Elucidating the Influence of Tumor Presence on the Polymersome Circulation Time in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Polymersome Preparation
2.3. Radiolabeling and Radionuclide Retention
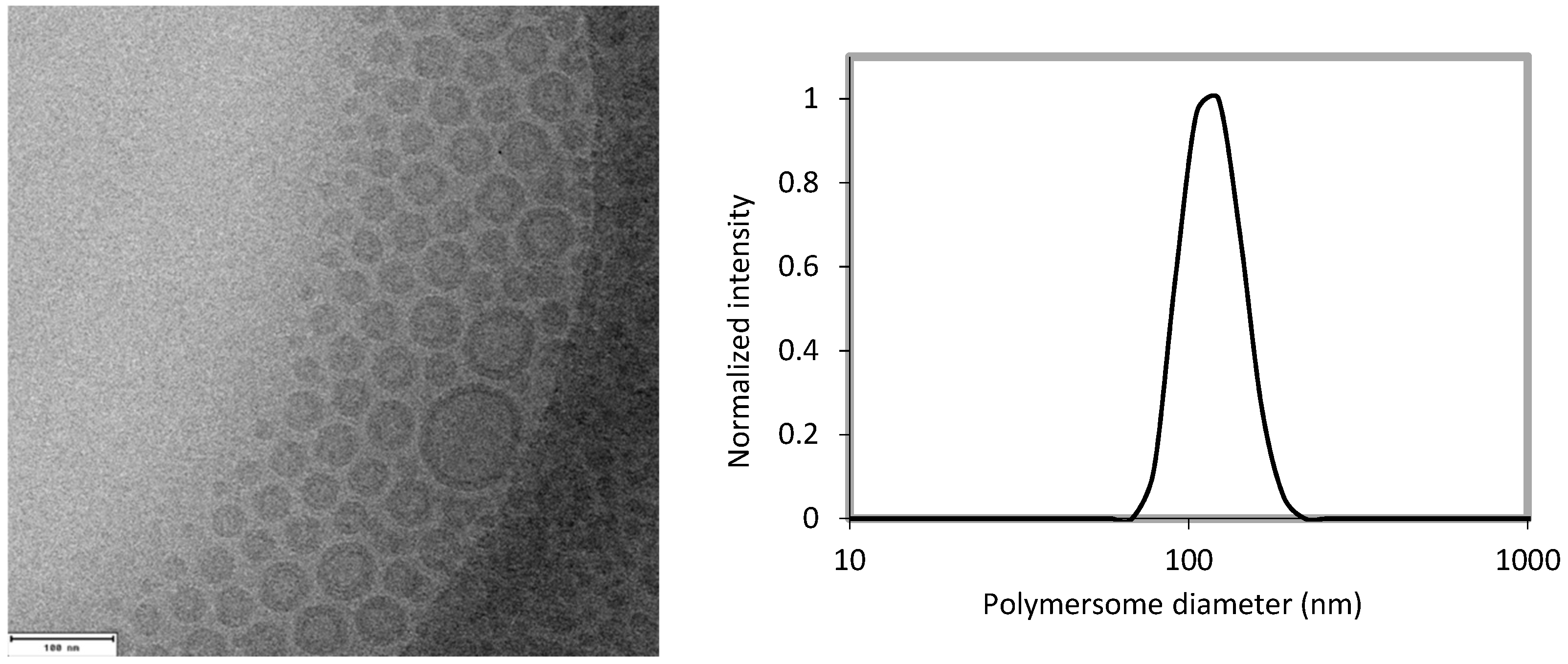
2.4. Cryogenic Transmission Electron Microscopy (Cryo-TEM) and Dynamic Light Scattering (DLS)
2.5. Cellular Uptake Experiments
2.6. Animals
2.7. Blood Clearance
2.8. Clodronate Liposomes
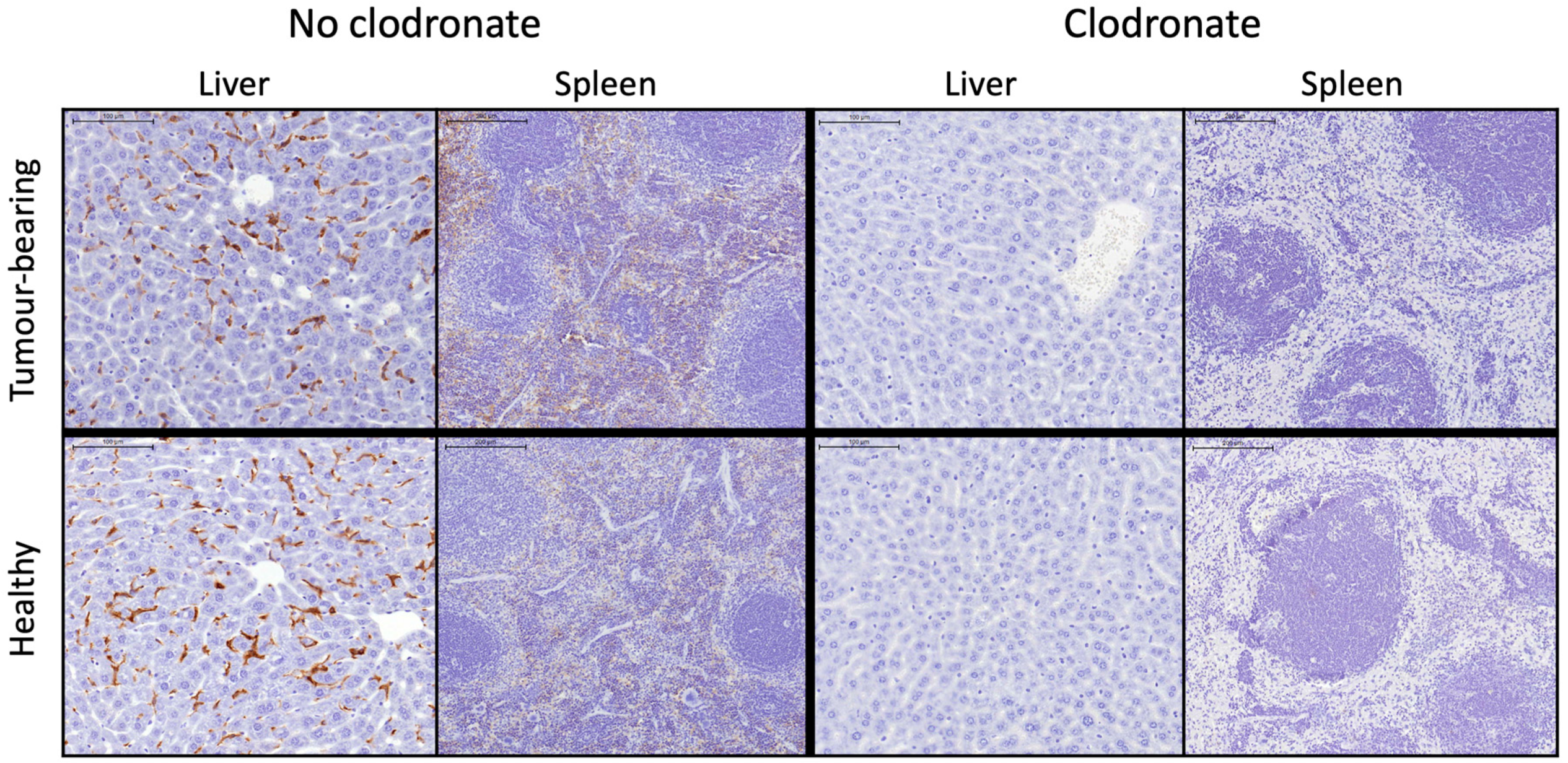
2.9. Immunohistochemistry
3. Results and Discussion
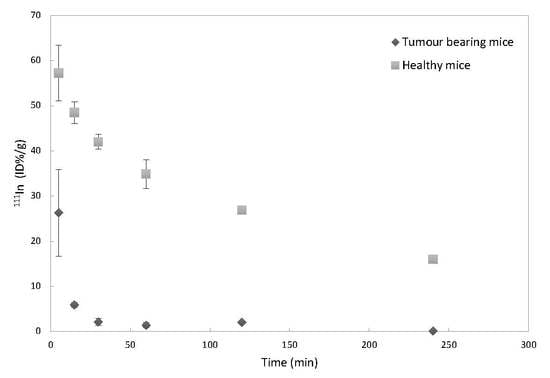
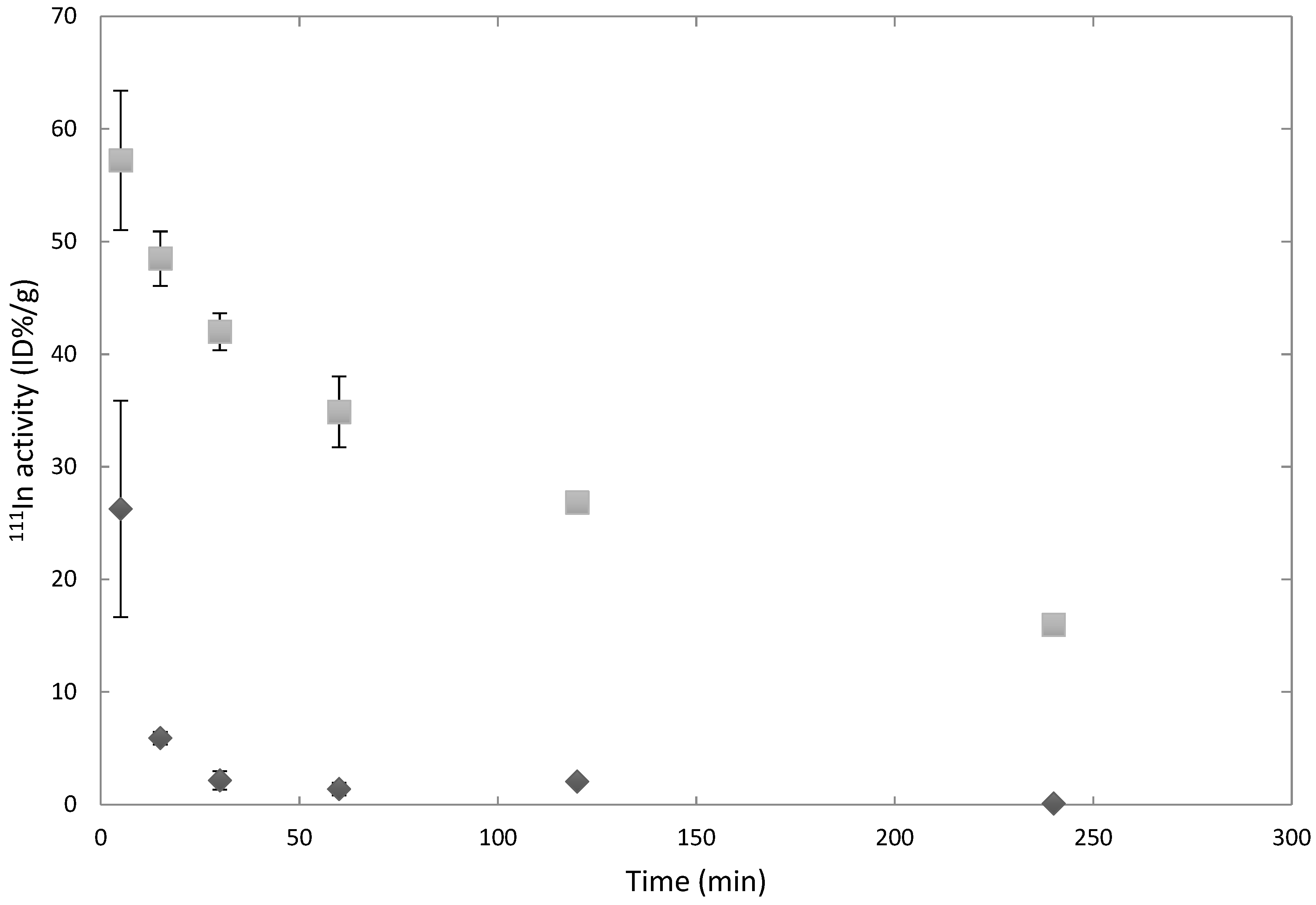
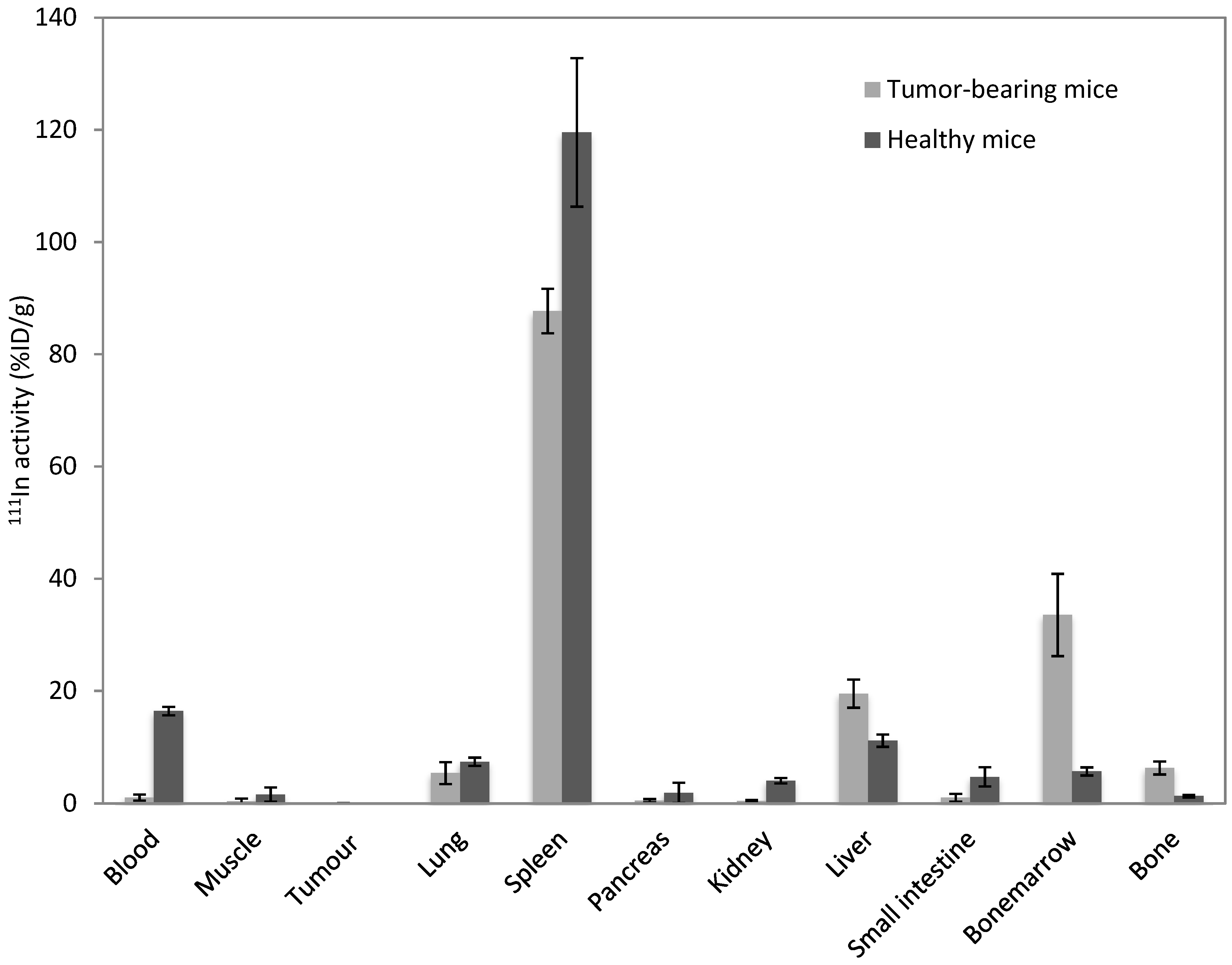
3.1. Circulation Time in Healthy Versus Tumor-Bearing Mice
3.2. Influence of Macrophages on Circulation Time
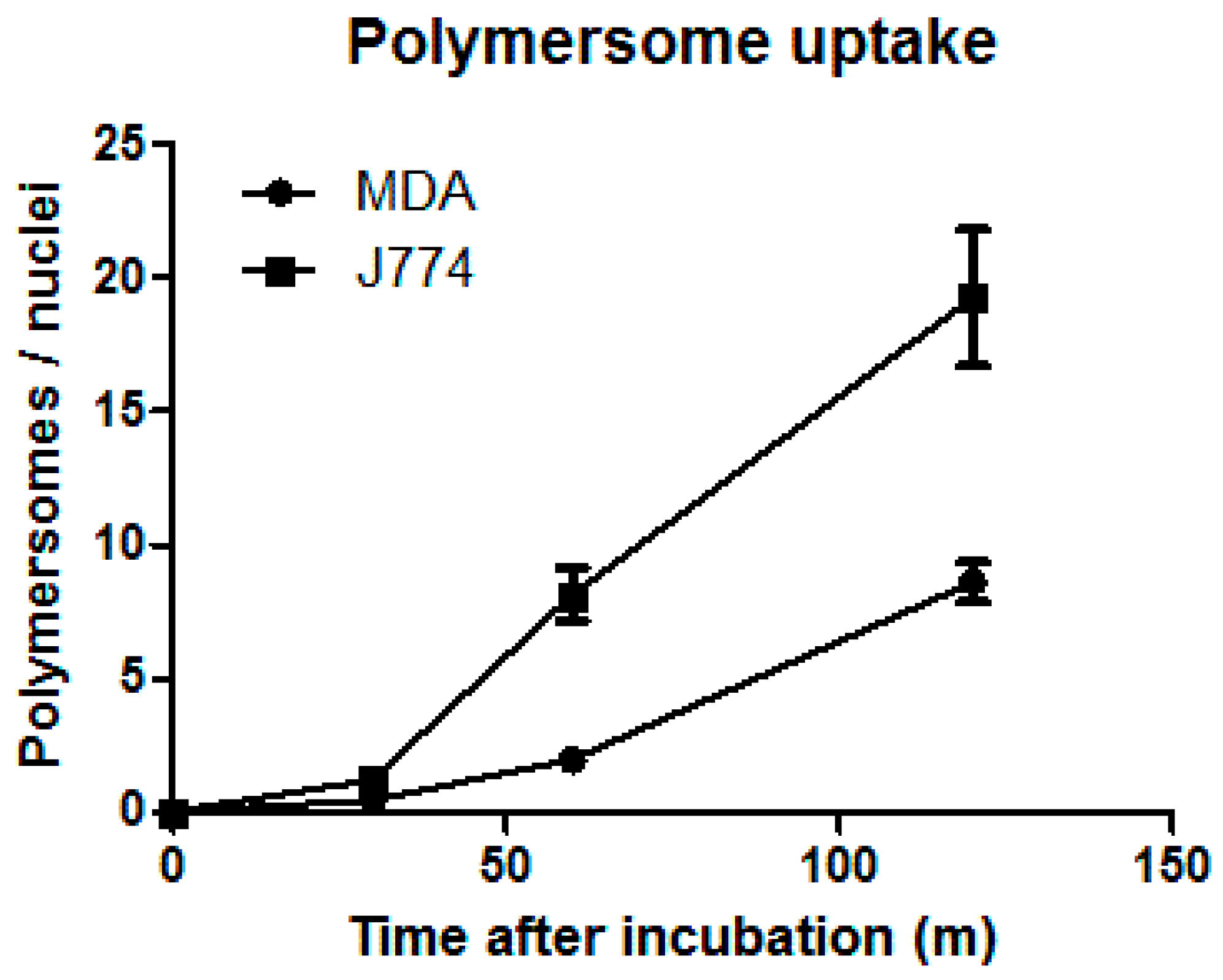
3.2.1. Cell Experiments
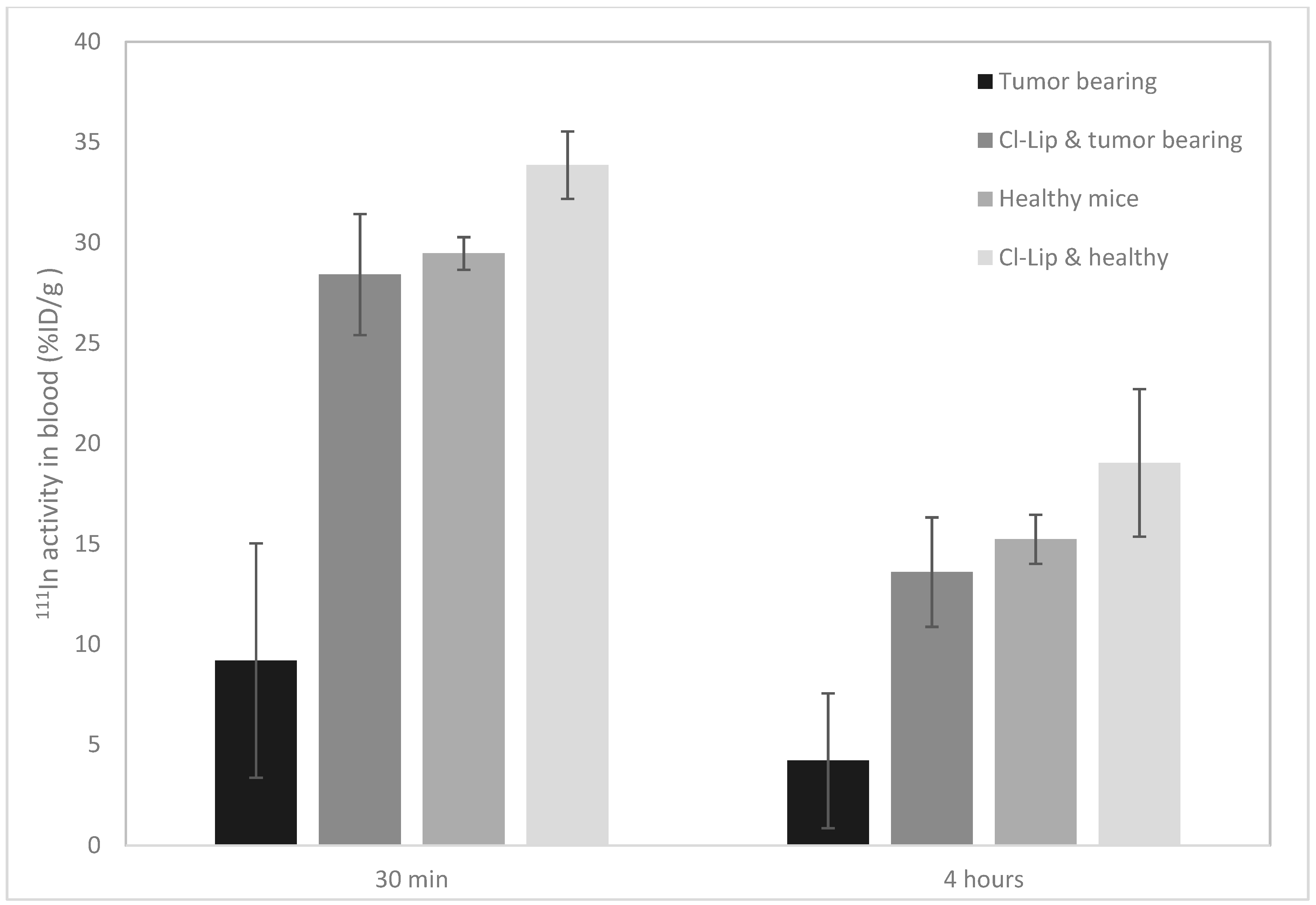
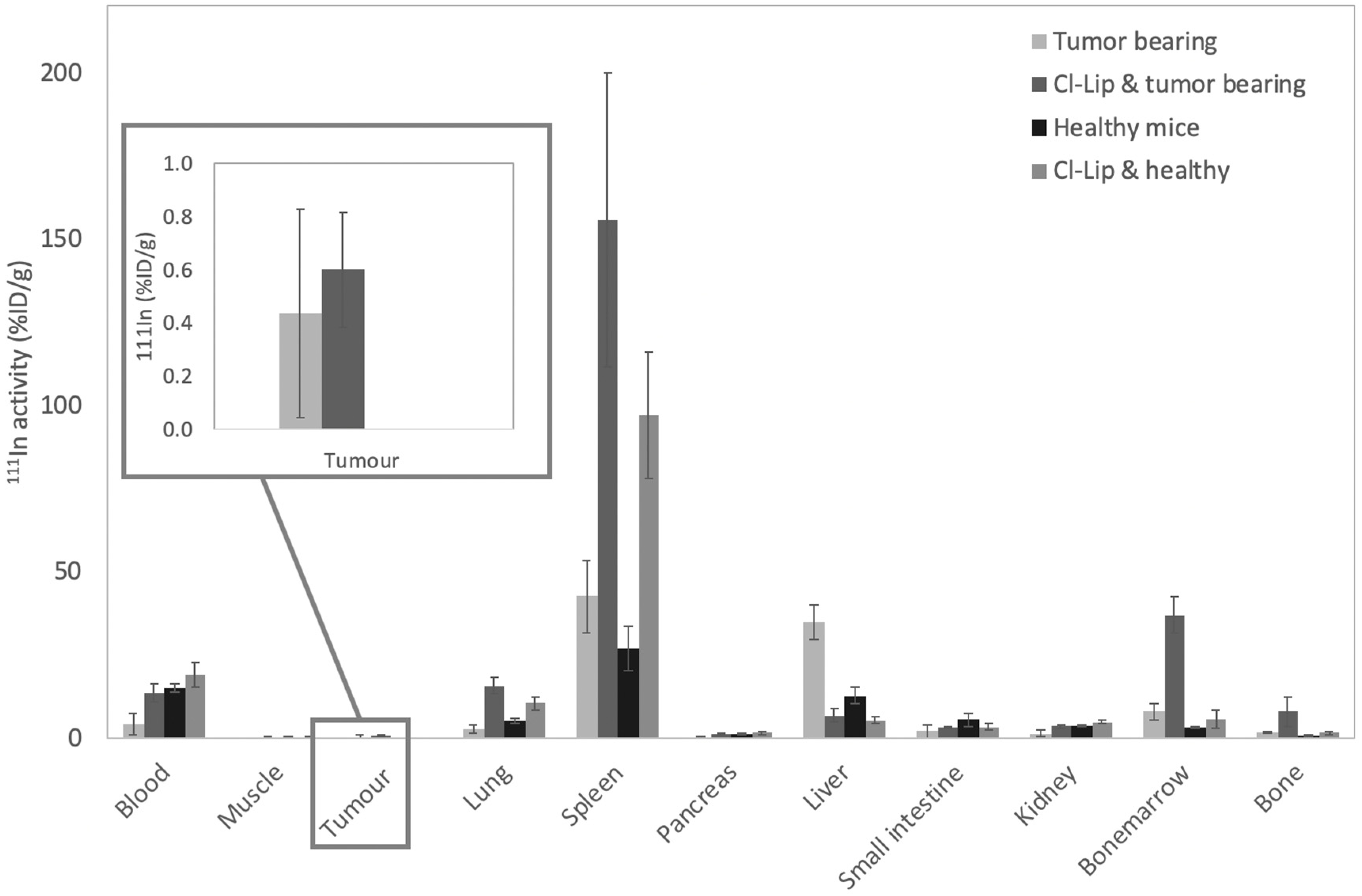
3.2.2. Animal Experiments
3.2.3. Comparison to Other Nanoparticle Systems
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, L.; Gu, F.; Chan, J.; Wang, A.; Langer, R.; Farokhzad, O. Nanoparticles in medicine: Therapeutic applications and developments. Clin. Pharmacol. Ther. 2008, 83, 761–769. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic. Bioeng. Transl. Med. 2016, 1, 10–29. [Google Scholar] [CrossRef]
- O’Brien, M.E.R.; Wigler, N.; Inbar, M.; Rosso, R.; Grischke, E.; Santoro, A.; Catane, R.; Kieback, D.G.; Tomczak, P.; Ackland, S.P.; et al. Reduced cardiotoxicity and comparable efficacy in a phase III trial of pegylated liposomal doxorubicin HCl (CAELYX™/Doxil®) versus conventional doxorubicin for first-line treatment of metastatic breast cancer. Ann. Oncol. 2004, 15, 440–449. [Google Scholar] [CrossRef]
