The Ratio of Nasal Cannula Gas Flow to Patient Inspiratory Flow on Trans-nasal Pulmonary Aerosol Delivery for Adults: An in Vitro Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experiment Set Up
2.2. Comparison between Groups
2.3. Statistical Analysis
3. Results
3.1. Inhaled Dose during Quiet and Distressed Breathing
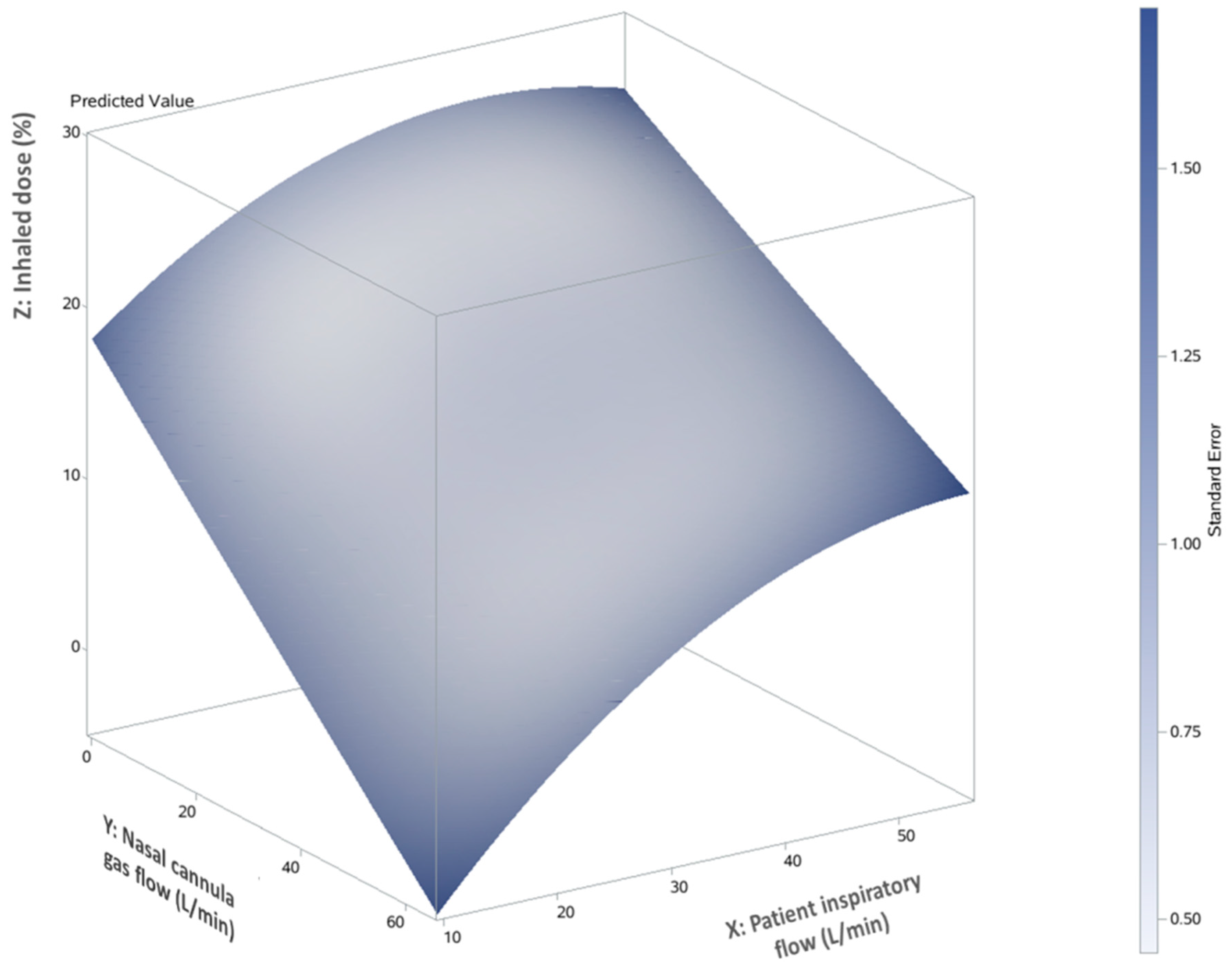
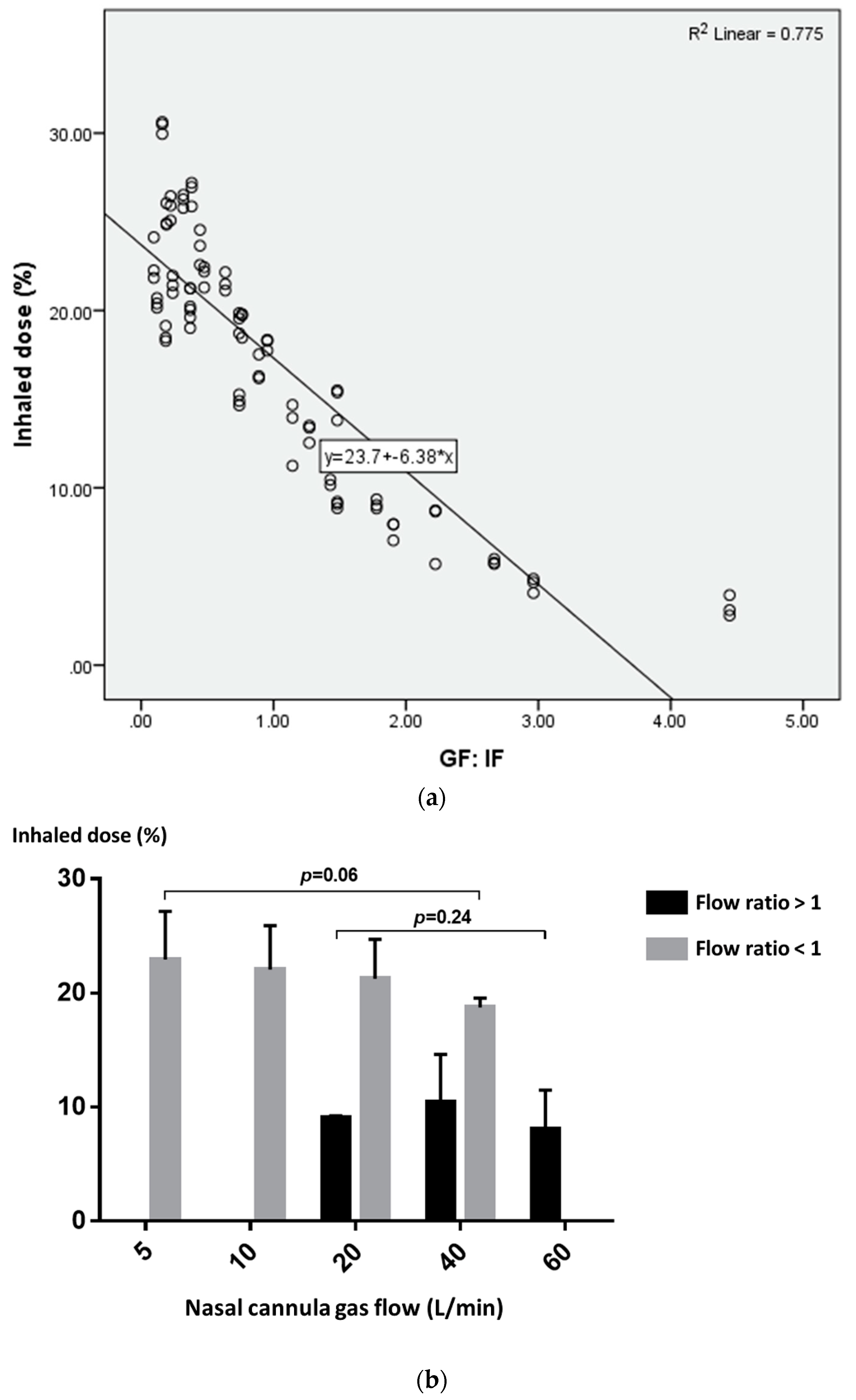
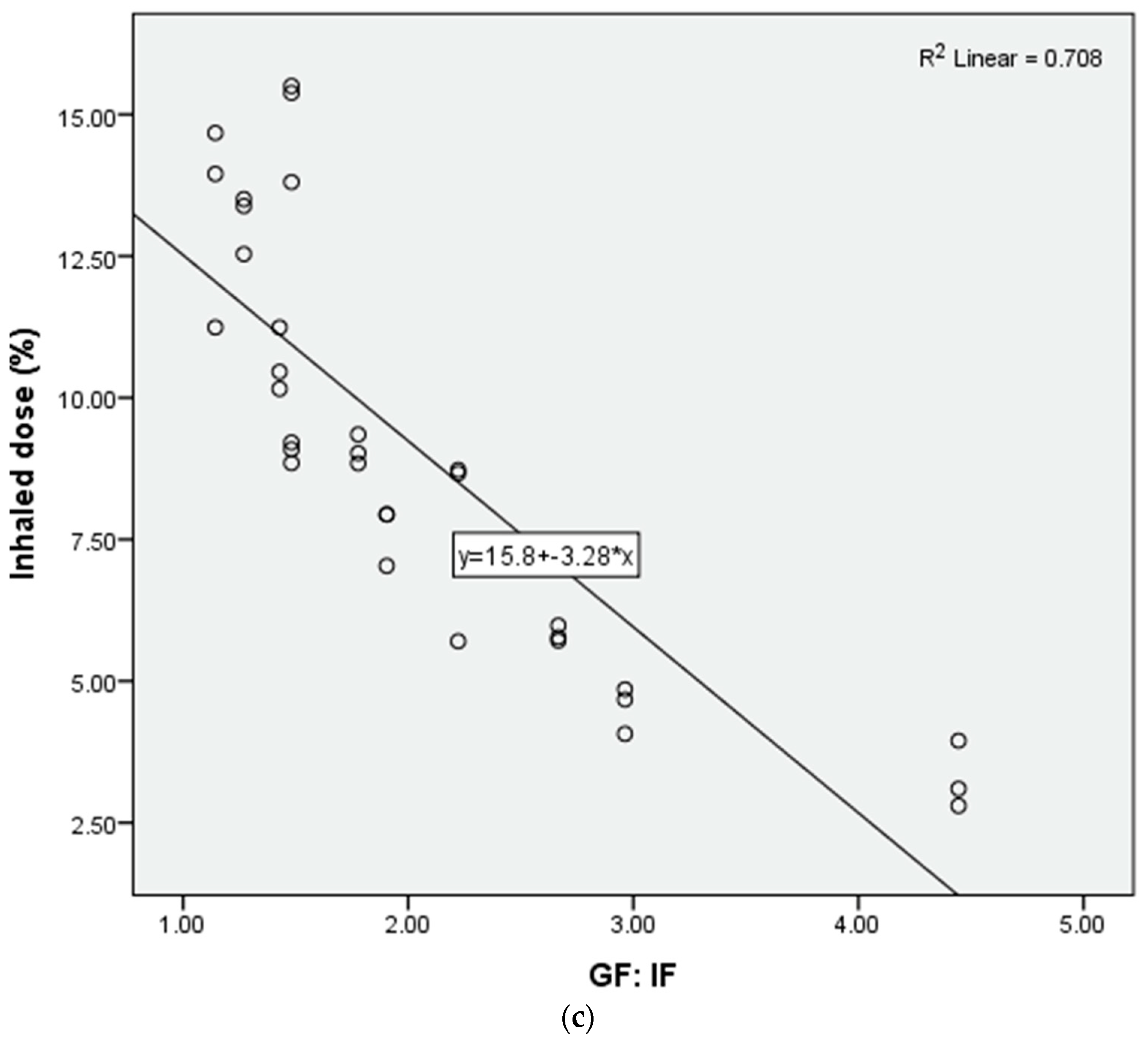
3.2. The Impact of Ratio of Nasal Cannula Gas Flow to Patient Inspiratory Flow on Inhaled Dose
3.3. Predictor of Inhaled Dose during Trans-Nasal Pulmonary Aerosol Delivery
4. Discussion
4.1. The Ratio of Nasal Cannula Gas Flow to Patient Inspiratory Flow in Trans-Nasal Aerosol Delivery
4.2. Clinical Implication
4.3. Limitation
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Helviz, Y.; Einav, S. A systematic review of the high-flow nasal cannula for adult patients. Crit. Care 2018, 22, 71. [Google Scholar] [CrossRef] [PubMed]
- Baudin, F.; Buisson, A.; Vanel, B.; Massenavette, B.; Pouyau, R.; Javouhey, E. Nasal high flow in management of children with status asthmaticus: A retrospective observational study. Ann. Intensive Care 2017, 7, 55. [Google Scholar] [CrossRef] [PubMed]
- Morgan, S.E.; Mosakowski, S.; Solano, P.; Hall, J.B.; Tung, A. High-flow nasal cannula and aerosolized β agonists for rescue therapy in children with bronchiolitis: A case series. Respir. Care 2015, 60, e161. [Google Scholar] [CrossRef]
- Valencia-Ramos, J.; Mirás, A.; Cilla, A.; Ochoa, C.; Arnaez, J. Incorporating a nebulizer system into high-flow nasal cannula improves comfort in infants with bronchiolitis. Respir. Care 2018, 63, 886–893. [Google Scholar] [CrossRef]
