Application of Size and Maturation Functions to Population Pharmacokinetic Modeling of Pediatric Patients
Abstract
:1. Introduction
2. Methods
2.1. Categorization of Pediatric Patients Based on Physiological Conditions
2.2. Data Collection for CsA, PHB and VAN
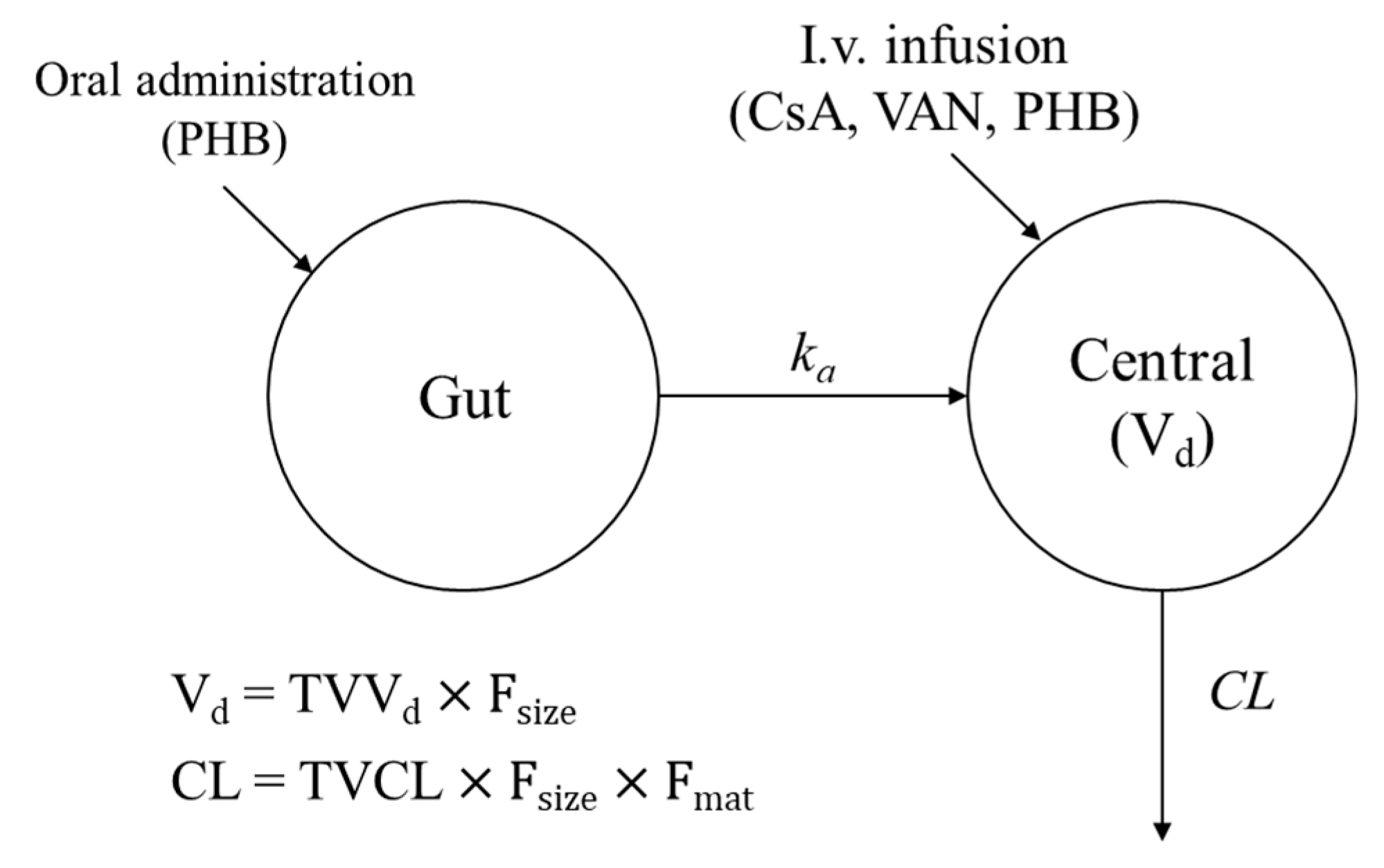
2.3. Development of a Structural Model
2.4. Incorporation of Size and Maturation Functions in the Structural Model
2.5. Steps for Covariate Searching
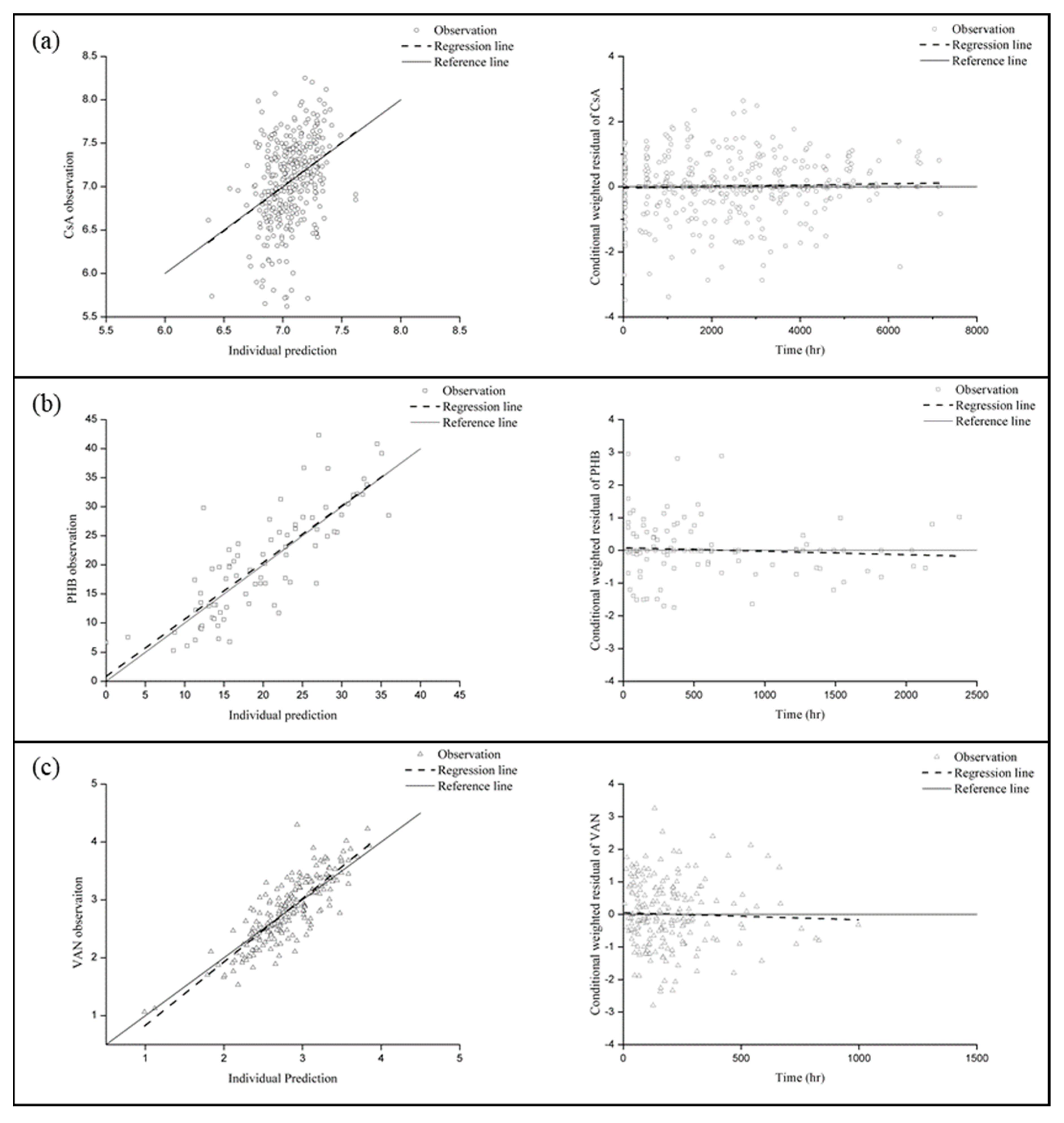
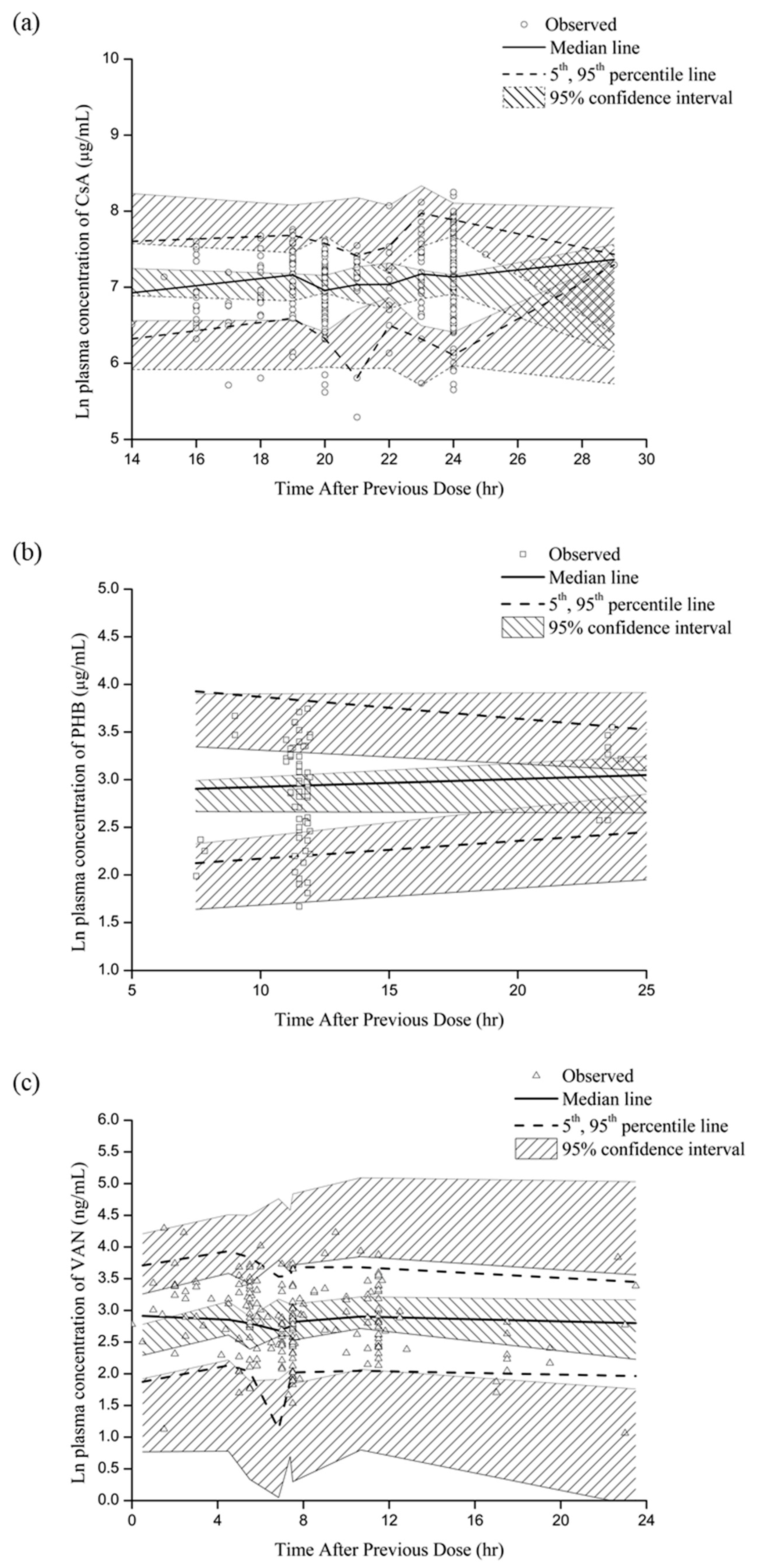
2.6. Model Evaluation
3. Results
3.1. Demographic Characteristics
3.2. Structural Model Development
3.3. Covariate Searching for Size and Maturation Functions
3.4. Final Model Selection and Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Anderson, B.J.; Holford, N.H.G. Mechanism-based concepts of size and maturity in pharmacokinetics. Annu. Rev. Pharmacol. Toxicol. 2008, 48, 303–332. [Google Scholar] [CrossRef] [PubMed]
- Holford, N.; Heo, Y.-A.; Anderson, B. A pharmacokinetic standard for babies and adults. J. Pharm. Sci. 2013, 102, 2941–2952. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, T. How children’s responses to drugs differ from adults. Br. J. Clin. Pharmacol. 2005, 59, 670–673. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, E.; Perez, R.; Hernandez, A.; Tejada, P.; Arteta, M.; Ramos, J.T. Factors and mechanisms for pharmacokinetic differences between pediatric population and adults. Pharmaceutics 2011, 3, 53–72. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.D. Children versus adults: Pharmacokinetic and adverse-effect differences. Epilepsia 2002, 43 (Suppl. 3), 53–59. [Google Scholar] [CrossRef]
- Lu, H.; Rosenbaum, S. Developmental pharmacokinetics in pediatric populations. J. Pediatr. Pharmacol. Ther. 2014, 19, 262–276. [Google Scholar]
