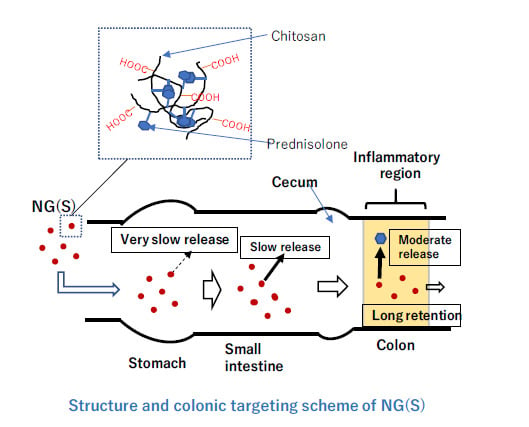
Nanogels of Succinylated Glycol Chitosan-Succinyl Prednisolone Conjugate: Preparation, In Vitro Characteristics and Therapeutic Potential
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instruments
2.3. Preparation of GCh-SP and S-GCh-SP
2.4. Preparation of FITC-Labeled NG(S)
2.5. Particle Characteristics
2.6. In Vitro Release Studies
2.7. HPLC Assay
2.8. Cell Culture
2.9. In Vitro Viability Studies Using Raw 264.7 Cells in the Presence of Several Substances
2.10. Cellular Uptake of FTC-NG(S)
2.11. Animals
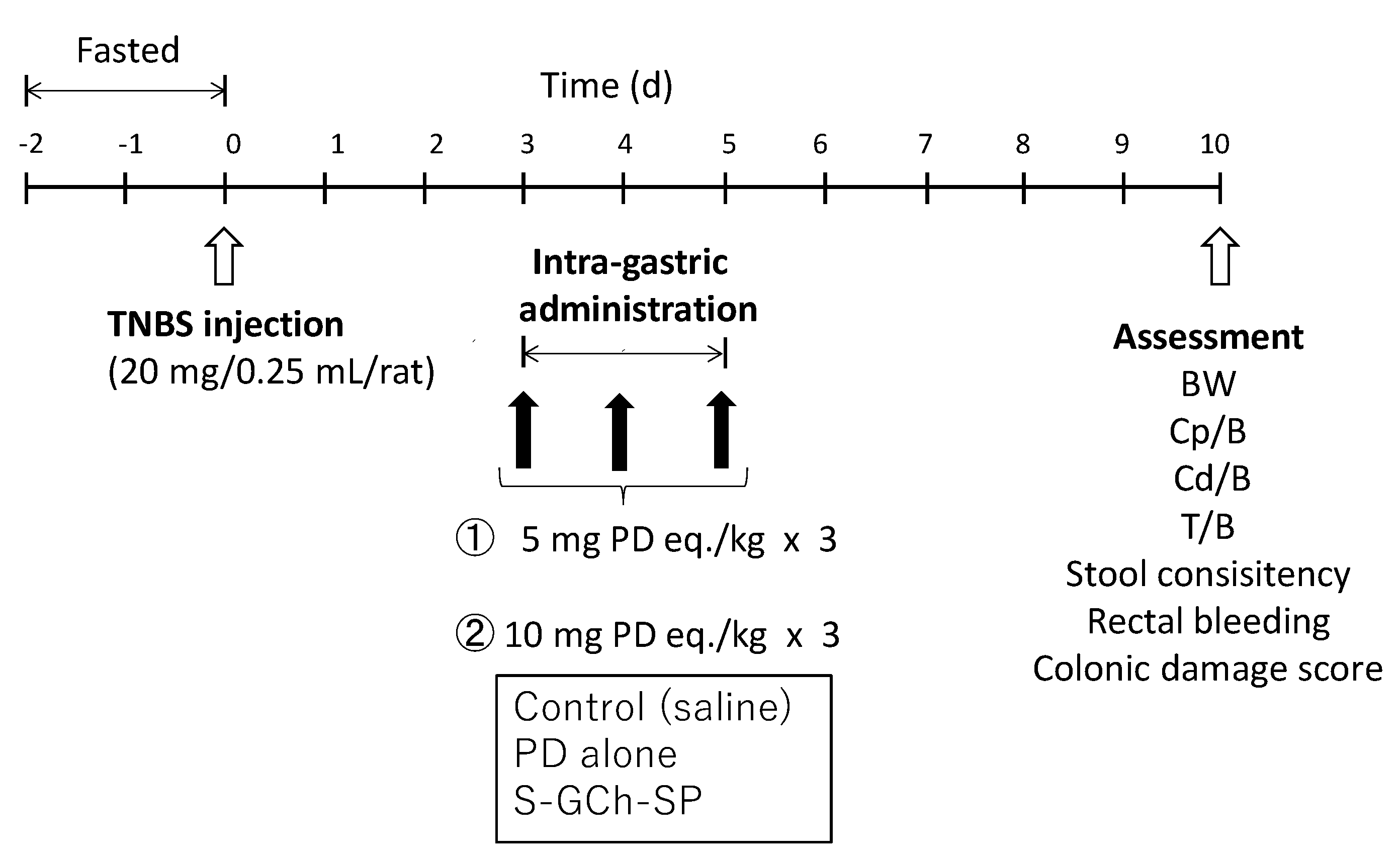
2.12. In Vivo Studies on Efficacy and Toxic Side Effects
2.13. Histopathological Study
2.14. Statistical Analysis
3. Results and Discussion
3.1. Preparation and Characterization of GCh-SP and S-GCh-SP
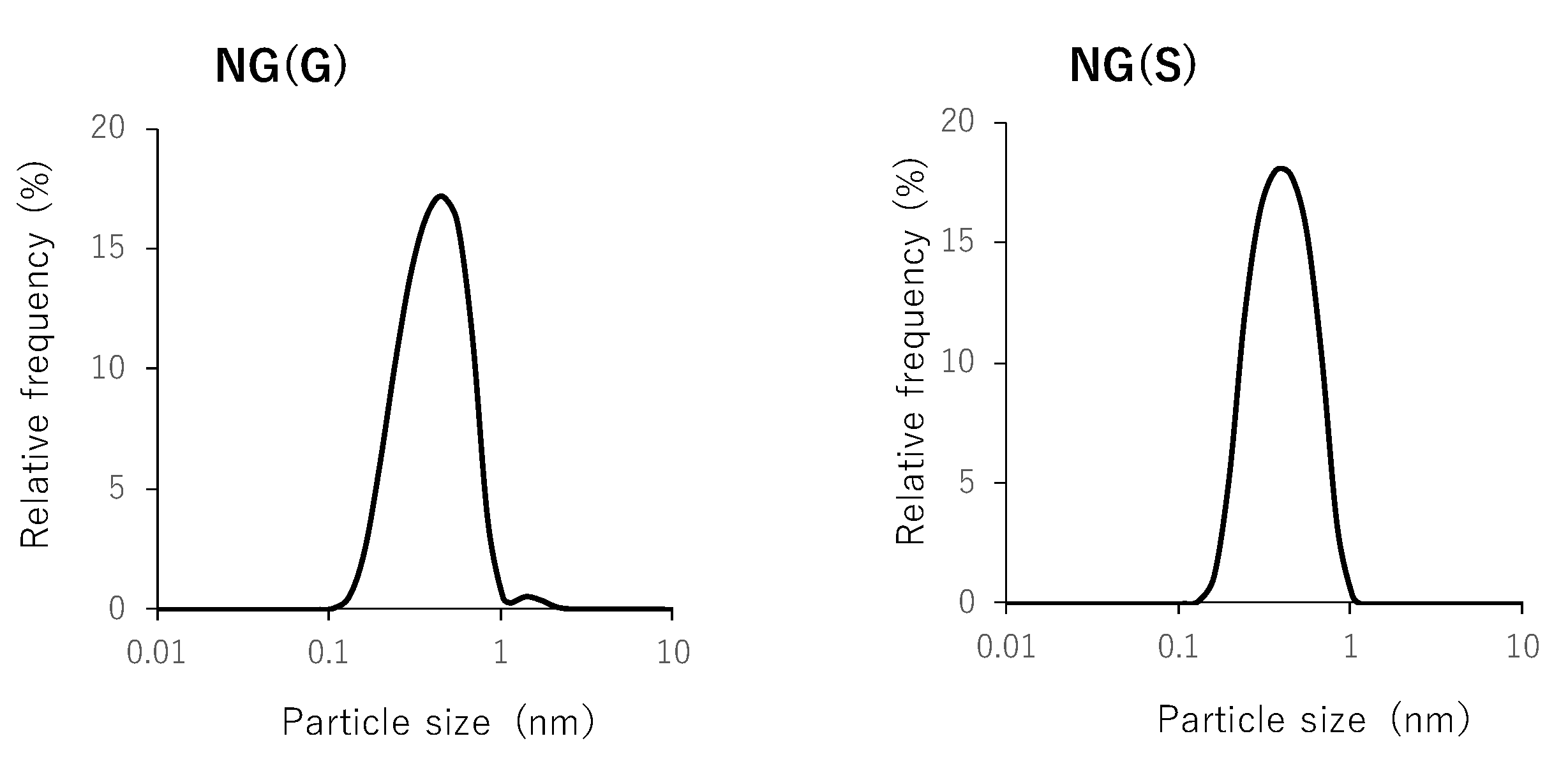
3.2. Particle Characteristics of NG(G) and NG(S)
3.3. Characteristics of FTC-NG(S)
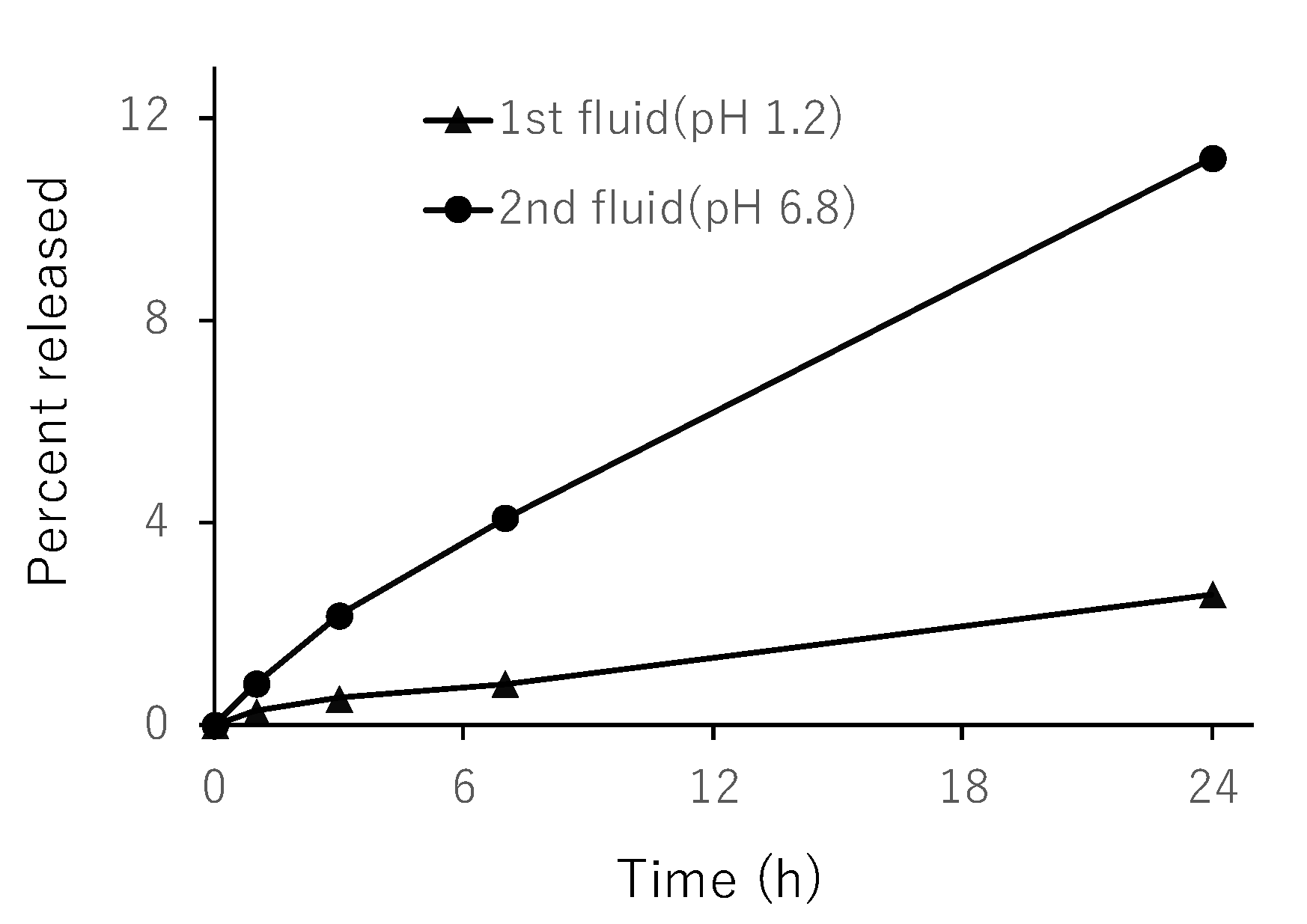
3.4. In Vitro Release from NG(S)
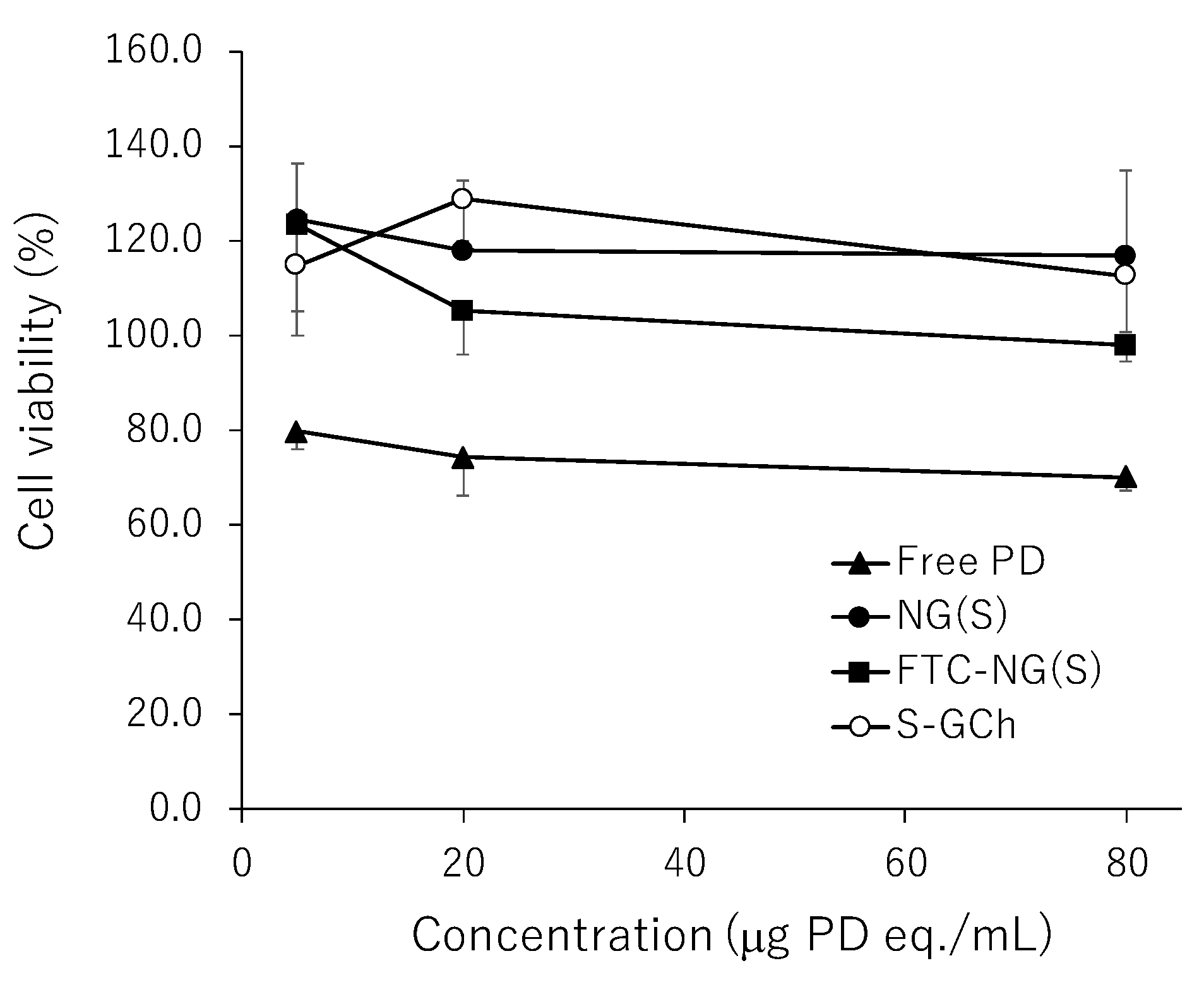
3.5. In Vitro Viabilities of Raw 264.7 Cells in Media with Different Substances
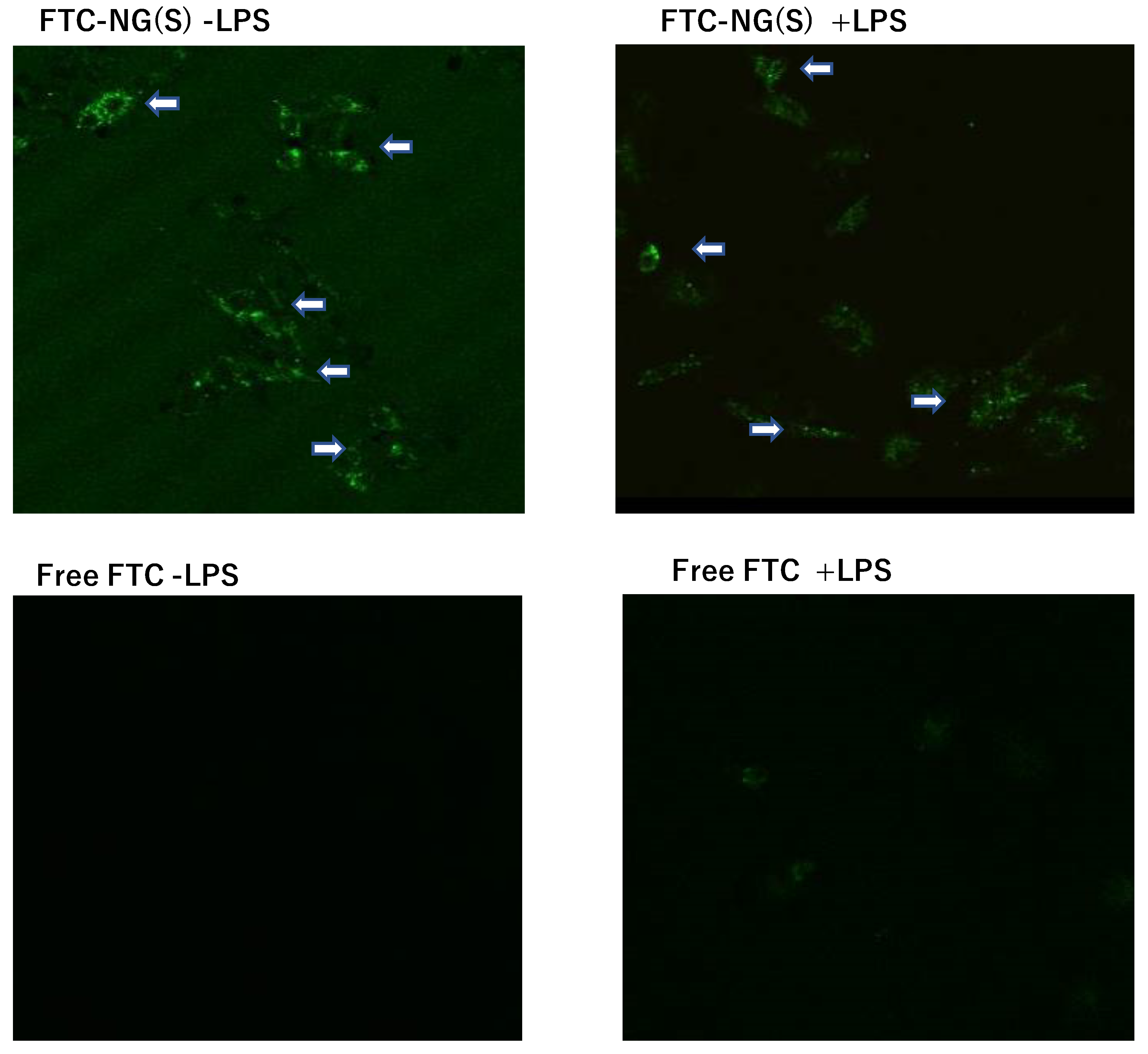
3.6. Cellular Uptake of FTC-NG(S) by Raw 264.7 Cells
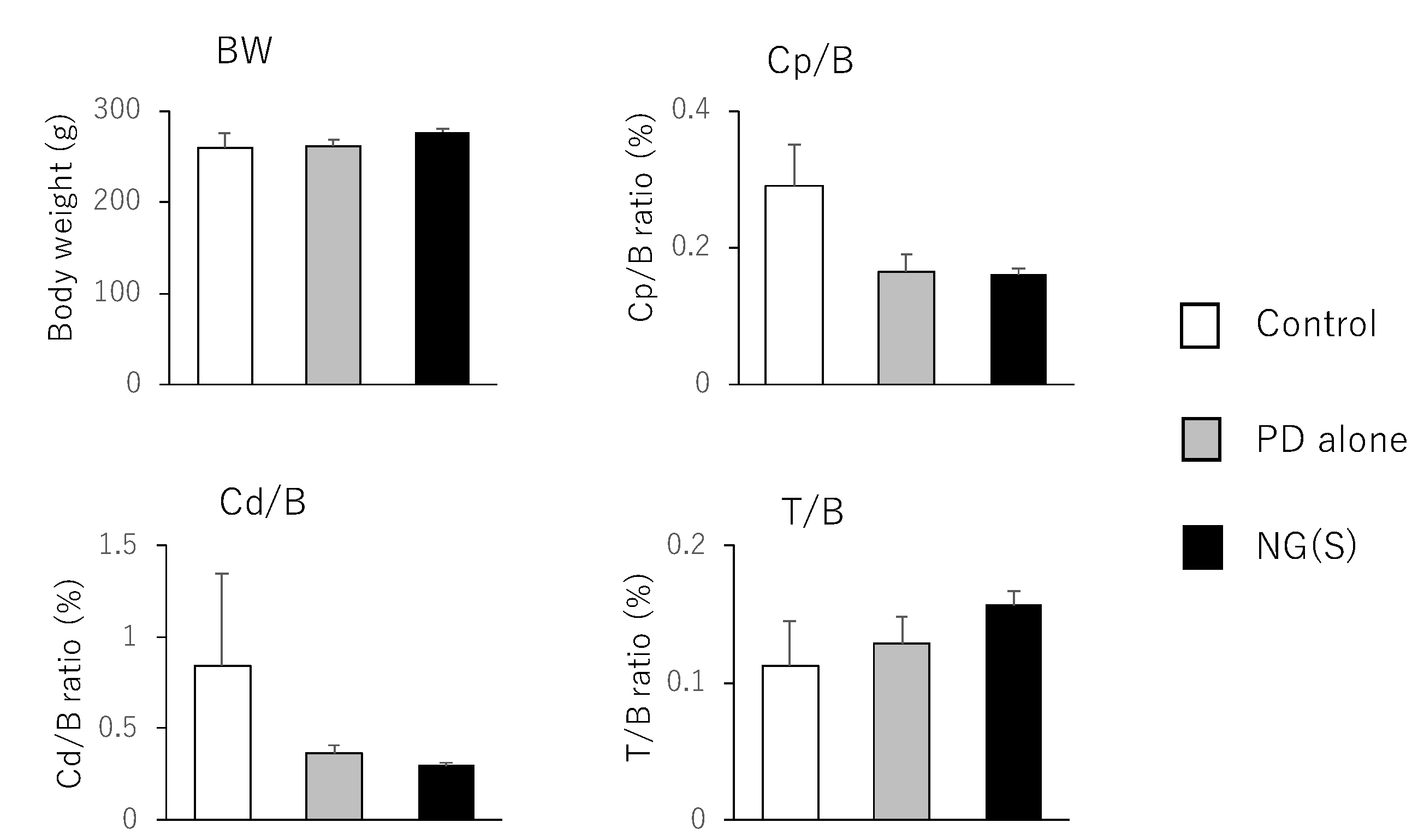
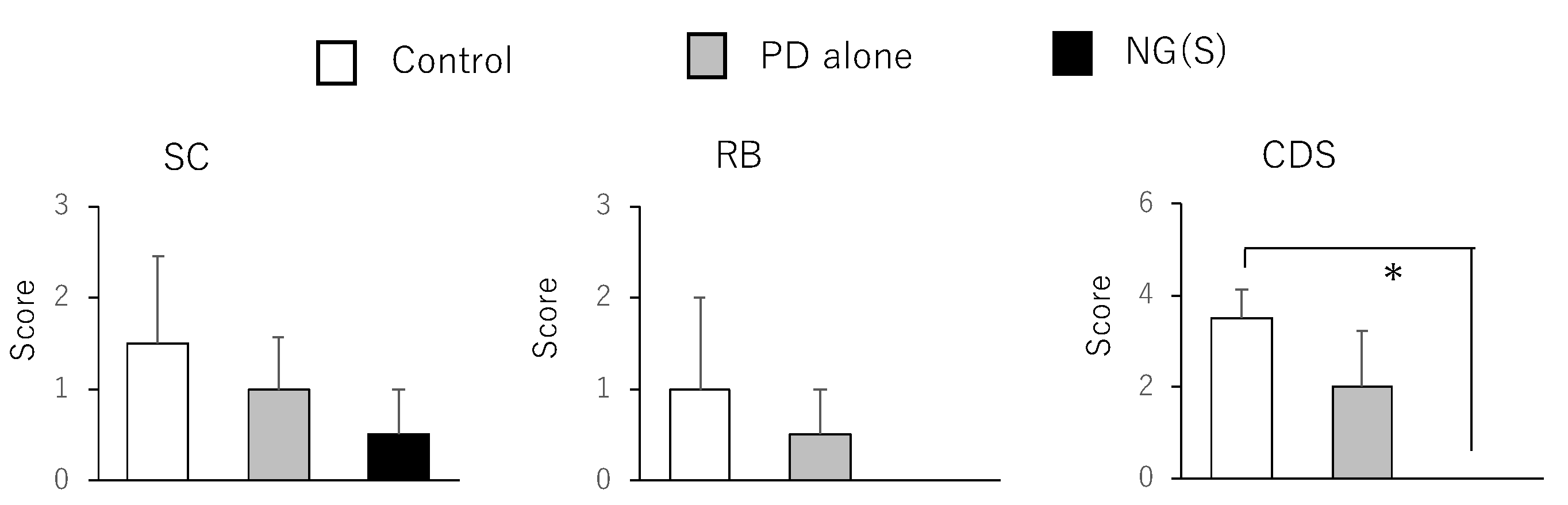
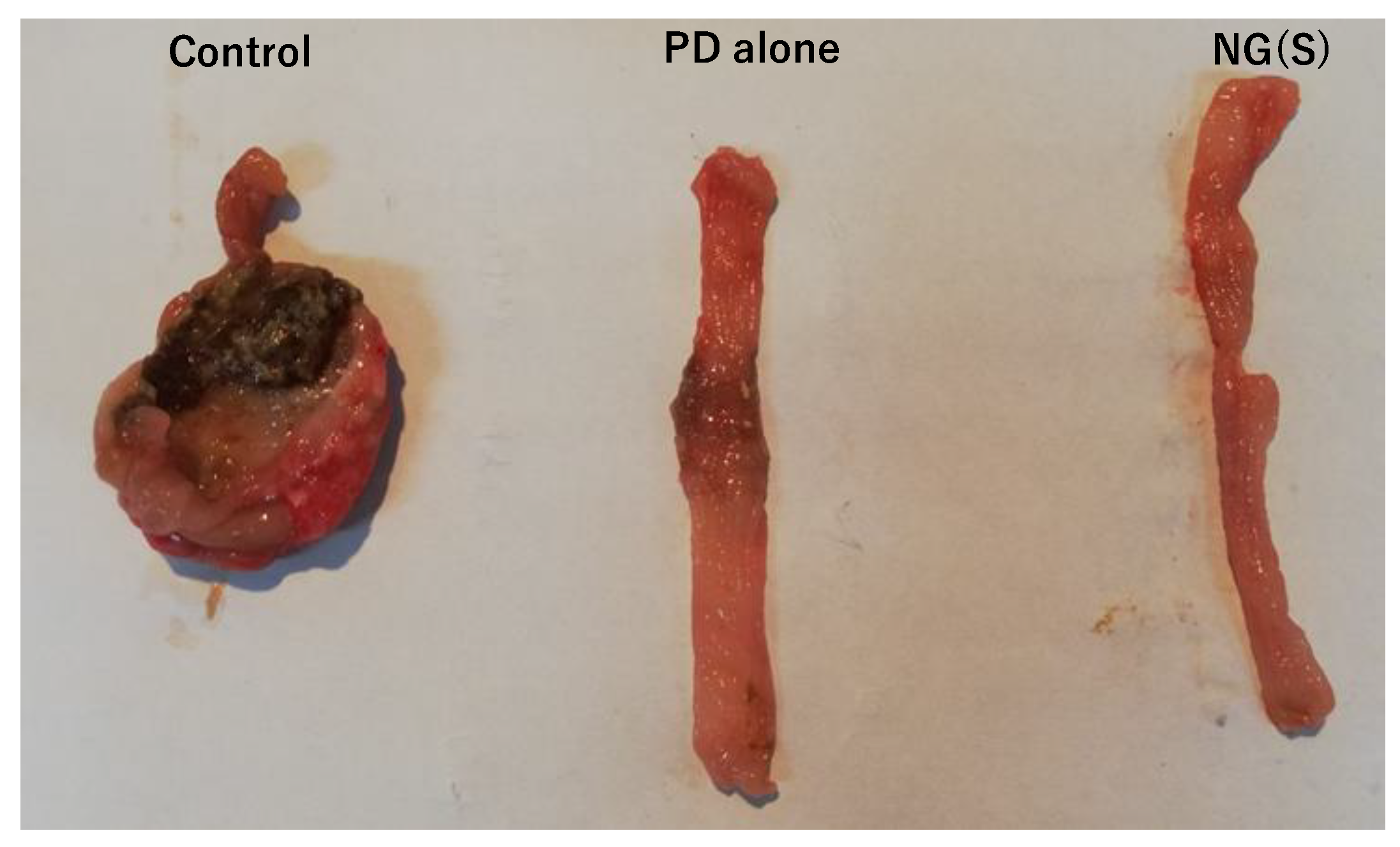
3.7. Efficacy and Toxic Side Effects of NG(S) in Rats with TNBS-Induced UC