- Bräunlich, J.; Wirtz, H. Oral versus nasal high-flow bronchodilator inhalation in chronic obstructive pulmonary disease. J. Aerosol. Med. Pulm. Drug Deliv. 2018, 31, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Reminiac, F.; Vecellio, L.; Bodet-Contentin, L.; Gissot, V.; Le Pennec, D.; Salmon Gandonnière, C.; Cabrera, M.; Dequin, P.F.; Plantier, L.; Ehrmann, S. Nasal high-flow bronchodilator nebulization: A randomization cross-over study. Ann. Intensive Care 2018, 8, 128. [Google Scholar] [CrossRef]
- Madney, Y.M.; Fathy, M.; Elberry, A.A.; Ravea, H.; Abdelrahim, M.E. Aerosol delivery through an adult high-flow nasal cannula circuit using low-flow oxygen. Respir. Care 2019. [CrossRef] [PubMed]
- Ammar, M.A.; Sasidhar, M.; Lam, S.W. Inhaled epoprostenol through noninvasive routes of ventilator support systems. Ann. Pharmacother. 2018, 52, 1173–1181. [Google Scholar] [CrossRef]
- Dugernier, J.; Hesse, M.; Jumetz, T.; Bialais, E.; Roeseler, J.; Depoortere, V.; Michotte, J.B.; Wittebole, X.; Ehrmann, S.; Laterre, P.F.; et al. Aerosol delivery with two nebulizers through high-flow nasal cannula: A randomized cross-over single-photon emission computed tomography-computed tomography study. J. Aerosol. Med. Pulm. Drug Deliv. 2017, 30, 349–358. [Google Scholar] [CrossRef]