- Food and Drug Administration, HHS. International conference on harmonisation; guidance on E11 clinical investigation of medicinal products in the pediatric population; availability. Notice. Fed. Regist. 2000, 65, 78493–78494. [Google Scholar]
- Blanco, J.G.; Harrison, P.L.; Evans, W.E.; Relling, M.V. Human cytochrome P450 maximal activities in pediatric versus adult liver. Drug Metab. Dispos. 2000, 28, 379–382. [Google Scholar]
- Alcorn, J.; McNamara, P.J. Pharmacokinetics in the newborn. Adv. Drug Deliv. Rev. 2003, 55, 667–686. [Google Scholar] [CrossRef]
- Miller, M.D.; Marty, M.A.; Arcus, A.; Brown, J.; Morry, D.; Sandy, M. Differences between children and adults: Implications for risk assessment at California EPA. Int. J. Toxicol. 2002, 21, 403–418. [Google Scholar] [CrossRef]
- Kahn, M.A.; Reddy, I.K. Pediatric and geriatric dosing. In Pharmaceutical and Clinical Calculations; CRC Press: Boca Raton, FL, USA, 2000; pp. 263–288. [Google Scholar]
- Munzenberger, P.J.; McKercher, P. Pediatric dosing—The pharmacist’s dilemma. Contemp. Pharm. Pract. 1980, 3, 11–14. [Google Scholar]
- Elias, G.P.; Antoniali, C.; Mariano, R.C. Comparative study of rules employed for calculation of pediatric drug dosage. J. Appl. Oral. Sci. 2005, 13, 114–119. [Google Scholar] [CrossRef]
- Batchelor, H.K.; Marriott, J.F. Paediatric pharmacokinetics: Key considerations. Br. J. Clin. Pharmacol. 2015, 79, 395–404. [Google Scholar] [CrossRef]
- Ginsberg, G.; Hattis, D.; Sonawane, B.; Russ, A.; Banati, P.; Kozlak, M.; Smolenski, S.; Goble, R. Evaluation of child/adult pharmacokinetic differences from a database derived from the therapeutic drug literature. Toxicol. Sci. 2002, 66, 185–200. [Google Scholar] [CrossRef]
