A Preliminary Investigation of Additive Manufacture to Fabricate Human Nail Plate Surrogates for Pharmaceutical Testing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
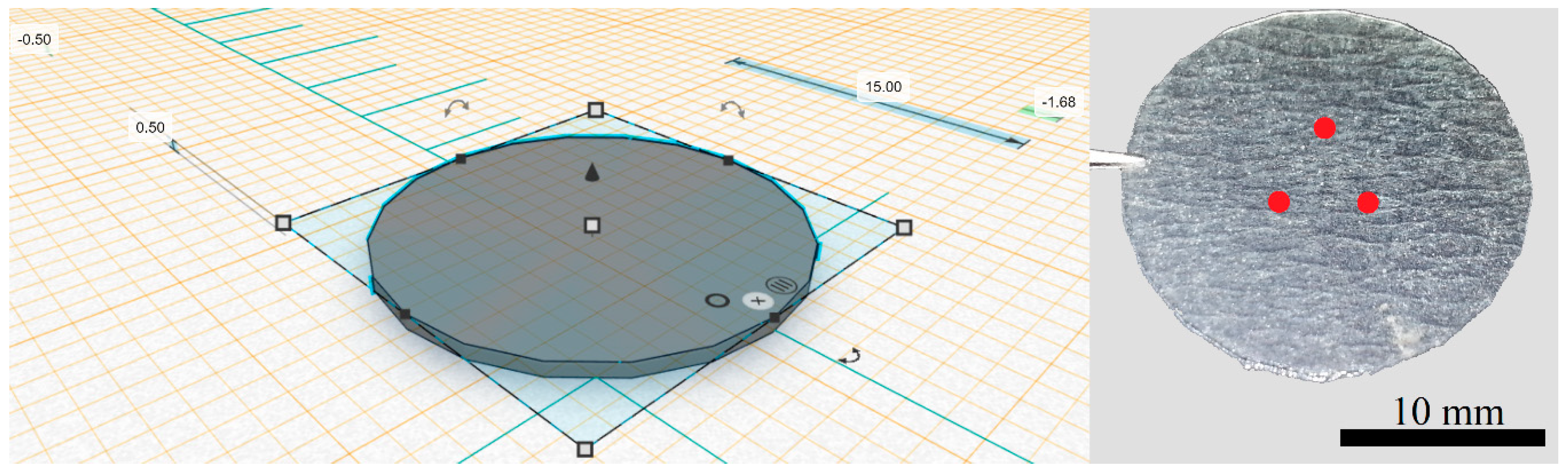
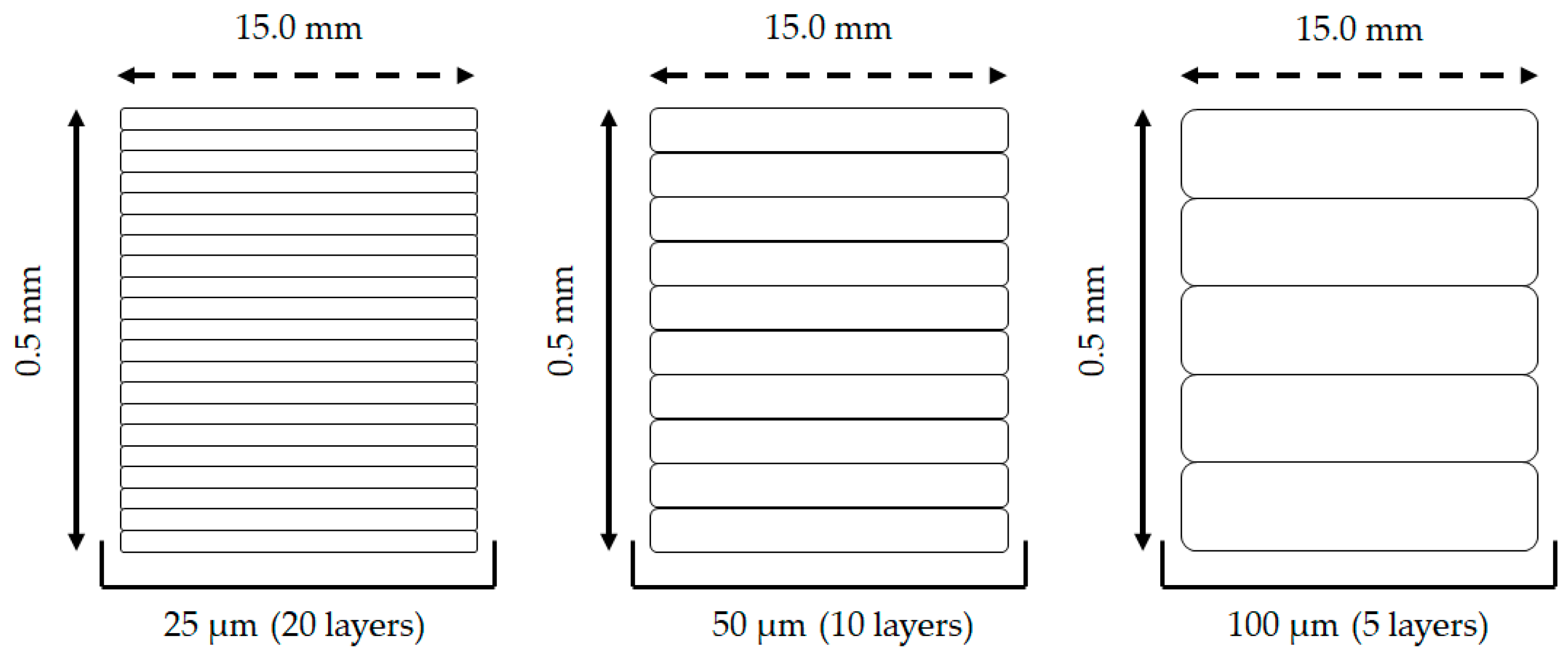
2.2. 3D Printing Prototype (Design and Printing Parameters)
2.3. High Performance Liquid Chromatographic (HPLC) Analysis
2.4. Permeation Study with Commercial Formulation in 3D Printed Polymeric Matrices
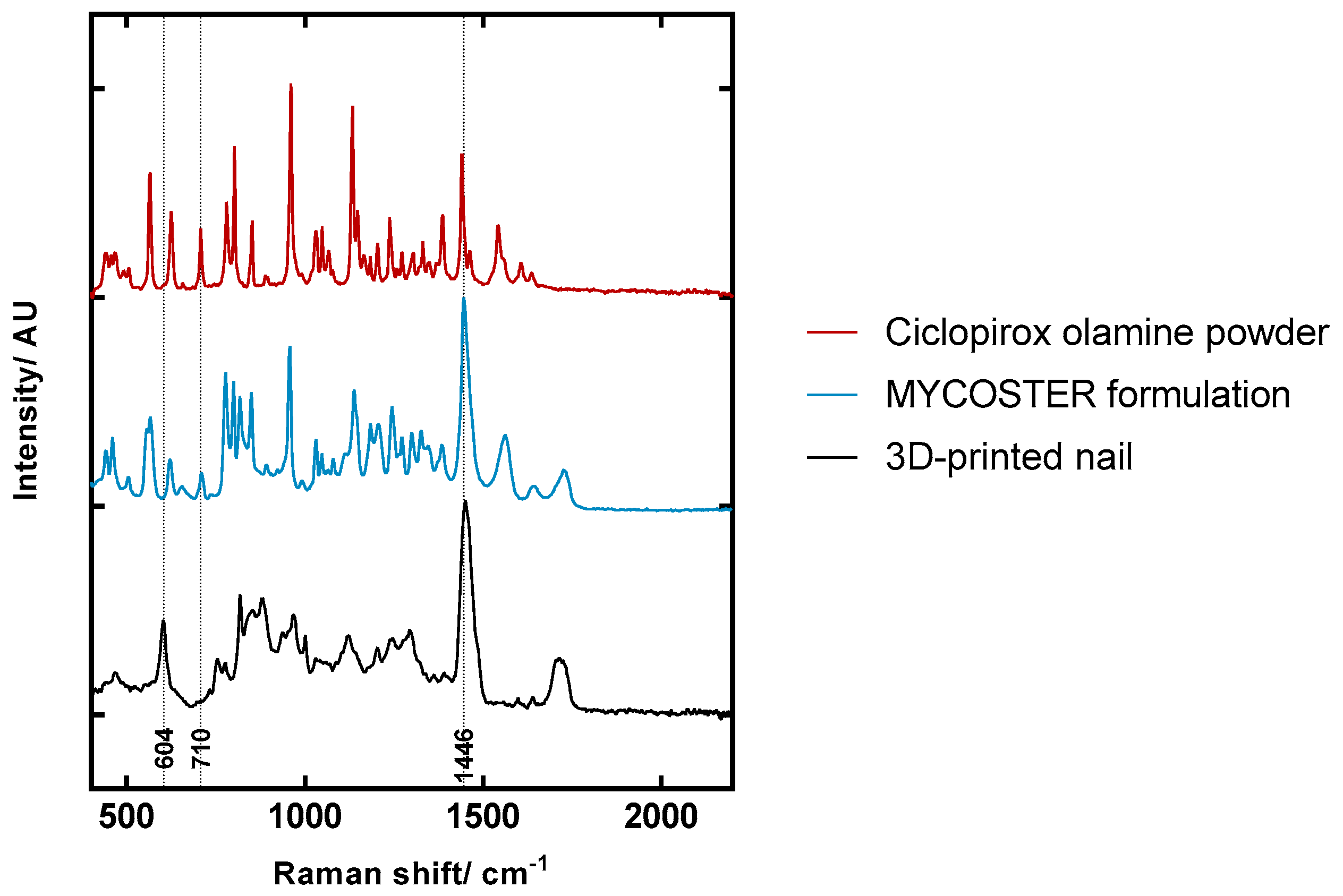
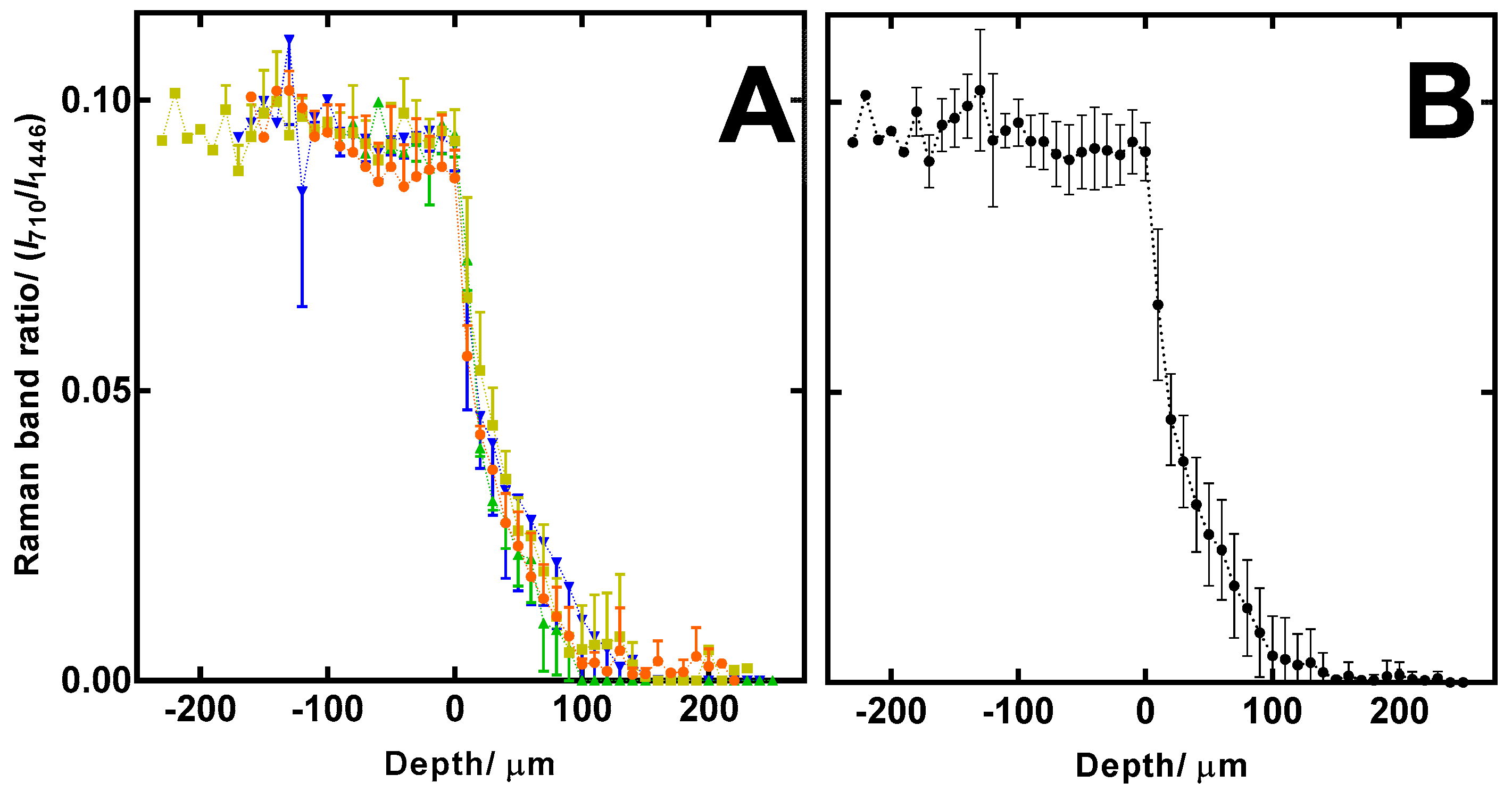
2.5. Confocal Raman Spectroscopy (CRS) Study of Ciclopirox Olamine in 3D Printed Polymeric Matrices
3. Results and Discussion
3.1. 3D Printing Prototype (Design and Printing Parameters)
3.2. Permeation Study with Commercial Formulation in 3D Printed Polymeric Matrices
3.3. Confocal Raman Spectroscopy (CRS) Study of Ciclopirox Olamine in 3D Printed Polymeric Matrices
