Improved In Vitro Model for Intranasal Mucosal Drug Delivery: Primary Olfactory and Respiratory Epithelial Cells Compared with the Permanent Nasal Cell Line RPMI 2650
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.1.1. Primary Cells
2.1.2. RPMI 2650 Cultivation
2.1.3. TEER Measurement
2.2. Permeation
2.3. Immunofluorescence Staining
2.4. Cryosectioning and Colorimetric Staining of Cell Culture Insert Membranes
2.5. Reverse Transcription PCR (RT PCR)
2.6. Dot Blot
2.7. Western Blot
2.8. Statistics
3. Results
3.1. Evaluation of Nasal Primary Cells and RPMI 2650 Concerning Olfactory Mucosa Model Characteristica-Monolayer, Tight Growth, Cilia and Mucus Production
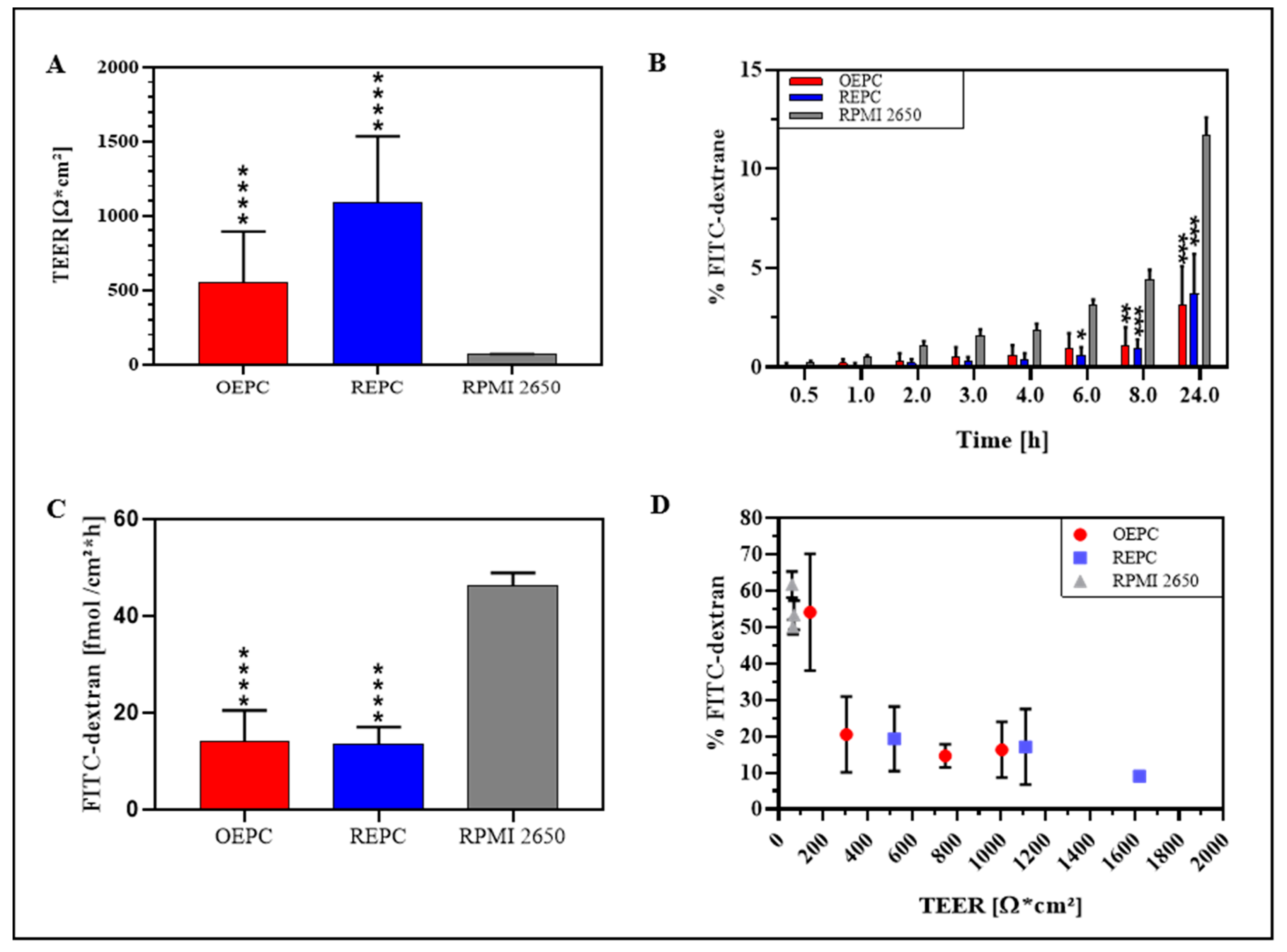
3.2. FITC-Dextran Permeation Thought RPMI 2650 and Nasal Primary Cell Barriers
4. Discussion
4.1. Comparison of OEPC and REPC: Differences in Barrier Formation and Marker Protein Expression
4.2. Primary Cell Model Evaluation: RPMI 2650 vs. Primary Nasal Cell Barrier
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Barnabas, W. Drug targeting strategies into the brain for treating neurological diseases. J. Neurosci. Methods 2019, 311, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Illum, L. Nasal drug delivery: New developments and strategies. Drug Discov. Today 2002, 7, 1184–1189. [Google Scholar] [CrossRef]
- Neuwelt, E.; Abbott, N.J.; Abrey, L.; Banks, W.A.; Blakley, B.; Engelhardt, B.; Grammas, P.; Nedergaard, M.; Nutt, J.; Pardridge, W.; et al. Strategies to advance translational research into brain barriers. Lancet Neurol. 2008, 7, 84–96. [Google Scholar] [CrossRef]
- Gao, H. Progress and perspectives on targeting nanoparticles for brain drug delivery. Acta Pharm. Sin. B 2016, 6, 268–286. [Google Scholar] [CrossRef] [PubMed]
- Illum, L. Nasal drug delivery—Possibilities, problems and solutions. J. Control. Release 2003, 87, 187–198. [Google Scholar] [CrossRef]
- Costantino, H.R.; Illum, L.; Brandt, G.; Johnson, P.H.; Quay, S.C. Intranasal delivery: Physicochemical and therapeutic aspects. Int. J. Pharm. 2007, 337, 1–24. [Google Scholar] [CrossRef]
- Lochhead, J.J.; Thorne, R.G. Intranasal delivery of biologics to the central nervous system. Adv. Drug Deliv. Rev. 2012, 64, 614–628. [Google Scholar] [CrossRef]
- Harkema, J.R.; Carey, S.A.; Wagner, J.G. The Nose Revisited: A Brief Review of the Comparative Structure, Function, and Toxicologic Pathology of the Nasal Epithelium. Toxicol. Pathol. 2006, 34, 252–269. [Google Scholar] [CrossRef]
- Gizurarson, S. Anatomical and histological factors affecting intranasal drug and vaccine delivery. Curr. Drug Deliv. 2012, 9, 566–582. [Google Scholar] [CrossRef]
- Paik, S.; Lehman, M.; Seiden, A.M.; Duncan, H.J. Olfactory Biopsy. Arch. Otolaryngol. Head Neck Surg. 1992, 118, 731–738. [Google Scholar] [CrossRef]
- Marttin, E.; Schipper, N.G.M.; Coos Verhoef, J.; Merkus, F.W.H.M. Nasal mucociliary clearance as a factor in nasal drug delivery. Adv. Drug Deliv. Rev. 1998, 29, 13–38. [Google Scholar] [CrossRef]
