Aerosolization Performance of Jet Nebulizers and Biopharmaceutical Aspects
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
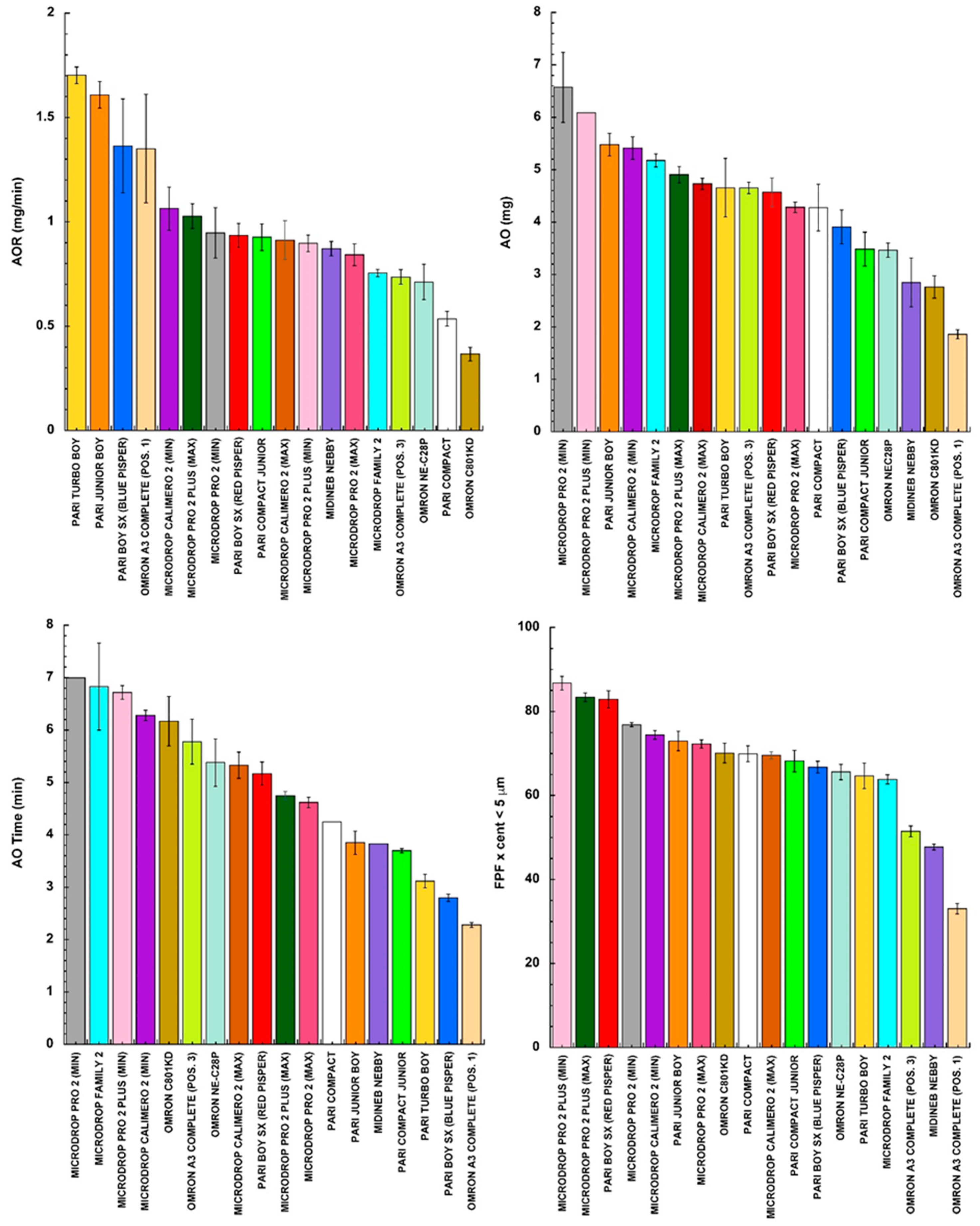
2.2.1. Determination of AOR and AO
2.2.2. Aerodynamic Assessment
2.2.3. Data Processing
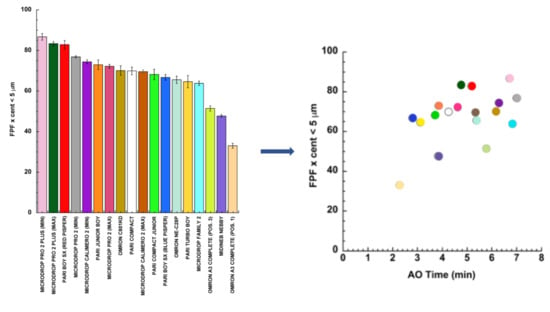
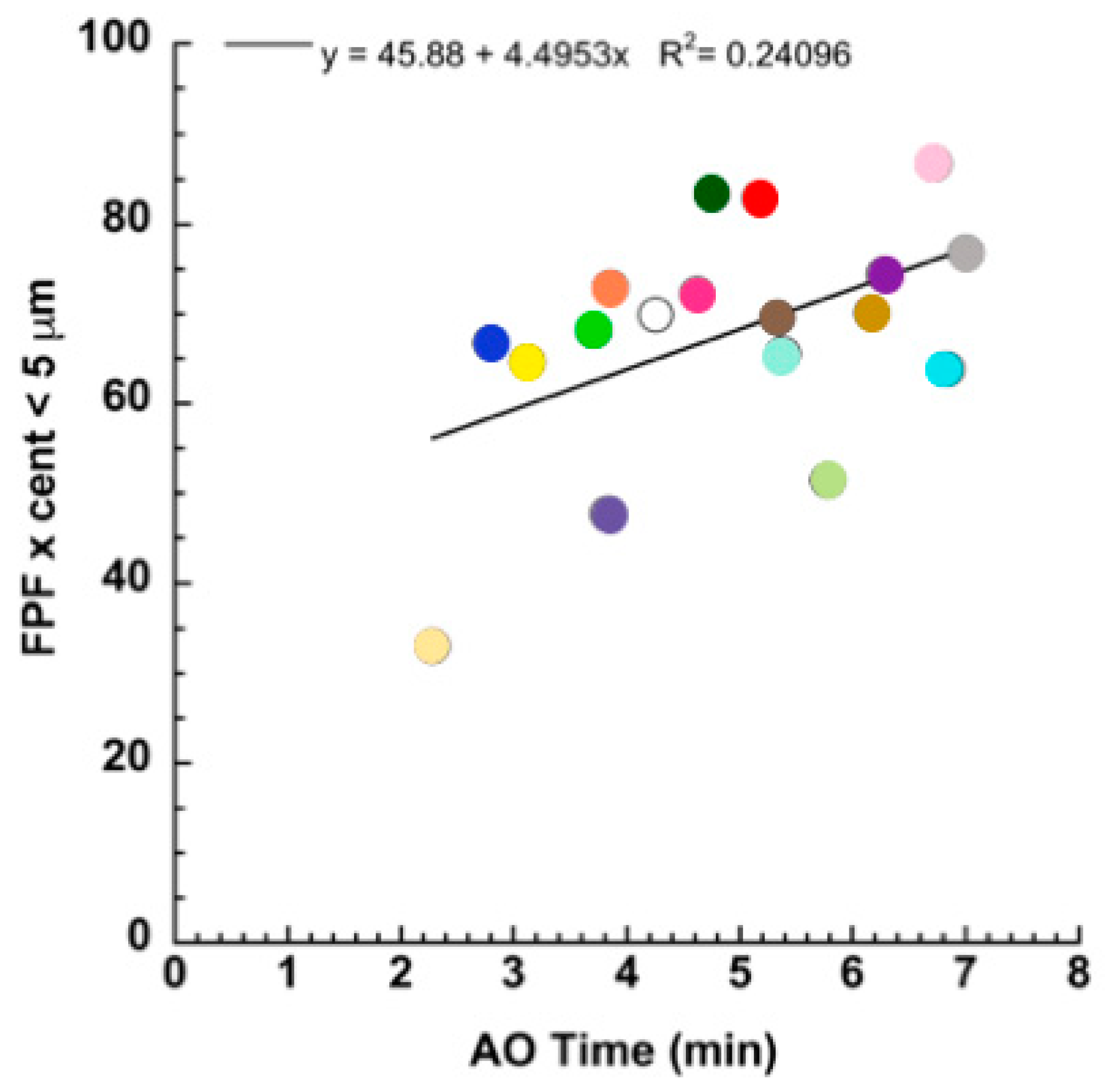
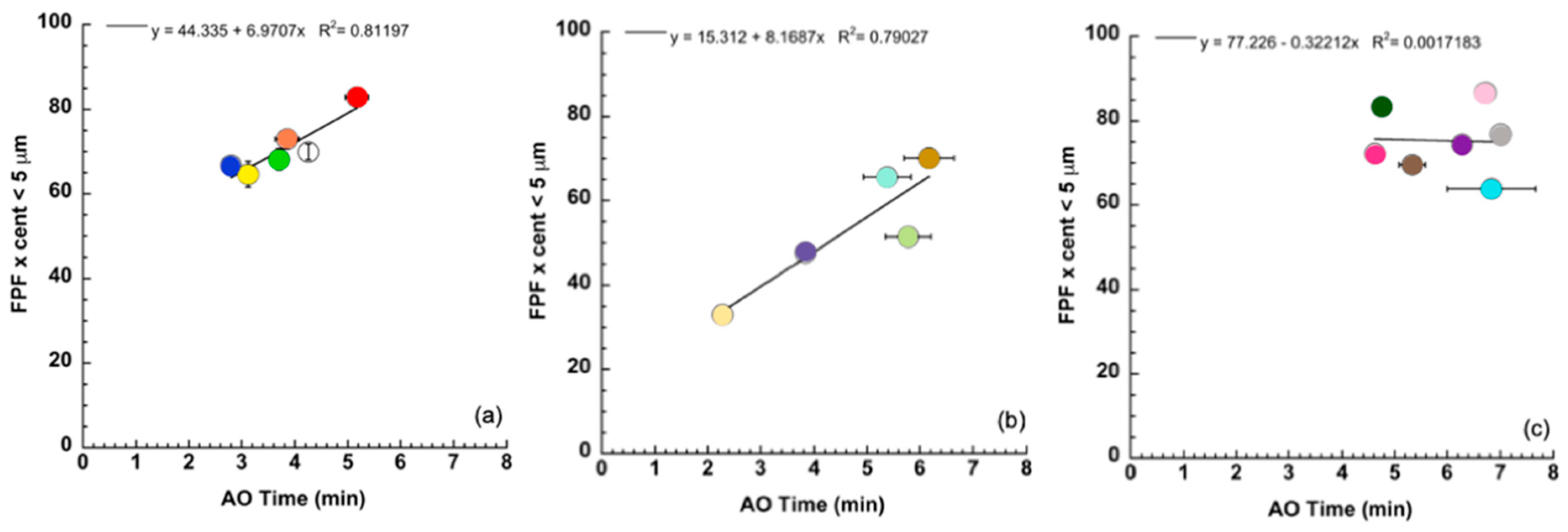
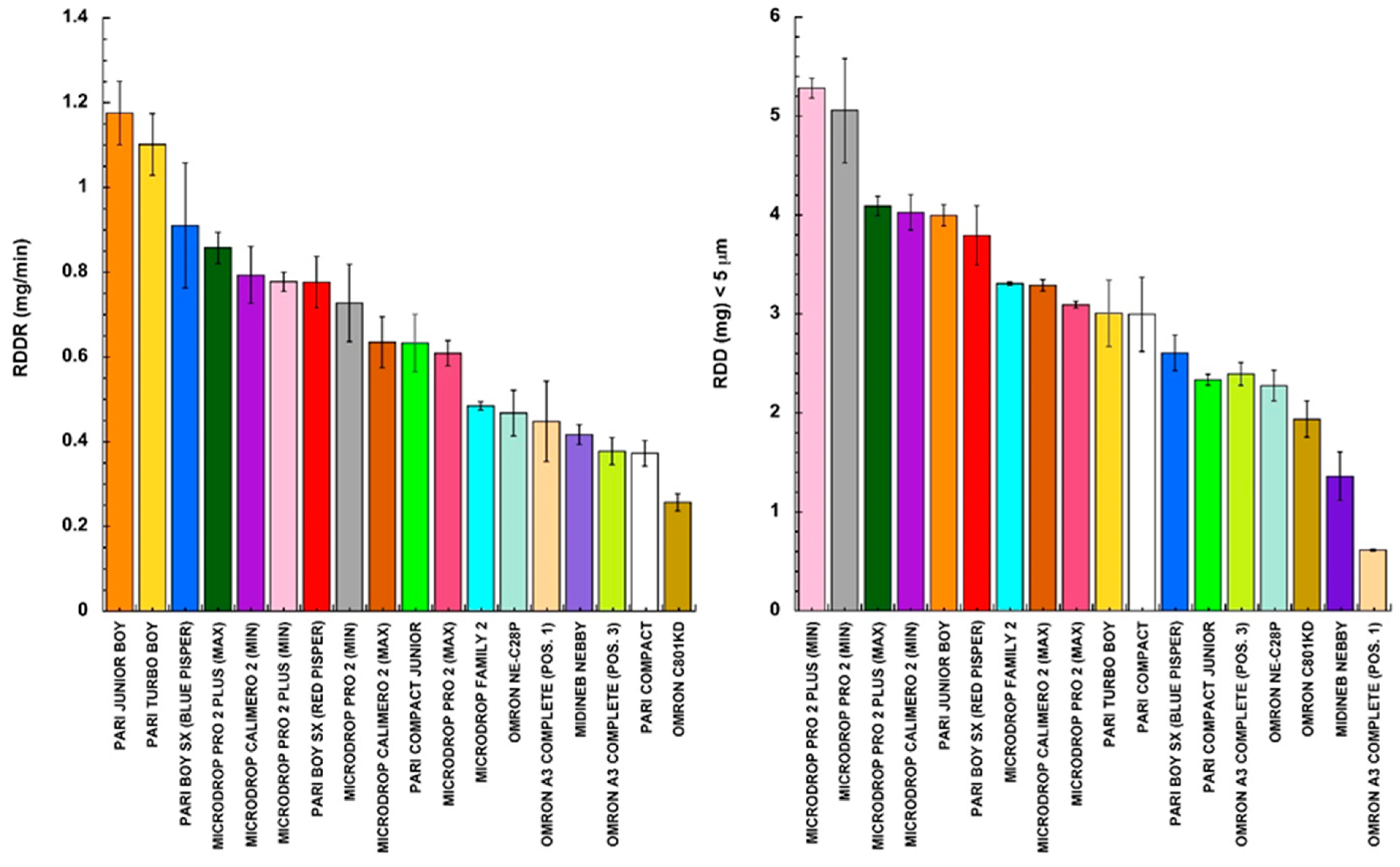
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stein, S.W.; Thiel, C.G. The history of therapeutic aerosols. J. Aerosol Med. Pulm. Drug Deliv. 2017, 30, 20–41. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.; Verma, R.; Garcia-Contreras, L. Inhalation drug delivery devices: Technology update. Med. Devices 2015, 8, 131–139. [Google Scholar]
- Mashat, M.; Clark, B.J.; Assi, K.H.; Chrystyn, H. In vitro aerodynamic characterization of the dose emitted during nebulization of tobramycin high strength solution by novel and jet nebulizer delivery systems. Pulm. Pharmacol. Ther. 2016, 37, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Lavorini, F.; Fontana, G.A.; Usmani, O.S. New Inhaler Devices—The Good, the Bad and the Ugly. Respiration 2014, 88, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Rangaraj, N.; Pailla, S.R.; Sampathi, S. Insight into pulmonary drug delivery: Mechanism of drug deposition to device characterization and regulatory requirements. Pulm. Pharmacol. Ther. 2019, 54, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Pitance, L.; Vecellio, L.; Leal, T.; Reychler, G.; Reychler, H.; Liistro, G. Delivery Efficacy of a Vibrating Mesh Nebulizer and a Jet Nebulizer under Different Configurations. J. Aerosol Med. Pulm. Drug Deliv. 