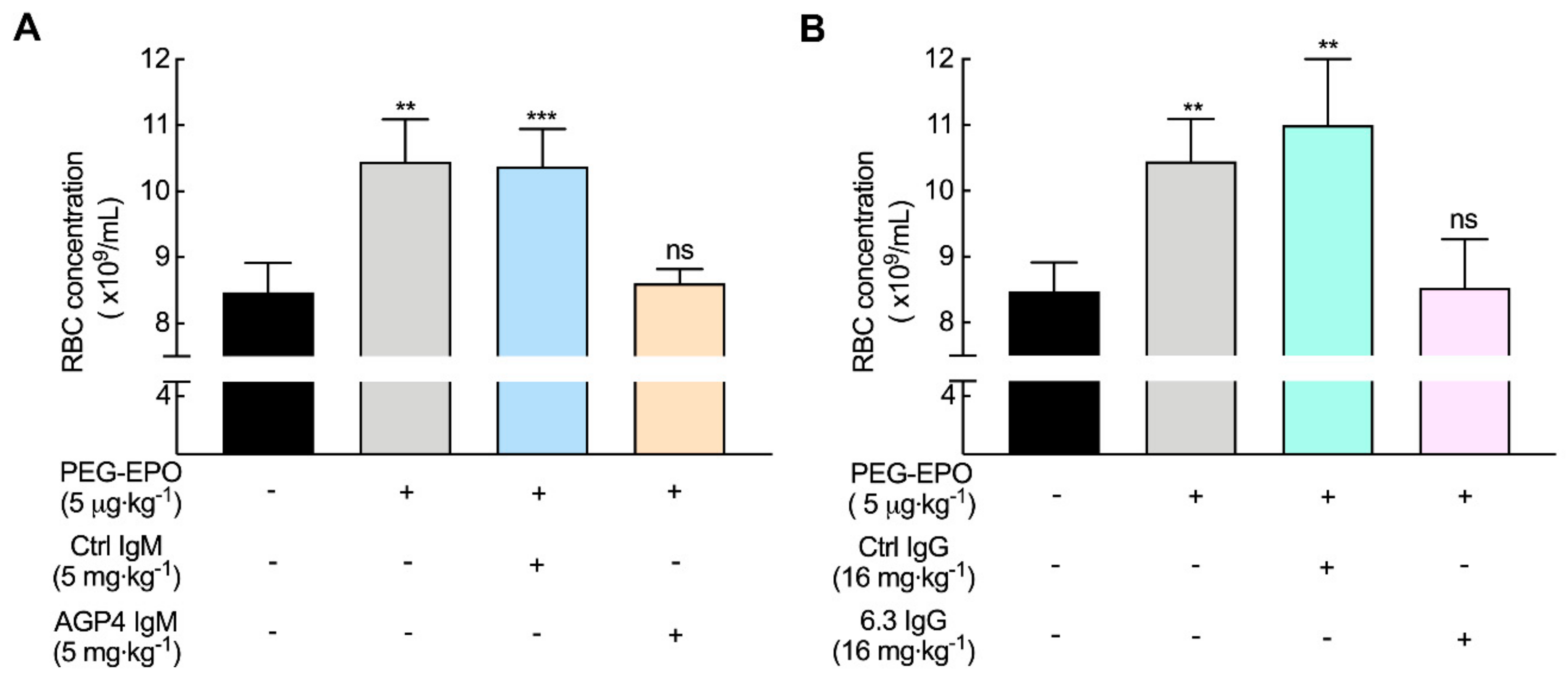
3.3. Anti-PEG Antibodies Can Decrease the Bioactivity of PEG-EPO in Mice
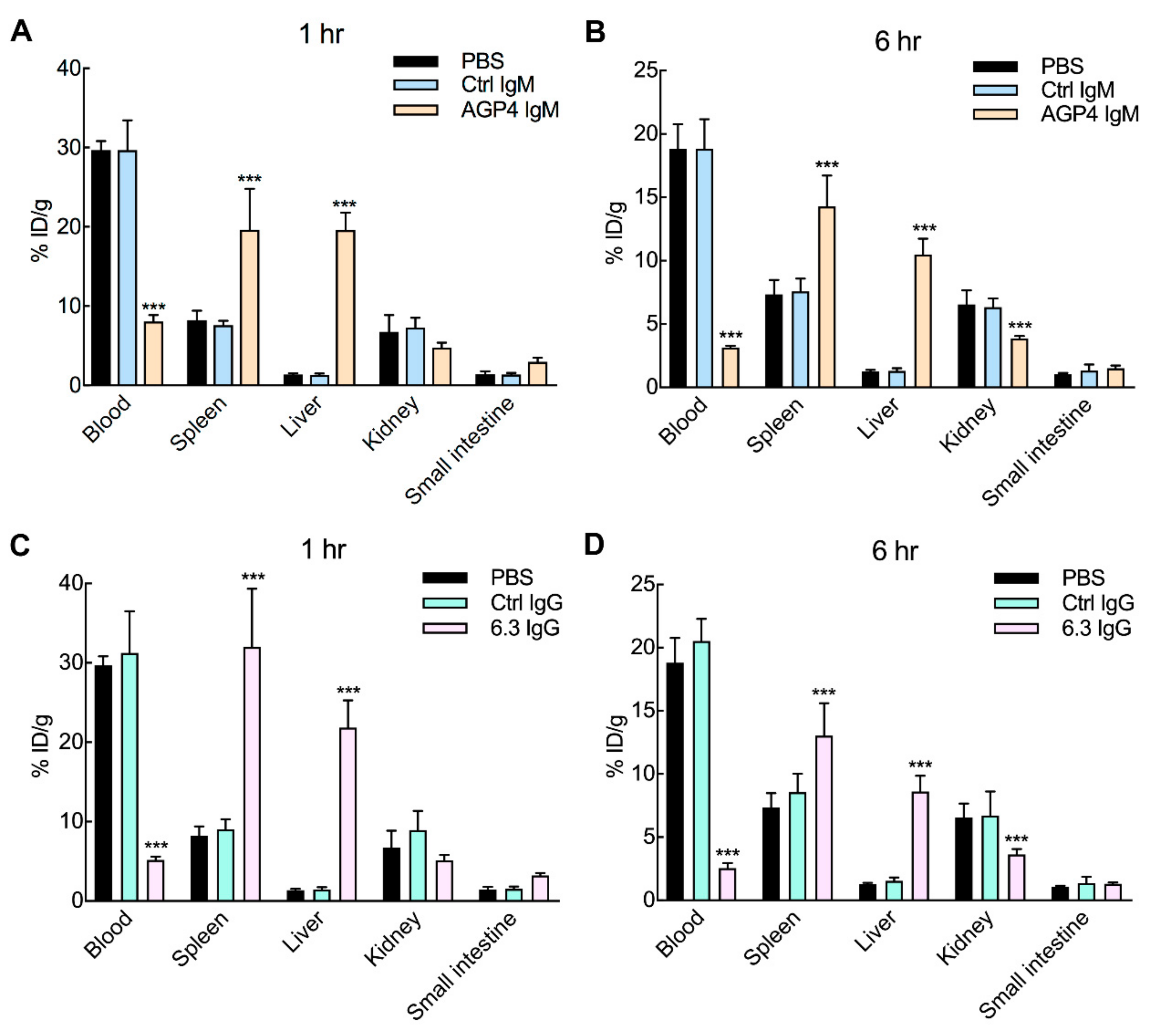
3.4. Anti-PEG Antibodies Promote PEG-EPO Accumulation in the Spleen and Liver
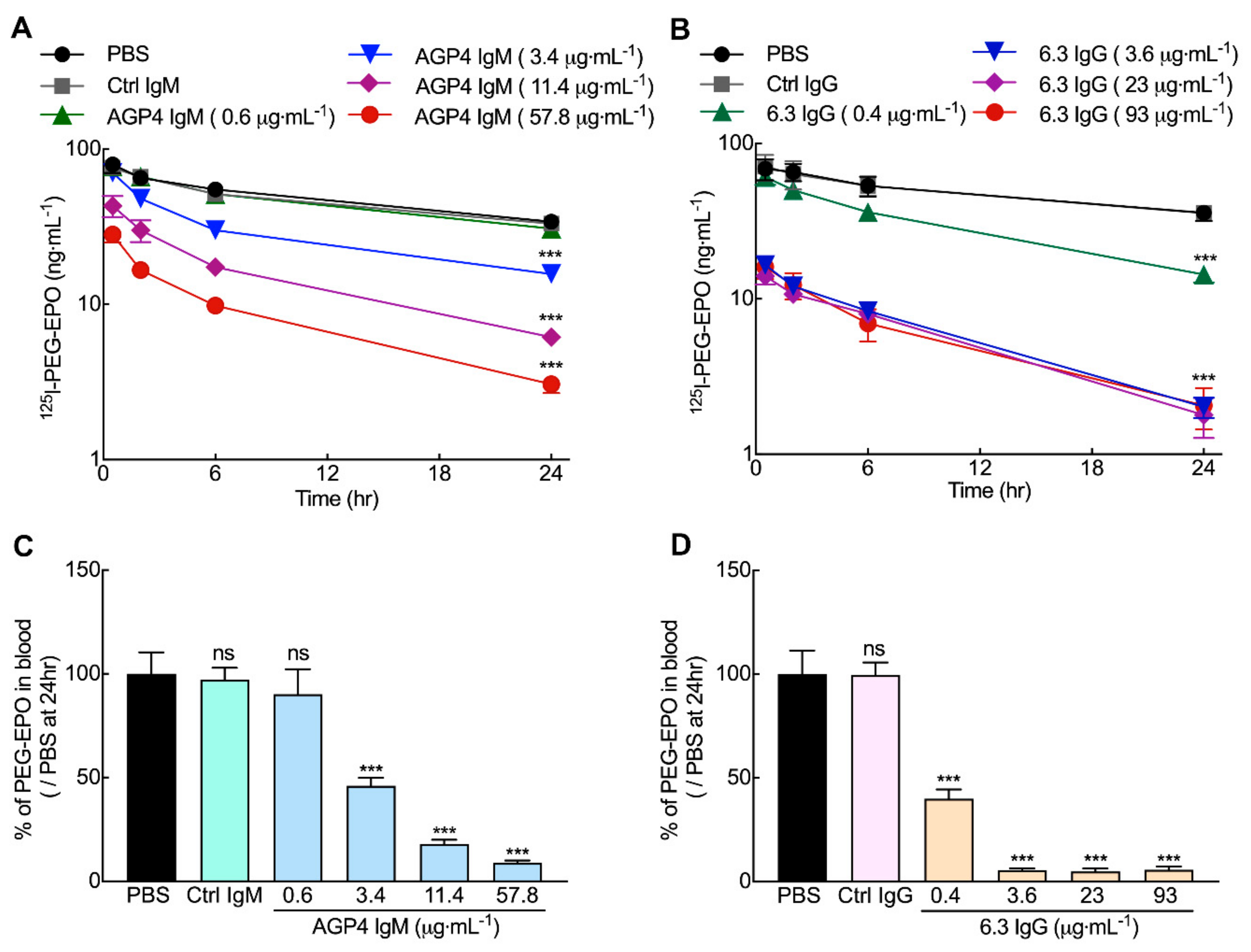
3.5. Anti-PEG Antibodies can Accelerate the Clearance of PEG-EPO in Mice
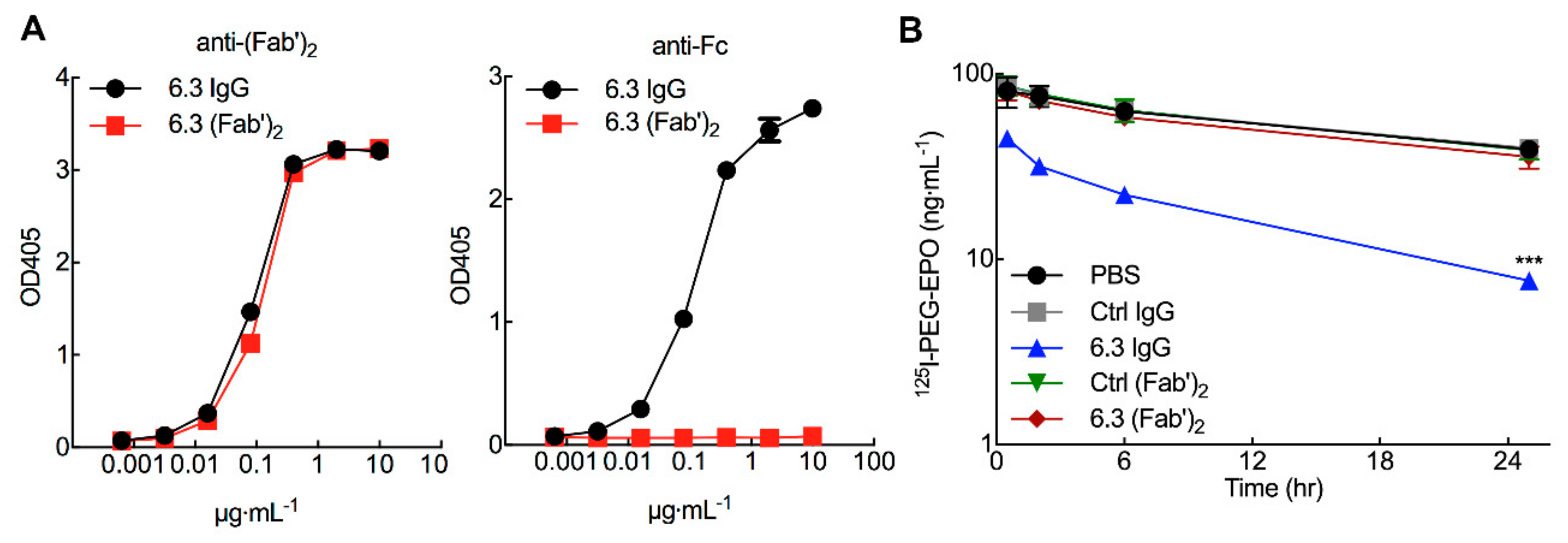
3.6. 125I-PEG-EPO Clearance is Mediated by the Fc Portion of Anti-PEG IgG
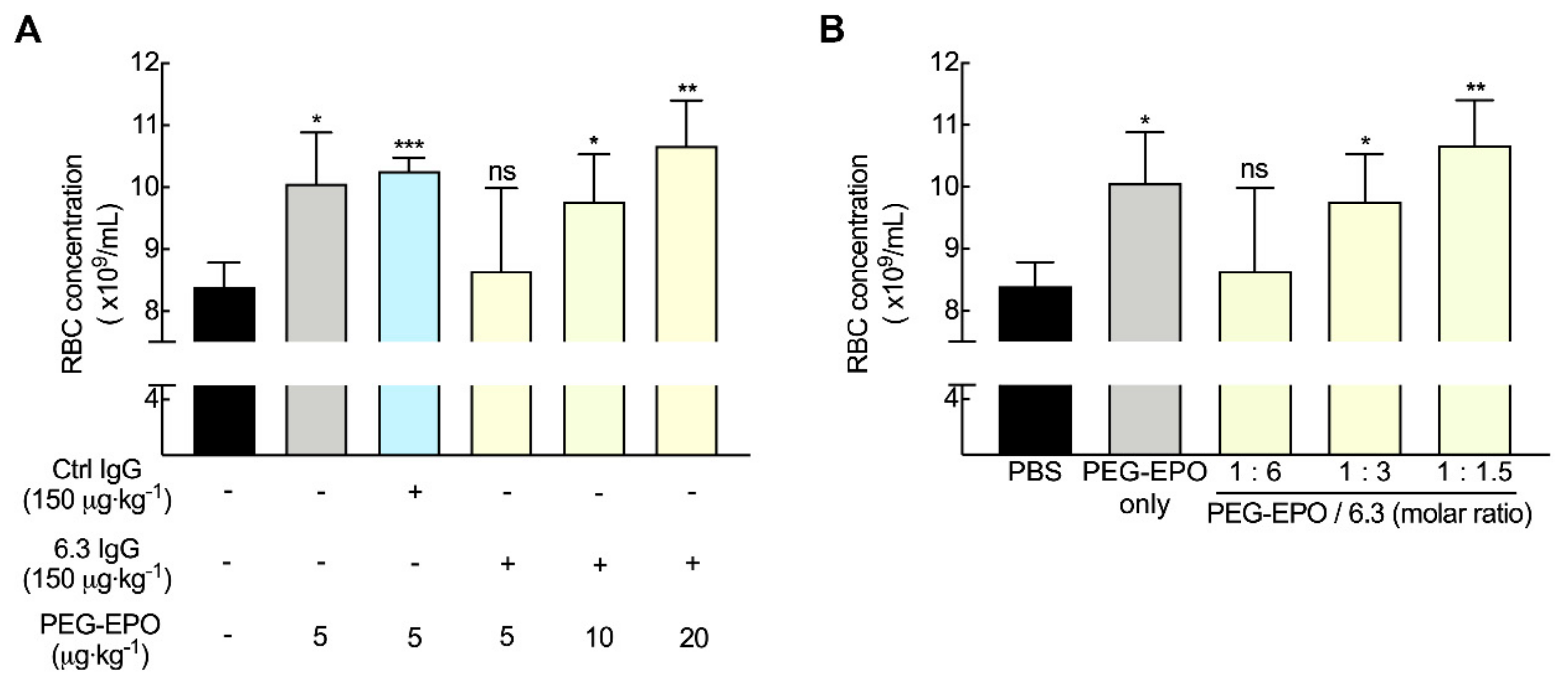
3.7. Fc-Mediated Clearance can be Compensated by Altering PEG-EPO Dose
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Swierczewska, M.; Lee, K.C.; Lee, S. What is the future of PEGylated therapies? Expert Opin. Emerg. Drugs 2015, 20, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.H.P.M.; Torres-Obreque, K.M.; Meneguetti, G.P.; Amaro, B.P.; Rangel-Yagui, C.O. Protein PEGylation for the design of biobetters: From reaction to purification processes. Braz. J. Pharm. Sci. 2018, 54, 1–17. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, X.-l.; Yuan, Y.-h.; Pu, J.; Liao, F. Site-Specific PEGylation of Therapeutic Proteins via Optimization of Both Accessible Reactive Amino Acid Residues and PEG Derivatives. BioDrugs Clin. Immunother. Biopharm. Gene Ther. 2012, 26, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Dozier, J.K.; Distefano, M.D. Site-Specific PEGylation of Therapeutic Proteins. Int. J. Mol. Sci. 2015, 16, 25831–25864. [Google Scholar] [CrossRef]
- Turecek, P.L.; Bossard, M.J.; Schoetens, F.; Ivens, I.A. PEGylation of Biopharmaceuticals: A Review of Chemistry and Nonclinical Safety Information of Approved Drugs. J Pharm. Sci. 2016, 105, 460–475. [Google Scholar] [CrossRef]
- Ganson, N.J.; Kelly, S.J.; Scarlett, E.; Sundy, J.S.; Hershfield, M.S. Control of hyperuricemia in subjects with refractory gout, and induction of antibody against poly(ethylene glycol) (PEG), in a phase Ι trial of subcutaneous PEGylated urate oxidase. Arthritis Res. Ther. 2005, 8, R12. [Google Scholar] [CrossRef]