- Réminiac, F.; Vecellio, L.; Loughlin, R.M.; Le Pennec, D.; Cabrera, M.; Vourc’h, N.H.; Fink, J.B.; Ehrmann, S. Nasal high flow nebulization in infants and toddlers: An in vitro and in vivo scintigraphic study. Pediatr. Pulmonol. 2017, 52, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Bhashyam, A.R.; Wolf, M.T.; Marcinkowski, A.L.; Saville, A.; Thomas, K.; Carcillo, J.A.; Corcoran, T.E. Aerosol delivery through nasal cannulas: An in vitro study. J. Aerosol. Med. Pulm. Drug Deliv. 2008, 21, 181–188. [Google Scholar] [CrossRef]
- Ari, A.; Harwood, R.; Sheard, M.; Dailey, P.; Fink, J.B. In vitro comparison of heliox and oxygen in aerosol delivery using pediatric high flow nasal cannula. Pediatr. Pulmonol. 2011, 46, 795–801. [Google Scholar] [CrossRef]
- Perry, S.A.; Kesser, K.C.; Geller, D.E.; Selhorst, D.M.; Rendle, J.K.; Hertzog, J.H. Influences of cannula size and flow rate on aerosol drug delivery through the Vapotherm humidified high-flow nasal cannula system. Pediatr. Crit. Care Med. 2013, 14, e250–e256. [Google Scholar] [CrossRef] [PubMed]
- Sunbul, F.S.; Fink, J.B.; Harwood, R.; Sheard, M.M.; Zimmerman, R.D.; Ari, A. Comparison of HFNC, bubble CPAP.; SiPAP on aerosol delivery in neonates: An in-vitro study. Pediatr. Pulmonol. 2015, 50, 1099–1106. [Google Scholar] [CrossRef]
- Réminiac, F.; Vecellio, L.; Heuzé-Vourc’h, N.; Petitcollin, A.; Respaud, R.; Cabrera, M.; Pennec, D.L.; Diot, P.; Ehrmann, S. Aerosol therapy in adults receiving high flow nasal cannula oxygen therapy. J. Aerosol. Med. Pulm. Drug Deliv. 2016, 29, 134–141. [Google Scholar] [CrossRef]
- Dailey, P.A.; Harwood, R.; Walsh, K.; Fink, J.B.; Thayer, T.; Gagnon, G.; Ari, A. Aerosol delivery through adult high flow nasal cannula with heliox and oxygen. Respir. Care 2017, 62, 1186–1192. [Google Scholar] [CrossRef]