- Holford, N.H.G. The target concentration approach to clinical drug development. Clin. Pharmacokinet. 1995, 29, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Tod, M.; Jullien, V.; Pons, G. Facilitation of drug evaluation in children by population methods and modelling. Clin. Pharmacokinet. 2008, 47, 231–243. [Google Scholar] [CrossRef]
- Savage, V.M.; Deeds, E.J.; Fontana, W. Sizing up allometric scaling theory. PLoS Comput. Biol. 2008, 4, e1000171. [Google Scholar] [CrossRef] [PubMed]
- Calvier, E.A.M.; Krekels, E.H.J.; Välitalo, P.A.J.; Rostami-Hodjegan, A.; Tibboel, D.; Danhof, M. Allometric scaling of clearance in paediatric patients: When does the magic of 0.75 fade? Clin. Pharmacokinet. 2017, 56, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Ghafoori, P.; Gobburu, J.V.S. Allometry is a reasonable choice in pediatric drug development. J. Clin. Pharmacol. 2017, 57, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Edginton, A.N.; Shah, B.; Sevestre, M.; Momper, J.D. The integration of allometry and virtual populations to predict clearance and clearance variability in pediatric populations over the age of 6 years. Clin. Pharmacokinet. 2013, 52, 693–703. [Google Scholar] [CrossRef] [PubMed]
- Anderson, B.J.; Holford, N.H.G. Tips and traps analyzing pediatric PK data. Paediatr. Anaesth. 2011, 21, 222–237. [Google Scholar] [CrossRef] [PubMed]
- Koren, G.; Lau, A.; Klein, J.; Golas, C.; Bologa-Campeanu, M.; Soldin, S.; MacLeod, S.M.; Prober, C. Pharmacokinetics and adverse effects of amphotericin B in infants and children. J. Pediatr. 1988, 113, 559–563. [Google Scholar] [CrossRef]
- Konstan, M.W.; Hoppel, C.L.; Chai, B.L.; Davis, P.B. Ibuprofen in children with cystic fibrosis: Pharmacokinetics and adverse effects. J. Pediatr. 1991, 118, 956–964. [Google Scholar] [CrossRef]