- Armstrong, J.K.; Hempel, G.; Koling, S.; Chan, L.S.; Fisher, T.; Meiselman, H.J.; Garratty, G. Antibody Against Poly(Ethylene Glycol) Adversely Affects PEG-Asparaginase Therapy in Acute Lymphoblastic Leukemia Patients. Cancer 2007, 110, 103–111. [Google Scholar] [CrossRef]
- Sundy, J.S.; Ganson, N.J.; Kelly, S.J.; Scarlett, E.L.; Rehrig, C.D.; Huang, W.; Hershfield, M.S. Pharmacokinetics and Pharmacodynamics of Intravenous PEGylated Recombinant Mammalian Urate Oxidase in Patients With Refractory Gout. Arthritis Rheum. 2007, 56, 1021–1028. [Google Scholar] [CrossRef]
- Sundy, J.S.; Baraf, H.S.; Yood, R.A.; Edwards, N.L.; Gutierrez-Urena, S.R.; Treadwell, E.L.; Vazquez- Mellado, J.; White, W.B.; Lipsky, P.E.; Horowitz, Z.; et al. Efficacy and Tolerability of Pegloticase for the Treatment of Chronic Gout in Patients Refractory to Conventional Treatment: Two Randomized Controlled Trials. Jama 2011, 306, 711–720. [Google Scholar]
- Hershfield, M.S.; Ganson, N.J.; Kelly, S.J.; Scarlett, E.L.; Jaggers, D.A.; Sundy, J.S. Induced and pre-existing anti-polyethylene glycol antibody in a trial of every 3-week dosing of pegloticase for refractory gout, including in organ transplant recipients. Arthritis Res. Ther. 2014, 16, R63. [Google Scholar] [CrossRef]
- Lipsky, P.E.; Calabrese, L.H.; Kavanaugh, A.; Sundy, J.S.; Wright, D.; Wolfson, M.; Becker, M.A. Pegloticase immunogenicity: The relationship between efficacy and antibody development in patients treated for refractory chronic gout. Arthritis Res. Ther. 2014, 16, R60. [Google Scholar] [CrossRef] [PubMed]
- Longo, N.; Harding, C.O.; Burton, B.K.; Grange, D.K.; Vockley, J.; Wasserstein, M.; Rice, G.M.; Dorenbaum, A.; Neuenburg, J.K.; Musson, D.G.; et al. Single-dose, subcutaneous recombinant phenylalanine ammonia lyase conjugated with polyethylene glycol in adult patients with phenylketonuria: An open-label, multicentre, phase 1 dose-escalation trial. Lancet 2014, 384, 37–44. [Google Scholar] [CrossRef]