- Graziadei, P.P.; Graziadei, G.M. Neurogenesis and neuron regeneration in the olfactory system of mammals. I. Morphological aspects of differentiation and structural organization of the olfactory sensory neurons. J. Neurocytol 1979, 8, 1–18. [Google Scholar] [CrossRef]
- Morrison, E.E.; Costanzo, R.M. Morphology of olfactory epithelium in humans and other vertebrates. Microsc. Res. Tech. 1992, 23, 49–61. [Google Scholar] [CrossRef]
- Mittal, D.; Ali, A.; Md, S.; Baboota, S.; Sahni, J.K.; Ali, J. Insights into direct nose to brain delivery: Current status and future perspective. Drug Deliv. 2014, 21, 75–86. [Google Scholar] [CrossRef]
- Jafek, B.W. Ultrastructure of human nasal mucosa. Laryngoscope 1983, 93, 1576–1599. [Google Scholar] [CrossRef]
- Anton, F.; Peppel, P. Central projections of trigeminal primary afferents innervating the nasal mucosa: A horseradish peroxidase study in the rat. Neuroscience 1991, 41, 617–628. [Google Scholar] [CrossRef]
- Deatly, A.M.; Haase, A.T.; Fewster, P.H.; Lewis, E.; Ball, M.J. Human herpes virus infections and Alzheimer’s disease. Neuropathol. Appl. Neurobiol. 1990, 16, 213–223. [Google Scholar] [CrossRef]
- Balin, B.J.; Broadwell, R.D.; Salcmant, M.; El-Kalliny, M. Entry of peripherally administered protein to the CNS in mouse, rat and squirrel monkey. J. Comp. Neurol. 1986, 251, 260–280. [Google Scholar] [CrossRef]
- Bahadur, S.; Pathak, K. Physicochemical and physiological considerations for efficient nose-to-brain targeting. Expert Opin. Drug Deliv. 2011, 9, 19–31. [Google Scholar] [CrossRef]
- Wengst, A.; Reichl, S. RPMI 2650 epithelial model and three-dimensional reconstructed human nasal mucosa as in vitro models for nasal permeation studies. Eur. J. Pharm. Biopharm. 2010, 74, 290–297. [Google Scholar] [CrossRef]
- Gonçalves, V.S.S.; Matias, A.A.; Poejo, J.; Serra, A.T.; Duarte, C.M.M. Application of RPMI 2650 as a cell model to evaluate solid formulations for intranasal delivery of drugs. Int. J. Pharm. 2016, 515, 1–10. [Google Scholar] [CrossRef]
- Kürti, L.; Veszelka, S.; Bocsik, A.; Dung, N.T.K.; Ózsvári, B.; Puskás, L.G.; Kittel, Á.; Szabó-Révész, P.; Deli, M.A. The effect of sucrose esters on a culture model of the nasal barrier. Toxicol. Vitr. 2012, 26, 445–454. [Google Scholar] [CrossRef]
- Nakamura, K.; Maitani, Y.; Takayama, K. The enhancing effect of nasal absorption of FITC-dextran 4400 by β-sitosterol β-D-glucoside in rabbits. J. Control. Release 2002, 79, 147–155. [Google Scholar] [CrossRef]
- Grainger, C.I.; Greenwell, L.L.; Martin, G.P.; Forbes, B. The permeability of large molecular weight solutes following particle delivery to air-interfaced cells that model the respiratory mucosa. Eur. J. Pharm. Biopharm. 2009, 71, 318–324. [Google Scholar] [CrossRef]
- Nicolazzo, J.A.; Reed, B.L.; Finnin, B.C. The Effect of Various in Vitro Conditions on the Permeability Characteristics of the Buccal Mucosa. J. Pharm. Sci. 2003, 92, 2399–2410. [Google Scholar] [CrossRef]
- Schmidt, M.C.; Peter, H.; Lang, S.R.; Ditzinger, G.; Merkle, H.P. In vitro cell models to study nasal mucosal permeability and metabolism. Adv. Drug Deliv. Rev. 1998, 29, 51–79. [Google Scholar] [CrossRef]
