Photodegradation Study of Sertindole by UHPLC-ESI-Q-TOF and Influence of Some Metal Oxide Excipients on the Degradation Process
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Sample Preparation
2.3. Irradiation Procedure
2.4. Analytical Procedure
2.5. Chemometric Analysis
2.6. In Silico Evaluation of Sertindole and its TPs Properties
3. Results and Discussion
3.1. Optimization of the LC–ESI–MS/MS Method
3.2. Quantitative Study of the Phototransformation Process
3.2.1. Calibration and Validation of the Method
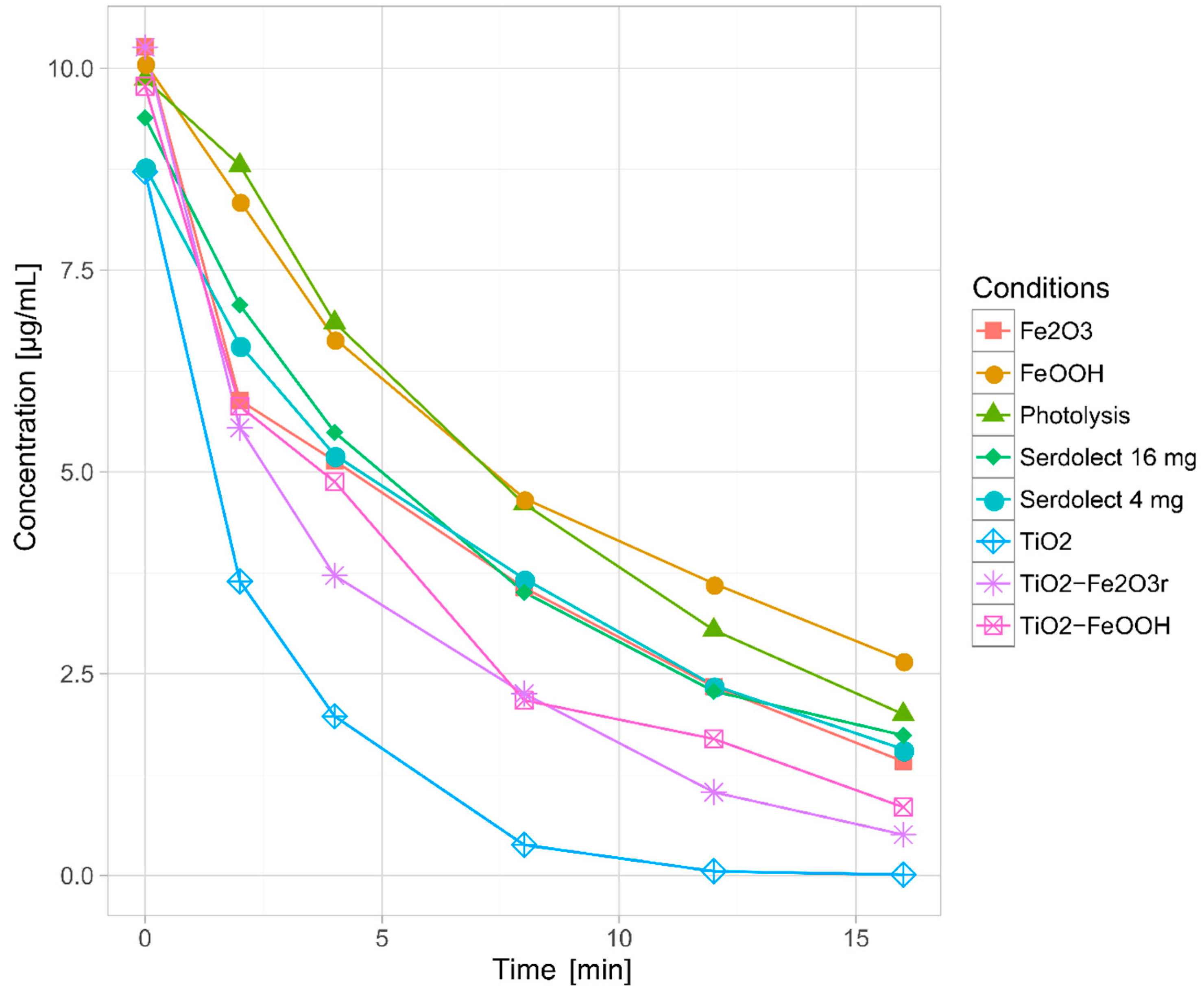
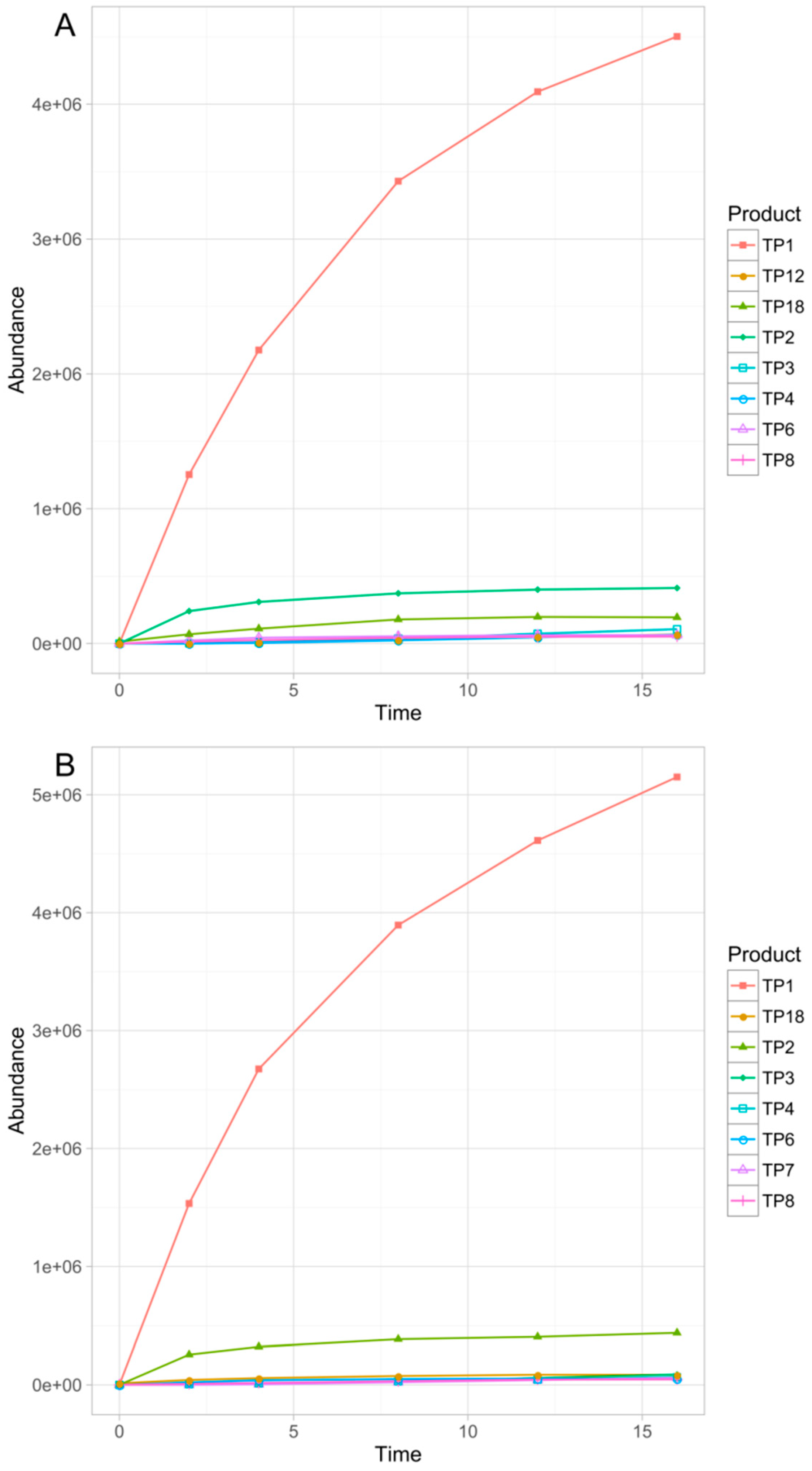
3.2.2. Phototransformation Kinetics
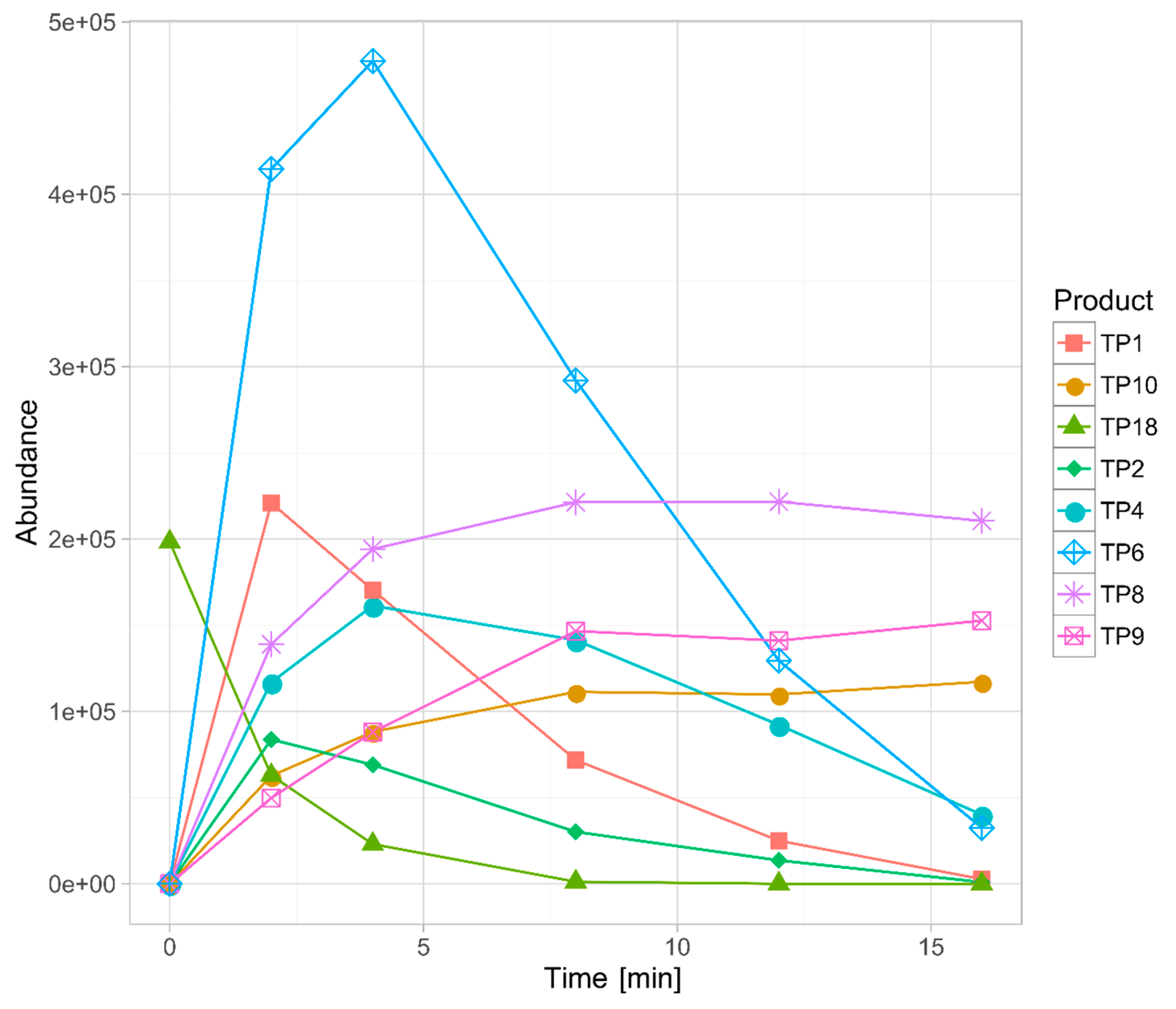
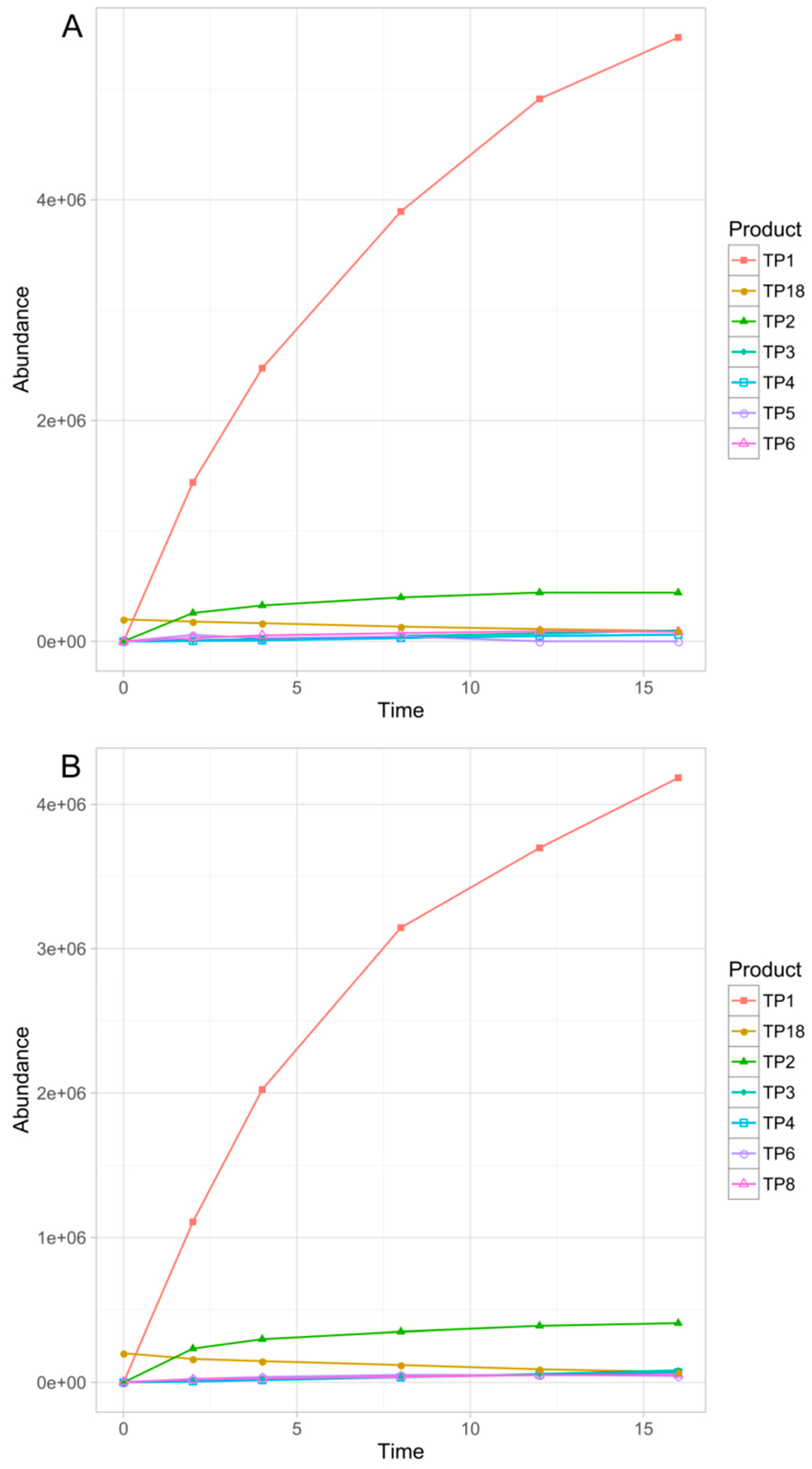
