Supercritical Solvent Impregnation of Different Drugs in Mesoporous Nanostructured ZnO
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of the MesoNsZnO Carrier
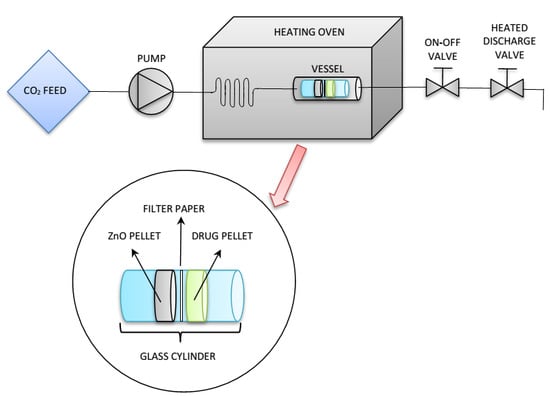
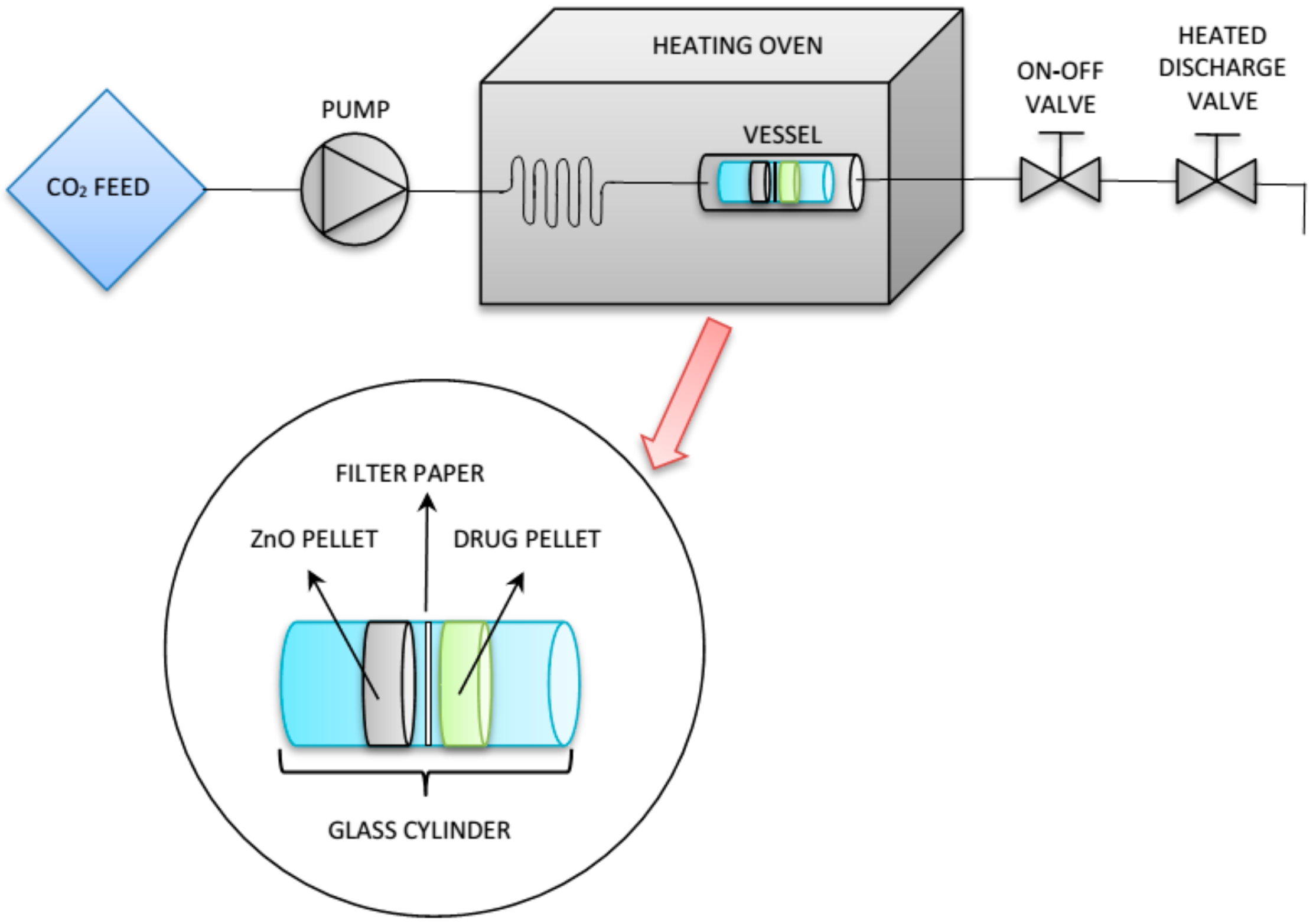
2.3. Drug Loading through SSI
2.4. Characterization
2.5. Evaluation of the Drug Loading and the Number of Molecular Layers on the Carrier Surface
3. Results
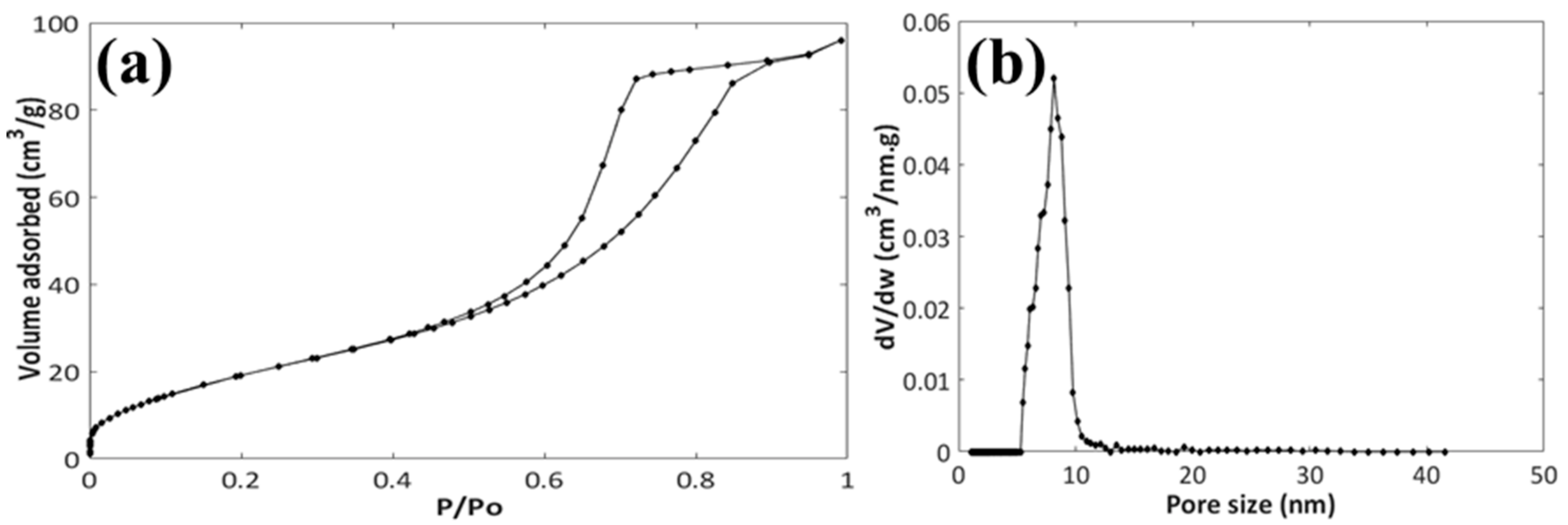
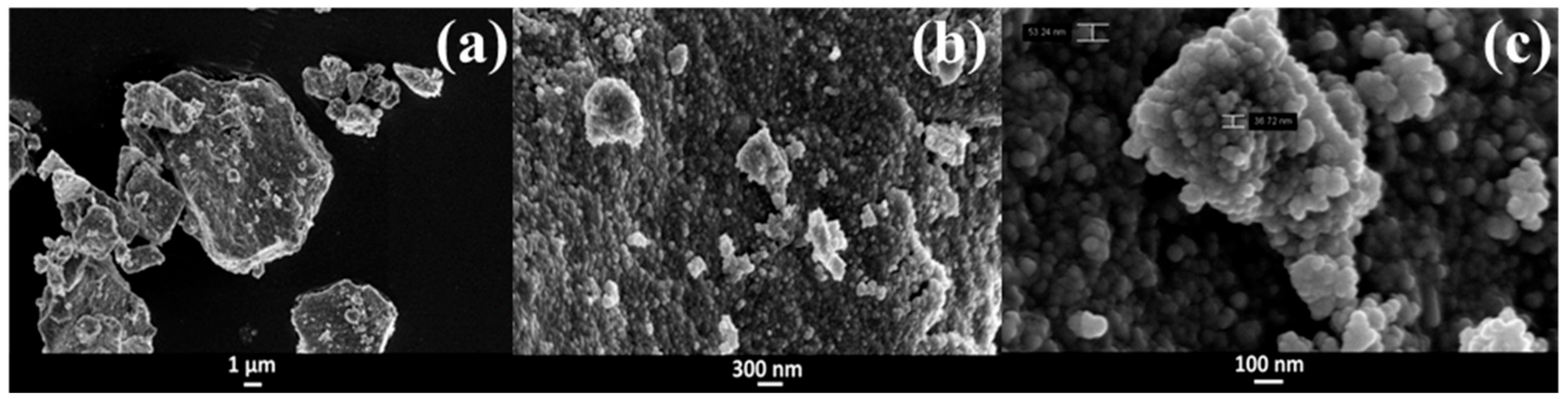
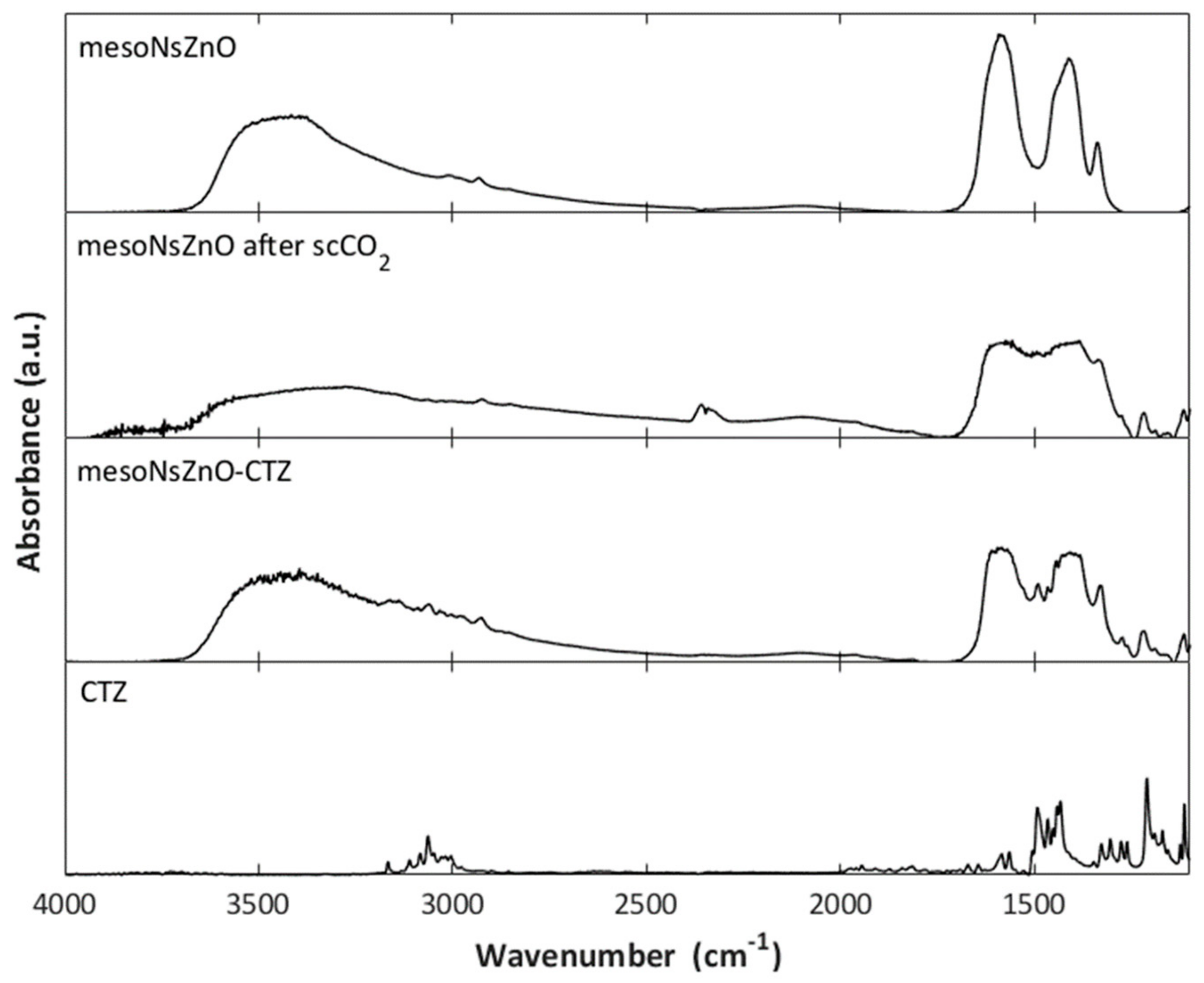
3.1. Characterization of the MesoNsZnO Carrier
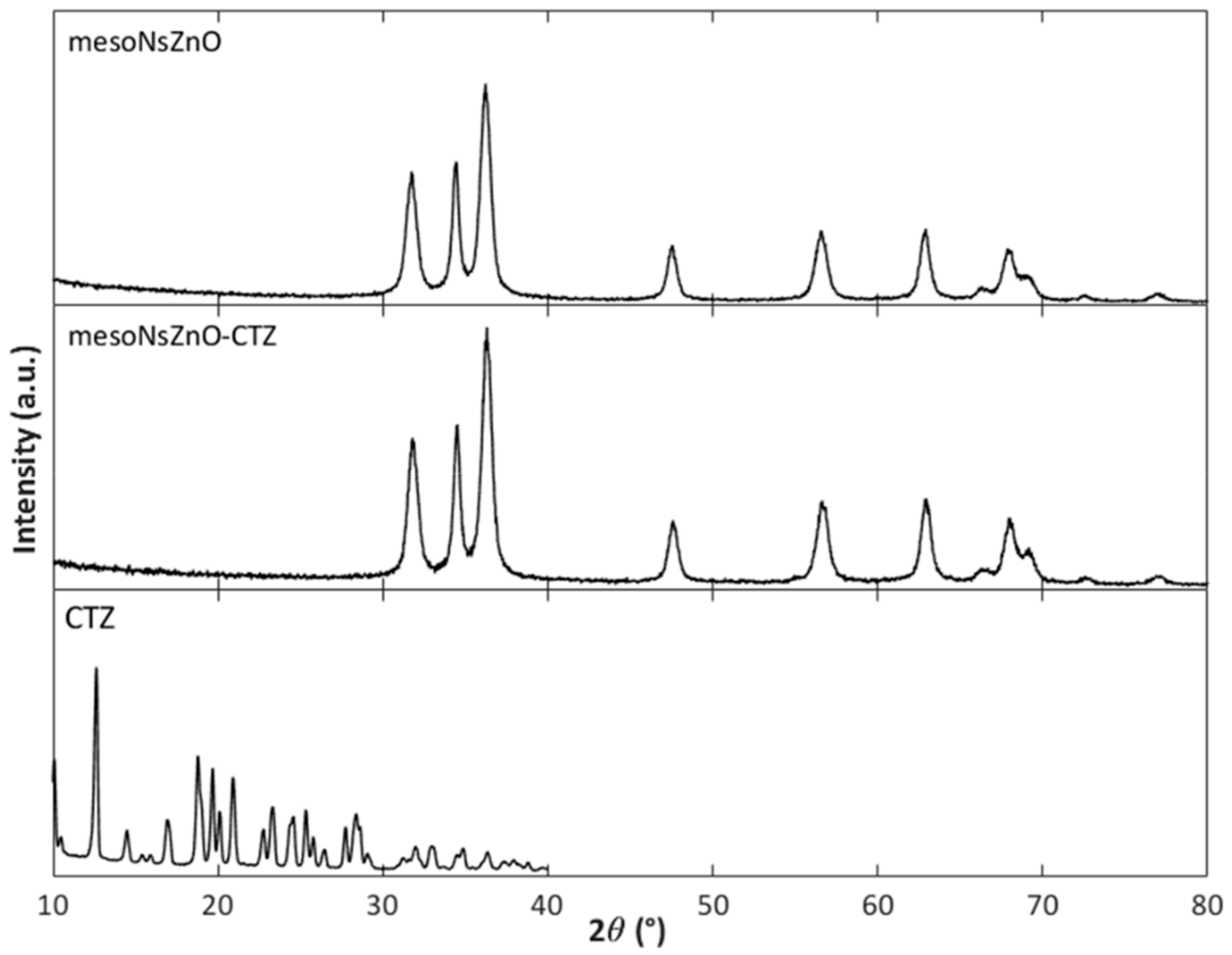
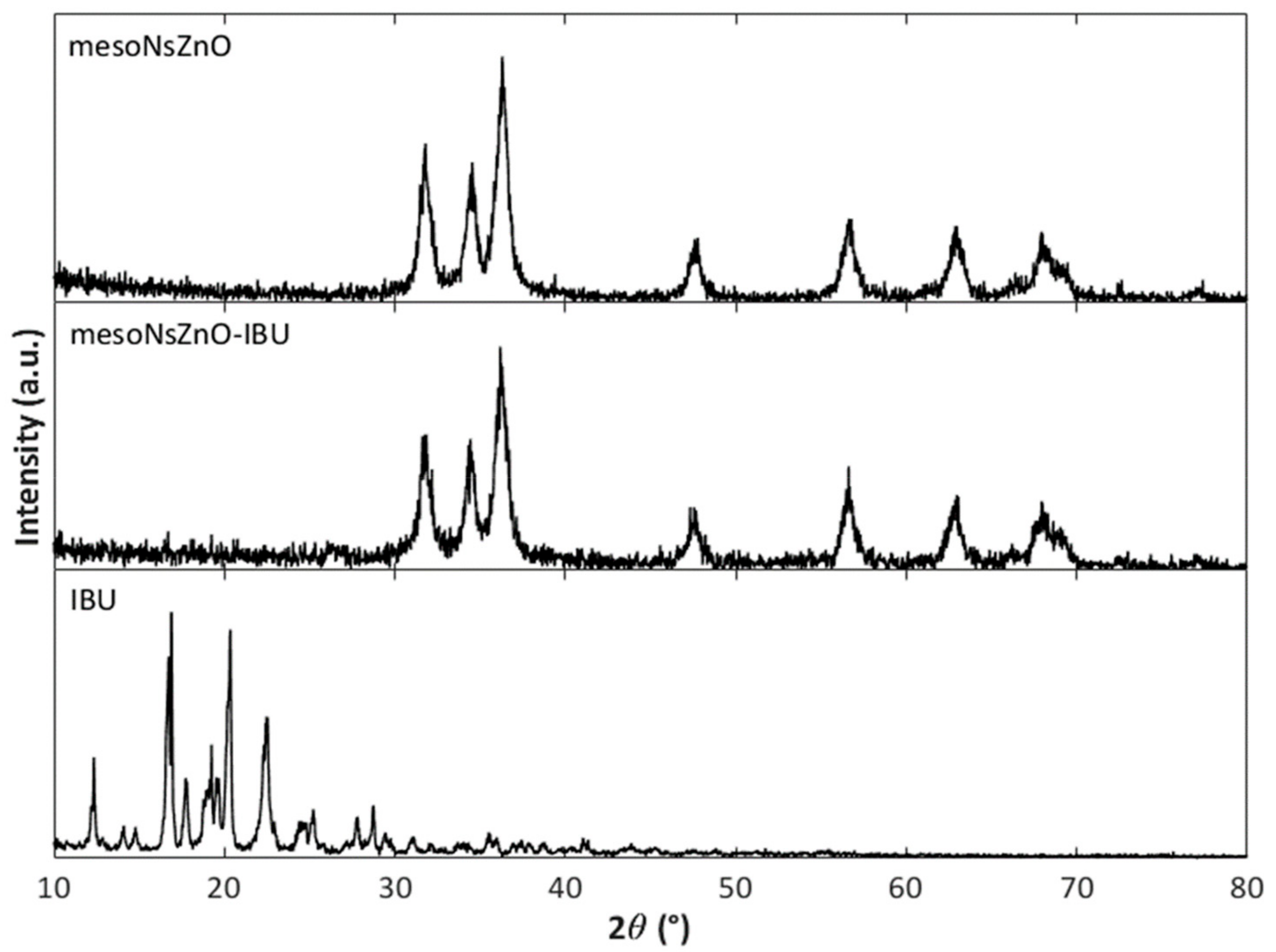
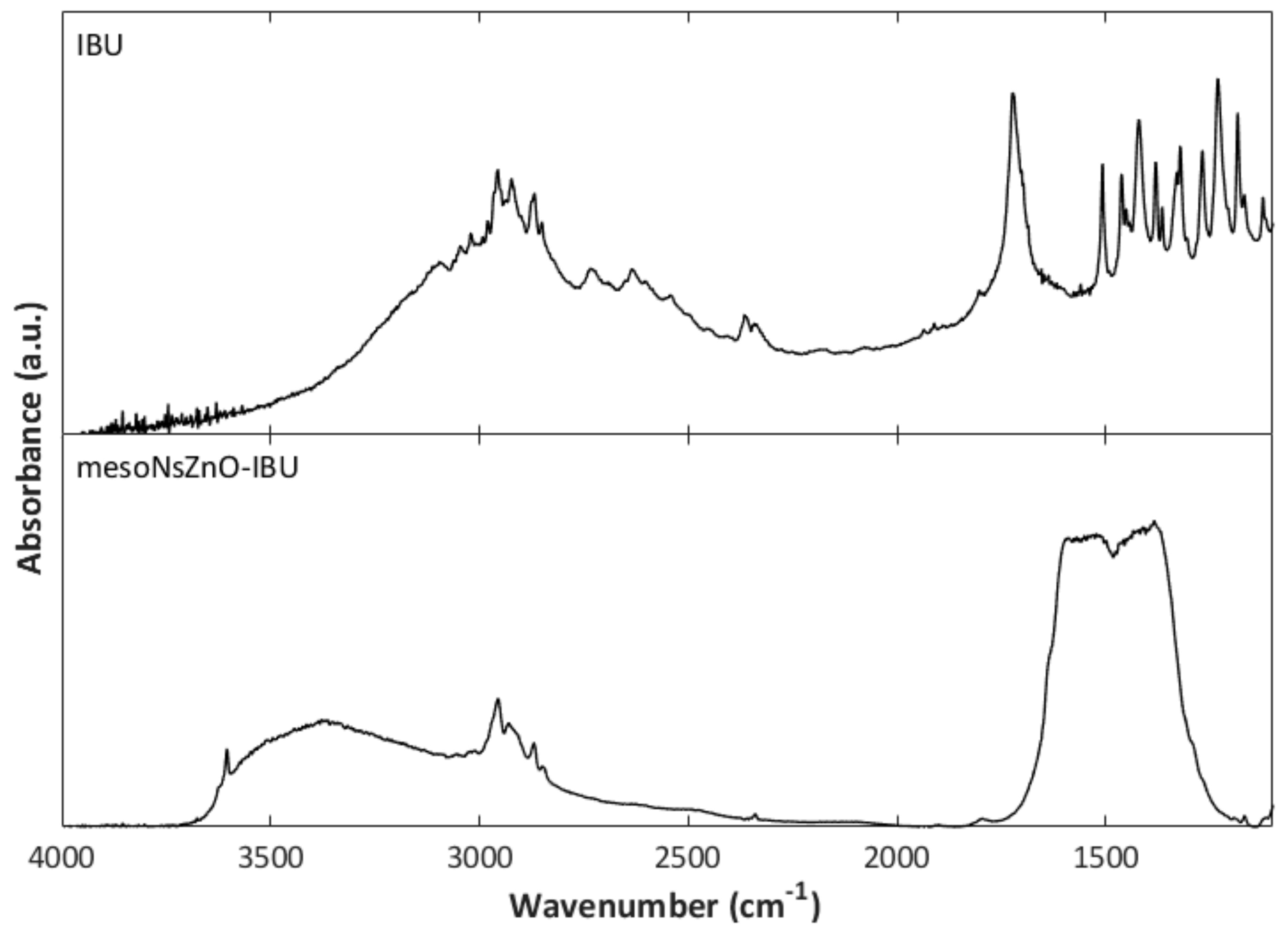
3.2. Drug Loading and Characterization of the MesoNsZnO Drug Systems
3.3. Effect of the scCO2 Treatment on the MesoNsZnO