- Kim, K.-J.; Malik, A.B. Protein transport across the lung epithelial barrier. Am. J. Physiol. Cell. Mol. Physiol. 2015, 284, L247–L259. [Google Scholar] [CrossRef]
- Moran, D.T.; Rowley, J.C., 3rd; Jafek, B.W. Electron microscopy of human olfactory epithelium reveals a new cell type: The microvillar cell. Brain Res. 1982, 253, 39–46. [Google Scholar] [CrossRef]
- Wadell, C.; Björk, E.; Camber, O. Nasal drug delivery—Evaluation of an in vitro model using porcine nasal mucosa. Eur. J. Pharm. Sci. 1999, 7, 197–206. [Google Scholar] [CrossRef]
- Samson, G.; De La Calera, A.G.; Dupuis-Girod, S.; Faure, F.; Decullier, E.; Paintaud, G.; Vignault, C.; Scoazec, J.Y.; Pivot, C.; Plauchu, H.; et al. Ex vivo study of bevacizumab transport through porcine nasal mucosa. Eur. J. Pharm. Biopharm. 2012, 80, 465–469. [Google Scholar] [CrossRef]
- Ladel, S.; Flamm, J.; Zadeh, A.S.; Filzwieser, D.; Walter, J.C.; Schlossbauer, P.; Kinscherf, R.; Lischka, K.; Luksch, H.; Schindowski, K. Allogenic fc domain-facilitated uptake of IgG in nasal Lamina propria: Friend or foe for intranasal CNS delivery? Pharmaceutics 2018, 10, 107. [Google Scholar] [CrossRef]
- Glorieux, S.; Van den Broeck, W.; van der Meulen, K.M.; Van Reeth, K.; Favoreel, H.W.; Nauwynck, H.J. In vitro culture of porcine respiratory nasal mucosa explants for studying the interaction of porcine viruses with the respiratory tract. J. Virol. Methods 2007, 142, 105–112. [Google Scholar] [CrossRef]
- Tulinski, P.; Fluit, A.C.; van Putten, J.P.M.; de Bruin, A.; Glorieux, S.; Wagenaar, J.A.; Duim, B. An Ex Vivo Porcine Nasal Mucosa Explants Model to Study MRSA Colonization. PLoS ONE 2013, 8, e53783. [Google Scholar] [CrossRef]
- Moore, G.E.; Sandberg, A.A. Studies of a human tumor cell line with a diploid karyotype. Cancer 1964, 17, 170–175. [Google Scholar] [CrossRef]
- Moorhead, P.S. Human tumor cell line with a quasi-diploid karyotype (RPMI 2650). Exp. Cell Res. 1965, 39, 190–196. [Google Scholar] [CrossRef]
- Hayflick, L.; Moorhead, P.S. The serial cultivation of human cell strains. Exp. Cell Res. 1961, 621, 585–621. [Google Scholar] [CrossRef]
- Mercier, C.; Perek, N.; Delavenne, X. Is RPMI 2650 a Suitable In Vitro Nasal Model for Drug Transport Studies? Eur. J. Drug Metab. Pharmacokinet. 2018, 43, 13–24. [Google Scholar] [CrossRef]
- Na, K.; Lee, M.; Shin, H.; Chung, S. In vitro nasal mucosa gland-like structure formation on a chip. Lab Chip 2017. [Google Scholar] [CrossRef]
- Mercier, C.; Hodin, S.; He, Z.; Perek, N.; Delavenne, X. Pharmacological Characterization of the RPMI 2650 Model as a Relevant Tool for Assessing the Permeability of Intranasal Drugs. Mol. Pharm. 2018, 15, 2246–2256. [Google Scholar] [CrossRef]
- Mercier, C.; Jacqueroux, E.; He, Z.; Hodin, S.; Perek, N.; Boudard, D.; Delavenne, X.; Constant, S. Pharmacological characterization of the 3D MucilAir™ nasal model. Eur. J. Pharm. Biopharm. 2019, 139, 186–196. [Google Scholar] [CrossRef]
- Moll, R.; Krepler, R.; Franke, W.W. Complex Cytokeratin Polypeptide Patterns Observed in Certain Human Carcinomas. Differentiation 1982, 23, 256–269. [Google Scholar] [CrossRef]