2010, 23, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Najlaha, M.; Parveenb, I.; Albed Alhnan, M.; Ahmedb, W.; Faheeme, A.; Phoenix, D.A.; Taylor, K.M.G.; Elhissi, A. The effects of suspension particle size on the performance of air-jet,ultrasonic and vibrating-mesh nebulisers. Int. J. Pharm. 2014, 4, 234–241. [Google Scholar] [CrossRef]
- Hatley, R.H.M.; Byrne, S.M. Variability in delivered dose and respirable delivered dose from nebulizers: Are current regulatory testing guidelines sufficient to produce meaningful information? Med. Devices 2017, 10, 17–28. [Google Scholar] [CrossRef]
- Kesten, S.; Israel, E.; Li, G.; Mitchell, J.; Wise, R.; Stern, T. Development of a novel digital breath-activated inhaler: Initial particle size characterization and clinical testing. Pulm. Pharmacol. Ther. 2018, 53, 27–32. [Google Scholar] [CrossRef]
- Hua, J.; Zhanga, R.; Bengb, H.; Denga, L.; Kea, Q.; Tana, W. Effects of flow pattern, device and formulation on particle size distribution of nebulized aerosol. Int. J. Pharm. 2019, 560, 35–46. [Google Scholar] [CrossRef]
- Buttini, F.; Rossi, I.; Di Cuia, M.; Ross, A.; Colombo, G.; Elviri, L.; Sonvico, F.; Balducci, A.G. Combinations of colistin solutions and nebulizers from lung infection management in cystic fibrosis patients. Int. J. Pharm. 2016, 502, 242–248. [Google Scholar] [CrossRef]