- Liu, Y.; Smith, C.A.; Yang, W.; Thompson, L.; Dash, A.; Counts, J.; Chung, M.; Molinelli, A.R.; Pei, D.; Kornegay, N.M.; et al. Pegaspargase Allergic Reactions Are Related to Anti-Pegaspargase Antibodies and to Intensity of Intrathecal Therapy. Blood 2018, 132 (Supp. 1), 2697. [Google Scholar] [CrossRef]
- Rau, R.E.; Dreyer, Z.; Choi, M.R.; Liang, W.; Skowronski, R.; Allamneni, K.P.; Devidas, M.; Raetz, E.A.; Adamson, P.C.; Blaney, S.M.; et al. Outcome of pediatric patients with acute lymphoblastic leukemia/lymphoblastic lymphoma with hypersensitivity to pegaspargase treated with PEGylated Erwinia asparaginase, pegcrisantaspase: A report from the Children’s Oncology Group. Pediatric Blood Cancer 2018, 65, e26873. [Google Scholar] [CrossRef] [PubMed]
- Richter, A.W.; Åkerblom, E. Antibodies against Polyethylene Glycol Produced in Animals by Immunization with Monomethoxy Polyethylene Glycol Modified Proteins. Int. Arch. Allergy Immunol. 1983, 70, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Richter, A.W.; Åkerblom, E. Polyethylene Glycol Reactive Antibodies in Man: Titer Distribution in Allergic Patients Treated with Monomethoxy Polyethylene Glycol Modified Allergens or Placebo, and in Healthy Blood Donors. Int. Arch. Allergy Immunol. 1984, 74, 36–39. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Ichihara, M.; Yoshioka, Y.; Ishida, T.; Nakagawa, S.; Kiwada, H. Intravenous Administration of Polyethylene Glycol-Coated (PEGylated) Proteins and PEGylated Adenovirus Elicits an Anti-PEG Immunoglobulin M Response. Biol. Pharm. Bull. 2012, 35, 1336–1342. [Google Scholar] [CrossRef]
- Mima, Y.; Hashimoto, Y.; Shimizu, T.; Kiwada, H.; Ishida, T. Anti-PEG IgM Is a Major Contributor to the Accelerated Blood Clearance of Polyethylene Glycol-Conjugated Protein. Mol. Pharm. 2015, 12, 2429–2435. [Google Scholar] [CrossRef]
- Ishida, T.; Ichihara, M.; Wang, X.; Yamamoto, K.; Kimura, J.; Majima, E.; Kiwada, H. Injection of PEGylated liposomes in rats elicits PEG-specific IgM, which is responsible for rapid elimination of a second dose of PEGylated liposomes. J. Control. Release 2006, 112, 15–25. [Google Scholar] [CrossRef]