- Emerich, D.F.; Thanos, C.G. Targeted nanoparticle-based drug delivery and diagnosis. J. Drug Target. 2007, 15, 163–183. [Google Scholar] [CrossRef]
- Hashizume, H.; Baluk, P.; Morikawa, S.; McLean, J.W.; Thurston, G.; Roberge, S.; Jain, R.K.; McDonald, D.M. Openings between defective endothelial cells explain tumor vessel leakiness. Am. J. Pathol. 2000, 156, 1363–1380. [Google Scholar] [CrossRef]
- Cheng, Z.; Al Zaki, A.; Hui, J.Z.; Muzykantov, V.R.; Tsourkas, A. Multifunctional nanoparticles: Cost versus benefit of adding targeting and imaging capabilities. Science 2012, 338, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Bolkestein, M.; de Blois, E.; Koelewijn, S.J.; Eggermont, A.M.M.; Grosveld, F.; de Jong, M.; Koning, G.A. Investigation of factors determining the enhanced permeability and retention effect in subcutaneous xenografts. J. Nucl. Med. 2016, 57, 601–607. [Google Scholar] [CrossRef]
- Shenoy, D.B.; Amiji, M.M. Poly(ethylene oxide)-modified poly(e-caprolactone) nanoparticles for targeted delivery of tamoxifen in breast cancer. Int. J. Pharm. 2005, 293, 261–270. [Google Scholar] [CrossRef]
- Ahmed, F.; Pakunlu, R.I.; Brannan, A.; Bates, F.; Minko, T.; Discher, D.E. Biodegradable polymersomes loaded with both paclitaxel and doxorubicin permeate and shrink tumors, inducing apoptosis in proportion to accumulated drug. J. Control. Release 2006, 116, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Charrois, G.J.R.; Allen, T.M. Drug release rate influences the pharmacokinetics, biodistribution, therapeutic activity, and toxicity of pegylated liposomal doxorubicin formulations in murine breast cancer. Biochim. Biophys. Acta-Biomembr. 2004, 1663, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Sawant, R.R.; Torchilin, V.P. Enhanced cytotoxicity of TATp-bearing paclitaxel-loaded micelles in vitro and in vivo. Int. J. Pharm. 2009, 374, 114–118. [Google Scholar] [CrossRef]