- O’Callaghan, C.; Barry, P.W. The science of nebulised drug delivery. Thorax 1997, 52, S31–S44. [Google Scholar] [CrossRef]
- Ali, M. Pulmonary Drug Delivery. In Handbook of Non-Invasive Drug Delivery Systems, 1st ed.; Kulkarni, V.S., Ed.; Elsevier Kindlington: Oxford, UK, 2010; Chapter 9; pp. 209–246. [Google Scholar]
- Hou, S.; Wu, J.; Li, X.; Shu, H. Practical, regulatory and clinical considerations for development of inhalation drug products. Asian J. Pharm. Sci. 2015, 10, 490–500. [Google Scholar] [CrossRef]
- Dhand, R.; Dolovich, M.; Chipps, B.; Myers, T.R.; Restrepo, R.; Rosen Farrar, J. The role of nebulized therapy in the management of COPD: Evidence and recommendations. COPD 2012, 9, 58–72. [Google Scholar] [CrossRef]
- Olveira, C.; Mûnoz, A.; Domen, A. Nebulized therapy. SEPAR Year. Arch. Bronconeumol. 2014, 50, 535–545. [Google Scholar] [CrossRef]
- Ari, A. Jet, Ultrasonic, and Mesh Nebulizers: An evaluation of nebulizers for better clinical outcomes. Eurasian J. Pulmonol. 2014, 16, 1–7. [Google Scholar] [CrossRef]
- Ari, A. Drug delivery interfaces: A way to optimize inhalation therapy in spontaneously breathing children. World J. Clin. Pediatr. 2016, 5, 281–287. [Google Scholar] [CrossRef]
- Martin, A.R.; Finlay, W.H. Nebulizers for drug delivery to the lungs. Expert Opin. Drug Deliv. 2014, 12, 889–900. [Google Scholar] [CrossRef]