- Andrews, L.M.; Hesselink, D.A.; van Gelder, T.; Koch, B.C.P.; Cornelissen, E.A.M.; Brüggemann, R.J.M.; van Schaik, R.H.N.; de Wildt, S.N.; Cransberg, K.; de Winter, B.C.M. A population pharmacokinetic model to predict the individual starting dose of tacrolimus following pediatric renal transplantation. Clin. Pharmacokinet. 2018, 57, 475–489. [Google Scholar] [CrossRef]
- Neely, M.N.; Rakhmanina, N.Y. Pharmacokinetic optimization of antiretroviral therapy in children and adolescents. Clin. Pharmacokinet. 2011, 50, 143–189. [Google Scholar] [CrossRef]
- Kadam, R.S.; Van Den Anker, J.N. Pediatric clinical pharmacology of voriconazole: Role of pharmacokinetic/pharmacodynamic modeling in pharmacotherapy. Clin. Pharmacokinet. 2016, 55, 1031–1043. [Google Scholar] [CrossRef]
- Svensson, E.M.; Yngman, G.; Denti, P.; McIlleron, H.; Kjellsson, M.C.; Karlsson, M.O. Evidence-based design of fixed-dose combinations: Principles and application to pediatric anti-tuberculosis therapy. Clin. Pharmacokinet. 2018, 57, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Béranger, A.; Benaboud, S.; Urien, S.; Moulin, F.; Bille, E.; Lesage, F.; Zheng, Y.; Genuini, M.; Gana, I.; Renolleau, S.; et al. Piperacillin population pharmacokinetics and dosing regimen optimization in critically ill children with normal and augmented renal clearance. Clin. Pharmacokinet. 2019, 58, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Ree, Y.S.; Back, H.M.; Yun, H.Y.; Ahn, J.H.; Son, E.S.; Han, J.W.; Lyu, C.J.; Rhie, S.J. Dose optimization based on population pharmacokinetic modeling of high-dose cyclosporine, a p-glycoprotein inhibitor, in combination with systemic chemotherapy in pediatric patients with retinoblastoma. J. Ocul. Pharmacol. Ther. 2018, 34, 647–655. [Google Scholar] [CrossRef]