- Peter, H.G. Cell Culture Sheets to Study Nasal Peptide Metabolism the Human Nasal RPMI 2650 Cell Line Model; ETH Zürich: Zürich, Switzerland, 1996. [Google Scholar]
- Stratford, R.E.; Lee, V.H.L. Aminopeptidase activity in homogenates of various absorptive mucosae m the albino rabbit: Implications in peptide delivery. Int. J. Pharm. 1986, 30, 73–82. [Google Scholar] [CrossRef]
- Tchao, R. Epithelial cell interaction in air-liquid interface culture. Vitro Cell. Dev. Biol. 1989, 25, 460–465. [Google Scholar] [CrossRef]
- de Jong, P.M.; van Sterkenburg, M.A.; Hesseling, S.C.; Kempenaar, J.A.; Mulder, A.A.; Mommaas, A.M.; Dijkman, J.H.; Ponec, M. Ciliogenesis in human bronchial epithelial cells cultured at the air-liquid interface. Am. J. Respir. Cell Mol. Biol. 1994, 10, 271–277. [Google Scholar] [CrossRef]
- Lee, M.K.; Yoo, J.W.; Lin, H.; Kim, Y.S.; Kim, D.D.; Choi, Y.M.; Park, S.K.; Lee, C.H.; Roh, H.J. Air-liquid interface culture of serially passaged human nasal epithelial cell monolayer for in vitro drug transport studies. Drug Deliv. J. Deliv. Target. Ther. Agents 2005, 12, 305–311. [Google Scholar] [CrossRef]
- Pezzulo, A.A.; Starner, T.D.; Scheetz, T.E.; Traver, G.L.; Tilley, A.E.; Harvey, B.-G.; Crystal, R.G.; McCray, P.B.; Zabner, J. The air-liquid interface and use of primary cell cultures are important to recapitulate the transcriptional profile of in vivo airway epithelia. Am. J. Physiol. Cell. Mol. Physiol. 2010, 300, L25–L31. [Google Scholar] [CrossRef]
- Chen, S.; Einspanier, R.; Schoen, J. Transepithelial electrical resistance (TEER): A functional parameter to monitor the quality of oviduct epithelial cells cultured on filter supports. Histochem. Cell Biol. 2015, 144, 509–515. [Google Scholar] [CrossRef]
- Van Itallie, C.M.; Anderson, J.M. Claudins and Epithelial Paracellular Transport. Annu. Rev. Physiol. 2005, 68, 403–429. [Google Scholar] [CrossRef]
- Flamm, J.; Boscher, M.; Maigler, F.; Akana, C.; Lindemann, J.; Kleiner, S.; Sommer, F.; Schindowski, K. Standardized refined intranasal admini- stration for region-specific intranasal drug deposition in mice established with 3D rapid prototypes under 3R criteria. Berl. Munch. Tierarztl. Wochenschr. 2018, 131, 408–416. [Google Scholar] [CrossRef]
- Röhm, M.; Carle, S.; Maigler, F.; Flamm, J.; Kramer, V.; Mavoungou, C.; Schmid, O.; Schindowski, K.; Schmid, O.; Mavoungou, C.; et al. A comprehensive screening platform for aerosolizable protein formulations for intranasal and pulmonary drug delivery. Int. J. Pharm. 2017, 532, 537–546. [Google Scholar] [CrossRef]
- Nieder, B.; Wagner, H.; Luksch, H. Development of output connections from the inferior colliculus to the optic tectum in barn owls. J. Comp. Neurol. 2003, 464, 511–524. [Google Scholar] [CrossRef]
- Troxler, R.F.; Offner, G.D.; Nunes, D.P.; Oppenheim, F.G.; Iontcheva, I. Molecular characterization of a major high molecular weight mucin from human sublingual gland. Glycobiology 2007, 7, 965–973. [Google Scholar] [CrossRef][Green Version]