- Alexis, F.; Pridgen, E.; Molnar, L.K.; Farokhzad, O.C. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol. Pharm. 2008, 5, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Brigger, I.; Dubernet, C.; Couvreur, P. Nanoparticles in cancer therapy and diagnosis. Adv. Drug Deliv. Rev. 2012, 64, 24–36. [Google Scholar] [CrossRef]
- Li, S.-D.; Huang, L. Pharmacokinetics and biodistribution of nanoparticles. Mol. Pharm. 2008, 5, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Lasic, D.D.; Needham, D. The “Stealth” liposome: A prototypical biomaterial. Chem. Rev. 1995, 95, 2601–2628. [Google Scholar] [CrossRef]
- Yoo, J.-W.; Chambers, E.; Mitragotri, S. Factors that control the circulation time of nanoparticles in blood: Challenges, solutions and future prospects. Curr. Pharm. Des. 2010, 16, 2298–2307. [Google Scholar] [CrossRef]
- Lu, W.-L.; Qi, X.-R.; Zhang, Q.; Li, R.-Y.; Wang, G.-L.; Zhang, R.-J.; Wei, S.-L. A PEGylated liposomal platform: Pharmacokinetics, pharmacodynamics, and toxicity in mice using doxorubicin as a model drug. J. Pharmacol. Sci. 2004, 95, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Klibanov, A.L.; Maruyama, K.; Torchilin, V.P.; Huang, L. Amphipathic polyethyleneglycols effectively prolong the circulation time of liposomes. FEBS Lett. 1990, 268, 235–237. [Google Scholar] [CrossRef]
- Litzinger, D.C.; Buiting, A.M.J.; van Rooijen, N.; Huang, L. Effect of liposome size on the circulation time and intraorgan distribution of amphipathic poly(ethylene glycol)-containing liposomes. Biochim. Biophys. Acta-Biomembr. 1994, 1190, 99–107. [Google Scholar] [CrossRef]
