Excipient Interactions in Glucagon Dry Powder Inhaler Formulation for Pulmonary Delivery
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Micronization of Glucagon
2.2.2. Preparation of Interactive Mixtures and Homogeneity Test
2.2.3. Drug Dispersion by Twin-Stage Impinger (TSI)
2.2.4. Quantitative Analysis of Glucagon by LC-MS/MS
2.2.5. Morphological Studies by SEM
2.2.6. ATR-FTIR
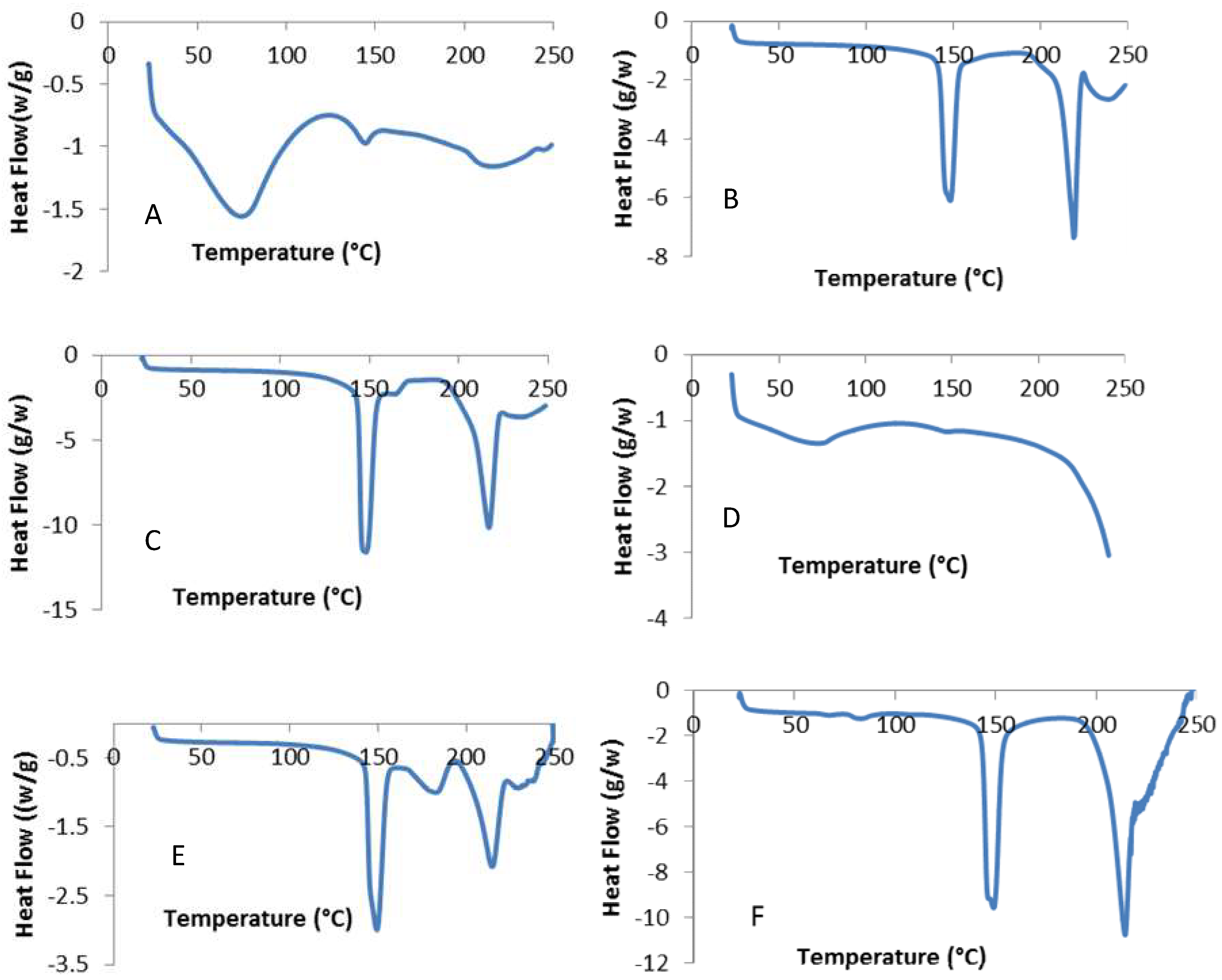
2.2.7. Differential Scanning Calorimetry (DSC)
2.2.8. Raman Spectroscopy
2.2.9. Statistical Analysis
3. Results and Discussions
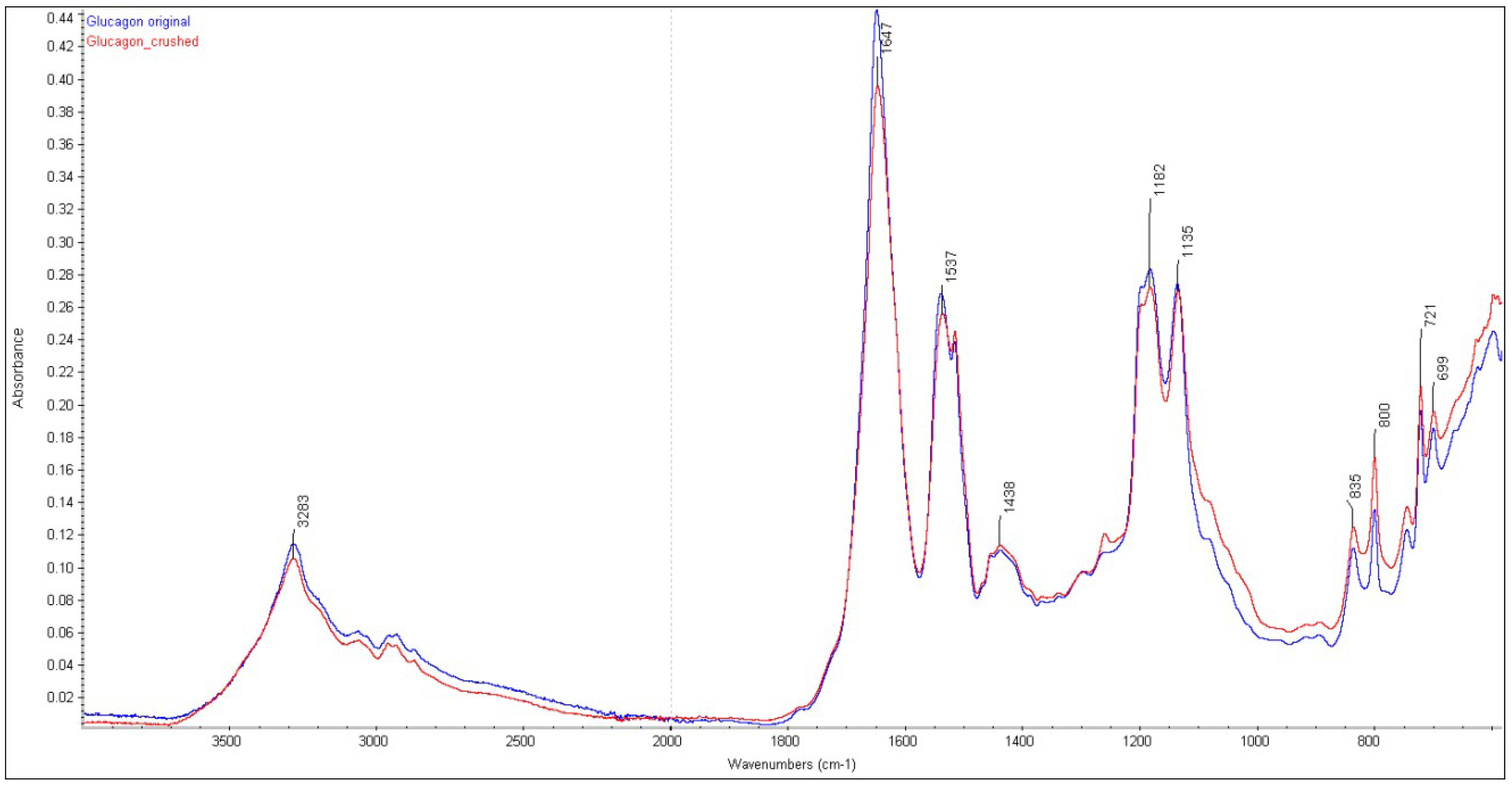
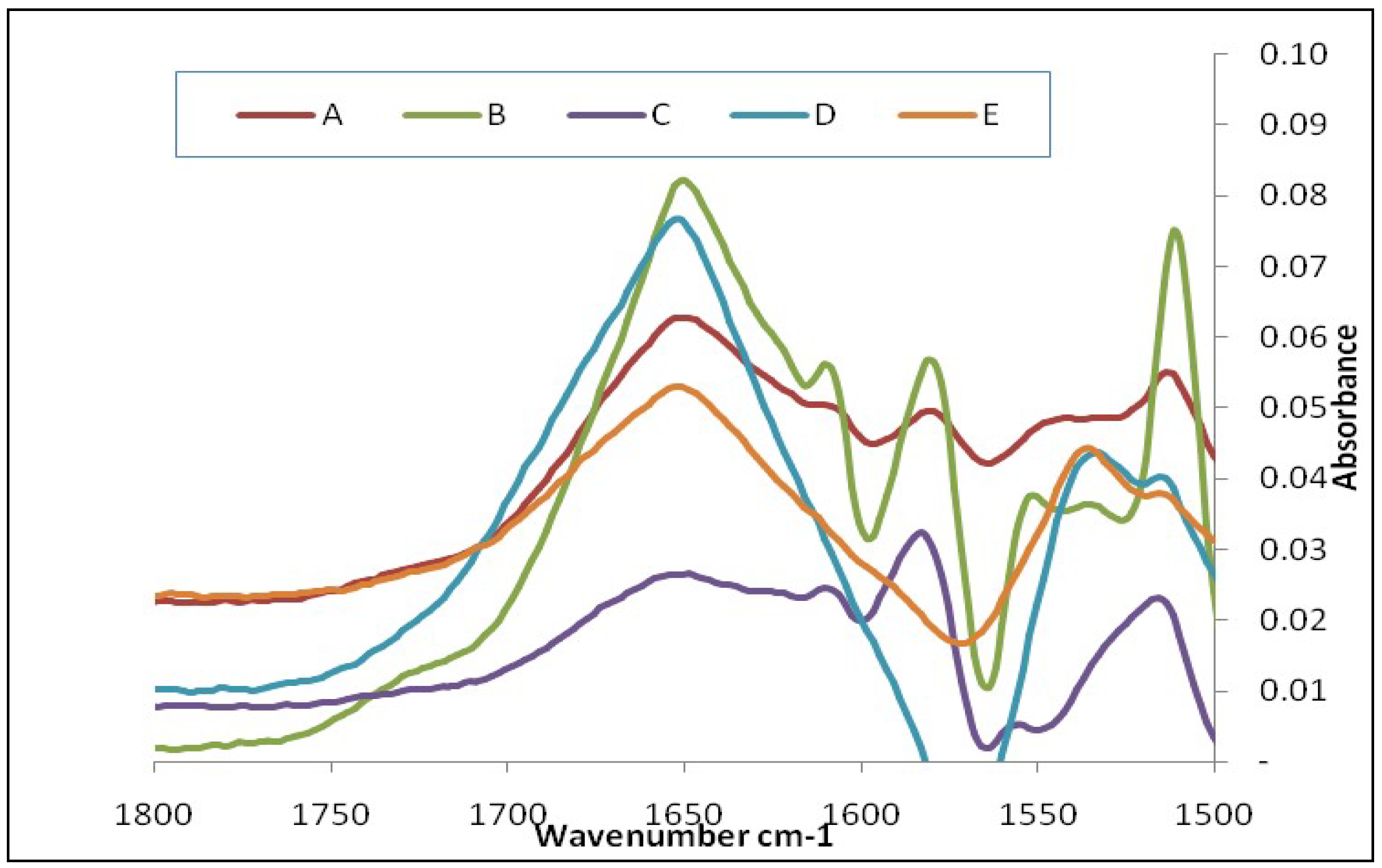
3.1. FTIR Analysis
3.2. Crystallinity of Powders
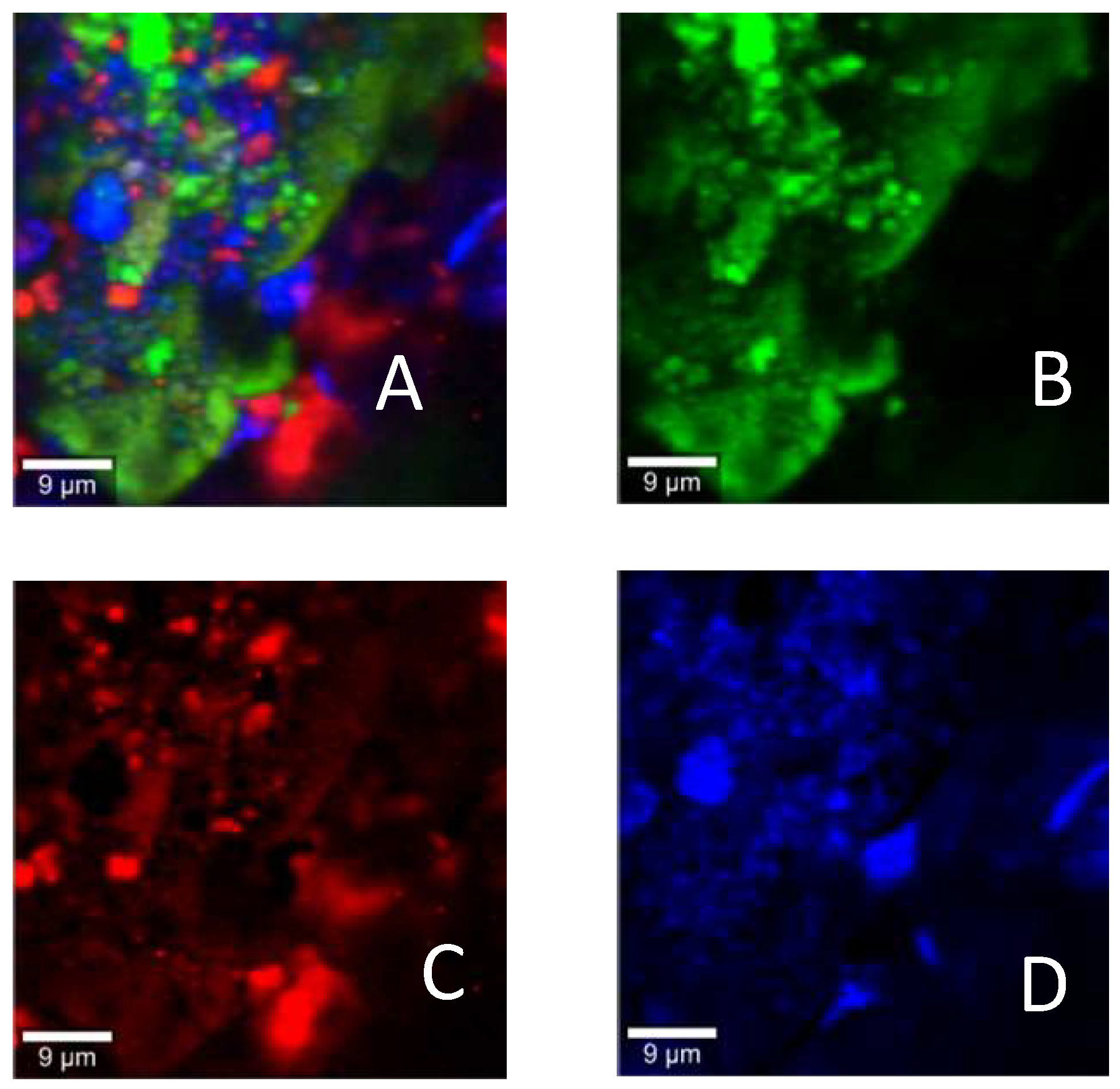
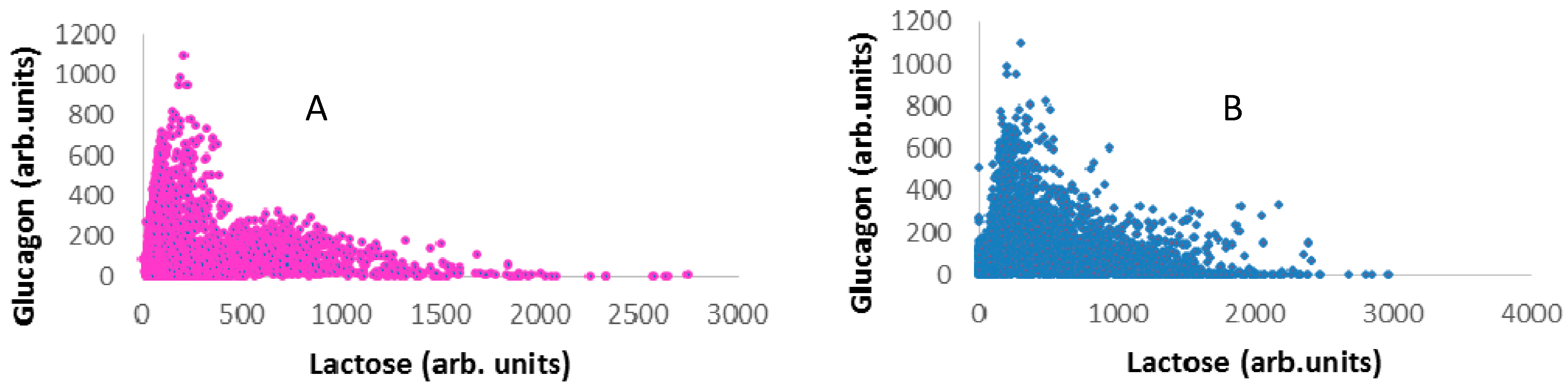