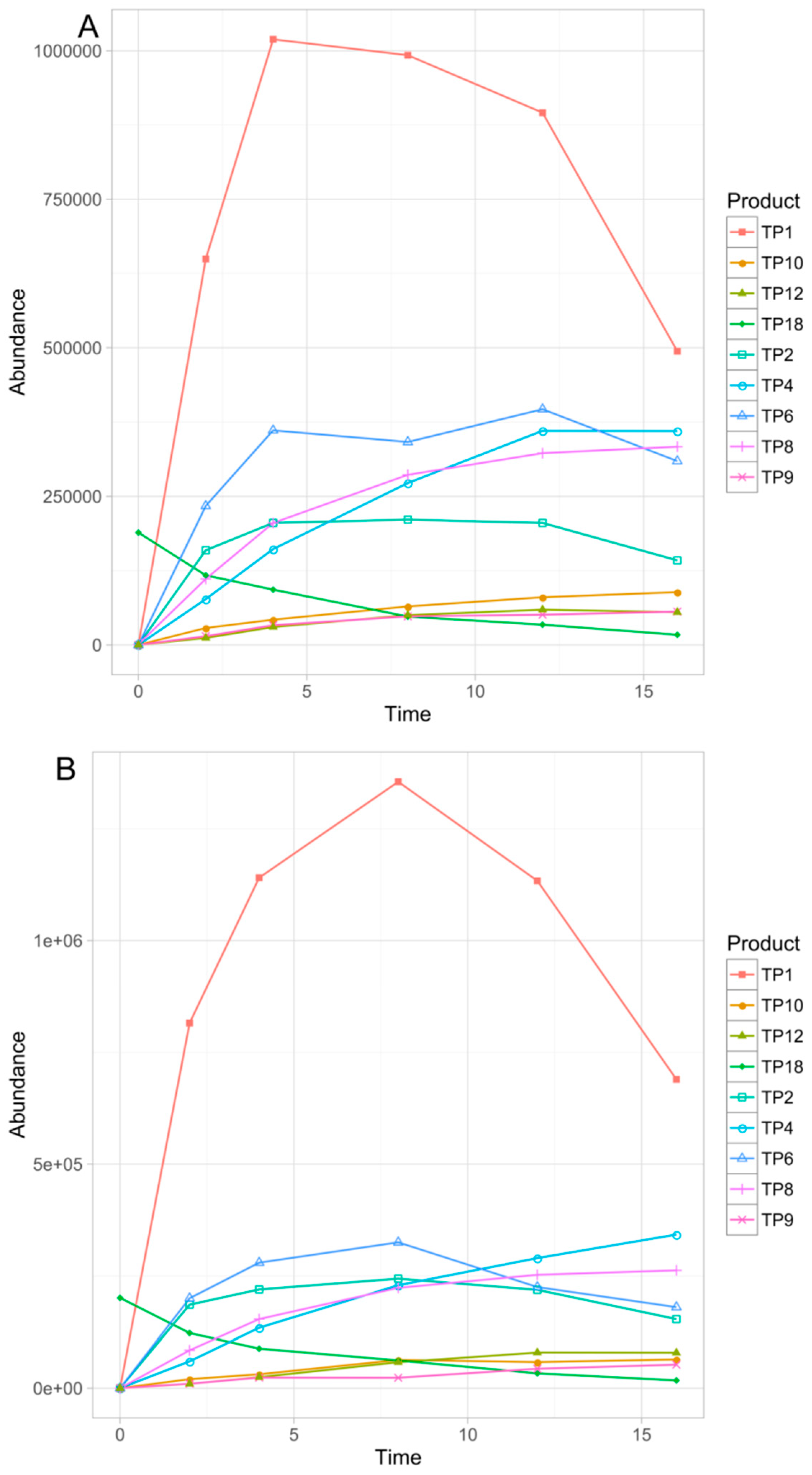
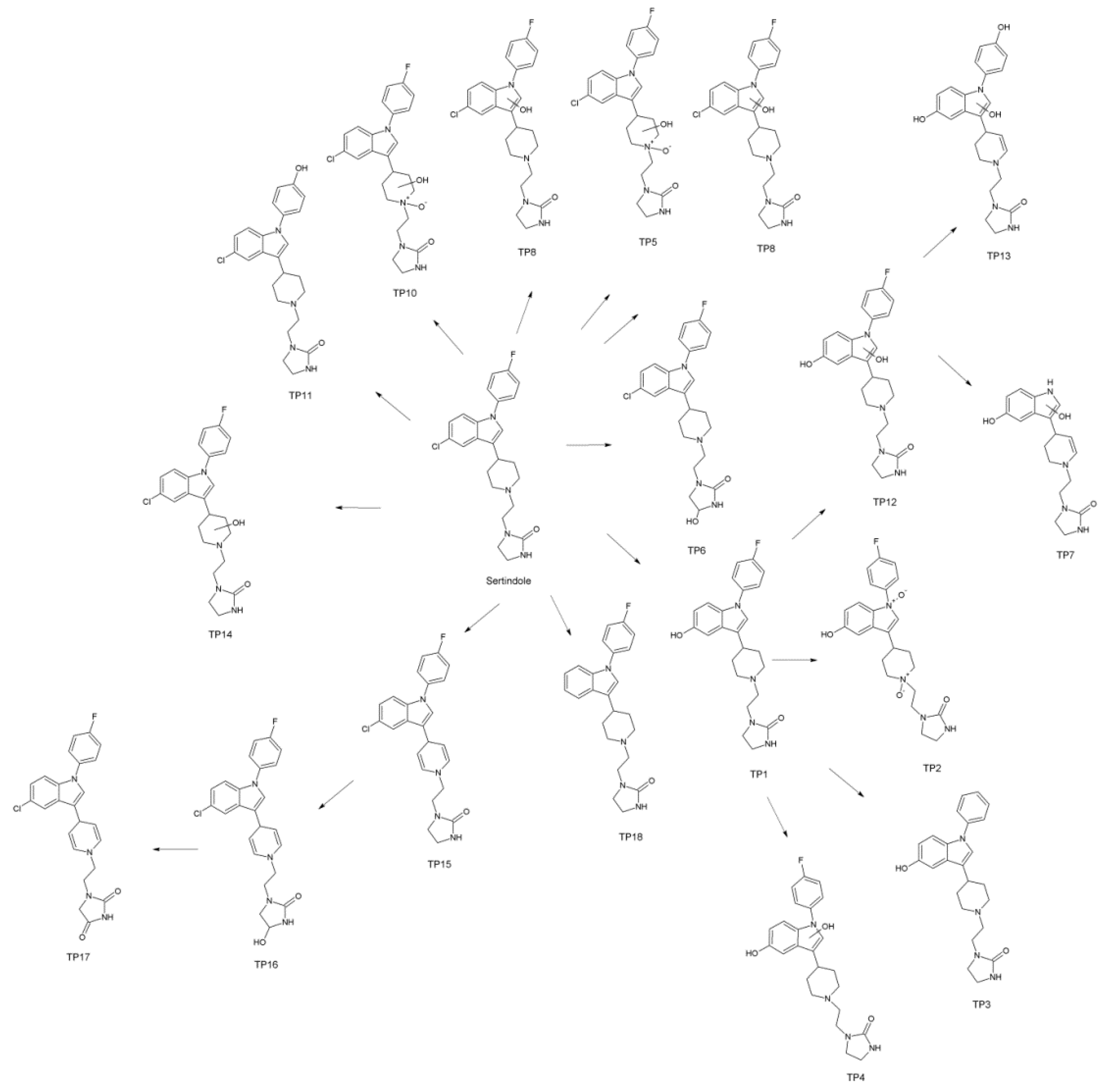
3.3. Identification of the Transformation Products
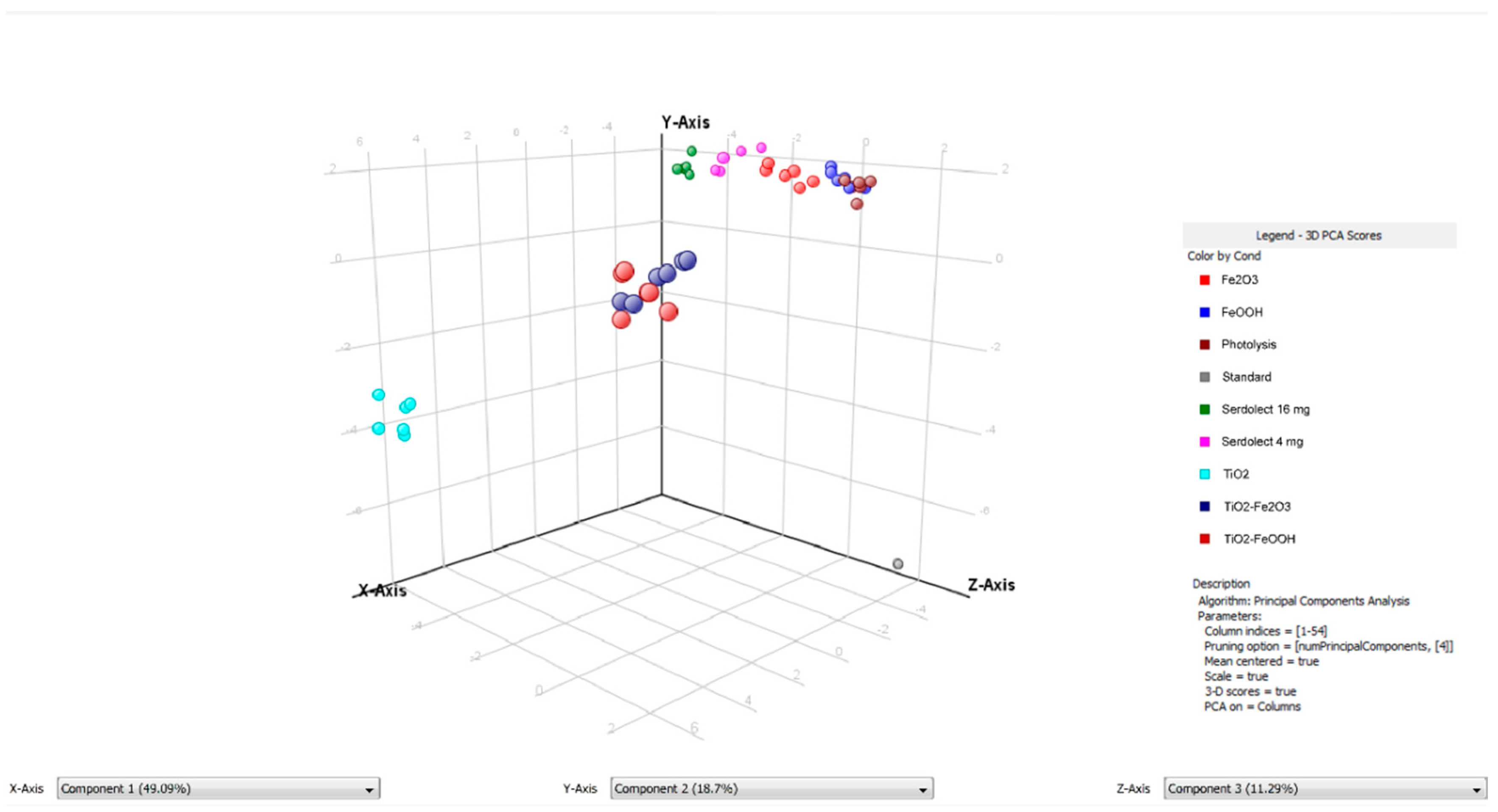
3.4. Chemometric Data Analysis
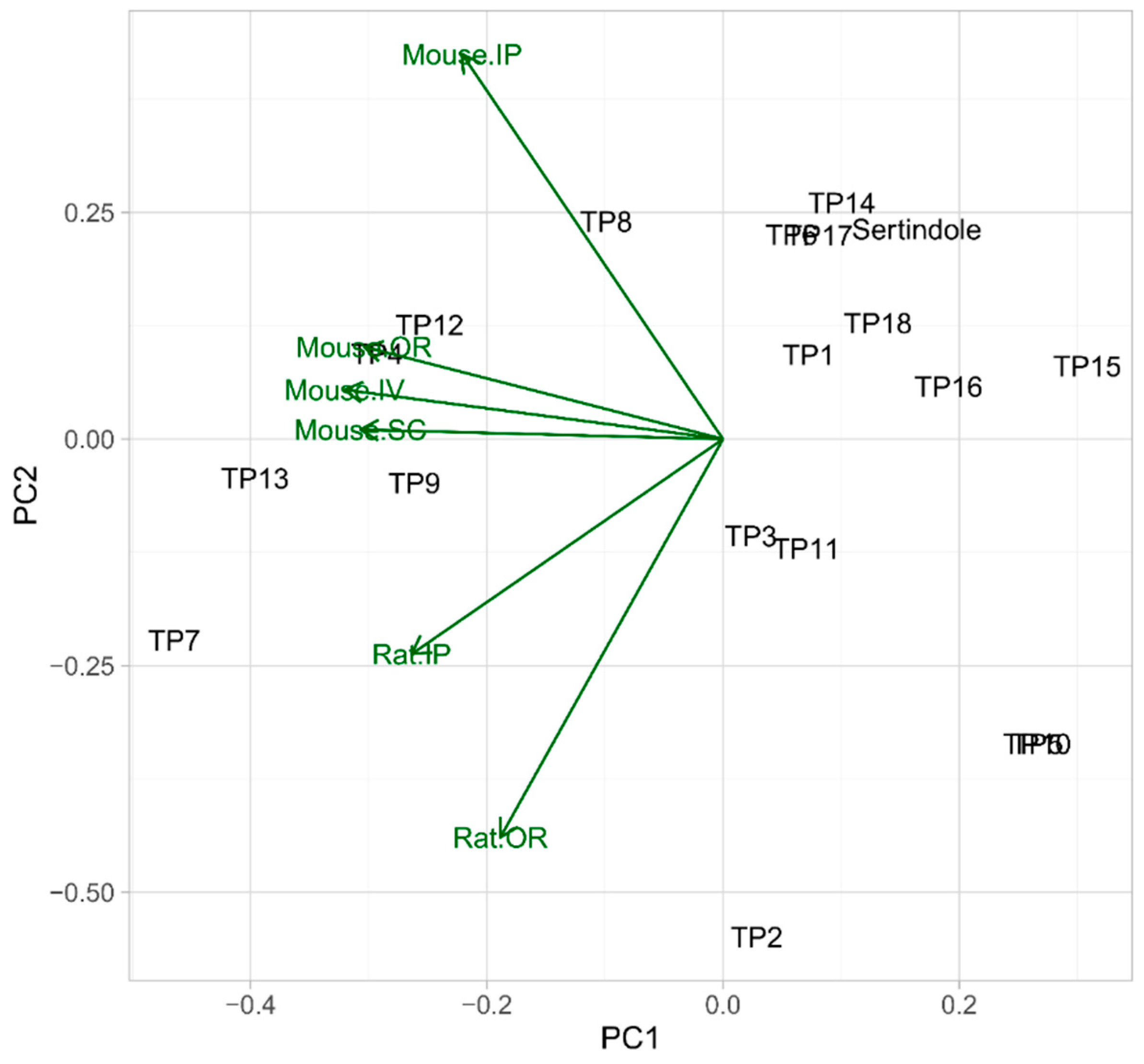
3.5. In Silico Evaluation of TPs Properties
3.5.1. Acute Toxicity to Rodents
3.5.2. Mutagenicity
3.5.3. hERG Inhibition
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Tonnesen, H.H. Photostability of Drugs and Drug Formulations, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2004; ISBN 978-1-4200-2359-6. [Google Scholar]
- Trawiński, J.; Skibiński, R. Studies on photodegradation process of psychotropic drugs: A review. Environ. Sci. Pollut. Res. 2017, 24, 1152–1199. [Google Scholar] [CrossRef] [PubMed]
- International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use. Stability Testing: Photostability Testing of New Drug Substances and Products: - Q1B_Guideline.pdf. Available online: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q1B/Step4/Q1B_Guideline.pdf (accessed on 7 July 2016).