- Brinkhuis, R.P.; Stojanov, K.; Laverman, P.; Eilander, J.; Zuhorn, I.S.; Rutjes, F.P.; Van Hest, J.C. Size dependent biodistribution and SPECT imaging of 111In-labeled polymersomes. Bioconjug. Chem. 2012, 23, 958–965. [Google Scholar] [CrossRef]
- Wang, G.; de Kruijff, R.M.; Abou, D.; Ramos, N.; Mendes, E.; Franken, L.E.; Wolterbeek, H.T.; Denkova, A.G. Pharmacokinetics of polymersomes composed of poly(butadiene-ethylene oxide); Healthy versus tumor-bearing mice. J. Biomed. Nanotechnol. 2016, 12, 320–328. [Google Scholar] [CrossRef]
- Kai, M.P.; Brighton, H.E.; Fromen, C.A.; Shen, T.W.; Luft, J.C.; Luft, Y.E.; Keeler, A.W.; Robbins, G.R.; Ting, J.P.Y.; Zamboni, W.C.; et al. Tumor presence induces global immune changes and enhances nanoparticle clearance. ACS Nano 2016, 10, 861–870. [Google Scholar] [CrossRef]
- Wang, G.; de Kruijff, R.M.; Rol, A.; Thijssen, L.; Mendes, E.; Morgenstern, A.; Bruchertseifer, F.; Stuart, M.C.A.; Wolterbeek, H.T.; Denkova, A.G. Retention studies of recoiling daughter nuclides of 225Ac in polymer vesicles. Appl. Radiat. Isot. 2014, 85, 45–53. [Google Scholar] [CrossRef]
- de Kruijff, R.M.; Drost, K.; Thijssen, L.; Morgenstern, A.; Bruchertseifer, F.; Lathouwers, D.; Wolterbeek, H.T.; Denkova, A.G. Improved 225Ac daughter retention in InPO4 containing polymersomes. Appl. Radiat. Isot. 2017, 128, 183–189. [Google Scholar] [CrossRef]
- Wang, G.; de Kruijff, R.M.; Stuart, M.C.A.; Mendes, E.; Wolterbeek, H.T.; Denkova, A.G. Polymersomes as radionuclide carriers loaded via active ion transport through the hydrophobic bilayer. Soft Matter 2013, 9, 727–734. [Google Scholar] [CrossRef]
- Photos, P.J.; Bacakova, L.; Discher, B.; Bates, F.S.; Discher, D.E. Polymer vesicles in vivo: Correlations with PEG molecular weight. J. Control. Release 2003, 90, 323–334. [Google Scholar] [CrossRef]