- Judge, A.; McClintock, K.; Phelps, J.R.; MacLachlan, I. Hypersensitivity and Loss of Disease Site Targeting Caused by Antibody Responses to PEGylated Liposomes. Mol. Ther. 2006, 13, 328–337. [Google Scholar] [CrossRef]
- Ishida, T.; Kiwada, H. Accelerated blood clearance (ABC) phenomenon upon repeated injection of PEGylated liposomes. Int. J. Pharm. 2008, 354, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Ichihara, M.; Hyodo, K.; Yamamoto, E.; Ishida, T.; Kiwada, H.; Ishihara, H.; Kikuchi, H. Accelerated blood clearance of PEGylated liposomes containing doxorubicin upon repeated administration to dogs. Int. J. Pharm. 2012, 436, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Cheng, X.; Su, Y.; Pei, Y.; Song, Y.; Jiao, J.; Huang, Z.; Ma, Y.; Dong, Y.; Yao, Y.; et al. Accelerated blood clearance phenomenon upon cross-administration of PEGylated nanocarriers in beagle dogs. Int. J. Nanomed. 2015, 10, 3533–3545. [Google Scholar]
- Moreno, A.; Pitoc, G.A.; Ganson, N.J.; Layzer, J.M.; Hershfield, M.S.; Tarantal, A.F.; Sullenger, B.A. Anti- PEG Antibodies Inhibit the Anticoagulant Activity of PEGylated Aptamers. Cell Chem. Biol. 2019, 26, 634–644.e3. [Google Scholar] [CrossRef]
- Chen, B.-M.; Su, Y.-C.; Chang, C.-J.; Burnouf, P.-A.; Chuang, K.-H.; Chen, C.-H.; Cheng, T.-L.; Chen, Y.-T.; Wu, J.-Y.; Roffler, S.R. Measurement of Pre-Existing IgG and IgM Antibodies against Polyethylene Glycol in Healthy Individuals. Anal. Chem. 2016, 88, 10661–10666. [Google Scholar] [CrossRef]
- Yang, Q.; Jacobs, T.M.; McCallen, J.D.; Moore, D.T.; Huckaby, J.T.; Edelstein, J.; Lai, S.K. Analysis of Pre-Existing IgG and IgM Antibodies against Polyethylene Glycol (PEG) in the General Population. Anal. Chem. 2016, 88, 11084–11812. [Google Scholar] [CrossRef]
- Garay, R.P.; El-Gewely, R.; Armstrong, J.K.; Garratty, G.; Richette, P. Antibodies against polyethylene glycol in healthy subjects and in patients treated with PEG-conjugated agents. Expert Opin. Drug Deliv. 2012, 9, 1319–1323. [Google Scholar] [CrossRef]