- Di Paola, A.; García-López, E.; Marcì, G.; Palmisano, L. A survey of photocatalytic materials for environmental remediation. J. Hazard. Mater. 2012, 211–212, 3–29. [Google Scholar] [CrossRef] [PubMed]
- Kakinoki, K.; Yamane, K.; Igarashi, M.; Yamamoto, M.; Teraoka, R.; Matsuda, Y. Evaluation of titanium dioxide as a pharmaceutical excipient for preformulation of a photo-labile drug: Effect of physicochemical properties on the photostability of solid-state nisoldipine. Chem. Pharm. Bull. (Tokyo) 2005, 53, 811–815. [Google Scholar] [CrossRef] [PubMed]
- Hyttel, J.; Nielsen, J.B.; Nowak, G. The acute effect of sertindole on brain 5-HT2, D2 and α1 receptors (ex vivo radioreceptor binding studies). J. Neural Transm. 1992, 89, 61–69. [Google Scholar] [CrossRef]
- Sánchez, C.; Arnt, J.; Dragsted, N.; Hyttel, J.; Lembøl, H.L.; Meier, E.; Perregaard, J.; Skarsfeldt, T. Neurochemical and in vivo pharmacological profile of sertindole, a limbic-selective neuroleptic compound. Drug Dev. Res. 1991, 22, 239–250. [Google Scholar] [CrossRef]
- Van Kammen, D.P.; McEvoy, J.P.; Targum, S.D.; Kardatzke, D.; Sebree, T.B. A randomized, controlled, dose-ranging trial of sertindole in patients with schizophrenia. Psychopharmacology (Berl.) 1996, 124, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Moore, N.A. Behavioural pharmacology of the new generation of antipsychotic agents. Br. J. Psychiatry 1999, 174, 5–11. [Google Scholar] [CrossRef]
- Canal-Raffin, M.; Déridet, E.; Titier, K.; Frakra, E.; Molimard, M.; Moore, N. Simplified ultraviolet liquid chromatographic method for determination of sertindole, dehydrosertindole and norsertindole, in human plasma. J. Chromatogr. B 2005, 814, 61–67. [Google Scholar] [CrossRef] [PubMed]
- El-Kosasy, A.M.; Hussein, L.A.; Sedki, N.G.; Salama, N.N. Micelle enhanced and native spectrofluorimetric methods for determination of sertindole using sodium dodecyl sulfate as sensitizing agent. Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 2016, 153, 422–427. [Google Scholar] [CrossRef] [PubMed]
- Tzeng, T.-B.; Stamm, G.; Chu, S. Sensitive method for the assay of sertindole in plasma by high-performance liquid chromatography and fluorimetric detection. J. Chromatogr. B. Biomed. Sci. Appl. 1994, 661, 299–306. [Google Scholar] [CrossRef]
- Choong, E.; Rudaz, S.; Kottelat, A.; Guillarme, D.; Veuthey, J.-L.; Eap, C.B. Therapeutic drug monitoring of seven psychotropic drugs and four metabolites in human plasma by HPLC–MS. J. Pharm. Biomed. Anal. 2009, 50, 1000–1008. [Google Scholar] [CrossRef] [PubMed]
- El-Ragehy, N.A.; Hassan, N.Y.; Abdelkawy, M.; Tantawy, M.A. Stability-indicating chromatographic methods for the determination of sertindole. J. Chromatogr. Sci. 2014, 52, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Trawiński, J.; Skibiński, R.; Komsta, Ł. Comparison of ESI and APCI sources in Q-TOF mass spectrometer in photodegradation study of selected psychotropic drugs. Acta Chromatogr. 2017, 29, 161–172. [Google Scholar] [CrossRef]
- Bandara, J.; Mielczarski, J.A.; Lopez, A.; Kiwi, J. 2. Sensitized degradation of chlorophenols on iron oxides induced by visible light comparison with titanium oxide. Appl. Catal. B Environ. 2001, 34, 321–333. [Google Scholar] [CrossRef]

| Parameters | Results |
|---|---|
| Linearity | |
| Concentration range (μg mL−1) | (0.5:20) |
| Slope | 4.5894 |
| SDa of slope | 0.1143 |
| Intercept | 0.4528 |
| SDa of intercept | 0.1809 |
| Correlation coefficient (r) | 0.9998 |
| LODb (μg mL−1) | 0.034 |
| LOQc (μg mL−1) | 0.100 |
| Precision (RSDd%) | |
| Intra-day (n = 12) | 1.74 |
| Inter-day (n = 18) | 2.53 |
| Accuracy | |
| Recovery (%) | 100.20 |
| RSDd (%) | 2.77 |
| Experiment | Model | Fit (r) | k ± SD (min−1) | t1/2 (min) |
|---|---|---|---|---|
| Direct photolysis | Pseudo-first-order | 0.9989 | 0.0996 ± 5.22 × 10−6 | 6.96 |
| TiO2 | Pseudo-first-order | 0.9989 | 0.4143 ± 3.42 × 10−3 | 1.67 |
| FeOOH yellow | Pseudo-first-order | 0.9963 | 0.0829 ± 8.13 × 10−4 | 8.36 |
| Fe2O3 red | Pseudo-first-order | 0.9874 | 0.1236 ± 8.96 × 10−4 | 5.61 |
| TiO2–FeOOH (yellow) | Pseudo-first-order | 0.9894 | 0.1524 ± 9.64 × 10−5 | 4.55 |