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kankala, R.K.; Zhang, Y.S.; Wang, S.-B.; Lee, C.-H.; Chen, A.-Z. Supercritical Fluid Technology: An Emphasis on Drug Delivery and Related Biomedical Applications. Adv. Healthc. Mater. 2017, 6, 1700433. [Google Scholar] [CrossRef] [PubMed]
- Girotra, P.; Singh, S.K.; Nagpal, K. Supercritical Fluid Technology: A Promising Approach in Pharmaceutical Research. Pharm. Dev. Technol. 2013, 18, 22–38. [Google Scholar] [CrossRef] [PubMed]
- Champeau, M.; Thomassin, J.-M.; Tassaing, T.; Jérôme, C. Drug Loading of Polymer Implants by Supercritical CO2 Assisted Impregnation: A Review. J. Control. Release 2015, 209, 248–259. [Google Scholar] [CrossRef] [PubMed]
- Gurikov, P.; Smirnova, I. Amorphization of Drugs by Adsorptive Precipitation from Supercritical Solutions: A Review. J. Supercrit. Fluids 2018, 132, 105–125. [Google Scholar] [CrossRef]
- Ivanovic, J.; Knauer, S.; Fanovich, A.; Milovanovic, S.; Stamenic, M.; Jaeger, P.; Zizovic, I.; Eggers, R. Supercritical CO2 Sorption Kinetics and Thymol Impregnation of PCL and PCL-HA. J. Supercrit. Fluids 2016, 107, 486–498. [Google Scholar] [CrossRef]
- Li, Y.; He, Z.-D.; Zheng, Q.-E.; Hu, C.; Lai, W.-F. Hydroxypropyl-β-Cyclodextrin for Delivery of Baicalin via Inclusion Complexation by Supercritical Fluid Encapsulation. Molecules 2018, 23, 1169. [Google Scholar] [CrossRef]
- Banchero, M.; Manna, L. The Use of Lysine to Enhance the Supercritical Complexation of Ketoprofen and Cyclodextrins. J. Supercrit. Fluids 2012, 67, 76–83. [Google Scholar] [CrossRef]
- García-Casas, I.; Montes, A.; Valor, D.; Pereyra, C.; de la Ossa, E.M. Impregnation of Mesoporous Silica with Mangiferin Using Supercritical CO2. J. Supercrit. Fluids 2018, 140, 129–136. [Google Scholar] [CrossRef]
- Gignone, A.; Manna, L.; Ronchetti, S.; Banchero, M.; Onida, B. Incorporation of Clotrimazole in Ordered Mesoporous Silica by Supercritical CO2. Microporous Mesoporous Mater. 2014, 200, 291–296. [Google Scholar] [CrossRef]