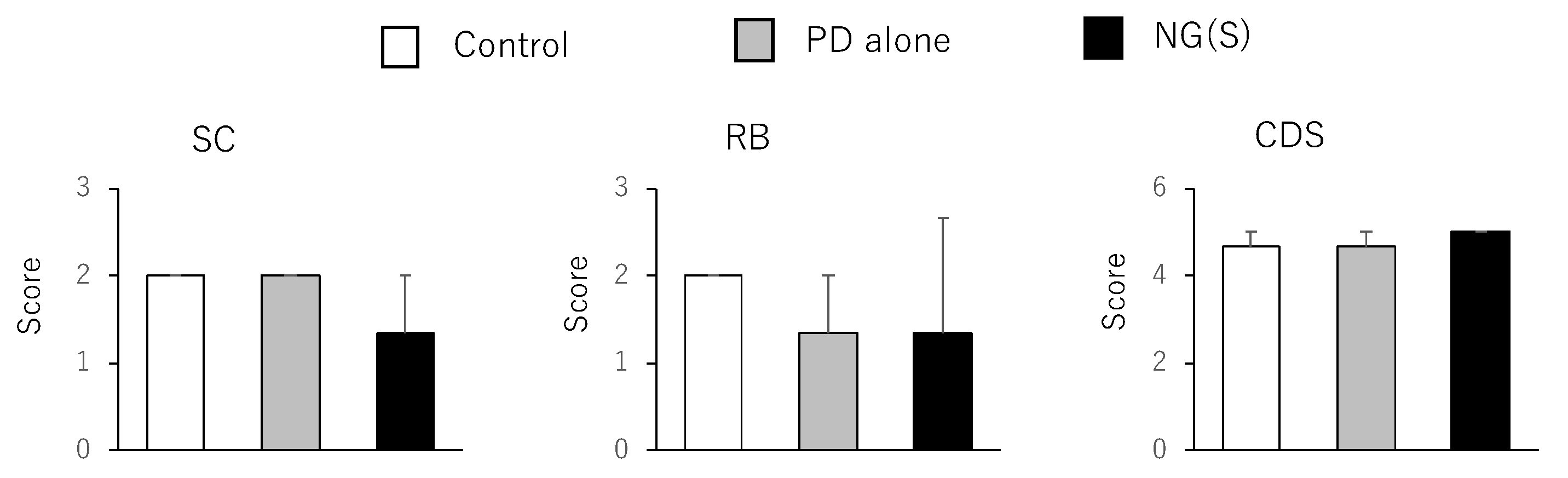
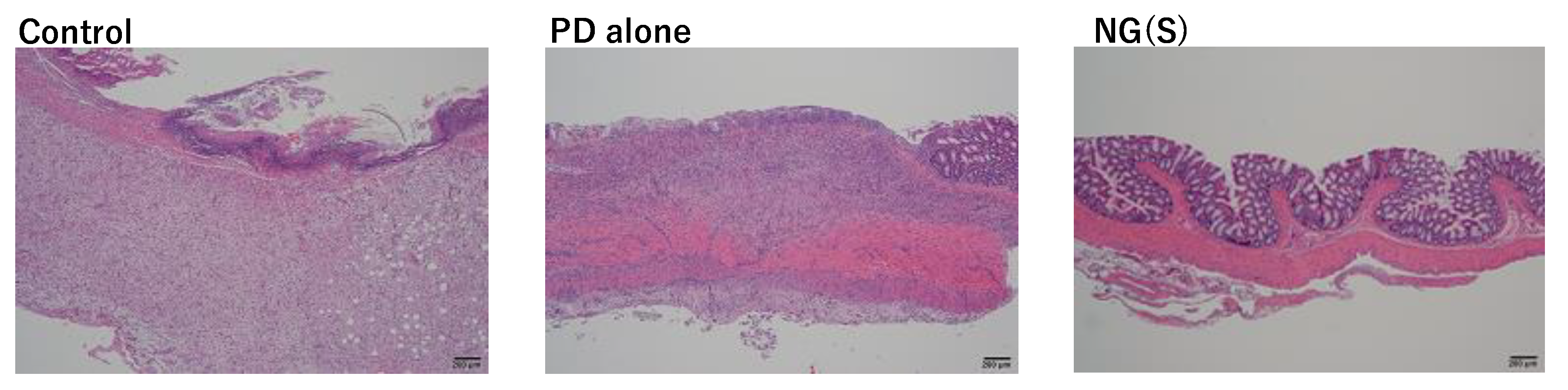
3.8. Histopathological Evaluation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lakatos, P.L.; Lakatos, L. Risk for colorectal cancer in ulcerative colitis: Changes, causes and management strategies. World J. Gastroenterol. 2008, 14, 3937–3947. [Google Scholar] [CrossRef] [PubMed]
- Nakata, Y.; Nakashima, Y.; Kubota, N.; Kaiga, T.; Mamiya, T.; Mihara, Y.; Yamasaki, Y.; Jinno, D.; Kobayashi, M.; Miyata, T.; et al. A case of fulminant ulcerative colitis complicated with toxic megacolon and perforation. J. Nihon Univ. Med. Assoc. 2013, 72, 26–29. [Google Scholar] [CrossRef][Green Version]
- Kirk, A.P.; Cason, J.; Fordham, J.N.; Brown, K.A.; Goddard, D.H.; Holborow, E.J.; Lennard-Jones, J.E. Polymorphonuclear leukocyte function in ulcerative colitis and Crohn’s disease. Dig. Dis. Sci. 1983, 28, 236–248. [Google Scholar] [CrossRef] [PubMed]
- Karlinger, K.; Györke, T.; Makö, E.; Mester, A.; Tarján, Z. The epidemiology and the pathogenesis of inflammatory bowel disease. Eur. J. Radiol. 2000, 35, 154–167. [Google Scholar] [CrossRef]
- Buc, M. Crohns disease and ulcerative colitis-current view on genetic determination, immunopathogenesis and biologic therapy. Epidemiol. Mikrobiol. Imunol. 2017, 66, 189–197. [Google Scholar] [PubMed]