- Ari, A.; de Andrade, A.D.; Sheard, M.; AIHamad, B.; Fink, J.B. Performance comparisons of jet and mesh nebulizers using different interfaces in simulated spontaneously breathing adults and children. J. Aerosol Med. Pulm. Drug Deliv. 2015, 28, 281–289. [Google Scholar] [CrossRef]
- Saeed, H.; Ali, A.M.A.; Elberry, A.A.; Eldin, A.S.; Rabea, H.; Abdelrahim, M.E.A. Modeling and optimization of nebulizers’ performance in non-invasive ventilation using different fill volumes: Comparative study between vibrating mesh and jet nebulizers. Pulm. Pharmacol. Ther. 2018, 50, 62–71. [Google Scholar] [CrossRef]
- Murayama, N.; Murayama, K. Comparison of the clinical efficacy of salbutamol with jet and mesh nebulizers in asthmatic children. Pulm. Med. 2018, 2018, 1–6. [Google Scholar] [CrossRef]
- Dunne, R.B.; Shortt, S. Comparison of bronchodilator administration with vibrating mesh nebulizer and standard jet nebulizer in the emergency department. Am. J. Emerg. Med. 2018, 36, 641–646. [Google Scholar] [CrossRef]
- Chang, K.H.; Moon, S.-H.; Oh, J.Y.; Yoon, Y.-S.; Gu, N.; Lim, C.-Y.; Park, B.J.; Nam, K.C. Comparison of salbutamol delivery efficiency for jet versus mesh nebulizer using mice. Pharmaceutics 2019, 11, 192. [Google Scholar] [CrossRef]
- Fink, J.B. Aerosol Drug Therapy. In Egan’s Fundamentals of Respiratory Care, 10th ed.; Kacmarek, R.M., Stoller, J.K., Heuer, A.J., Eds.; Elsevier: Mosby, St., Louis, MI, USA, 2012; Chapter 36; pp. 844–886. [Google Scholar]