- Liu, Y.; Reidler, H.; Pan, J.; Milunic, D.; Qin, D.; Chen, D.; Vallejo, Y.R.; Yin, R. A double antigen bridging immunogenicity ELISA for the detection of antibodies to polyethylene glycol polymers. J.Pharm. Toxicol. Methods 2011, 64, 238–245. [Google Scholar] [CrossRef]
- Ganson, N.J.; Povsic, T.J.; Sullenger, B.A.; Alexander, J.H.; Zelenkofske, S.L.; Sailstad, J.M.; Rusconi, C.P.; Hershfield, M.S. Pre-existing anti–polyethylene glycol antibody linked to first-exposure allergic reactions to pegnivacogin, a PEGylated RNA aptamer. J. Allergy Clin. Immunol. 2016, 137, 1610–1613.e1617. [Google Scholar] [CrossRef]
- Lubich, C.; Allacher, P.; de la Rosa, M.; Bauer, A.; Prenninger, T.; Horling, F.M.; Siekmann, J.; Oldenburg, J.; Scheiflinger, F.; Reipert, B.M. The Mystery of Antibodies Against Polyethylene Glycol (PEG) - What do we Know? Pharm. Res. 2016, 33, 2239–2249. [Google Scholar] [CrossRef]
- Povsic, T.J.; Lawrence, M.G.; Lincoff, A.M.; Mehran, R.; Rusconi, C.P.; Zelenkofske, S.L.; Huang, Z.; Sailstad, J.; Armstrong, P.W.; Steg, P.G.; et al. Pre-existing anti-PEG antibodies are associated with severe immediate allergic reactions to pegnivacogin, a PEGylated aptamer. J. Allergy Clin. Immunol. 2016, 138, 1712–1715. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.-L.; Wu, P.-Y.; Wu, M.-F.; Chern, J.-W.; Roffler, S.R. Accelerated Clearance of Polyethylene Glycol-Modified Proteins by Anti-Polyethylene Glycol IgM. Bioconjugate Chem. 1999, 10, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.-L.; Chen, B.-M.; Chern, J.-W.; Wu, M.-F.; Roffler, S.R. Efficient Clearance of Poly(ethylene glycol)-Modified Immunoenzyme with Anti-PEG Monoclonal Antibody for Prodrug Cancer Therapy. Bioconjugate Chem. 2000, 11, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.-L.; Cheng, C.-M.; Chen, B.-M.; Tsao, D.-A.; Chuang, K.-H.; Hsiao, S.-W.; Lin, Y.-H.; Roffler, S.R. Monoclonal Antibody-Based Quantitation of Poly(ethylene glycol)-Derivatized Proteins, Liposomes, and Nanoparticles. Bioconjugate Chem. 2005, 16, 1225–1231. [Google Scholar] [CrossRef]
- Ishida, T.; Maeda, R.; Ichihara, M.; Irimura, K.; Kiwada, H. Accelerated clearance of PEGylated liposomes in rats after repeated injections. J. Control. Release 2003, 88, 35–42. [Google Scholar] [CrossRef]