- Shah, A.S.; Ben-Shahar, Y.; Moninger, T.O.; Kline, J.N.; Welsh, M.J. Motile Cilia of Human Airway Epithelia Are Chemosensory. Science 2009, 325, 1131–1134. [Google Scholar] [CrossRef]
- Heijink, I.H.; Brandenburg, S.M.; Noordhoek, J.A.; Postma, D.S.; Slebos, D.J.; Van Oosterhout, A.J.M. Characterisation of cell adhesion in airway epithelial cell types using electric cell-substrate impedance sensing. Eur. Respir. J. 2010, 35, 894–903. [Google Scholar] [CrossRef]
- Crowe, T.P.; Greenlee, M.H.W.; Kanthasamy, A.G.; Hsu, W.H. Mechanism of intranasal drug delivery directly to the brain. Life Sci. 2018, 195, 44–52. [Google Scholar] [CrossRef]
- Baker, H.; Spencer, R.F. Transneuronal transport of peroxidase-conjugated wheat germ agglutinin (WGA-HRP) from the olfactory epithelium to the brain of the adult rat. Exp. Brain Res. 1986, 63, 461–473. [Google Scholar] [CrossRef]
- Jorissen, M.; Van Der Schueren, B.; Van Den Berghe, H.; Cassiman, J.J. Contribution of in vitro culture methods for respiratory epithelial cells to the study of the physiology of the respiratory tract. Eur. Respir. J. 1991, 4, 210–217. [Google Scholar]
- Bhowmick, R.; Gappa-Fahlenkamp, H. Cells and Culture Systems Used to Model the Small Airway Epithelium. Lung 2016, 194, 419–428. [Google Scholar] [CrossRef]
- Even-Tzur, N.; Jaffa, A.; Gordon, Z.; Gottlieb, R.; Kloog, Y.; Einav, S.; Wolf, M.; Elad, D. Air-liquid interface culture of nasal epithelial cells on denuded amniotic membranes. Cell. Mol. Bioeng. 2010, 3, 307–318. [Google Scholar] [CrossRef]
- Jeliazkova-Mecheva, V.V.; Bobilya, D.J. A porcine astrocyte/endothelial cell co-culture model of the blood-brain barrier. Brain Res. Protoc. 2003, 12, 91–98. [Google Scholar] [CrossRef]
- Pardeshi, C.V.; Belgamwar, V.S. Direct nose to brain drug delivery via integrated nerve pathways bypassing the blood–brain barrier: An excellent platform for brain targeting. Expert Opin. Drug Deliv. 2013, 10, 957–972. [Google Scholar] [CrossRef]
- Rath, T.; Kuo, T.T.; Baker, K.; Qiao, S.W.; Kobayashi, K.; Yoshida, M.; Roopenian, D.; Fiebiger, E.; Lencer, W.I.; Blumberg, R.S. The immunologic functions of the neonatal FC receptor for IGG. J. Clin. Immunol. 2013, 33, 9–17. [Google Scholar] [CrossRef]
- Gänger, S.; Schindowski, K. Tailoring formulations for intranasal Nose-to-Brain delivery via the olfactory area: A review on physico-chemical characteristics and mucociliary clearance of the nasal olfactory mucosa. Pharmaceutics 2018, 10, 116. [Google Scholar] [CrossRef]
- Dolberg, A.M.; Reichl, S. Expression of P-glycoprotein in excised human nasal mucosa and optimized models of RPMI 2650 cells. Int. J. Pharm. 2016, 508, 22–33. [Google Scholar] [CrossRef]
- Berger, J.T.; Voynow, J.A.; Peters, K.W.; Rose, M.C. Respiratory carcinoma cell lines MUC genes and glycoconjugates. Am. J. Respir. Cell Mol. Biol. 1999, 20, 500–510. [Google Scholar] [CrossRef]
- Kreft, M.E.; Lasi, E.; Kristan, K. The Characterization of the Human Nasal Epithelial Cell Line RPMI 2650 Under Different Culture Conditions and Their Optimization for an Appropriate in vitro Nasal Model. Pharm. Res. 2015, 32, 665–679. [Google Scholar] [CrossRef]
