Influenza Virus-Like Particles Presenting both Toxoplasma gondii ROP4 and ROP13 Enhance Protection against T. gondii Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Parasites and Cells
2.2. Generation of T. gondii VLPs
2.3. Characterization of T. gondii VLPs
2.4. Immunization and Challenge Infection
2.5. T. gondii-Specific Antibodies Response in Serum
2.6. Mouse Sample Collection
2.7. Analysis of Antibody Secreting Cells and Cytokines
2.8. Flow Cytometry
2.9. Purification of T. gondii ME49 Cyst
2.10. Statistical Analysis
3. Results
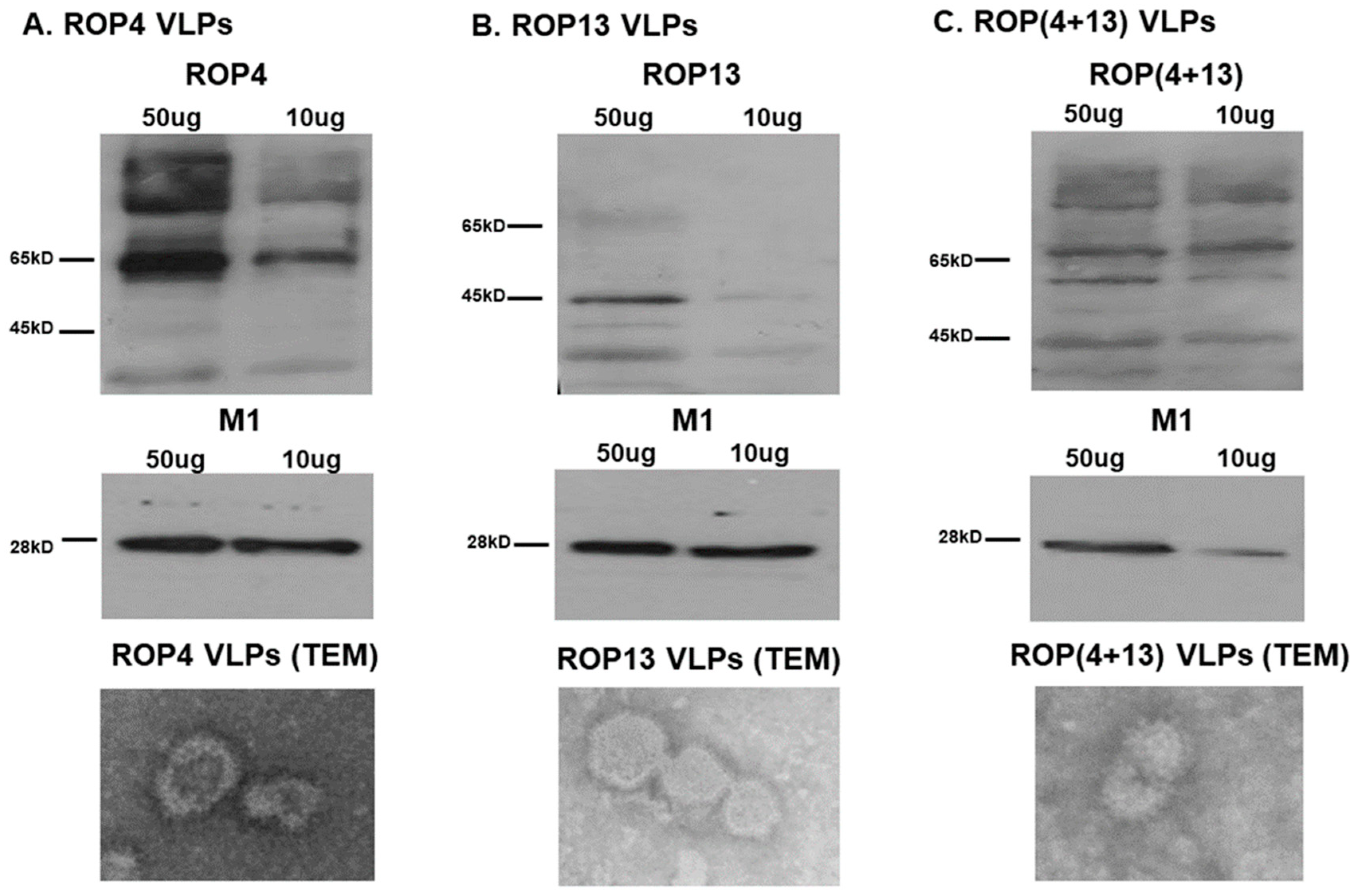
3.1. Characterization of VLPs
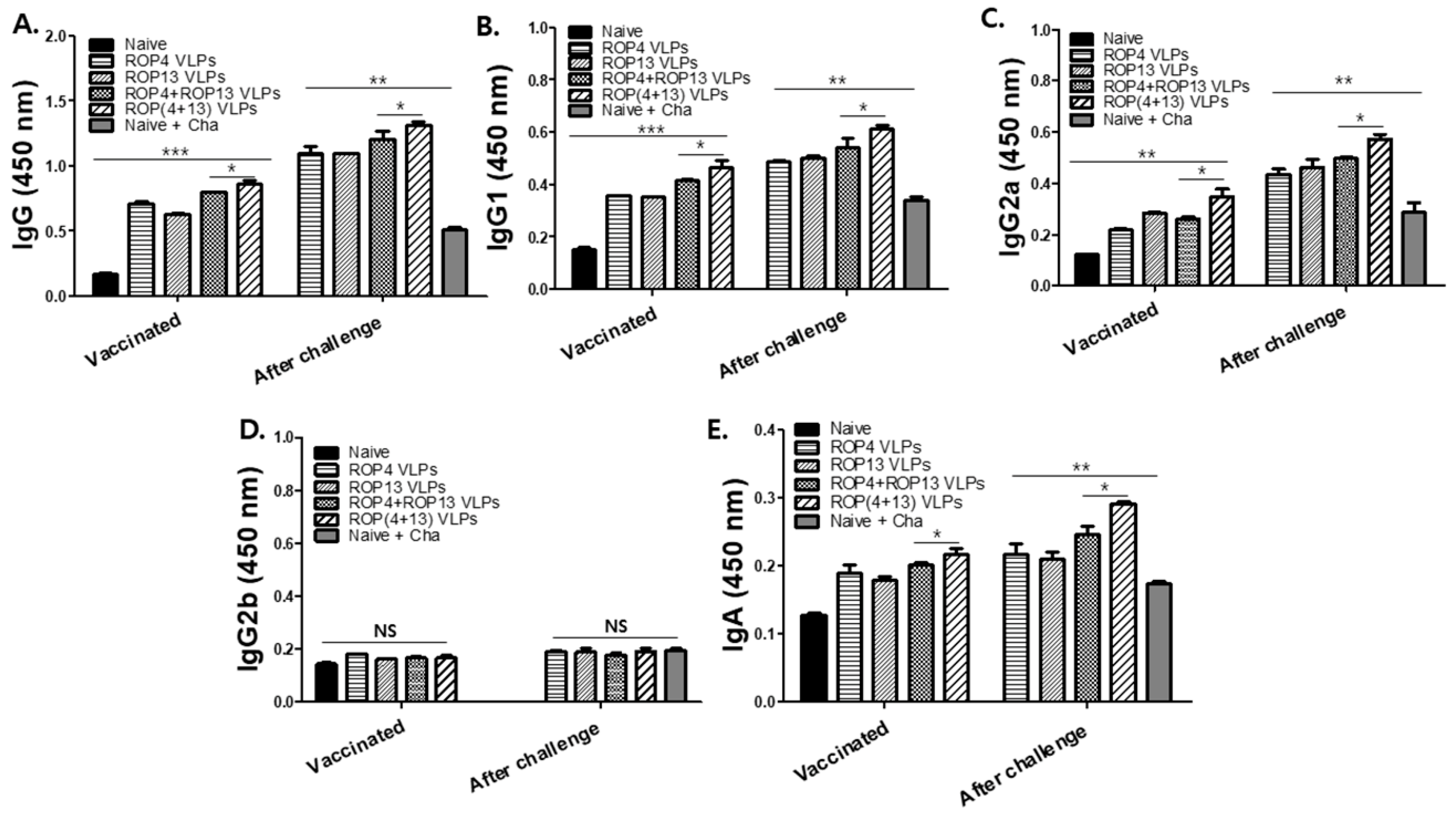
3.2. T. gondii-Specific Antibody Response in Serum