- Hsieh, Y.-C.; Wang, H.-E.; Lin, W.-W.; Roffler, S.R.; Cheng, T.-C.; Su, Y.-C.; Li, J.-J.; Chen, C.-C.; Huang, C.-H.; Chen, B.-M.; et al. Pre-existing anti-polyethylene glycol antibody reduces the therapeutic efficacy and pharmacokinetics of PEGylated liposomes. Theranostics 2018, 8, 3164–3175. [Google Scholar] [CrossRef]
- Grenier, P.; Viana, I.M.d.O.; Lima, E.M.; Bertrand, N. Anti-polyethylene glycol antibodies alter the protein corona deposited on nanoparticles and the physiological pathways regulating their fate in vivo. J. Control. Release 2018, 287, 121–131. [Google Scholar] [CrossRef]
- Park, E.J.; Choi, J.; Lee, K.C.; Na, D.H. Emerging PEGylated non-biologic drugs. Expert Opin. Emerg. Drugs 2019, 24, 107–119. [Google Scholar] [CrossRef]
- Curran, M.P.; McCormack, P.L. Methoxy Polyethylene Glycol-Epoetin Beta: A Review of its Use in the Management of Anaemia Associated with Chronic Kidney Disease. Drugs 2008, 68, 1139–1156. [Google Scholar] [CrossRef]
- Ohashi, N.; Sakao, Y.; Yasuda, H.; Kato, A.; Fujigaki, Y. Methoxy polyethylene glycol-epoetin beta for anemia with chronic kidney disease. Int. J. Nephrol. Renov. Disease 2012, 5, 53–60. [Google Scholar] [CrossRef]
- Su, Y.-C.; Chen, B.-M.; Chuang, K.-H.; Cheng, T.-L.; Roffler, S.R. Sensitive Quantification of PEGylated Compounds by Second-Generation Anti-Poly(ethylene glycol) Monoclonal Antibodies. Bioconjugate Chem. 2010, 21, 1264–1270. [Google Scholar] [CrossRef] [PubMed]
- Gross, A.W.; Lodish, H.F. Cellular Trafficking and Degradation of Erythropoietin and Novel Erythropoiesis Stimulating Protein (NESP). J. Biol. Chem. 2006, 281, 2024–2032. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, T.N.M.; Tsomides, T.J. In Vitro Radiolabeling of Peptides and Proteins (Volume 00, Issue 1, Unit 3.3.1–3.3.19). In Current Protocols in Protein Science; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1995. [Google Scholar]
- Andrew Sarah, M.; Titus Julie, A. Fragmentation of Immunoglobulin G. Curr. Protoc. Immunol. 2001, 21, 2.8.1–2.8.10. [Google Scholar]