3.3. Raman Mapping
4. Aerosolization of Glucagon from DPI Formulations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Reno, F.E.; Edwards, C.N.; Bendix Jensen, M.; Torok-Batho, M.; Esdaile, D.J.; Piche, C.; Triest, M.; Carballo, D. Needle-free nasal delivery of glucagon for treatment of diabetes-related severe hypoglycemia: toxicology of polypropylene resin used in delivery device. Cutaneous Ocul. Toxicol. 2016, 35, 242–247. [Google Scholar] [CrossRef]
- Suico, J.; Hövelmann, U.; Zhang, S.; Shen, T.; Bergman, B.; Sherr, J.; Zijlstra, E.; Frier, B.; Plum-Moerschel, L. Nasal glucagon: A viable alternative to treat insulin-induced hypoglycaemia in adults with type 1 diabetes. Can. J. Diabetes 2018, 42, S53. [Google Scholar] [CrossRef]
- Imam, S.S.; Aqil, M.; Gupta, H. Pulmonary Vaccine Delivery Systems: A Novel Approach for Immunization. Curr. Drug Ther. 2014, 9, 166–172. [Google Scholar] [CrossRef]
- Islam, N.; Rahman, S. Pulmonary drug delivery: Implication for new strategy for pharmacotherapy for neurodegenerative disorders. Drug Discov. Ther. 2008, 2, 264–276. [Google Scholar]
- Rudolph, C.; Lausier, J.; Naundorf, S.; Muller, R.H.; Rosenecker, J. In vivo gene delivery to the lung using polyethylenimine and fractured polyamidoamine dendrimers. J. Gene Med. 2000, 2, 269–278. [Google Scholar] [CrossRef]
- Endo, K.; Amikawa, S.; Matsumoto, A.; Sahashi, N.; Onoue, S. Erythritol-based dry powder of glucagon for pulmonary administration. Int. J. Pharm. 2005, 290, 63–71. [Google Scholar] [CrossRef]