- Mack, D.R. Ongoing advances in inflammatory bowel diseases, including maintenance therapies, biologic agents, and biology of disease. Curr. Opin. Pediatr. 1998, 10, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Varshosaz, J.; Emami, J.; Fassihi, A.; Tavakoli, N.; Minaiyan, M.; Ahmadi, F.; Mahzouni, P.; Dorkoosh, F. Effectiveness of budesonide-succinate-dextran conjugate as a novel prodrug of budesonide against acetic acid-induced colitis in rats. Int. J. Colorectal. Dis. 2010, 25, 1159–1165. [Google Scholar] [CrossRef]
- Bamba, S.; Tsujikawa, T.; Inatomi, O.; Nakahara, T.; Koizumi, Y.; Saitoh, Y.; Sasaki, M.; Fujiyama, Y.; Andoh, A. Factors affecting the efficacy of cyclosporin A therapy for refractory ulcerative colitis. J. Gastroenterol. Hepatol. 2010, 25, 494–498. [Google Scholar] [CrossRef]
- Van Hogezand, R.A. Medical management of patients with difficult-to-treat inflammatory bowel disease. Neth. J. Med. 1994, 45, 55–59. [Google Scholar]
- Reddy, S.I.; Friedman, S.; Telford, J.J.; Strate, L.; Ookubo, R.; Banks, P.A. Are patients with inflammatory bowel disease receiving optimal care? Am. J. Gastroenterol. 2005, 100, 1357–1361. [Google Scholar] [CrossRef]
- Kondo, T.; Amano, K. Era of steroid sparing in the management of immune-mediated inflammatory diseases. Immunol. Med. 2018, 41, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.C.; Sarsour, K.; Gale, S.; Pethö-Schramm, A.; Jick, S.S.; Meier, C.R. Incidence and risk of glucocorticoid-associated adverse effects in patients with rheumatoid arthritis. Arthritis Care Res. 2019, 71, 498–511. [Google Scholar] [CrossRef] [PubMed]