- Garratty, G. Progress in Modulating the RBC Membrane To Produce Transfusable Universal/Stealth Donor RBCs. Transfus. Med. Rev. 2004, 18, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Myler, H.; Hruska, M.W.; Srinivasan, S.; Cooney, E.; Kong, G.; Dodge, R.; Krishna, M.; Zhu, J.; Felix, T.; Gleason, C.; et al. Anti-PEG antibody bioanalysis: A clinical case study with PEG-IFN-λ-1a and PEG-IFN-α2a in naive patients. Bioanalysis 2015, 7, 1093–1106. [Google Scholar] [CrossRef] [PubMed]
- Fishbane, S.; Pannier, A.; Liogier, X.; Jordan, P.; Dougherty, F.C.; Reigner, B. Pharmacokinetic and Pharmacodynamic Properties of Methoxy Polyethylene Glycol-Epoetin Beta Are Unaffected by the Site of Subcutaneous Administration. J. Clin. Pharmacol. 2007, 47, 1390–1397. [Google Scholar] [CrossRef]
- Guilliams, M.; Bruhns, P.; Saeys, Y.; Hammad, H.; Lambrecht, B.N. The function of Fcr receptors in dendritic cells and macrophages. Nature Rev. Immunol. 2014, 14, 94–108. [Google Scholar] [CrossRef]
- Oh, D.J. Anaphylaxis Following the Intravenous Administration of Continuous Erythropoietin receptor Activator in a Haemodialysis Patient. Nephrology 2014, 19, 304. [Google Scholar] [CrossRef]
| Brand Name | Component | PEG (kDa) | PEG Number | Disease | Approval Year |
|---|---|---|---|---|---|
| Adagen® | Adenosine deaminase | 5 | 11-17 | Severe immunodeficiency | 1990 |
| Oncaspar® | L-Asparaginase | 5 | 69-82 | Leukemia | 1994 |
| Doxil® | Liposomal doxorubicin | 2 | multiple | Cancer | 1995 |
| PEG-Intron® | Interferon alfa-2b | 12 | 1 | Hepatitis C | 2001 |
| PEGASYS® | Interferon alfa-2a | 40 | 1 (branched) | Hepatitis | 2001 |
| Neulasta® | G-CSF | 20 | 1 | Neutropenia | 2002 |
| Somavert® | Antagonist (GHR) | 5 | 4-6 | Acromegaly | 2003 |
| Macugen® | Anti-VEGF aptamer | 20 | 2 | Macular degeneration | 2004 |
| Mircera® | Epoetin beta | 30 | 1 | Anemia | 2007 |
| Cimzia® | Anti-TNFα Fab | 40 | 1 (branched) | Rheumatoid arthritis | 2008 |
| Krystexxa® | Uricase | 10 | 9-11 | Gout | 2010 |