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- De Berker, D. Nail anatomy. Clin. Derm. 2013, 31, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Vejnovic, I.; Simmler, L.; Betz, G. Investigation of different formulations for drug delivery through the nail plate. Int. J. Pharm. 2010, 386, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Mertin, D.; Lippold, B.C. In-vitro Permeability of the Human Nail and of a Keratin Membrane from Bovine Hooves: Prediction of the Penetration Rate of Antimycotics through the Nail Plate and their Efficacy. J. Pharm. Pharmacol. 1997, 49, 866–872. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.M.; Vora, Z.M. Formulation development and optimization of transungual drug delivery system of terbinafine hydrochloride for the treatment of onychomycosis. Drug Del. Trans. Res. 2016, 6, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Mertin, D.; Lippold, B.C. In-vitro Permeability of the Human Nail and of a Keratin Membrane from Bovine Hooves: Penetration of Chloramphenicol from Lipophilic Vehicles and a Nail Lacquer. J. Pharm. Pharmacol. 1997, 49, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Laubé, F.; Poupon, A.; Zinck, P.; Müller-Goymann, C.; Reichl, S.; Nardello-Rataj, V. Physicochemical investigations of native nails and synthetic models for a better understanding of surface adhesion of nail lacquers. Eur. J. Pharm Sci. 2019, 131, 208–217. [Google Scholar] [CrossRef] [PubMed]
- George, S.C.; Thomas, S. Transport phenomena through polymeric systems. Prog. Polym. Sci. 2001, 26, 985–1017. [Google Scholar] [CrossRef]
- Zaleski, R.; Stefaniak, W.; Maciejewska, M.; Goworek, J. Porosity of polymer materials by various techniques. J. Porous Mat. 2008, 16, 691. [Google Scholar] [CrossRef]
- Hilton, S.; Penny, M.; Sil Dos Santos, B.; Patel, B. Three-Dimensional Printing of Impregnated Plastics for Chemical Reactions. WO2017158336A1, 21 September 2019. [Google Scholar]