- Girlich, C.; Scholmerich, J. Topical delivery of steroids in inflammatory bowel disease. Curr. Drug Deliv. 2012, 9, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Lamprecht, A.; Ubrich, N.; Yamamoto, H.; Schäfer, U.; Takeuchi, H.; Maincent, P.; Kawashima, Y.; Lehr, C.M. Biodegradable nanoparticles for targeted drug delivery in treatment of inflammatory bowel disease. J. Pharmacol. Exp. Ther. 2001, 299, 775–781. [Google Scholar]
- Yano, H.; Hirayama, F.; Kamada, M.; Arima, H.; Uekama, K. Colon-specific delivery of prednisolone-appended alpha-cyclodextrin conjugate: Alleviation of systemic side effect after oral administration. J. Control. Release 2002, 79, 103–112. [Google Scholar] [CrossRef]
- Tozaki, H.; Odoriba, T.; Okada, N.; Fujita, T.; Terabe, A.; Suzuki, T.; Okabe, S.; Muranishi, S.; Yamamoto, A. Chitosan capsules for colon-specific drug delivery: Enhanced localization of 5-aminosalicylic acid in the large intestine accelerates healing of TNBS-induced colitis in rats. J. Control. Release 2002, 82, 51–61. [Google Scholar] [CrossRef]
- Onishi, H.; Oosegi, T.; Machida, Y. Efficacy and toxicity of Eudragit-coated chitosan-succinyl-prednisolone conjugate microspheres using rats with 2, 4, 6-trinitrobenzenesulfonic acid-induced colitis. Int. J. Pharm. 2008, 358, 296–302. [Google Scholar] [CrossRef]