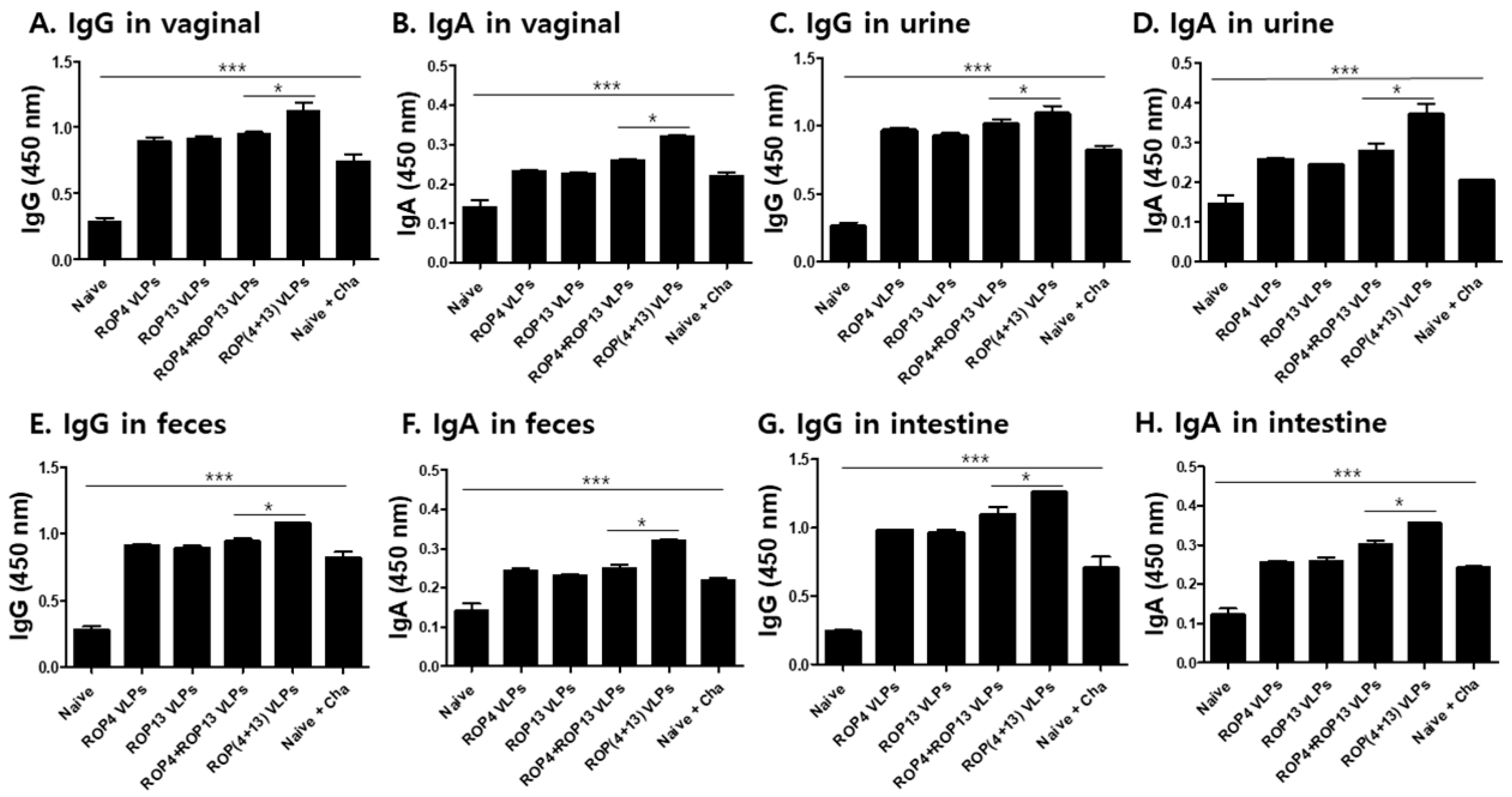
3.3. IgG and IgA Antibody Responses in Mucosal Samples
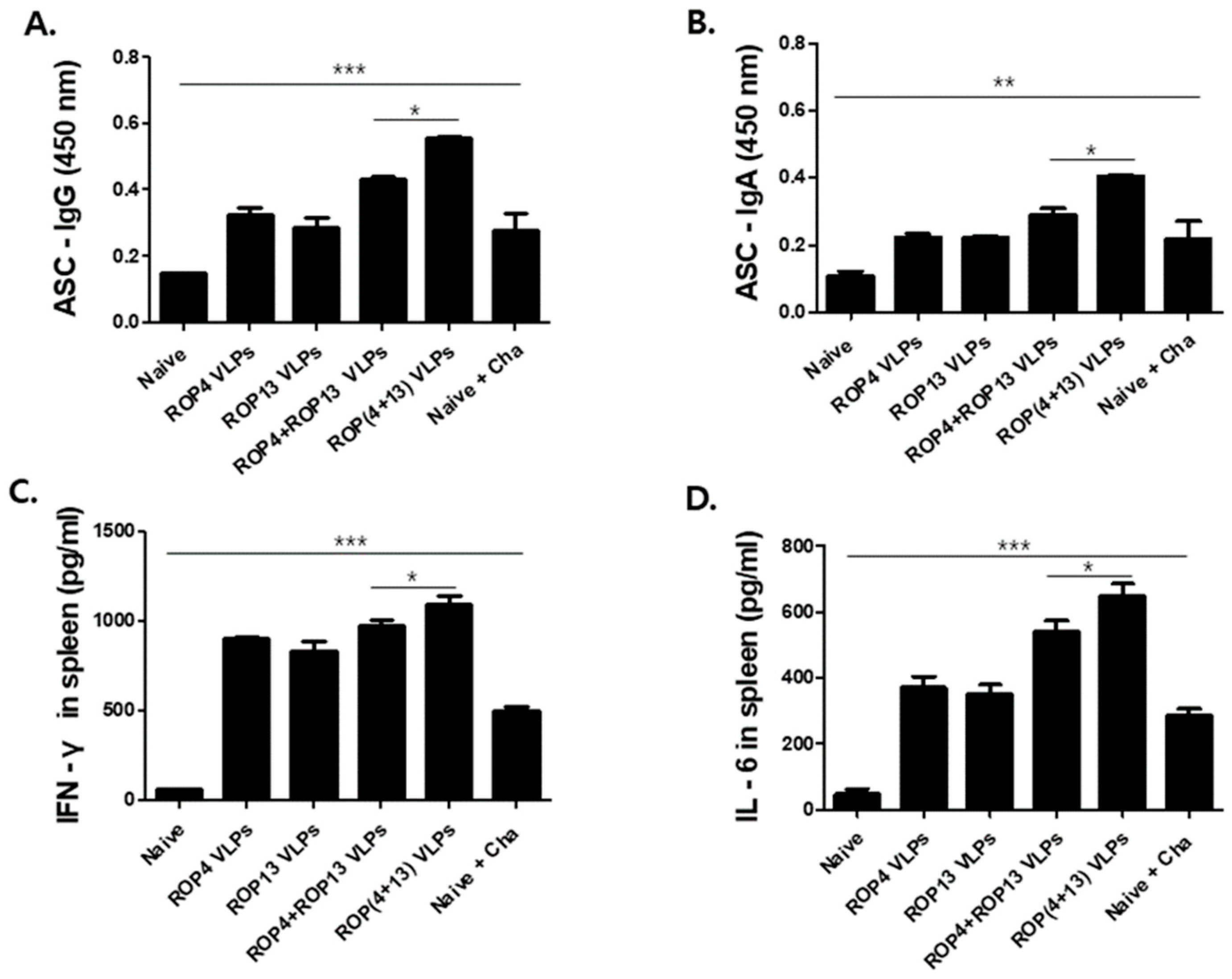
3.4. Antibody Secreting Cells and Cytokine Response
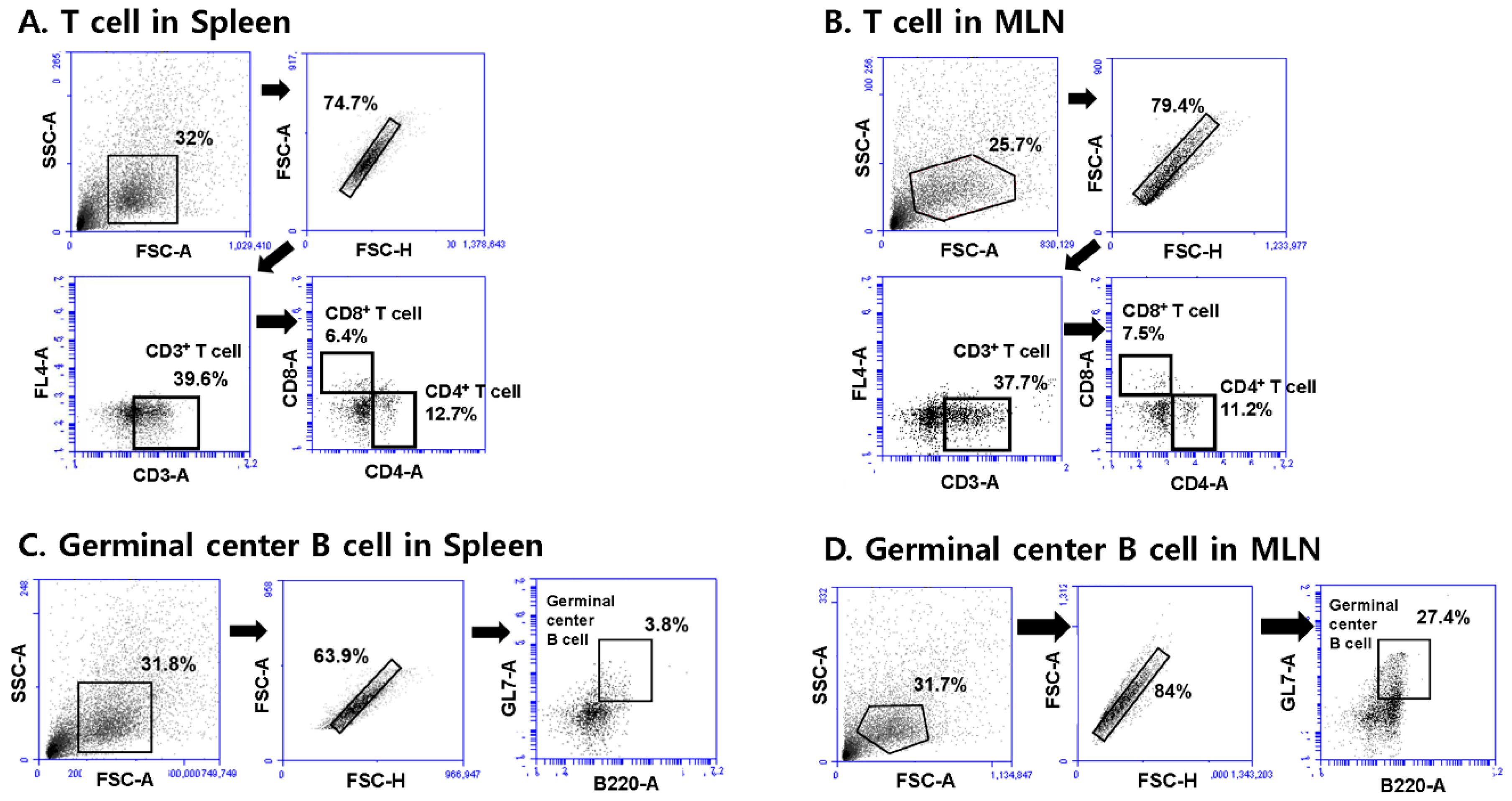
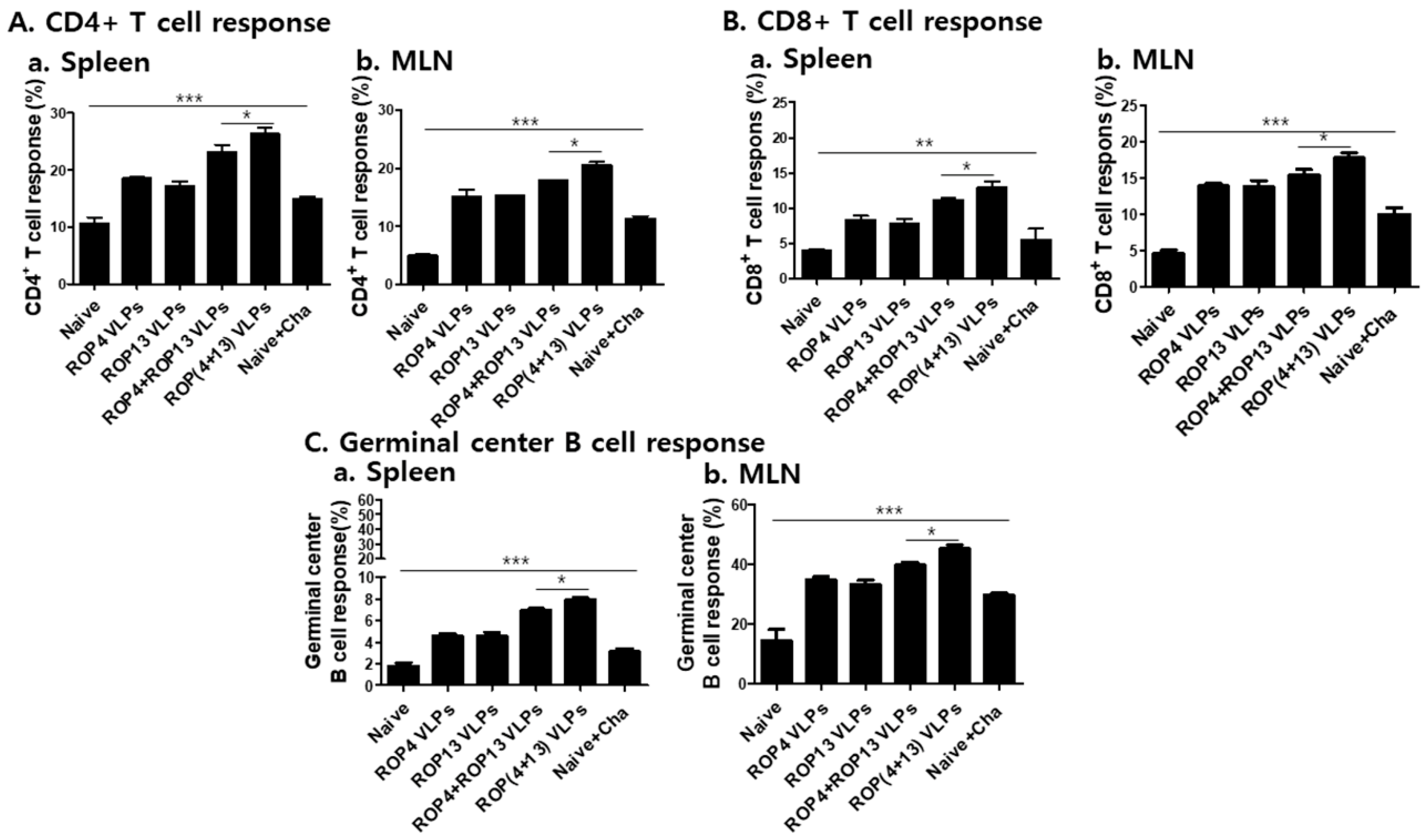
3.5. T. gondii VLPs Immunization Induces T and B Cell Responses
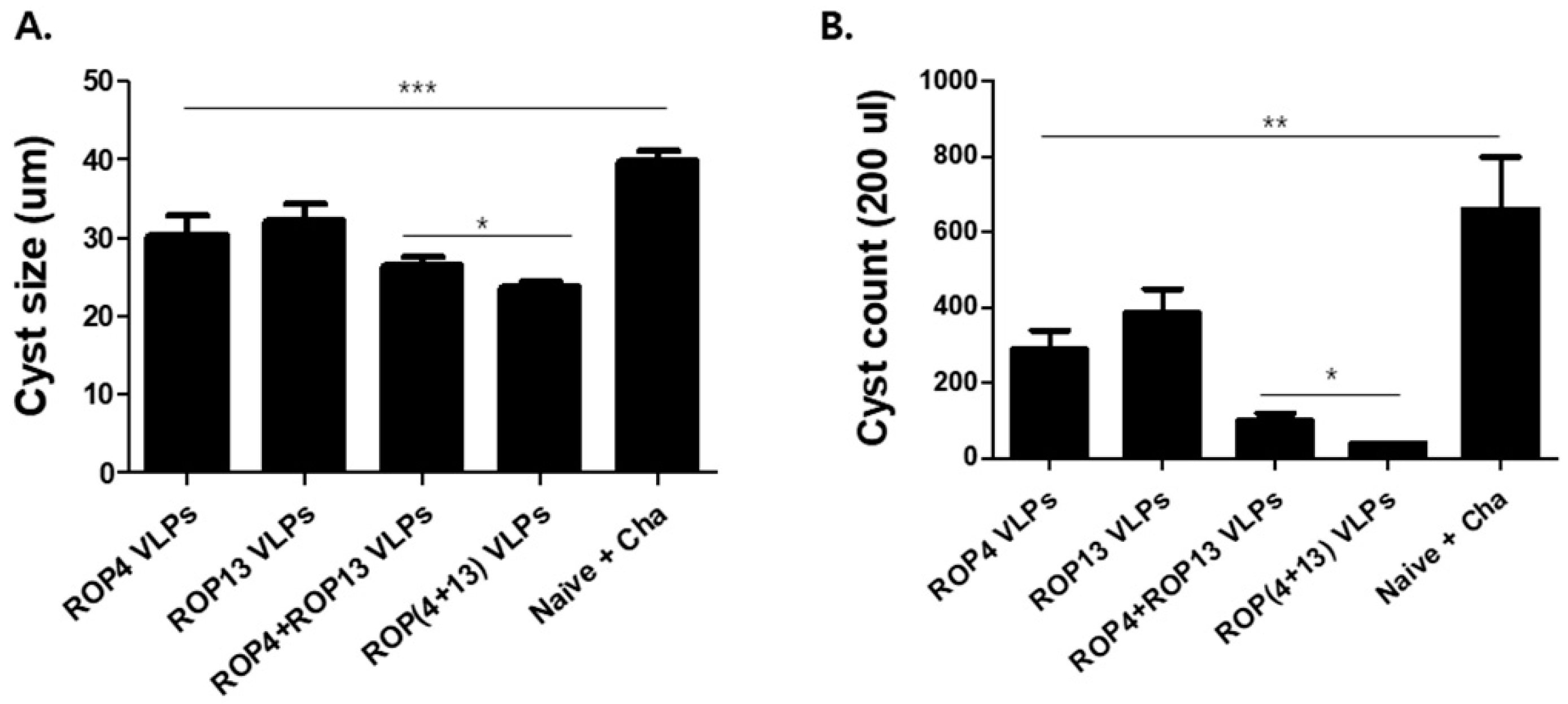