- Sethi, S.; Malik, M.A.; Goswami, S.; Saxena, P.; Srivastava, A.; Kashyap, S.; Pushker, N.; Bajaj, M.S.; Bakhshi, S.; Kaur, J. Expression of P-glycoprotein in human retinoblastoma and its clinical significance. Tumor Biol. 2014, 35, 11735–11740. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.S.; Haddad, G.; Thormer, P.S.; DeBoer, G.; Lin, Y.P.; Ondrusek, N.; Yeger, H.; Ling, V. P-glycoprotein expression as a predictor of the outcome of therapy for nerobalstoma. N. Engl. J. Med. 1991, 325, 1608–1614. [Google Scholar] [CrossRef]
- Glauser, T.; Shinnar, S.; Gloss, D.; Alldredge, B.; Arya, R.; Bainbridge, J.; Bare, M.; Bleck, T.; Dodson, E.; Garrity, L.; et al. Evidence-based guideline: Treatment of convulsive status epilepticus in children and adults: Report of the guideline committee of the American epilepsy society. Epilepsy Curr. 2016, 16, 48–61. [Google Scholar] [CrossRef]
- Lindbom, L.; Pihlgren, P.; Jonsson, E.N.; Jonsson, N. PsN-Toolkit—A collection of computer intensive statistical methods for non-linear mixed effect modeling using NONMEM. Comput. Methods Programs Biomed. 2005, 79, 241–257. [Google Scholar] [CrossRef]
- Jonsson, E.N.; Karlsson, M.O. Xpose—An S-PLUS based population pharmacokinetic/pharmacodynamic model building aid for NONMEM. Comput. Methods Programs Biomed. 1999, 58, 51–64. Available online: http://www.ncbi.nlm.nih.gov/pubmed/10195646 (accessed on 6 April 2016). [CrossRef]
- Jonsson, E.N.; Karlsson, M.O. Automated covariate model building within NONMEM. Pharm. Res. 1998, 15, 1463–1468. [Google Scholar] [CrossRef]
- Ette, E.I.; Williams, P.J. Population pharmacokinetics I: Background, concepts, and models. Ann. Pharmacother. 2004, 38, 1702–1706. [Google Scholar] [CrossRef]
- Post, T.M.; Freijer, J.I.; Ploeger, B.A.; Danhof, M. Extensions to the visual predictive check to facilitate model performance evaluation. J. Pharmacokinet. Pharmacodyn. 2008, 35, 185–202. [Google Scholar] [CrossRef]
- Efron, B.; Tibshirani, R. An Introduction to the Bootstrap; Chapman & Hall: London, UK, 1994. [Google Scholar]