- Kaur, K.; Kim, K. Studies of chitosan/organic acid/Eudragit RS/RL-coated system for colonic delivery. Int. J. Pharm. 2009, 366, 140–148. [Google Scholar] [CrossRef]
- Xiao, B.; Si, X.; Zhang, M.; Merlin, D. Oral administration of pH-sensitive curcumin-loaded microparticles for ulcerative colitis therapy. Colloids Surf. B Biointerfaces 2015, 135, 379–385. [Google Scholar] [CrossRef]
- Lamprecht, A.; Schäfer, U.; Lehr, C.M. Size-dependent bioadhesion of micro- and nanoparticulate carriers to the inflamed colonic mucosa. Pharm. Res. 2001, 18, 788–793. [Google Scholar] [CrossRef]
- Löfberg, R.; OstergaardThomsen, O.; Langholz, E.; Schiöler, L.; Danielsson, A.; Suhr, O.; Graffner, H.; Påhlman, L.; Matzen, P.; Møller-Petersen, J.F.; et al. Budesonide versus prednisolone retention enemas in active distal ulcerative colitis. Aliment. Pharmacol. Ther. 1994, 8, 623–669. [Google Scholar]
- Lee, F.I.; Jewell, D.P.; Mani, V.; Keighley, M.R.; Kingston, R.D.; Record, C.O.; Grace, R.H.; Daniels, S.; Patterson, J.; Smith, K. A randomised trial comparing mesalazine and prednisolone foam enemas in patients with acute distal ulcerative colitis. Gut 1996, 38, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Onishi, H.; Ikeuchi-Takahashi, Y.; Kawano, K.; Hattori, Y. Preparation of chondroitin sulfate-glycyl-prednisolone conjugate nanogel and its efficacy in rats with ulcerative colitis. Biol. Pharm. Bull. 2019, in press. [Google Scholar] [CrossRef] [PubMed]