- Rideau, E.; Dimova, R.; Schwille, P.; Wurm, F.R.; Landfester, K. Liposomes and polymersomes: A comparative review towards cell mimicking. Chem. Soc. Rev. 2018, 47, 8571–8610. [Google Scholar] [CrossRef] [PubMed]
- Discher, B.M.; Won, Y.-Y.; Ege, D.S.; Lee, J.C.-M.; Bates, F.S.; Discher, D.E.; Hammer, D.A. Polymersomes: Tough vesicles made from diblock copolymers. Science 1999, 284, 1143–1146. [Google Scholar] [CrossRef] [PubMed]
- Zeisberger, S.M.; Odermatt, B.; Marty, C.; Zehnder-Fjällman, A.H.M.; Ballmer-Hofer, K.; Schwendener, R.A. Clodronate-liposome-mediated depletion of tumor-associated macrophages: A new and highly effective antiangiogenic therapy approach. Br. J. Cancer 2006, 95, 272–281. [Google Scholar] [CrossRef]
- Maeda, H.; Wu, J.; Sawa, T.; Matsumura, Y.; Hori, K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J. Control. Release 2000, 65, 271–284. [Google Scholar] [CrossRef]
- de Kruijff, R.M.; van der Meer, A.J.G.M.; Windmeijer, C.A.A.; Kouwenberg, J.J.M.; Morgenstern, A.; Bruchertseifer, F.; Sminia, P.; Denkova, A.G. The therapeutic potential of polymersomes loaded with 225Ac evaluated in 2D and 3D in vitro glioma models. Eur. J. Pharm. Biopharm. 2018, 127, 85–91. [Google Scholar] [CrossRef]
- Kiessling, R.; Wasserman, K.; Horiguchi, S.; Kono, K.; Sjoè Berg, J.; Pisa, P.; Petersson, M. Tumor-induced immune dysfunction. Cancer Immunol. Immunother. 1999, 48, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.W.; Roberts, R.A.; Robbins, G.R.; Perry, J.L.; Kai, M.P.; Chen, K.; Bo, T.; Napier, M.E.; Ting, J.P.Y.; DeSimone, J.M.; et al. Nanoparticle clearance is governed by Th1/Th2 immunity and strain background. J. Clin. Invest. 2013, 123, 3061–3073. [Google Scholar] [CrossRef] [PubMed]