- Mohmmed, S.A.; Vianna, M.E.; Hilton, S.T.; Boniface, D.R.; Ng, Y.-L.; Knowles, J.C. Investigation to test potential stereolithography materials for development of an in vitro root canal model. Microscopy Res. Tech. 2016, 80, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.-W.; Lee, S.J.; Ko, I.K.; Kengla, C.; Yoo, J.J.; Atala, A. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat. Biotech. 2016, 34, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Pourchet, L.J.; Thepot, A.; Albouy, M.; Courtial, E.J.; Boher, A.; Blum, L.J.; Marquette, C.A. Human Skin 3D Bioprinting Using Scaffold-Free Approach. Ad. Health. Mat. 2017, 6, 1601101. [Google Scholar] [CrossRef] [PubMed]
- Hakimi, N.; Cheng, R.; Leng, L.; Sotoudehfar, M.; Ba, P.Q.; Bakhtyar, N.; Amini-Nik, S.; Jeschke, M.G.; Günther, A. Handheld skin printer: In situ formation of planar biomaterials and tissues. Lab Chip 2018, 18, 1440–1451. [Google Scholar] [CrossRef] [PubMed]
- Formlabs. A Guide to Post-Curing Formlabs Resins. Available online: https://archive-media.formlabs.com/upload/A-Guide-to-Post-Curing-Formlabs-Resins.pdf (accessed on 27 January 2019).