- Oosegi, T.; Onishi, H.; Machida, Y. Gastrointestinal distribution and absorption behavior of Eudragit-coated chitosan-prednisolone conjugate microspheres in rats with TNBS-induced colitis. Int. J. Pharm. 2008, 348, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Onishi, H.; Saito, Y.; Sasatsu, M.; Machida, Y. Kinetic analysis of in vitro and in vivo release of prednisolone from the conjugate of glycol-chitosan and succinyl-prednisolone. Int. J. Pharm. 2011, 410, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Onishi, H.; Matsuyama, M. Conjugate between chondroitin sulfate and prednisolone with a glycine linker: Preparation and in vitro conversion analysis. Chem. Pharm. Bull. 2013, 61, 902–912. [Google Scholar] [CrossRef] [PubMed]
- Xiao, B.; Xu, Z.; Viennois, E.; Zhang, Y.; Zhang, Z.; Zhang, M.; Han, M.K.; Kang, Y.; Merlin, D. Orally targeted delivery of tripeptide KPV via hyaluronic acid-functionalized nanoparticles efficiently alleviates ulcerative colitis. Mol. Ther. 2017, 25, 1628–1640. [Google Scholar] [CrossRef]
- Onishi, H. Pharmacokinetic evaluation of chitosan-succinyl-prednisolone conjugate microparticles as a colonic delivery system: Comparison with enteric-coated conjugate microparticles. Health 2014, 6, 611157. [Google Scholar] [CrossRef][Green Version]