- Owens, D.E., III; Peppas, N.A. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int. J. Pharm. 2006, 307, 93–102. [Google Scholar] [CrossRef]
- Avgoustakis, K.; Beletsi, A.; Panagi, Z.; Klepetsanis, P.; Livaniou, E.; Evangelatos, G.; Ithakissios, D.S. Effect of copolymer composition on the physicochemical characteristics, in vitro stability, and biodistribution of PLGA-mPEG nanoparticles. Int. J. Pharm. 2003, 259, 115–127. [Google Scholar] [CrossRef]
- Porter, C.J.H.; Moghimi, S.M.; Illum, L.; Davis, S.S. The polyoxyethylene/polyoxypropylene block co-polymer Poloxamer-407 selectively redirects intravenously injected microspheres to sinusoidal endothelial cells of rabbit bone marrow. FEBS Lett. 1992, 305, 62–66. [Google Scholar] [CrossRef]
- Tsoi, K.M.; MacParland, S.A.; Ma, X.-Z.; Spetzler, V.N.; Echeverri, J.; Ouyang, B.; Fadel, S.M.; Sykes, E.A.; Goldaracena, N.; Kaths, J.M.; et al. Mechanism of hard-nanomaterial clearance by the liver. Nat. Mater. 2016, 15, 1212–1221. [Google Scholar] [CrossRef]
- Terry, S.Y.A.; Boerman, O.C.; Gerrits, D.; Franssen, G.M.; Metselaar, J.M.; Lehmann, S.; Oyen, W.J.G.; Gerdes, C.A.; Abiraj, K. 111In-anti-F4/80-A3-1 antibody: A novel tracer to image macrophages. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1430–1438. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bertrand, N.; Leroux, J.-C. The journey of a drug-carrier in the body: An anatomo-physiological perspective. J. Control. Release 2012, 161, 152–163. [Google Scholar] [CrossRef]
- Van Rooijen, N.; Sanders, A. Liposome mediated depletion of macrophages: Mechanism of action, preparation of liposomes and applications. J. Immunol. Methods 1994, 174, 83–93. [Google Scholar] [CrossRef]