- Seifert, G.; Krämer, I.; Rossi, A.; Kamin, W. Vergleichende untersuchung von aktuell in Deutschland vermarkteten druckluftverneblern und den damit erzeugten aerosolen. Krankenhauspharmazie 2019, 40, 241–249. [Google Scholar]
- European Committee for Standardization. CEN: EN 13544-1:2007+ A1:2009. Respiratory Therapy Equipment: Nebulizing Systems and Their Components; British Standard Institute: London, UK, 2010. [Google Scholar]
- Preparation for nebulization: Characterization. In European Pharmacopoeia, 9th ed.; EDQM, Council of Europe: Strasbourg, France, 2016; Chapter 2.9.44.
- Hastedt, J.E.; Backman, P.; Clark, A.R.; Doub, W.; Hickey, A.; Hochhaus, G.; Kuehl, P.J.; Lehr, C.-M.; Mauser, P.; McConville, J.; et al. Scope and relevance of a pulmonary biopharmaceutical classification system AAPS/FDA/USP Workshop March 16–17th, 2015 in Baltimore, MD. AAPS Open 2016, 2, 2–20. [Google Scholar] [CrossRef]
- Walz-Jung, H.; Kamin, W.; Krämer, I. No differences? Aerosol characteristics of 9 jet nebulizers tested in vitro. Poster n. 3570. European Respiratory Society, ERS. In Proceedings of the 24th International Congress, Munich, Germany, 6–10 September 2014. [Google Scholar]
| Nebulizer | Manufacturer | Batch (S/N) | Configuration | Identification Color | |
|---|---|---|---|---|---|
| Pari Compact | Pari | 2W17C10078 | White |  | |
| Pari Compact Junior | Pari | 2W17A13163 | Light green |  | |
| Pari Boy SX | Pari | 2W17B01844 | Blue Pisper * | Blue |  |
| Red Pisper * | Red |  | |||
| Pari JuniorBoy SX | Pari | 2W17B08883 | Orange |  | |