- British Pharmacopoeia Commission. British Pharmacopoeia 2017; TSO: London, UK, 2017. [Google Scholar]
- Oliveira, G.; Hadgraft, J.; Lane, M.E. The role of vehicle interactions on permeation of an active through model membranes and human skin. Int. J. Cosmetic Sci. 2012, 34, 536–545. [Google Scholar] [CrossRef] [PubMed]
- Bu, W.; Fan, X.; Sexton, H.; Heyman, I. A direct LC/MS/MS method for the determination of ciclopirox penetration across human nail plate in in vitro penetration studies. J. Pharm. Biomed. Anal. 2010, 51, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.; Turner, R.; Wevrett, S.R. Use of in vitro performance models in the assessment of drug delivery across the human nail for nail disorders. Exp. Op. Drug Del. 2018, 15, 983–989. [Google Scholar] [CrossRef] [PubMed]
- Täuber, A.; Müller-Goymann, C.C. In vitro permeation and penetration of ciclopirox olamine from poloxamer 407-based formulations – comparison of isolated human stratum corneum, bovine hoof plates and keratin films. Int. J. Pharm. 2015, 489, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Hui, X.; Baker, S.J.; Wester, R.C.; Barbadillo, S.; Cashmore, A.K.; Sanders, V.; Hold, K.M.; Akama, T.; Zhang, Y.K.; Plattner, J.J. In Vitro Penetration of a Novel Oxaborole Antifungal (AN2690) Into the Human Nail Plate. J. Pharm. Sci. 2007, 96, 2622–2631. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.S.; Karashima, T.; Yamamoto, M.; Hamaguchi, H. Molecular-level pursuit of yeast mitosis by time- and space-resolved Raman spectroscopy. J. Raman Spect. 2003, 34, 1–3. [Google Scholar] [CrossRef]
| Platform Coating | Removal of Printout from Building Platform | Inspection of 3D Printed Scaffold 1 (Mean ± SD) |
|---|---|---|
| None 2 | Printout breakage and platform damage on attempt | h (0.51 ± 0.02 mm) O.D. (-) 3 |
| White masking tape 50 mm (Eurocel Ltd., Alfreton, UK) | Accessible removal of the printout from the plate. Extended peeling of the coating during printing | h (0.28 ± 0.22 mm) O.D. (9.3 ± 5.5 mm) |
| Blue masking tape HB 850 12 mm (Hi-Bond Tapes Ltd., Corby, UK) | Printout did not adhere to the coating | - |
| Kapton tape 50 mm (3D FilaPrint, Essex, UK) | Unmanageable removal of the coating from the plate. Hindrance to detach printout from coating | h (0.48 ± 0.03 mm) O.D. (15.0 ± 0.01 mm) |
| Scotch-Blue™ painter’s tape for multi-surfaces 24 mm (3M, Flemington, NJ, USA) | Accessible removal of the printout from the plate. Simple separation of the printout from coating | h (0.50 ± 0.01 mm) O.D. (15.0 ± 0.02 mm) |
| 3D Nail Thickness (mm) | 3D Nail Length (mm) | Printing Temperature (°C) | Printing Resolution (μm) | Inspection of 3D Printed Scaffold (Mean ± SD) |