- Van Rooijen, N.; Bakker, J.; Sanders, A. Transient suppression of macrophage functions by liposome-encapsulated drugs. Trends Biotechnol. 1997, 15, 178–185. [Google Scholar] [CrossRef]
- Aichele, P.; Zinke, J.; Grode, L.; Schwendener, R.A.; Kaufmann, S.H.E.; Seiler, P. Macrophages of the splenic marginal zone are essential for trapping of blood-borne particulate antigen but dispensable for induction of specific T cell responses. J. Immunol. 2003, 171, 1148–1155. [Google Scholar] [CrossRef] [PubMed]
- Demoy, M.; Gibaud, S.; Andreux, J.P.; Weingarten, C.; Gouritin, B.; Couvreur, P. Splenic trapping of nanoparticles: Complementary approaches for in situ studies. Pharm. Res. 1997, 14, 463–468. [Google Scholar] [CrossRef]
- Demoy, M.; Andreux, J.P.; Weingarten, C.; Gouritin, B.; Guilloux, V.; Couvreur, P. Splenic capture of nanoparticles: Influence of animal species and surface characteristics. Pharm. Res. 1999, 16, 37–41. [Google Scholar] [CrossRef]
- Gordon, S.; Pluddemann, A.; Martinez Estrada, F. Macrophage heterogeneity in tissues: Phenotypic diversity and functions. Immunol. Rev. 2014, 262, 36–55. [Google Scholar] [CrossRef] [PubMed]
- Dawidczyk, C.M.; Russell, L.M.; Hultz, M.; Searson, P.C. Tumor accumulation of liposomal doxorubicin in three murine models: Optimizing delivery efficiency. Nanomedicine 2017, 13, 1637–1644. [Google Scholar] [CrossRef]
- Wong, A.D.; Ye, M.; Ulmschneider, M.B.; Searson, P.C. Quantitative analysis of the enhanced permeation and retention (EPR) effect. PLoS One 2015, 10, e0123461. [Google Scholar] [CrossRef]
- Ngoune, R.; Peters, A.; von Elverfeldt, D.; Winkler, K.; Pütz, G. Accumulating nanoparticles by EPR: A route of no return. J. Control. Release 2016, 238, 58–70. [Google Scholar] [CrossRef]