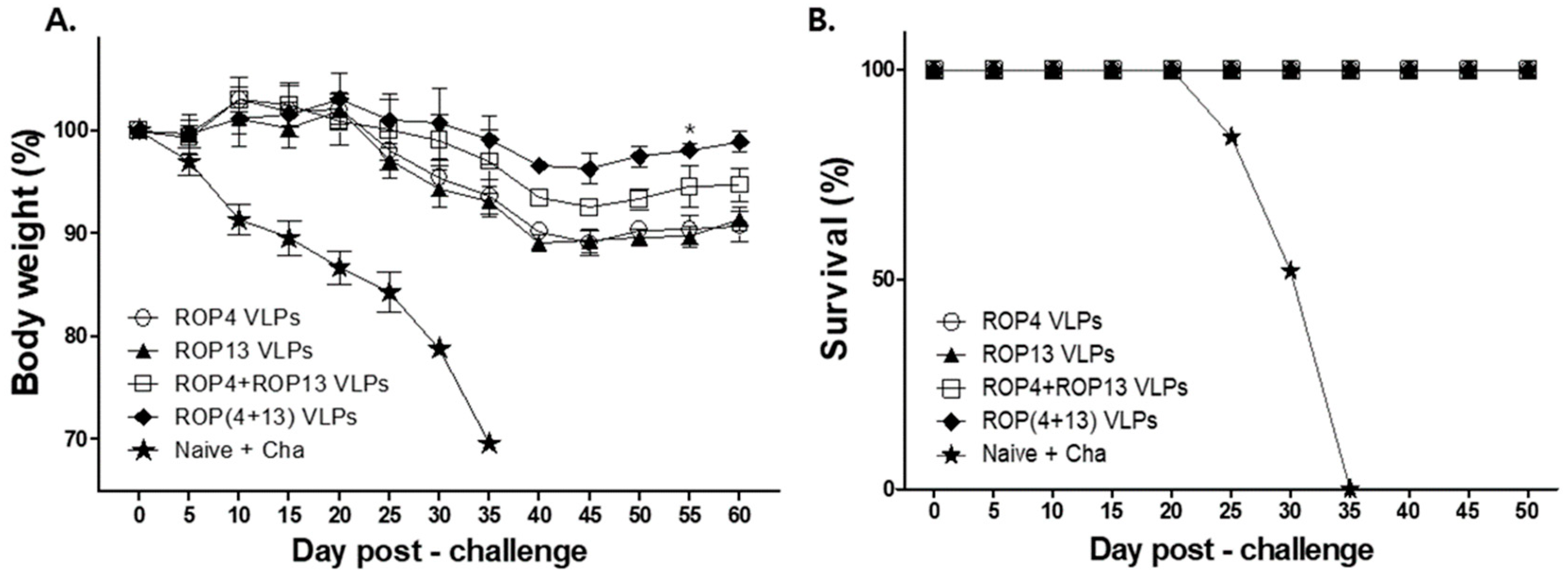
3.6. T. gondii VLPs Immunization Induces Protection
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Luft, B.J.; Remington, J.S. Toxoplasmic Encephalitis in AIDS. Clin. Infect. Dis. 1992, 15, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Lopes, F.M.R.; Gonçalves, D.D.; Mitsuka-Breganó, R.; Freire, R.L.; Navarro, I.T. Toxoplasma Gondii Infection in Pregnancy. Braz. J. Infect. Dis. 2007, 11, 496–506. [Google Scholar] [CrossRef] [PubMed]
- Saadatnia, G.; Golkar, M. A Review on Human Toxoplasmosis. Scand. J. Infect. Dis. 2012, 44, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Pappas, G.; Roussos, N.; Falagas, M.E. Toxoplasmosis Snapshots: Global Status of Toxoplasma Gondii Seroprevalence and Implications for Pregnancy and Congenital Toxoplasmosis. Int. J. Parasitol. 2009, 39, 1385–1394. [Google Scholar] [CrossRef] [PubMed]
- Parasites—Toxoplasmosis (Toxoplasma Infection) Epidemiology & Risk Factors. Available online: https://www.cdc.gov/parasites/toxoplasmosis/epi.html (accessed on 4 September 2018).