- Hamada, M.; Nagai, T.; Kai, N.; Tanoue, Y. Influences of different dissolving method of succinic anhydride on the succinylation level of some proteins. J. Natl. Fish. Univ. 1999, 145–150. [Google Scholar]
- Kato, Y.; Onishi, H.; Machida, Y. Biological fate of highly-succinylated N-succinyl-chitosan and antitumor characteristics of its water-soluble conjugate with mitomycin C at IV and IP administration into tumor-bearing mice. Biol. Pharm. Bull. 2000, 23, 1497–1503. [Google Scholar] [CrossRef][Green Version]
- Morris, G.P.; Beck, P.L.; Herridge, M.S.; Depew, W.T.; Szewczuk, M.R.; Wallace, J.L. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology 1989, 96, 795–803. [Google Scholar] [CrossRef]
- Tozaki, H.; Fujita, T.; Odoriba, T.; Terabe, A.; Okabe, S.; Muranishi, S.; Yamamoto, A. Validation of a pharmacokinetic model of colon-specific drug delivery and the therapeutic effects of chitosan capsules containing 5-aminosalicylic acid on 2,4,6-trinitrobenzenesulphonic acid-induced colitis in rats. J. Pharm. Pharmacol. 1999, 51, 1107–1112. [Google Scholar] [CrossRef] [PubMed]



| Preparation | GCh (mg) | SP (mg) | WSC (mg) | PD Content * (mg) |
|---|---|---|---|---|
| GCh-SP | 100 | 100 | 500 | 23.0 + 2.2 |
| Preparation | GCh-SP (mg) | SA (mg) | PD Content * (mg) |
|---|---|---|---|
| S-GCh-SP | 150 | 1050 | 13.7 ± 1.4 |