- Madney, Y.M.; Fathy, M.; Elberry, A.A.; Rabea, H.; Abdelrahim, M.E.A. Nebulizers and spacers for aerosol delivery through adult nasal cannula at low oxygen flow rate: An in-vitro study. J. Drug Deliv. Sci. Technol. 2017, 39, 260–265. [Google Scholar] [CrossRef]
- Li, J.; Gong, L.; Ari, A.; Fink, J.B. Decrease the flow setting to improve trans-nasal pulmonary aerosol delivery via “high-flow nasal cannula” to infants and toddlers. Pediatr. Pulmonol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, T.E.; Saville, A.; Adams, P.S.; Johnston, D.J.; Czachowski, M.R.; Domnina, Y.A.; Lin, J.H.; Weiner, D.J.; Huber, A.S.; Sanchez De Toledo, J.; et al. Deposition studies of aerosol delivery by nasal cannula to infants. Pediatr. Pulmonol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Ari, A. Aerosol drug delivery through high flow nasal cannula. Curr. Pharm. Biotechnol. 2017, 18, 877–882. [Google Scholar] [CrossRef]
- Golshahi, L.; Longest, P.W.; Azimi, M.; Syed, A.; Hindle, M. Intermittent aerosol delivery to the lungs during high-flow nasal cannula therapy. Respir. Care 2014, 59, 1476–1486. [Google Scholar] [CrossRef] [PubMed]
- Hess, D.R. Aerosol therapy during noninvasive ventilation or high-flow nasal cannula. Respir. Care 2015, 60, 880–891. [Google Scholar] [CrossRef] [PubMed]



| Breathing Pattern | Vt (mL) | RR (bpm) | I:E | Ti (s) | Aerosol Inhalation Time (RR × Ti) (s) | Inspiratory Flow (L/min) | Nasal Cannula Gas Flow (L/min) | Ratio of Nasal Cannula Gas Flow to Patient Inspiratory Flow |
|---|---|---|---|---|---|---|---|---|