| Sylatron® | Interferon alfa-2b | 12 | 1 | Melanoma | 2011 |
| Omontys® | Analog of erythropoietin | 40 | 1 (branched) | Anemia | 2012 |
| Movantik® | Antagonist (C34H53NO11) | 0.3 | 1 | Opioid-induced constipation | 2014 |
| Plegridy® | Interferon beta-1a | 20 | 1 | Multiple sclerosis | 2014 |
| Adynovate® | Factor VIII | 20 | 1 (branched) | Hemophilia A | 2015 |
| Onivyde® | Liposomal irinotecan | 2 | multiple | Cancer | 2015 |
| Rebinyn® | Factor IX | 40 | 1 | Hemophilia B | 2017 |
| Palynziq® | Phenylalanine ammonia lyase | 20 | 9 | Phenylketonuria | 2018 |
| Jivi® | Factor VIII | 60 | 1 (branched) | Hemophilia A | 2018 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, T.-C.; Chen, B.-M.; Lin, W.-W.; Yu, P.-H.; Chiu, Y.-W.; Chen, Y.-T.; Wu, J.-Y.; Cheng, T.-L.; Hwang, D.-Y.; Roffler, S. Both IgM and IgG Antibodies against Polyethylene Glycol Can Alter the Biological Activity of Methoxy Polyethylene Glycol-Epoetin Beta in Mice. Pharmaceutics 2020, 12, 15. https://doi.org/10.3390/pharmaceutics12010015
Chang T-C, Chen B-M, Lin W-W, Yu P-H, Chiu Y-W, Chen Y-T, Wu J-Y, Cheng T-L, Hwang D-Y, Roffler S. Both IgM and IgG Antibodies against Polyethylene Glycol Can Alter the Biological Activity of Methoxy Polyethylene Glycol-Epoetin Beta in Mice. Pharmaceutics. 2020; 12(1):15. https://doi.org/10.3390/pharmaceutics12010015
Chicago/Turabian StyleChang, Tien-Ching, Bing-Mae Chen, Wen-Wei Lin, Pei-Hua Yu, Yi-Wen Chiu, Yuan-Tsong Chen, Jer-Yuarn Wu, Tian-Lu Cheng, Daw-Yang Hwang, and Steve Roffler. 2020. "Both IgM and IgG Antibodies against Polyethylene Glycol Can Alter the Biological Activity of Methoxy Polyethylene Glycol-Epoetin Beta in Mice" Pharmaceutics 12, no. 1: 15. https://doi.org/10.3390/pharmaceutics12010015
APA StyleChang, T.-C., Chen, B.-M., Lin, W.-W., Yu, P.-H., Chiu, Y.-W., Chen, Y.-T., Wu, J.-Y., Cheng, T.-L., Hwang, D.-Y., & Roffler, S. (2020). Both IgM and IgG Antibodies against Polyethylene Glycol Can Alter the Biological Activity of Methoxy Polyethylene Glycol-Epoetin Beta in Mice. Pharmaceutics, 12(1), 15. https://doi.org/10.3390/pharmaceutics12010015