| TiO2–Fe2O3 (red) | Pseudo-first-order | 0.9950 | 0.1871 ± 8.61 × 10−4 | 3.70 |
| Serdolect 4 mg | Pseudo-first-order | 0.9984 | 0.1076 ± 2.60 × 10−3 | 6.44 |
| Serdolect 16 mg | Pseudo-first-order | 0.9948 | 0.1052 ± 1.61 × 10−3 | 6.59 |
| Cpd. | tR (min) | Elemental | Mass (m/z) | Error (ppm) | DBE | Fragmentation (MS/MS) | Occurrence | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Formula (M+H)+ | Exp. | Theo. | Mass (m/z) | Elemental Formula | DPa | Tb | Fyc | Frd | TFye | TFrf | S4g | S16h | ||||
| Sert | 7.06 | C24H27ClFN4O | 441.1858 | 441.1852 | 1.36 | 13 | 355.1379 | C21H21ClFN2 | + | + | + | + | + | + | + | + |
| 329.1200 | C19H19ClFN2 | |||||||||||||||
| 298.0765 | C18H14ClFN | |||||||||||||||
| 270.0464 | C16H10ClFN | |||||||||||||||
| 196.1443 | C10H18N3O | |||||||||||||||
| 168.1131 | C8H14N3O | |||||||||||||||
| 142.0976 | C6H12N3O | |||||||||||||||
| 113.0713 | C5H9N2O | |||||||||||||||
| 99.0562 | C4H7N2O | |||||||||||||||
| 70.0655 | C4H8N | |||||||||||||||
| TP1 | 5.50 | C24H28FN4O2 | 423.2194 | 423.2191 | 0.71 | 13 | 337.1704 | C21H22FN2O | + | + | + | + | + | + | + | + |
| 311.1584 | C19H20FN2O | |||||||||||||||
| 266.0951 | C17H13FNO | |||||||||||||||
| 196.1443 | C10H18N3O | |||||||||||||||
| 168.1123 | C8H14N3O | |||||||||||||||
| 142.0976 | C6H12N3O | |||||||||||||||
| 113.0713 | C5H9N2O | |||||||||||||||
| 84.0810 | C5H10N | |||||||||||||||
| 70.0653 | C4H8N | |||||||||||||||
| TP2 | 5.48 | C24H28FN4O4 | 455.2086 | 455.2089 | −0.66 | 13 | 439.2115 | C24H28FN4O3 | + | + | + | + | + | + | + | + |
| 421.1996 | C24H26FN4O2 | |||||||||||||||
| 194.1250 | C10H16N3O | |||||||||||||||
| 113.0712 | C5H9N2O | |||||||||||||||
| TP3 | 5.31 | C24H29N4O2 | 405.2283 | 405.2285 | −0.49 | 13 | 319.1767 | C21H23N2O | + | − | + | + | + * | + * | + | + |
| 196.1445 | C10H18N3O | |||||||||||||||
| 113.0712 | C5H9N2O | |||||||||||||||
| 84.0807 | C5H10N | |||||||||||||||
| 71.0622 | C3H7N2 | |||||||||||||||
| TP4 | 4.72 | C24H26FN4O3 | 437.1983 | 437.1983 | 0 | 14 | 339.1500 | C20H20FN2O | + | + | + | + | + | + | + | + |
| 296.1094 | C18H15FNO2 | |||||||||||||||
| 194.1286 | C10H16N3O | |||||||||||||||
| 168.1123 | C8H14N3O | |||||||||||||||
| 142.0950 | C6H12N3O | |||||||||||||||
| 113.0705 | C5H9N2O | |||||||||||||||
| TP5 | 5.40 | C24H27ClFN4O3 | 473.1742 | 473.1750 | −1.69 | 13 | 445.1759 | C23H27ClFN4O2 | + | + * | + | + * | + * | + * | − | + * |
| 439.1637 | C24H25ClFN4O | |||||||||||||||
| 387.1273 | C21H21ClFN2O2 | |||||||||||||||
| 359.1309 | C20H21ClFN2O | |||||||||||||||
| 316.0954 | C18H16ClFNO | |||||||||||||||
| 194.1297 | C10H16N3O | |||||||||||||||
| 182.0912 | C8H12N3O2 | |||||||||||||||
| 113.0709 | C5H9N2O | |||||||||||||||
| 85.0768 | C4H9N2 | |||||||||||||||
| 71.0616 | C3H7N2 | |||||||||||||||
| TP6 | 6.89 | C24H27ClFN4O2 | 457.1805 | 457.1801 | 0.87 | 13 | 439.1635 | C24H25ClFN4O | + | + | + | + | + | + | + | + |
| 355.1355 | C21H21ClFN2 | |||||||||||||||
| 194.1282 | C10H16N3O | |||||||||||||||
| 168.1125 | C8H14N3O | |||||||||||||||
| 129.0674 | C5H9N2O2 | |||||||||||||||
| 113.0709 | C5H9N2O | |||||||||||||||
| 111.0553 | C5H7N2O | |||||||||||||||
| 84.0784 | C5H10N | |||||||||||||||
| TP7 | 2.42 | C18H23N4O3 | 343.1778 | 343.1765 | 4.08 | 10 | 257.131 | C15H17N2O2 | + | − | + * | + * | + * | + * | + * | + |
| 245.1286 | C14H17N2O2 | |||||||||||||||
| 214.0869 | C13H12NO2 | |||||||||||||||
| 201.0787 | C12H11NO2 | |||||||||||||||
| 194.1282 | C10H16N3O | |||||||||||||||
| 168.1123 | C8H14N3O | |||||||||||||||
| 142.0981 | C6H12N3O | |||||||||||||||
| 113.0705 | C5H9N2O | |||||||||||||||
| TP8 | 5.93 | C24H27ClFN4O2 | 457.1810 | 457.1801 | 1.97 | 13 | 371.1311 | C21H21ClFN2O | + * | + | + * | + | + | + | + | + * |
| 345.1139 | C19H19ClFN2O | |||||||||||||||
| 328.0858 | C19H16ClFNO | |||||||||||||||
| 194.1247 | C10H16N3O | |||||||||||||||
| 113.0713 | C5H9N2O | |||||||||||||||
| 70.0657 | C4H8N | |||||||||||||||
| TP9 | 5.28 | C24H27ClFN4O4 | 489.1704 | 489.1699 | 1.02 | 13 | 471.1599 | C24H25ClFN4O3 | + * | + | − | + * | + | + | − | − |
| 443.1607 | C23H25ClFN4O2 | |||||||||||||||
| 387.1255 | C21H21ClFN4O2 | |||||||||||||||
| 361.1126 | C19H19ClFN2O2 | |||||||||||||||