| Quiet breathing | 300 | 15 | 1: 2 | 1.33 | 20 | 13.5 | 5, 10, 20,40,60 | 0.37, 0.74, 1.48, 2.96, 4.44 |
| 500 | 15 | 1: 2 | 1.33 | 20 | 22.5 | 5, 10, 20,40,60 | 0.22, 0.44, 0.88, 1.78, 2.67 | |
| 700 | 15 | 1: 2 | 1.33 | 20 | 31.5 | 5, 10, 20,40,60 | 0.16, 0.32, 0.64, 1.27, 1.90 | |
| Distressed breathing | 450 | 30 | 1: 1 | 1 | 30 | 27 | 5, 10, 20,40,60 | 0.19, 0.37, 0.74, 1.48, 2.22 |
| 700 | 30 | 1: 1 | 1 | 30 | 42 | 5, 10, 20,40,60 | 0.12, 0.24, 0.48, 0.95, 1.43 | |
| 700 | 30 | 1:1.5 | 0.8 | 24 | 52.5 | 5, 10, 20,40,60 | 0.10, 0.19, 0.38, 0.76, 1.14 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Gong, L.; Fink, J.B. The Ratio of Nasal Cannula Gas Flow to Patient Inspiratory Flow on Trans-nasal Pulmonary Aerosol Delivery for Adults: An in Vitro Study. Pharmaceutics 2019, 11, 225. https://doi.org/10.3390/pharmaceutics11050225
Li J, Gong L, Fink JB. The Ratio of Nasal Cannula Gas Flow to Patient Inspiratory Flow on Trans-nasal Pulmonary Aerosol Delivery for Adults: An in Vitro Study. Pharmaceutics. 2019; 11(5):225. https://doi.org/10.3390/pharmaceutics11050225
Chicago/Turabian StyleLi, Jie, Lingyue Gong, and James B. Fink. 2019. "The Ratio of Nasal Cannula Gas Flow to Patient Inspiratory Flow on Trans-nasal Pulmonary Aerosol Delivery for Adults: An in Vitro Study" Pharmaceutics 11, no. 5: 225. https://doi.org/10.3390/pharmaceutics11050225
APA StyleLi, J., Gong, L., & Fink, J. B. (2019). The Ratio of Nasal Cannula Gas Flow to Patient Inspiratory Flow on Trans-nasal Pulmonary Aerosol Delivery for Adults: An in Vitro Study. Pharmaceutics, 11(5), 225. https://doi.org/10.3390/pharmaceutics11050225