- Hooker, A.C.; Staatz, C.E.; Karlsson, M.O. Conditional Weighted Residuals (CWRES): A model diagnostic for the FOCE method. Pharm. Res. 2007, 24, 2187–2197. [Google Scholar] [CrossRef]
- Fahr, A. Cyclosporin clinical pharmacokinetics. Clin. Pharmacokinet. 1993, 24, 472–495. [Google Scholar] [CrossRef]
- Filippi, L.; la Marca, G.; Cavallaro, G.; Fiorini, P.; Favelli, F.; Malvagia, S.; Donzelli, G.; Guerrini, R. Phenobarbital for neonatal seizures in hypoxic ischemic encephalopathy: A pharmacokinetic study during whole body hypothermia. Epilepsia 2011, 52, 794–801. [Google Scholar] [CrossRef]
- Roberts, J.K.; Stockmann, C.; Constance, J.E.; Stiers, J.; Spigarelli, M.G.; Ward, R.M.; Sherwin, C.M.T. Pharmacokinetics and pharmacodynamics of antibacterials, antifungals, and antivirals used most frequently in neonates and infants. Clin. Pharmacokinet. 2014, 53, 581–610. [Google Scholar] [CrossRef] [PubMed]
- Vinks, A.; Emoto, C.; Fukuda, T. Modeling and simulation in pediatric drug therapy: Application of pharmacometrics to define the right dose for children. Clin. Pharmacol. Ther. 2015, 98, 298–308. [Google Scholar] [CrossRef] [PubMed]
| Patient Characteristics | Number or Mean ± Standard Deviation (SD; Range) | ||
|---|---|---|---|
| CsA | PHB | VAN | |
| No. of patients | 34 | 28 | 93 |
| Gender | |||
| Male | 20 | 11 | 57 |
| Female | 14 | 17 | 36 |
| Age | |||
| Gestational age | - | 36.7 ± 4.4 (23.6–41.7) weeks | 31.9 ± 4.7 (22.9–40.3) weeks |
| Postnatal age | 26.8 ± 17.8 (1–79) months | 32.4 ± 30.7 (3–150) days | 9.3 ± 12.4 (0.1–80.4) weeks |
| Post-conceptional age | - | 41.3 ± 3.9 (31–51.1) weeks | 41.2 ± 14.2 (25.6–110) weeks |
| Body weight (kg) | 12.9 ± 3.8 (5–24) | 3.3 ± 1 (1–6.9) | 3.2 ± 2.6 (0.4–14.9) |
| Birth weight (kg) | - | 2.64 ± 0.87 (0.4–3.81) | - |
| Height (cm) | 87.4 ± 14.4 (55–123) | 50.6 ± 5.8 (31–63.2) | 56.8 ± 5.4 (49.2–82.6) |