| Pari TurboBoy SX | Pari | 2W16H00598 | Yellow |  | |
| Microdrop Family 2 | Flaem Nuova | 16A 155 0873 | Light blue |  | |
| Microdrop Calimero 2 | Flaem Nuova | 16AF450652 | Ampoule Valve MAX | Brown |  |
| Ampoule Valve MIN | Purple |  | |||
| Microdrop Pro 2 | Flaem Nuova | 15 A7870439 | Ampoule Valve MAX | Fuchsia |  |
| Ampoule Valve MIN | Grey |  | |||
| Microdrop Pro 2 Plus | Flaem Nuova | Engineering sample | Ampoule Valve MAX | Dark green |  |
| Ampoule Valve MIN | Pink |  | |||
| Omron C801KD | Omron Healthcare | 20160600989VF | Yellow-green |  | |
| Omron NE-C28P | Omron Healthcare | 20160905635UF | Green water |  | |
| Omron A3 Complete | 3A Healthcare | 201702/00279F | Ampoule Position 1 | Blush |  |
| Ampoule Position 3 | Lemon green |  | |||
| Midineb Nebby | 3A Healthcare | 16/30635 | Lilac |  | |
| Nebulizer | Configuration | MMAD (μm) | GSD |
|---|---|---|---|
| Pari Compact | 3.21 ± 0.15 | 2.27 ± 0.03 | |
| Pari Compact Junior | 3.38 ± 0.19 | 2.22 ± 0.01 | |
| Pari Boy SX | Blue Pisper | 3.48 ± 0.09 | 2.21 ± 0.03 |
| Red Pisper | 2.56 ± 0.12 | 1.99 ± 0.02 | |
| Pari Junior Boy SX | 3.14 ± 0.12 | 2.10 ± 0.04 | |
| Pari Turbo Boy SX | 3.67 ± 0.20 | 2.19 ± 0.05 | |
| Microdrop Family 2 | 3.65 ± 0.07 | 2.12 ± 0.01 | |
| Microdrop Calimero 2 | Ampoule Valve MAX | 3.28 ± 0.09 | 2.19 ± 0.03 |
| Ampoule Valve MIN | 2.90 ± 0.03 | 2.22 ± 0.05 | |
| Microdrop Pro 2 | Ampoule Valve MAX | 3.14 ± 0.06 | 2.10 ± 0.02 |
| Ampoule Valve MIN | 2.84 ± 0.07 | 2.10 ± 0.05 | |