| 210.1219 | C10H16N3O2 | |||||||||||||||
| 129.0662 | C5H9N2O2 | |||||||||||||||
| 113.0707 | C5H9N2O | |||||||||||||||
| 111.0547 | C5H7N2O | |||||||||||||||
| 69.0505 | C3H5N2 | |||||||||||||||
| TP10 | 5.09 | C24H27ClFN4O3 | 473.1735 | 473.1750 | −3.17 | 13 | 455.1604 | C23H27ClFN4O2 | + * | + | + * | + * | + | + | + * | + * |
| 387.1265 | C21H21ClFN2O2 | |||||||||||||||
| 326.0723 | C19H14ClFNO | |||||||||||||||
| 300.0601 | C17H12ClFNO | |||||||||||||||
| 210.1259 | C10H16N3O2 | |||||||||||||||
| 194.1274 | C10H16N3O | |||||||||||||||
| 142.0974 | C6H12N3O | |||||||||||||||
| 113.0708 | C5H9N2O | |||||||||||||||
| TP11 | 6.03 | C24H28ClN4O2 | 439.1891 | 439.1895 | −0.91 | 13 | 353.1431 | C21H22ClN2O | + * | + * | + * | + * | + * | + * | + * | + * |
| 196.1421 | C10H18N3O | |||||||||||||||
| 113.0712 | C5H9N2O | |||||||||||||||
| TP12 | 4.54 | C24H28FN4O3 | 439.2139 | 439.2140 | −0.23 | 13 | 353.1632 | C21H22FN2O2 | + * | + * | + * | + * | + | + | + | + * |
| 327.1488 | C19H20FN2O2 | |||||||||||||||
| 310.1222 | C19H17FNO2 | |||||||||||||||
| 194.1247 | C10H16N3O | |||||||||||||||
| 113.0707 | C5H9N2O | |||||||||||||||
| TP13 | 3.89 | C24H27N4O4 | 435.2036 | 435.2027 | 2.07 | 14 | 194.1297 | C10H16N3O | + * | − | + * | + * | + * | + * | + * | + * |
| 168.1122 | C8H14N3O | |||||||||||||||
| 113.0705 | C5H9N2O | |||||||||||||||
| TP14 | 6.35 | C24H27ClFN4O2 | 457.1802 | 457.1801 | 0.22 | 13 | 142.0964 | C6H12N3O | − | + * | + * | + * | + * | + * | + * | + * |
| 113.0709 | C5H9N2O | |||||||||||||||
| TP15 | 6.57 | C24H23ClFN4O | 437.1516 | 437.1539 | −5.26 | 15 | 325.0877 | C19H15ClFN2 | + * | + * | + * | + * | + * | + * | + * | + * |
| 308.0628 | C19H12ClFN | |||||||||||||||
| 113.0710 | C5H9N2O | |||||||||||||||
| TP16 | 6.35 | C24H23ClFN4O2 | 453.1474 | 453.1488 | −3.09 | 15 | 435.1362 | C24H21ClFN4O | − | + * | − | − | + * | + * | + * | − |
| 351.1076 | C21H17ClFN2 | |||||||||||||||
| 325.0861 | C19H15ClFN2 | |||||||||||||||
| 308.0643 | C19H12ClFN | |||||||||||||||
| 129.0654 | C5H9N2O2 | |||||||||||||||
| 113.0707 | C5H9N2O | |||||||||||||||
| 111.0547 | C5H7N2O | |||||||||||||||
| TP17 | 6.66 | C24H21ClFN4O2 | 451.1304 | 451.1332 | −6.21 | 16 | 325.0805 | C19H15ClFN2 | + * | + * | − | − | + * | + * | + * | − |
| 308.0537 | C19H12ClFN | |||||||||||||||
| 258.0515 | C15H10ClFN | |||||||||||||||
| 127.0502 | C5H7N2O2 | |||||||||||||||
| 99.0568 | C4H7N2O | |||||||||||||||
| TP18 | 6.52 | C24H28FN4O | 407.2229 | 407.2242 | −3.19 | 13 | 321.1769 | C21H22FN2 | + | + | + | + | + | + | + | + |
| 196.1423 | C10H18N3O | |||||||||||||||
| 113.0710 | C5H9N2O | |||||||||||||||
| Compound | Mutagenicity | hERG inhibition |
|---|---|---|
| Sertindole | 0.39 | 0.95 |
| TP1 | 0.4 | 0.71 |
| TP2 | 0.58 | 0.33 |
| TP3 | 0.4 | 0.57 |
| TP4 | 0.4 | 0.37 |
| TP5 | 0.48 | 0.79 |
| TP6 | 0.38 | 0.87 |
| TP7 | 0.41 | 0.06 |
| TP8 | 0.26 | 0.92 |
| TP9 | 0.25 | 0.68 |
| TP10 | 0.48 | 0.79 |
| TP11 | 0.26 | 0.75 |
| TP12 | 0.26 | 0.72 |
| TP13 | 0.36 | 0.21 |
| TP14 | 0.37 | 0.88 |
| TP15 | 0.78 | 0.53 |
| TP16 | 0.84 | 0.37 |
| TP17 | 0.7 | 0.4 |
| TP18 | 0.45 | 0.92 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trawiński, J.; Skibiński, R. Photodegradation Study of Sertindole by UHPLC-ESI-Q-TOF and Influence of Some Metal Oxide Excipients on the Degradation Process. Pharmaceutics 2019, 11, 299. https://doi.org/10.3390/pharmaceutics11070299
Trawiński J, Skibiński R. Photodegradation Study of Sertindole by UHPLC-ESI-Q-TOF and Influence of Some Metal Oxide Excipients on the Degradation Process. Pharmaceutics. 2019; 11(7):299. https://doi.org/10.3390/pharmaceutics11070299
Chicago/Turabian StyleTrawiński, Jakub, and Robert Skibiński. 2019. "Photodegradation Study of Sertindole by UHPLC-ESI-Q-TOF and Influence of Some Metal Oxide Excipients on the Degradation Process" Pharmaceutics 11, no. 7: 299. https://doi.org/10.3390/pharmaceutics11070299
APA StyleTrawiński, J., & Skibiński, R. (2019). Photodegradation Study of Sertindole by UHPLC-ESI-Q-TOF and Influence of Some Metal Oxide Excipients on the Degradation Process. Pharmaceutics, 11(7), 299. https://doi.org/10.3390/pharmaceutics11070299