| Body surface area (m2) | - | - | 0.2 ± 0.1 (0.1–0.5) |
| Serum creatinine (mg/dL) | 0.34 ± 0.09 (0.2–0.8) | 0.6 ± 0.59 (0.2–3.8) | 0.4 ± 0.3 (0.1–3.37) |
| GFR (mL/min/1.73 m2) | 142.8 ± 39.2 (63.4–250.4) | - | - |
| Cystatin-C (mg/L) | - | - | 1.8 ± 0.5 (0.7–3.6) |
| AST (IU/L) | 33.8 ± 9.0 (21–85) | 64 ± 102.7 (11–676) | - |
| ALT (IU/L) | 20.7 ± 112 (7–70) | 65.7 ± 117.7 (7–765) | - |
| Blood urea nitrogen (mg/dL) | 10.7 ± 3.8 (1.9–20.6) | - | - |
| Total bilirubin (mg/dL) | 0.28 ± 0.33 (0.1–4.3) | 3.8 ± 3.3 (0.2–14.5) | - |
| Direct bilirubin (mg/dL) | - | 2.2 ± 2.7 (0.1–12.7) | - |
| Serum albumin (g/dL) | 4.5 ± 0.3 (3.4–5.2) | - | 2.7 ± 0.6 (1.6–4.9) |
| Total protein (g/dL) | - | - | 4.4 ± 0.8 (1.7–6.9) |
| Haematocrit (%) | 31.7 ± 3.4 (23.8–40.8) | - | - |
| Total cholesterol (mg/dL) | 167.3 ± 29.7 (102–240) | - | - |
| Chemotherapy cycles (CTx) | 5 ± 2.8 (1–12) | - | - |
| Drug | Objective Function Value (ΔOFV) | ||
|---|---|---|---|
| Structural Model * | Structural Model + Size Scaling | Structural Model + Size Scaling + Maturation Function | |
| CsA | −121.986 (-) | −153.115 (−31.129) | −155.075 (−33.089) |
| PHB | 475.849 (-) | 451.087 (−24.762) | 400.966 (−74.883) |
| VAN | 106.068 (-) | 24.258 (−81.81) | −28.042 (−134.11) |
| Parameters | CsA | PHB | VAN | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Population Mean (%RSE) | IIV (CV%) (%RSE) | Bootstrap (n = 2000) 5th–95th Percentile | Population Mean (%RSE) | IIV (CV%) (%RSE) | Bootstrap (n = 2000) 5th–95th Percentile | Population Mean (%RSE) | IIV (CV%) (%RSE) | Bootstrap (n = 2000) 5th–95th Percentile | |
| CL (L/hr) | 21.3 (4.4%) | 16.8% (17.5%) | 19.8–22.9 | 0.569 (5.0%) | 40.8% (1.2%) | 0.34–4.82 | 69.4 (13.7%) | 10.4% (68.2%) | 49.5–89.2 |