- Srinivasan, B.; Kolli, A.R.; Esch, M.B.; Abaci, H.E.; Shuler, L.; Hickman, J.J.; Srinivasan, B.; Kolli, A.R.; Esch, M.B.; Abaci, H.E.; et al. TEER Measurement Techniques for in vitro barrier model systems. J. Lab. Autom. 2016, 20, 107–126. [Google Scholar] [CrossRef]
- Engström, B.; Ekblom, A.; Hansson, P. The Olfactory and Respiratory Epithelium in Rhesus and Squirrel Monkeys Studied with Freeze-fracture Technique. Acta Otolaryngol. 2009, 108, 259–267. [Google Scholar] [CrossRef]
- Holcomb, J.D.; Graham, S.; Calof, A.L. Neuronal homeostasis in mammalian olfactory epithelium: A review. Am. J. Rhinol. 1996, 10, 125–134. [Google Scholar] [CrossRef]
- Yoo, J.-W.; Kim, Y.-S.; Lee, S.-H.; Lee, M.-K.; Roh, H.-J.; Jhun, B.-H.; Lee, C.-H.; Kim, D.-D. Serially passaged human nasal epithelial cell monolayer for in vitro drug transport studies. Pharm. Res. 2003, 20, 1690–1696. [Google Scholar] [CrossRef]
- Kim, N.; Hee, D.; Suh, M.; Ho, J.; Oh, S.; Kyun, M. Effect of lipopolysaccharide on diesel exhaust particle-induced junctional dysfunction in primary human nasal epithelial cells. Environ. Pollut. 2019, 248, 736–742. [Google Scholar] [CrossRef]
- Farshori, P.; Kachar, B. Redistribution and Phosphorylation of Occludin During Opening and Resealing of Tight Junctions in Cultured Epithelial Cells. J. Membr. Biol. 1999, 170, 147–156. [Google Scholar] [CrossRef]
- Bhat, M.; Toledo-Velasquez, D.; Wang, L.; Malanga, C.J.; Ma, J.K.; Rojanasakul, Y. Regulation of tight junction permeability by calcium mediators and cell cytoskeleton in rabbit tracheal epithelium. Pharm. Res. 1993, 10, 991–997. [Google Scholar] [CrossRef]
- Piperno, G.; Fuller, M.T. Monoclonal antibodies specific for an acetylated form of alpha-tubulin recognize the antigen in cilia and flagella from a variety of organisms. J. Cell Biol. 1985, 101, 2085–2094. [Google Scholar] [CrossRef]
- Quinones, G.B.; Danowski, B.A.; Devaraj, A.; Singh, V.; Ligon, L.A. The posttranslational modification of tubulin undergoes a switch from detyrosination to acetylation as epithelial cells become polarized. Mol. Biol. Cell 2011, 22, 1045–1057. [Google Scholar] [CrossRef]
- Zholos, A.V.; Atherton-Watson, H.; Elborn, J.S.; Ennis, M.; de Courcey, F.; Danahay, H.L.; Canning, P.; Williams, M.T.S. Development of primary human nasal epithelial cell cultures for the study of cystic fibrosis pathophysiology. Am. J. Physiol. Physiol. 2012, 303, C1173–C1179. [Google Scholar] [CrossRef]
- Jorissen, M.; Bessems, A. Normal ciliary beat frequency after ciliogenesis in nasal epithelial cells cultured sequentially as monolayer and in suspension. Acta Otolaryngol. 1995, 115, 66–70. [Google Scholar] [CrossRef]
- Salathe, M.; Bookman, R.J. Coupling of [Ca2+] i and ciliary beating in cultured tracheal epithelial cells. J. Cell Sci. 1995, 108, 431–440. [Google Scholar]
- Yager, J.; Chen, T.M.; Dulfano, M.J. Measurement of frequency of ciliary beats of human respiratory epithelium. Chest 1978, 73, 627–633. [Google Scholar] [CrossRef]