| Microdrop Pro 2 Plus | Ampoule Valve MAX | 2.47 ± 0.09 | 2.02 ± 0.03 |
| Ampoule Valve MIN | 2.14 ± 0.08 | 2.12 ± 0.02 | |
| Omron C801KD | 3.25 ± 0.15 | 2.04 ± 0.02 | |
| Omron NE-C28P | 3.63 ± 0.12 | 2.05 ± 0.03 | |
| Omron A3 Complete | Ampoule Position 1 | 6.76 ± 0.16 | 2.54 ± 0.02 |
| Ampoule Position 3 | 4.44 ± 0.06 | 2.12 ± 0.03 | |
| Midineb Nebby | 4.97 ± 0.01 | 2.16 ± 0.03 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adorni, G.; Seifert, G.; Buttini, F.; Colombo, G.; Stecanella, L.A.; Krämer, I.; Rossi, A. Aerosolization Performance of Jet Nebulizers and Biopharmaceutical Aspects. Pharmaceutics 2019, 11, 406. https://doi.org/10.3390/pharmaceutics11080406
Adorni G, Seifert G, Buttini F, Colombo G, Stecanella LA, Krämer I, Rossi A. Aerosolization Performance of Jet Nebulizers and Biopharmaceutical Aspects. Pharmaceutics. 2019; 11(8):406. https://doi.org/10.3390/pharmaceutics11080406
Chicago/Turabian StyleAdorni, Greta, Gerrit Seifert, Francesca Buttini, Gaia Colombo, Luciano A. Stecanella, Irene Krämer, and Alessandra Rossi. 2019. "Aerosolization Performance of Jet Nebulizers and Biopharmaceutical Aspects" Pharmaceutics 11, no. 8: 406. https://doi.org/10.3390/pharmaceutics11080406
APA StyleAdorni, G., Seifert, G., Buttini, F., Colombo, G., Stecanella, L. A., Krämer, I., & Rossi, A. (2019). Aerosolization Performance of Jet Nebulizers and Biopharmaceutical Aspects. Pharmaceutics, 11(8), 406. https://doi.org/10.3390/pharmaceutics11080406