- Laitinen, R.; Löbmann, K.; Strachan, C.J.; Grohganz, H.; Rades, T. Emerging Trends in the Stabilization of Amorphous Drugs. Int. J. Pharm. 2013, 453, 65–79. [Google Scholar] [CrossRef]
- Mishra, P.K.; Mishra, H.; Ekielski, A.; Talegaonkar, S.; Vaidya, B. Zinc Oxide Nanoparticles: A Promising Nanomaterial for Biomedical Applications. Drug Discov. Today 2017, 22, 1825–1834. [Google Scholar] [CrossRef] [PubMed]
- Pasquet, J.; Chevalier, Y.; Couval, E.; Bouvier, D.; Bolzinger, M.-A. Zinc Oxide as a New Antimicrobial Preservative of Topical Products: Interactions with Common Formulation Ingredients. Int. J. Pharm. 2015, 479, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Laurenti, M.; Cauda, V. Gentamicin-Releasing Mesoporous ZnO Structures. Materials 2018, 11, 314. [Google Scholar] [CrossRef] [PubMed]
- Leone, F.; Gignone, A.; Ronchetti, S.; Cavalli, R.; Manna, L.; Banchero, M.; Onida, B. A Green Organic-Solvent-Free Route to Prepare Nanostructured Zinc Oxide Carriers of Clotrimazole for Pharmaceutical Applications. J. Clean. Prod. 2018, 172, 1433–1439. [Google Scholar] [CrossRef]
- Leone, F.; Cataldo, R.; Mohamed, S.; Manna, L.; Banchero, M.; Ronchetti, S.; Mandras, N.; Tullio, V.; Cavalli, R.; Onida, B. Nanostructured ZnO as Multifunctional Carrier for a Green Antibacterial Drug Delivery System—A Feasibility Study. Nanomaterials 2019, 9, 407. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Long, J.; Liu, J.; Cao, Y. The Toxicity of ZnO Nanomaterials to HepG2 Cells: The Influence of Size and Shape of Particles: Toxicity of ZnO Nanomaterials to HepG2 Cells. J. Appl. Toxicol. 2019, 39, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Wischke, C.; Schwendeman, S.P. Principles of Encapsulating Hydrophobic Drugs in PLA/PLGA Microparticles. Int. J. Pharm. 2008, 364, 298–327. [Google Scholar] [CrossRef] [PubMed]
- Kikic, I.; De Zordi, N.; Moneghini, M.; Solinas, D. Solubility Estimation of Drugs in Ternary Systems of Interest for the Antisolvent Precipitation Processes. J. Supercrit. Fluids 2010, 55, 616–622. [Google Scholar] [CrossRef]
- Yamini, Y.; Moradi, M. Measurement and Correlation of Antifungal Drugs Solubility in Pure Supercritical CO2 Using Semiempirical Models. J. Chem. Thermodyn. 2011, 43, 1091–1096. [Google Scholar] [CrossRef]
- Dean, J.R.; Kane, M.; Khundker, S.; Dowle, C.; Tranter, R.L.; Jones, P. Estimation and Determination of Steroid Solubility in Supercritical Carbon Dioxide. Analyst 1995, 120, 2153–2157. [Google Scholar] [CrossRef]
- PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 15 May 2019).
- Chemicalize. ChemAxon 2014. Available online: http://www.chemicalize.org (accessed on 15 May 2019).