|---|---|---|---|---|
| 0.50 | 15.0 | 28 | 100 | h (0.50 ± 0.01 mm); O.D. (15.0 ± 0.1 mm) |
| 50 | h (0.50 ± 0.01 mm); O.D. (15.1 ± 0.2 mm) | |||
| 25 | h (0.50 ± 0.01 mm); O.D. (15.0 ± 0.2 mm) 1 | |||
| 20 | 100 | h (0.50 ± 0.01 mm); O.D. (15.1 ± 0.1 mm) | ||
| 50 | h (0.51 ± 0.01 mm); O.D. (15.0 ± 0.1 mm) | |||
| 25 | h (0.51 ± 0.02 mm); O.D. (15.3 ± 0.2 mm) | |||
| 20.0 | 28 | 100 | h (0.50 ± 0.01 mm); O.D. (20.1 ± 0.1 mm) | |
| 50 | h (0.51 ± 0.01 mm); O.D. (20.3 ± 0.2 mm) | |||
| 25 | h (0.51 ± 0.02 mm); O.D. (20.3 ± 0.2 mm) | |||
| 20 | 100 | h (0.51 ± 0.01 mm); O.D. (20.2 ± 0.1 mm) | ||
| 50 | h (0.51 ± 0.02 mm); O.D. (20.3 ± 0.2 mm) | |||
| 25 | h (0.52 ± 0.02 mm); O.D. (20.3 ± 0.2 mm) | |||
| 0.30 | 15.0 | 28 | 100 | h (0.32 ± 0.01 mm); O.D. (15.0 ± 0.1 mm) |
| 50 | h (0.32 ± 0.02 mm); O.D. (15.0 ± 0.1 mm) | |||
| 25 | h (0.29 ± 0.01 mm); O.D. (15.1 ± 0.2 mm) | |||
| 20 | 100 | h (0.32 ± 0.02 mm); O.D. (15.1 ± 0.1 mm) | ||
| 50 | h (0.29 ± 0.02 mm); O.D. (15.1 ± 0.2 mm) | |||
| 25 | h (0.29 ± 0.02 mm); O.D. (15.1 ± 0.2 mm) | |||
| 20.0 | 28 | 100 | h (0.31 ± 0.02 mm); O.D. (20.1 ± 0.1 mm) | |
| 50 | h (0.32 ± 0.01 mm); O.D. (20.3 ± 0.2 mm) | |||
| 25 | h (0.32 ± 0.02 mm); O.D. (20.3 ± 0.2 mm) | |||
| 20 | 100 | h (0.33 ± 0.02 mm); O.D. (20.1 ± 0.2 mm) | ||
| 50 | h (0.28 ± 0.03 mm); O.D. (20.2 ± 0.3 mm) | |||
| 25 | h (0.28 ± 0.02 mm); O.D. (20.3 ± 0.2 mm) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sil, B.C.; Patel, A.; Crowther, J.M.; Moore, D.J.; Hadgraft, J.; Hilton, S.T.; Lane, M.E. A Preliminary Investigation of Additive Manufacture to Fabricate Human Nail Plate Surrogates for Pharmaceutical Testing. Pharmaceutics 2019, 11, 250. https://doi.org/10.3390/pharmaceutics11060250
Sil BC, Patel A, Crowther JM, Moore DJ, Hadgraft J, Hilton ST, Lane ME. A Preliminary Investigation of Additive Manufacture to Fabricate Human Nail Plate Surrogates for Pharmaceutical Testing. Pharmaceutics. 2019; 11(6):250. https://doi.org/10.3390/pharmaceutics11060250
Chicago/Turabian StyleSil, Bruno C., Avnish Patel, Jonathan M. Crowther, David J. Moore, Jonathan Hadgraft, Stephen T. Hilton, and Majella E. Lane. 2019. "A Preliminary Investigation of Additive Manufacture to Fabricate Human Nail Plate Surrogates for Pharmaceutical Testing" Pharmaceutics 11, no. 6: 250. https://doi.org/10.3390/pharmaceutics11060250
APA StyleSil, B. C., Patel, A., Crowther, J. M., Moore, D. J., Hadgraft, J., Hilton, S. T., & Lane, M. E. (2019). A Preliminary Investigation of Additive Manufacture to Fabricate Human Nail Plate Surrogates for Pharmaceutical Testing. Pharmaceutics, 11(6), 250. https://doi.org/10.3390/pharmaceutics11060250