- Bateman, A.C.; Karasin, A.I.; Olsen, C.W. Differentiated swine airway epithelial cell cultures for the investigation of influenza A virus infection and replication. Influ. Other Respir. Viruses 2013, 7, 139–150. [Google Scholar] [CrossRef]
- Delgado-Ortega, M.; Olivier, M.; Sizaret, P.-Y.; Simon, G.; Meurens, F. Newborn pig trachea cell line cultured in air-liquid interface conditions allows a partial in vitro representation of the porcine upper airway tissue. BMC Cell Biol. 2014, 15, 14. [Google Scholar] [CrossRef]
- Getchell, M.L.; Getchell, T.V. Fine structural aspects of secretion and extrinsic innervation in the olfactory mucosa. Microsc. Res. Tech. 1992, 23, 111–127. [Google Scholar] [CrossRef]
- Solbu, T.T.; Holen, T. Aquaporin pathways and mucin secretion of bowman’s glands might protect the olfactory mucosa. Chem. Senses 2012, 37, 35–46. [Google Scholar] [CrossRef]
- Kim, C.-H.; Song, K.S.; Kim, S.-S.; Kim, H.-U.; Seong, J.-K.; Yoon, J.-H. Expression of MUC5AC mRNA in the Goblet Cells of Human Nasal Mucosa. Laryngoscope 2000, 110, 2110–2113. [Google Scholar] [CrossRef]
- Aust, M.R.; Madsen, C.S.; Jennings, A.; Kasperbauer, J.L.; Gendler, S.J. Mucin mRNA Expression in Normal and Vasomotor Inferior Turbinates. Am. J. Rhinol. 1997, 11, 293–302. [Google Scholar] [CrossRef]
- Kennel, C.; Gould, E.A.; Larson, E.D.; Salcedo, E.; Vickery, T.W.; Restrepo, D.; Ramakrishnan, V.R. Differential Expression of Mucins in Murine Olfactory Versus Respiratory Epithelium. bioRxiv 2019, 1–32. [Google Scholar] [CrossRef]
- Reichl, S.; Becker, K. Cultivation of RPMI 2650 cells as an in-vitro model for human transmucosal nasal drug absorption studies: Optimization of selected culture conditions. J. Pharm. Pharmacol. 2012, 64, 1621–1630. [Google Scholar] [CrossRef]
- Pozzoli, M.; Sonvico, F.; Ong, H.X.; Traini, D.; Bebawy, M.; Young, P.M. Optimization of RPMI 2650 Cells as a Model for Nasal Mucosa. Respir. Drug 2014, 2, 739–742. [Google Scholar]
- De Fraissinette, A.; Brun, R.; Felix, H.; Vonderscher, J.; Rummelt, A. Evaluation of the human cell line RPMI 2650 as an in vitro nasal model. Rhinology 1995, 33, 194–198. [Google Scholar]
- Werner, U.; Kissel, T. In-vitro cell culture models of the nasal epithelium: A comparative histochemical investigation of their suitability for drug transport studies. Pharm. Res. 1996, 13, 978–988. [Google Scholar] [CrossRef]
- Yoon, J.-H.; Moon, H.-J.; Seong, J.-K.; Kim, C.-H.; Lee, J.-J.; Choi, J.Y.; Song, M.S.; Kim, S.-H. Mucociliary differentiation according to time in human nasal epithelial cell culture. Differentiation 2002, 70, 77–83. [Google Scholar] [CrossRef][Green Version]
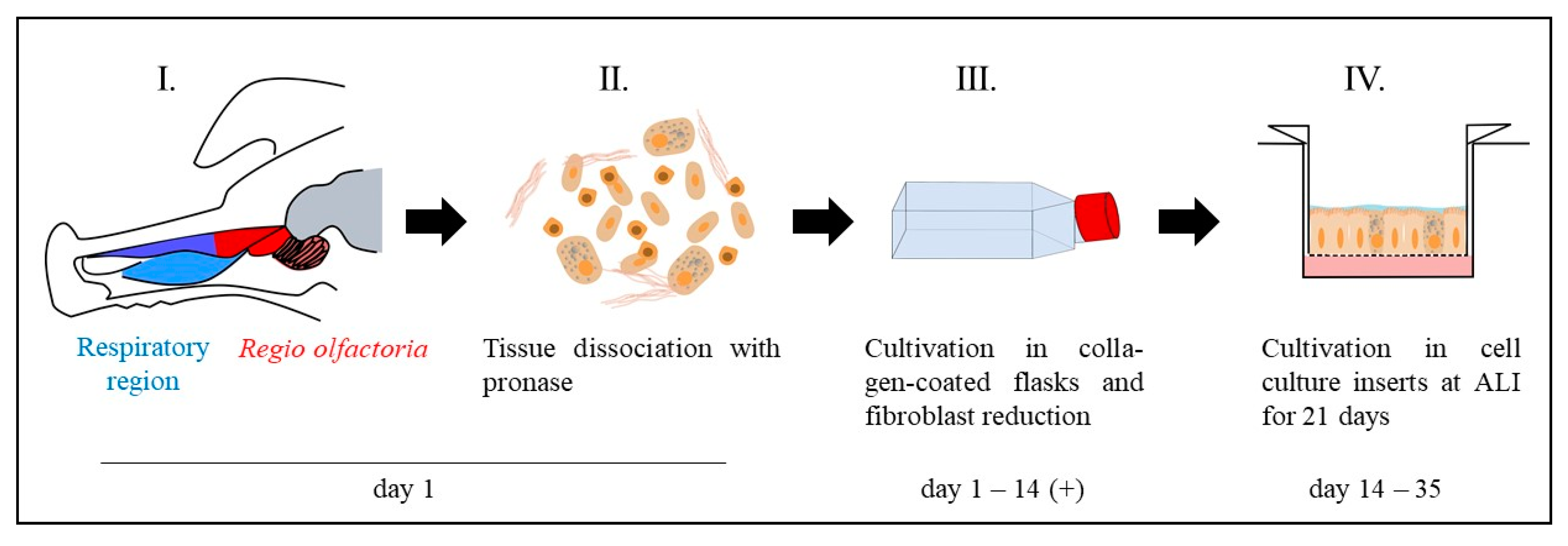
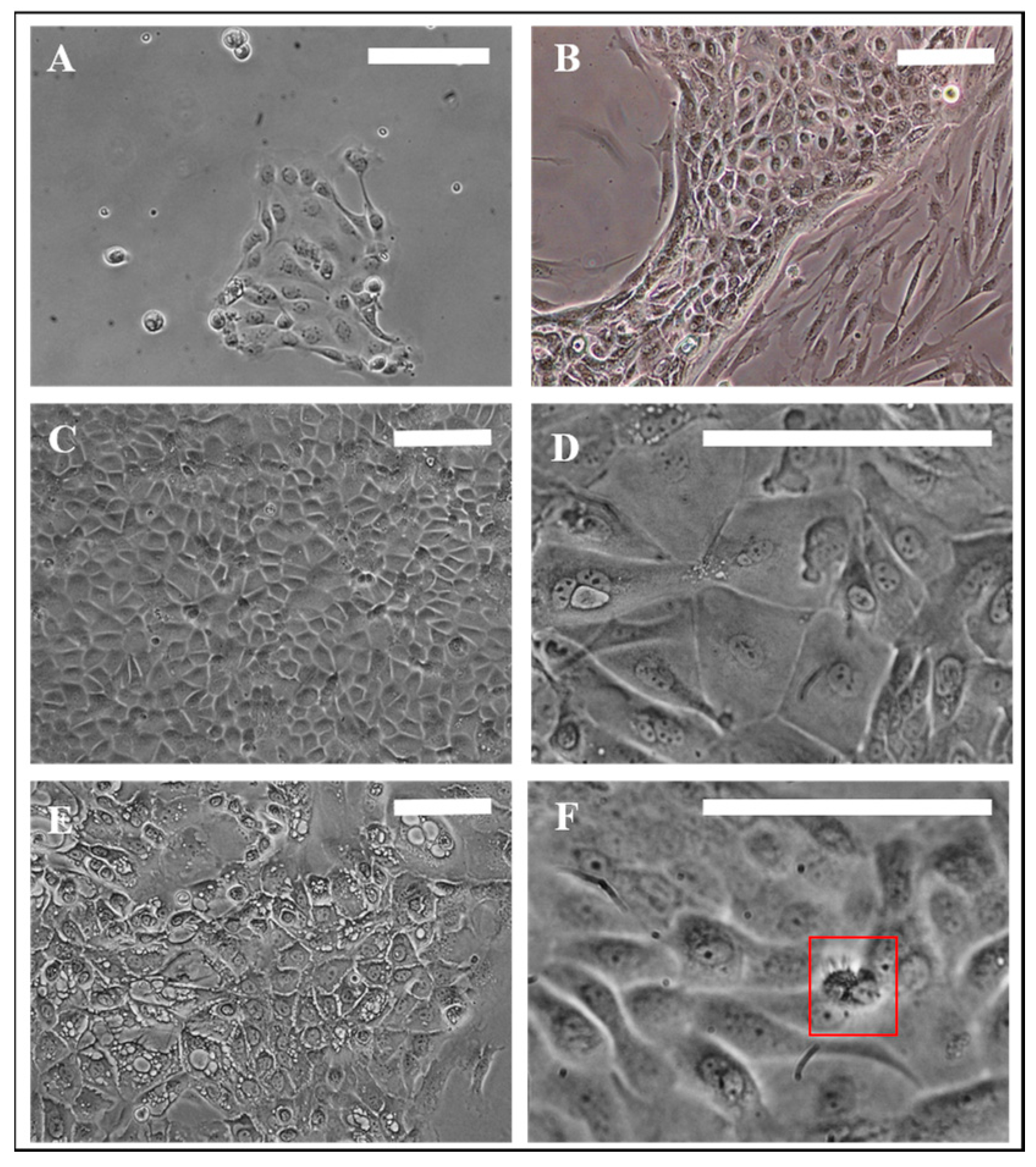
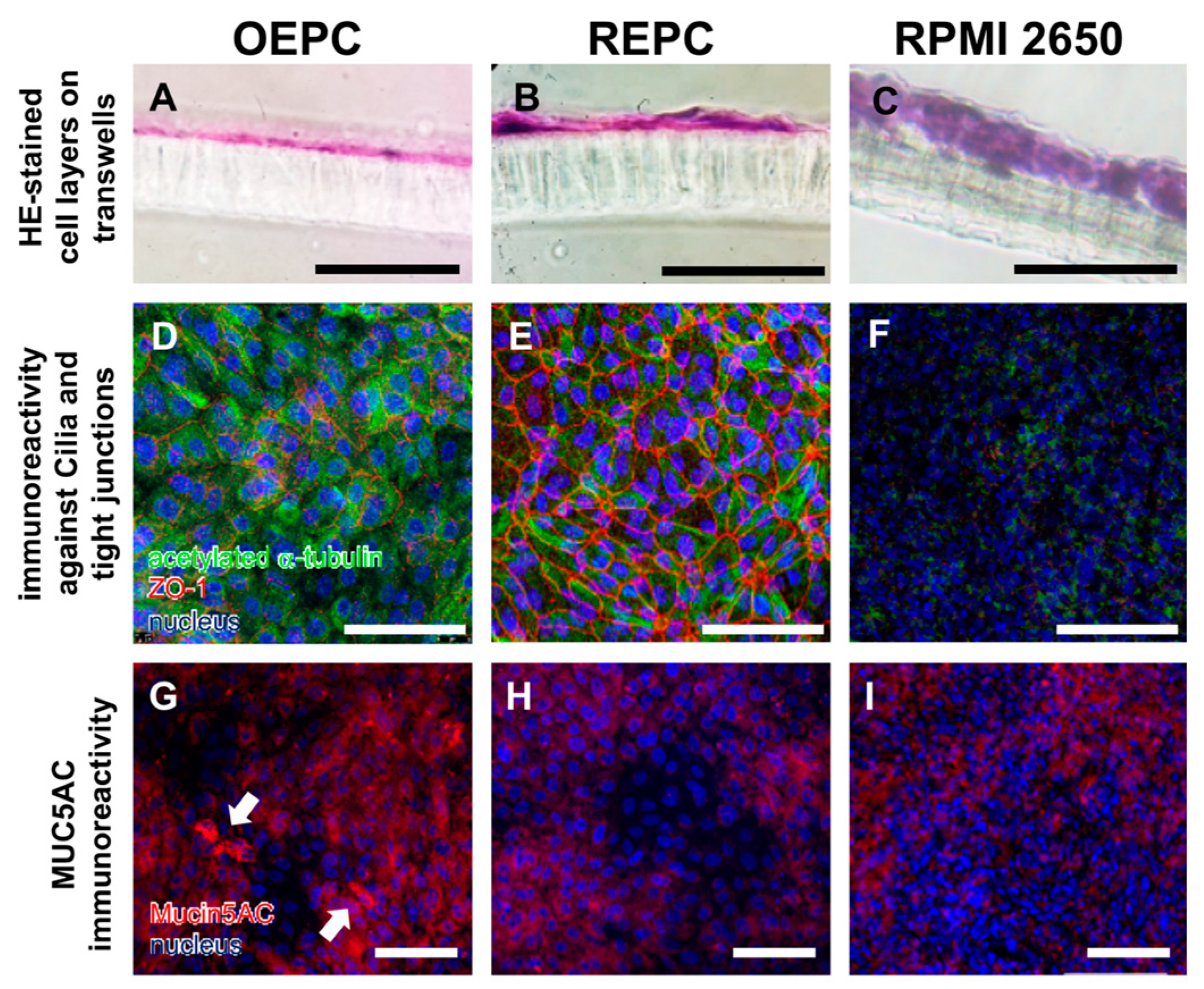
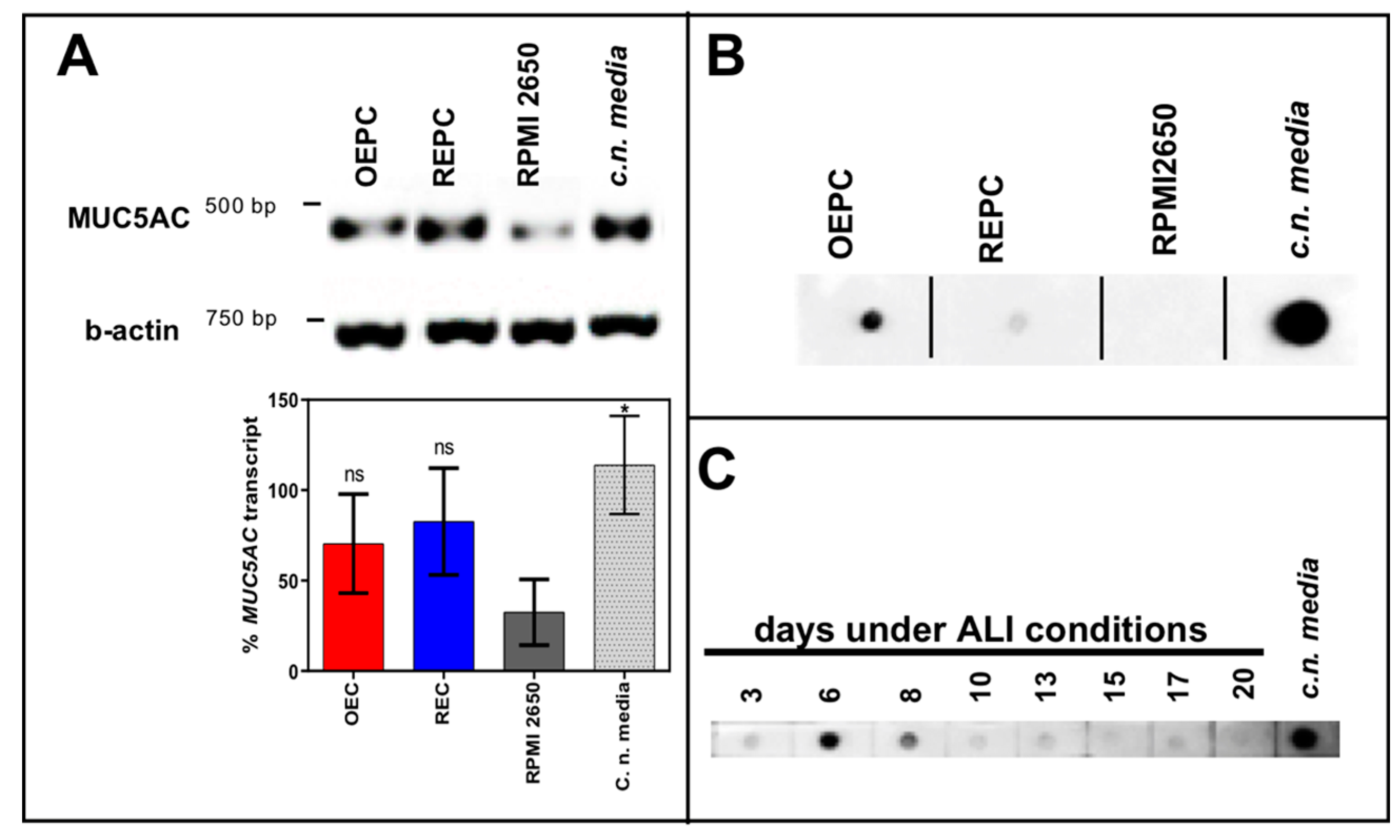
| Name | Composition |
|---|---|
| Primary culture adhesion medium | DMEM:F12 (1:1), 20% FBS, 2 mM Gln, 1% NEAA, 0.4 U/mL Penicillin-0.4 µg/mL Streptomycin, 0.6 I.U Gentamycinsulfate |
| Primary culture medium | DMEM:F12 (1:1), 10% FBS, 2 mM Gln, 1% NEAA, 0.4 U/mL Penicillin-0.4 µg/mL Streptomycin, 0.6 I.U Gentamycinsulfate |
| Pronase medium | EBSS + 1.4 mg/mL Pronase + 0.4 U/mL Penicillin-0.4 µg/mL Streptomycin, 0.6 I.U Gentamycinsulfate |