- Mitra, S.; Subia, B.; Patra, P.; Chandra, S.; Debnath, N.; Das, S.; Banerjee, R.; Kundu, S.C.; Pramanik, P.; Goswami, A. Porous ZnO Nanorod for Targeted Delivery of Doxorubicin: In Vitro and In Vivo Response for Therapeutic Applications. J. Mater. Chem. 2012, 22, 24145–24154. [Google Scholar] [CrossRef]
- Banchero, M.; Manna, L.; Ronchetti, S.; Campanelli, P.; Ferri, A. Supercritical Solvent Impregnation of Piroxicam on PVP at Various Polymer Molecular Weights. J. Supercrit. Fluids 2009, 49, 271–278. [Google Scholar] [CrossRef]
- Ni, M.; Xu, Q.-Q.; Yin, J.-Z. Preparation of Controlled Release Nanodrug Ibuprofen Supported on Mesoporous Silica Using Supercritical Carbon Dioxide. J. Mater. Res. 2012, 27, 2902–2910. [Google Scholar] [CrossRef]
- Velaga, S.P.; Ghaderi, R.; Carlfors, J. Preparation and Characterisation of Hydrocortisone Particles Using a Supercritical Fluids Extraction Process. Int. J. Pharm. 2002, 231, 155–166. [Google Scholar] [CrossRef]
- Yoganathan, R.; Mammucari, R.; Foster, N.R. Impregnation of Ibuprofen into Polycaprolactone Using Supercritical Carbon Dioxide. In Journal of Physics: Conference Series; IOP Publishing: Bristol, UK, 2010; Volume 215, p. 012087. [Google Scholar]
- Corrigan, O.I.; Crean, A.M. Comparative Physicochemical Properties of Hydrocortisone/PVP Composites Prepared Using Supercritical Carbon Dioxide by the GAS Anti-Solvent Recrystallization Process, by Coprecipitation and by Spray Drying. Int. J. Pharm. 2002, 245, 75–82. [Google Scholar] [CrossRef]
- Thommes, M.; Köhn, R.; Fröba, M. Sorption and Pore Condensation Behavior of Pure Fluids in Mesoporous MCM-48 Silica, MCM-41 Silica, SBA-15 Silica and Controlled-Pore Glass at Temperatures above and below the Bulk Triple Point. Appl. Surf. Sci. 2002, 196, 239–249. [Google Scholar] [CrossRef]
- Kamari, Y.; Ghiaci, M. Preparation and Characterization of Ibuprofen/Modified Chitosan/TiO2 Hybrid Composite as a Controlled Drug-Delivery System. Microporous Mesoporous Mater. 2016, 234, 361–369. [Google Scholar] [CrossRef]
- Pudney, P.D.A.; Mutch, K.J.; Zhu, S. Characterising the Phase Behaviour of Stearic Acid and Its Triethanolamine Soap and Acid-Soap by Infrared Spectroscopy. Phys. Chem. Chem. Phys. 2009, 11, 5010–5018. [Google Scholar] [CrossRef]
- Acharya, M.; Mishra, S.; Sahoo, R.N.; Mallick, S. Infrared Spectroscopy for Analysis of Co-Processed Ibuprofen and Magnesium Trisilicate at Milling and Freeze Drying. Acta Chim. Slov. 2017, 64, 45–54. [Google Scholar] [CrossRef]
- Graedel, T.E. Corrosion Mechanisms for Zinc Exposed to the Atmosphere. J. Electrochem. Soc. 1989, 136, 193C–203C. [Google Scholar] [CrossRef]
- Galhotra, P. Carbon Dioxide Adsorption on Nanomaterials. Ph.D. Thesis, University of Iowa, Iowa City, IA, USA, 2010. [Google Scholar]