- Montazeri, M.; Mehrzadi, S.; Sharif, M.; Sarvi, S.; Tanzifi, A.; Aghayan, S.A.; Daryani, A. Drug Resistance in Toxoplasma Gondii. Front. Microbiol. 2018, 9, 2587. [Google Scholar] [CrossRef]
- Wang, P.Y.; Yuan, Z.G.; Petersen, E.; Li, J.; Zhang, X.X.; Li, X.Z.; Li, H.X.; Lv, Z.C.; Cheng, T.; Ren, D.; et al. Protective Efficacy of a Toxoplasma Gondii Rhoptry Protein 13 Plasmid DNA Vaccine in Mice. Clin. Vaccine Immunol. 2012, 19, 1916–1920. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, A.; Li, Y.; Li, X.; Cong, H.; He, S.; Zhou, H. Immunization with a DNA Vaccine Encoding Toxoplasma Gondii Superoxide Dismutase (TgSOD) Induces Partial Immune Protection Against Acute Toxoplasmosis in BALB/C Mice. BMC Infect. Dis. 2017, 17. [Google Scholar] [CrossRef]
- Wang, H.L.; Wang, Y.J.; Pei, Y.J.; Bai, J.Z.; Yin, L.T.; Guo, R.; Yin, G.R. DNA Vaccination with a Gene Encoding Toxoplasma Gondii Rhoptry Protein 17 Induces Partial Protective Immunity Against Lethal Challenge in Mice. Parasite 2016, 23. [Google Scholar] [CrossRef][Green Version]
- Czarnewski, P.; Araújo, E.C.; Oliveira, M.C.; Mineo, T.W.; Silva, N.M. Recombinant TgHSP70 Immunization Protects Against Toxoplasma Gondii Brain Cyst Formation by Enhancing Inducible Nitric Oxide Expression. Front. Cell. Infect. Microbiol. 2017, 7. [Google Scholar] [CrossRef]
- Lee, D.; Kim, A.; Lee, S.; Quan, F. Cross-Protection Induced by Toxoplasma Gondii Virus-Like Particle Vaccine upon Intraperitoneal Route Challenge. Acta Trop. 2016, 164, 77–83. [Google Scholar] [CrossRef]
- Quan, F.; Lee, Y.; Kim, K.; Kim, M.; Kang, S. Progress in Developing Virus-Like Particle Influenza Vaccines. Expert Rev. Vaccines 2016, 15, 1281–1293. [Google Scholar] [CrossRef] [PubMed]
- Quan, F.; Huang, C.; Compans, R.W.; Kang, S. Virus-Like Particle Vaccine Induces Protective Immunity Against Homologous and Heterologous Strains of Influenza Virus. J. Virol. 2007, 81, 3514–3524. [Google Scholar] [CrossRef]
- Quan, F.; Kim, Y.; Lee, S.; Yi, H.; Kang, S.; Bozja, J.; Moore, M.L.; Compans, R.W. Viruslike Particle Vaccine Induces Protection Against Respiratory Syncytial Virus Infection in Mice. J. Infect. Dis. 2011, 204, 987–995. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kang, H.; Lee, D.; Quan, F. Protective Immunity Induced by Incorporating Multiple Antigenic Proteins of Toxoplasma Gondii into Influenza Virus-Like Particles. Front. Immunol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Kim, A.; Lee, S.; Quan, F. Virus-Like Particles Vaccine Containing Clonorchis Sinensis Tegumental Protein Induces Partial Protection Against Clonorchis Sinensis Infection. Parasites Vectors 2017, 10. [Google Scholar] [CrossRef]
- Gomez-Puertas, P.; Albo, C.; Perez-Pastrana, E.; Vivo, A.; Portela, A. Influenza Virus Matrix Protein is the Major Driving Force in Virus Budding. J. Virol. 2000, 74, 11538–11547. [Google Scholar] [CrossRef] [PubMed]
- Latham, T.; Galarza, J.M. Formation of Wild-Type and Chimeric Influenza Virus-Like Particles Following Simultaneous Expression of Only Four Structural Proteins. J. Virol. 2001, 75, 6154–6165. [Google Scholar] [CrossRef]
- Alizadeh, P.; Ahmadpour, E.; Daryani, A.; Kazemi, T.; Spotin, A.; Mahami-Oskouei, M.; Flynn, R.J.; Azadi, Y.; Rajabi, S.; Sandoghchian, S. IL-17 and IL-22 Elicited by a DNA Vaccine Encoding ROP13 Associated with Protection Against Toxoplasma Gondii in BALB/C Mice. J. Cell. Physiol. 2019, 234, 10782–10788. [Google Scholar] [CrossRef]
- Lee, D.H.; Lee, S.H.; Kim, A.R.; Quan, F.S. Virus-Like Nanoparticle Vaccine Confers Protection against Toxoplasma Gondii. PLoS ONE 2016, 11, e0161231. [Google Scholar] [CrossRef]