| RPMI 2650 medium | MEM, 10% FBS, 2 mM Gln, 0.4 U/mL Penicillin-0.4 µg/mL Streptomycin |
| Antibody | Antigen | Immunogen | Host | Source, Cat. # |
|---|---|---|---|---|
| Anti-MUC5AC Antibody (45M1) | Peptide core of gastric mucin M1 (Mucin 5AC) | M1 mucin | mouse | Novus biologicals, Centennial, CO, USA, Cat. #NBP2-15196 |
| Anti-ZO-1 (ZMD. 437) | ZO-1 | synthetic peptide derived from the N-terminal region of human, dog, mouse, and rat ZO-1 | rabbit | Thermo Fisher Scientific, Dreieich, Germany, Cat. #40-2300 |
| Anti-acetylated tubulin (6-11B-1) | Acetylated tubulin | acetylated tubulin from the outer arm of Strongylocentrotus purpuratus | mouse | Sigma Aldrich, Taufkirchen, Germany, Cat. #T7451 |
| Anti-β Actin (AC-15) | β Actin | not specified | mouse | Sigma Aldrich, Taufkirchen, Germany, Cat. #A5441 |
| Anti-murine IgG-Alexa Fluor® 488 | whole molecule mouse IgG | not specified | Goat | Jackson Immuno Research Europe Ltd., Cambridgeshire, UK, Cat. #115-545-003 |
| Anti-rabbit IgG-Rhodamine Red™-X | whole molecule rabbit IgG | not specified | donkey | Jackson Immuno Research Europe Ltd., Cambridgeshire, UK, Cat. #711-295-152 |
| Anti-murine IgG-HRP | whole molecule mouse IgG | not specified | goat | Sigma Aldrich, Taufkirchen, Germany, Cat. #AP5278 |
| Anti-rabbit IgG-HRP | Whole molecule rabbit IgG | not specified | Goat | Jackson Immuno Research Europe Ltd., Cambridgeshire, UK, Cat. #111-035-003 |
| mRNA Targets | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| MUC5AC | CCGGGCCTGTGCAACTA | GTTCCCAAACTCGATAGGGC |
| β-actin | GACACCAGGGCGTGATGG | GCAGCTCGTAGCTCTTCTCC |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ladel, S.; Schlossbauer, P.; Flamm, J.; Luksch, H.; Mizaikoff, B.; Schindowski, K. Improved In Vitro Model for Intranasal Mucosal Drug Delivery: Primary Olfactory and Respiratory Epithelial Cells Compared with the Permanent Nasal Cell Line RPMI 2650. Pharmaceutics 2019, 11, 367. https://doi.org/10.3390/pharmaceutics11080367
Ladel S, Schlossbauer P, Flamm J, Luksch H, Mizaikoff B, Schindowski K. Improved In Vitro Model for Intranasal Mucosal Drug Delivery: Primary Olfactory and Respiratory Epithelial Cells Compared with the Permanent Nasal Cell Line RPMI 2650. Pharmaceutics. 2019; 11(8):367. https://doi.org/10.3390/pharmaceutics11080367
Chicago/Turabian StyleLadel, Simone, Patrick Schlossbauer, Johannes Flamm, Harald Luksch, Boris Mizaikoff, and Katharina Schindowski. 2019. "Improved In Vitro Model for Intranasal Mucosal Drug Delivery: Primary Olfactory and Respiratory Epithelial Cells Compared with the Permanent Nasal Cell Line RPMI 2650" Pharmaceutics 11, no. 8: 367. https://doi.org/10.3390/pharmaceutics11080367
APA StyleLadel, S., Schlossbauer, P., Flamm, J., Luksch, H., Mizaikoff, B., & Schindowski, K. (2019). Improved In Vitro Model for Intranasal Mucosal Drug Delivery: Primary Olfactory and Respiratory Epithelial Cells Compared with the Permanent Nasal Cell Line RPMI 2650. Pharmaceutics, 11(8), 367. https://doi.org/10.3390/pharmaceutics11080367