- Smirnova, I.; Suttiruengwong, S.; Arlt, W. Aerogels: Tailor-Made Carriers for Immediate and Prolonged Drug Release. KONA Powder Part. J. 2005, 23, 86–97. [Google Scholar] [CrossRef]
- Qian, K.K.; Bogner, R.H. Application of Mesoporous Silicon Dioxide and Silicate in Oral Amorphous Drug Delivery Systems. J. Pharm. Sci. 2012, 101, 444–463. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.L.; McKenna, G.B. Vitrification and Crystallization of Organic Liquids Confined to Nanoscale Pores. Chem. Mater. 1996, 8, 2128–2137. [Google Scholar] [CrossRef]

| Drug | Chemical Structure | logP 1 | HBDA 2 | van der Waals Volume(Å3) 2 |
|---|---|---|---|---|
| Ibuprofen (IBU) | 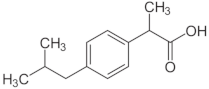 | 3.97 | 3 | 211.80 |
| Clotrimazole (CTZ) | 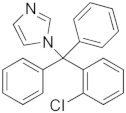 | 6.1 | 1 | 306.59 |
| Hydrocortisone (HC) |  | 1.61 | 8 | 347.26 |
| Drug | T (°C) | P (MPa) | Contact Time (h) |
|---|---|---|---|
| Clotrimazole (CTZ) | 100 | 25 | 12 |
| Hydrocortisone (HC) | 45 | 13 | 8 |
| Ibuprofen (IBU) | 35 | 10 | 3, 8, 24 |
| Drug | |||
|---|---|---|---|
| Ibuprofen (IBU) | 89.20 | 35.44 | 64.57 |
| Clotrimazole (CTZ) | 117.61 | 66.11 | 75.55 |
| Hydrocortisone (HC) | - | 47.67 | 97.02 |
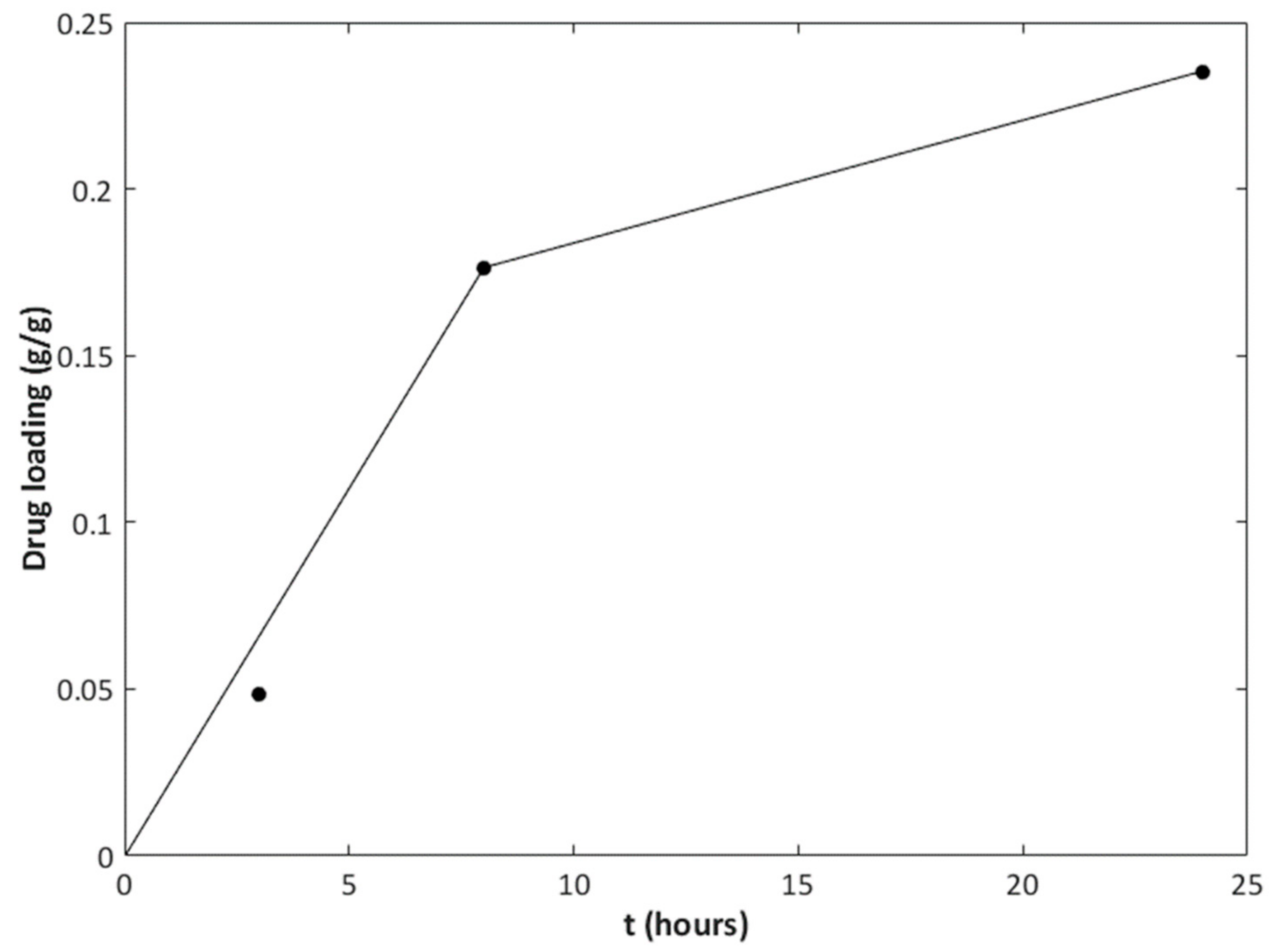
| Drug | T (°C) | P (MPa) | Contact Time (h) | Drug Loading (g/g) |
|---|---|---|---|---|
| Clotrimazole (CTZ) | 100 | 25 | 12 | 0.092 |
| Hydrocortisone (HC) | 45 | 13 | 8 | nil |
| Ibuprofen (IBU) | 35 | 10 | 3 | 0.048 |
| 8 | 0.18 | |||
| 24 | 0.24 |
| Drug | Contact Time (h) | NML1 | NML2 | NML3 |
|---|---|---|---|---|
| Clotrimazole (CTZ) | 12 | 2.5 | 1.4 | 1.6 |
| Ibuprofen (IBU) | 3 | 1.7 | 0.66 | 1.2 |
| 8 | 6.1 | 2.4 | 4.4 | |
| 24 | 8.2 | 3.2 | 5.9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banchero, M.; Mohamed, S.S.Y.; Leone, F.; Lopez, F.; Ronchetti, S.; Manna, L.; Onida, B. Supercritical Solvent Impregnation of Different Drugs in Mesoporous Nanostructured ZnO. Pharmaceutics 2019, 11, 340. https://doi.org/10.3390/pharmaceutics11070340
Banchero M, Mohamed SSY, Leone F, Lopez F, Ronchetti S, Manna L, Onida B. Supercritical Solvent Impregnation of Different Drugs in Mesoporous Nanostructured ZnO. Pharmaceutics. 2019; 11(7):340. https://doi.org/10.3390/pharmaceutics11070340
Chicago/Turabian StyleBanchero, Mauro, Sara S. Y. Mohamed, Federica Leone, Francesca Lopez, Silvia Ronchetti, Luigi Manna, and Barbara Onida. 2019. "Supercritical Solvent Impregnation of Different Drugs in Mesoporous Nanostructured ZnO" Pharmaceutics 11, no. 7: 340. https://doi.org/10.3390/pharmaceutics11070340
APA StyleBanchero, M., Mohamed, S. S. Y., Leone, F., Lopez, F., Ronchetti, S., Manna, L., & Onida, B. (2019). Supercritical Solvent Impregnation of Different Drugs in Mesoporous Nanostructured ZnO. Pharmaceutics, 11(7), 340. https://doi.org/10.3390/pharmaceutics11070340