- Lee, S.; Kim, A.; Lee, D.; Rubino, I.; Choi, H.; Quan, F. Protection Induced by Virus-Like Particles Containing Toxoplasma Gondii Microneme Protein 8 Against Highly Virulent RH Strain of Toxoplasma Gondii Infection. PLoS ONE 2017, 12, e0175644. [Google Scholar] [CrossRef]
- Choi, H.; Yoo, D.; Bondy, B.J.; Quan, F.; Compans, R.W.; Kang, S.; Prausnitz, M.R. Stability of Influenza Vaccine Coated Onto Microneedles. Biomaterials 2012, 33, 3756–3769. [Google Scholar] [CrossRef] [PubMed]
- Quan, F.; Compans, R.W.; Kang, S. Oral Vaccination with Inactivated Influenza Vaccine Induces Cross-Protective Immunity. Vaccine 2012, 30, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Hossain, J.; Yoo, D.; Lipatov, A.S.; Davis, C.T.; Quan, F.; Chen, L.; Hogan, R.J.; Donis, R.O.; Compans, R.W. Protective Immunity Against H5N1 Influenza Virus by a Single Dose Vaccination with Virus-Like Particles. Virology 2010, 405, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Dlugonska, H. Toxoplasma Rhoptries: Unique Secretory Organelles and Source of Promising Vaccine Proteins for Immunoprevention of Toxoplasmosis. J. Biomed. Biotechnol. 2008, 2008, 632424. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Lee, S.; Chu, K.; Lee, D.; Quan, F. Virus-Like Particles Expressing Toxoplasma Gondii Rhoptry Protein 18 Induces Better Protection than Rhoptry Protein 4 Against T. Gondii Infection. Korean J. Parasitol. 2018, 56, 429. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kang, H.; Lee, D.; Kang, S.; Quan, F. Virus-Like Particle Vaccines Expressing Toxoplasma Gondii Rhoptry Protein 18 and Microneme Protein 8 Provide Enhanced Protection. Vaccine 2018, 36, 5692–5700. [Google Scholar] [CrossRef] [PubMed]
- Lycke, N. Recent Progress in Mucosal Vaccine Development: Potential and Limitations. Nat. Rev. Immunol. 2012, 12, 592–605. [Google Scholar] [CrossRef] [PubMed]
- Liesenfeld, O. Oral Infection of C57BL/6 Mice with Toxoplasma Gondii: A New Model of Inflammatory Bowel Disease? J. Infect. Dis. 2002, 185, S96–S101. [Google Scholar] [CrossRef]
- Kwa, S.F.; Beverley, P.; Smith, A.L. Peyer’s Patches are Required for the Induction of Rapid Th1 Responses in the Gut and Mesenteric Lymph Nodes during an Enteric Infection. J. Immunol. 2006, 176, 7533–7541. [Google Scholar] [CrossRef]
- Mesin, L.; Ersching, J.; Victora, G.D. Germinal Center B Cell Dynamics. Immunity 2016, 45, 471–482. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, H.-J.; Lee, S.-H.; Kim, M.-J.; Chu, K.-B.; Lee, D.-H.; Chopra, M.; Choi, H.-J.; Park, H.; Jin, H.; Quan, F.-S. Influenza Virus-Like Particles Presenting both Toxoplasma gondii ROP4 and ROP13 Enhance Protection against T. gondii Infection. Pharmaceutics 2019, 11, 342. https://doi.org/10.3390/pharmaceutics11070342
Kang H-J, Lee S-H, Kim M-J, Chu K-B, Lee D-H, Chopra M, Choi H-J, Park H, Jin H, Quan F-S. Influenza Virus-Like Particles Presenting both Toxoplasma gondii ROP4 and ROP13 Enhance Protection against T. gondii Infection. Pharmaceutics. 2019; 11(7):342. https://doi.org/10.3390/pharmaceutics11070342
Chicago/Turabian StyleKang, Hae-Ji, Su-Hwa Lee, Min-Ju Kim, Ki-Back Chu, Dong-Hun Lee, Manika Chopra, Hyo-Jick Choi, Hyunwoo Park, Hui Jin, and Fu-Shi Quan. 2019. "Influenza Virus-Like Particles Presenting both Toxoplasma gondii ROP4 and ROP13 Enhance Protection against T. gondii Infection" Pharmaceutics 11, no. 7: 342. https://doi.org/10.3390/pharmaceutics11070342
APA StyleKang, H.-J., Lee, S.-H., Kim, M.-J., Chu, K.-B., Lee, D.-H., Chopra, M., Choi, H.-J., Park, H., Jin, H., & Quan, F.-S. (2019). Influenza Virus-Like Particles Presenting both Toxoplasma gondii ROP4 and ROP13 Enhance Protection against T. gondii Infection. Pharmaceutics, 11(7), 342. https://doi.org/10.3390/pharmaceutics11070342