- Gref, R.; Minamitake, Y.; Peracchia, M.; Trubetskoy, V.; Torchilin, V.; Langer, R. Biodegradable long-circulating polymeric nanospheres. Science 1994, 263, 1600–1603. [Google Scholar] [CrossRef]
- Peracchia, M.T. Stealth nanoparticles for intravenous administration. STP Pharma Sci. 2003, 13, 155–161. [Google Scholar]
- Gabizon, A.; Papahadjopoulos, D. Liposome formulations with prolonged circulation time in blood and enhanced uptake by tumors (phospholipid vesicles/drug delivery systems/cancer therapy/glycolipids. Proc. Natl. Acad. Sci. USA 1988, 85, 6949–6953. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Kruijff, R.M.; Raavé, R.; Kip, A.; Molkenboer-Kuenen, J.; Roobol, S.J.; Essers, J.; Heskamp, S.; Denkova, A.G. Elucidating the Influence of Tumor Presence on the Polymersome Circulation Time in Mice. Pharmaceutics 2019, 11, 241. https://doi.org/10.3390/pharmaceutics11050241
de Kruijff RM, Raavé R, Kip A, Molkenboer-Kuenen J, Roobol SJ, Essers J, Heskamp S, Denkova AG. Elucidating the Influence of Tumor Presence on the Polymersome Circulation Time in Mice. Pharmaceutics. 2019; 11(5):241. https://doi.org/10.3390/pharmaceutics11050241
Chicago/Turabian Stylede Kruijff, Robin M., René Raavé, Annemarie Kip, Janneke Molkenboer-Kuenen, Stefan J. Roobol, Jeroen Essers, Sandra Heskamp, and Antonia G. Denkova. 2019. "Elucidating the Influence of Tumor Presence on the Polymersome Circulation Time in Mice" Pharmaceutics 11, no. 5: 241. https://doi.org/10.3390/pharmaceutics11050241
APA Stylede Kruijff, R. M., Raavé, R., Kip, A., Molkenboer-Kuenen, J., Roobol, S. J., Essers, J., Heskamp, S., & Denkova, A. G. (2019). Elucidating the Influence of Tumor Presence on the Polymersome Circulation Time in Mice. Pharmaceutics, 11(5), 241. https://doi.org/10.3390/pharmaceutics11050241