| Vd (L) | 218 (25.5%) | 12.3% (110.7%) | 91.6–344.8 | 5.51 (2.1%) | 78.7% (6.8%) | 1.87–13.53 | 3.23 (6.1%) | 52.8% (15.9%) | 2.9–3.6 |
| TM50 (week) | - | - | - | 48.2 (2.1%) | - | 37.6–84.8 | 33.3 * | - | - |
| Hill coefficient | - | - | - | 5.99 (1.2%) | - | 1.6–8.3 | 3.68 * | - | - |
| ka (hr−1) | - | - | - | 50 * | - | - | - | - | - |
| Bioavailability | - | - | - | 0.724 (7.2%) | - | 0.58–0.87 | - | - | - |
| Proportional Error | |||||||||
| Residual variability | 46.8% (5.9%) | - | 42.2–51.3% | 35.6% (3.0%) | - | 27.8–43.4% | 40.8% (6.3%) | - | 36.3–45.3% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Back, H.-m.; Lee, J.B.; Han, N.; Goo, S.; Jung, E.; Kim, J.; Song, B.; An, S.H.; Kim, J.T.; Rhie, S.J.; et al. Application of Size and Maturation Functions to Population Pharmacokinetic Modeling of Pediatric Patients. Pharmaceutics 2019, 11, 259. https://doi.org/10.3390/pharmaceutics11060259
Back H-m, Lee JB, Han N, Goo S, Jung E, Kim J, Song B, An SH, Kim JT, Rhie SJ, et al. Application of Size and Maturation Functions to Population Pharmacokinetic Modeling of Pediatric Patients. Pharmaceutics. 2019; 11(6):259. https://doi.org/10.3390/pharmaceutics11060259
Chicago/Turabian StyleBack, Hyun-moon, Jong Bong Lee, Nayoung Han, Sungwoo Goo, Eben Jung, Junyeong Kim, Byungjeong Song, Sook Hee An, Jung Tae Kim, Sandy Jeong Rhie, and et al. 2019. "Application of Size and Maturation Functions to Population Pharmacokinetic Modeling of Pediatric Patients" Pharmaceutics 11, no. 6: 259. https://doi.org/10.3390/pharmaceutics11060259
APA StyleBack, H.-m., Lee, J. B., Han, N., Goo, S., Jung, E., Kim, J., Song, B., An, S. H., Kim, J. T., Rhie, S. J., Ree, Y. S., Chae, J.-w., Kim, J., & Yun, H.-y. (2019). Application of Size and Maturation Functions to Population Pharmacokinetic Modeling of Pediatric Patients. Pharmaceutics, 11(6), 259. https://doi.org/10.3390/pharmaceutics11060259