| Preparation | Particle Size * (nm) | Zeta Potential * (mV) |
|---|---|---|
| NG(G) | 408 ± 3 | 42.3 ± 0.4 |
| NG(S) | 396 ± 1 | −27.9 ± 0.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, H.; Ichikawa, A.; Ikeuchi-Takahashi, Y.; Hattori, Y.; Onishi, H. Nanogels of Succinylated Glycol Chitosan-Succinyl Prednisolone Conjugate: Preparation, In Vitro Characteristics and Therapeutic Potential. Pharmaceutics 2019, 11, 333. https://doi.org/10.3390/pharmaceutics11070333
Zhou H, Ichikawa A, Ikeuchi-Takahashi Y, Hattori Y, Onishi H. Nanogels of Succinylated Glycol Chitosan-Succinyl Prednisolone Conjugate: Preparation, In Vitro Characteristics and Therapeutic Potential. Pharmaceutics. 2019; 11(7):333. https://doi.org/10.3390/pharmaceutics11070333
Chicago/Turabian StyleZhou, Haiyan, Atsuko Ichikawa, Yuri Ikeuchi-Takahashi, Yoshiyuki Hattori, and Hiraku Onishi. 2019. "Nanogels of Succinylated Glycol Chitosan-Succinyl Prednisolone Conjugate: Preparation, In Vitro Characteristics and Therapeutic Potential" Pharmaceutics 11, no. 7: 333. https://doi.org/10.3390/pharmaceutics11070333
APA StyleZhou, H., Ichikawa, A., Ikeuchi-Takahashi, Y., Hattori, Y., & Onishi, H. (2019). Nanogels of Succinylated Glycol Chitosan-Succinyl Prednisolone Conjugate: Preparation, In Vitro Characteristics and Therapeutic Potential. Pharmaceutics, 11(7), 333. https://doi.org/10.3390/pharmaceutics11070333