- Onoue, S.; Yamamoto, K.; Kawabata, Y.; Hirose, M.; Mizumoto, T.; Yamada, S. Novel dry powder inhaler formulation of glucagon with addition of citric acid for enhanced pulmonary delivery. Int. J. Pharm. 2009, 382, 144–150. [Google Scholar] [CrossRef]
- Onoue, S.; Kuriyama, K.; Uchida, A.; Mizumoto, T.; Yamada, S. Inhalable Sustained-Release Formulation of Glucagon: In Vitro Amyloidogenic and Inhalation Properties, and In Vivo Absorption and Bioactivity. Pharm. Res. 2011, 28, 1157–1166. [Google Scholar] [CrossRef]
- Marriott, C.; Frijlink, H.W. Lactose as a carrier for inhalation products: Breathing new life into an old carrier. Adv. Drug Deliv. Rev. 2012, 64, 217–219. [Google Scholar] [CrossRef]
- Young, P.M.; Kwok, P.; Adi, H.; Chan, H.-K.; Traini, D. Lactose Composite Carriers for Respiratory Delivery. Pharm. Res. 2009, 26, 802–810. [Google Scholar] [CrossRef]
- Zhou, Q.; Denman, J.A.; Gengenbach, T.; Das, S.; Qu, L.; Zhang, H.; Larson, I.; Stewart, P.J.; Morton, D.A.V. Characterization of the surface properties of a model pharmaceutical fine powder modified with a pharmaceutical lubricant to improve flow via a mechanical dry coating approach. J. Pharm. Sci. 2011, 100, 3421–3430. [Google Scholar] [CrossRef]
- Tuli, R.A.; George, G.A.; Dargaville, T.R.; Islam, N. Studies on the Effect of the Size of Polycaprolactone Microspheres for the Dispersion of Salbutamol Sulfate from Dry Powder Inhaler Formulations. Pharm. Res. 2012, 29, 2445–2455. [Google Scholar] [CrossRef]
- Begat, P.; Price, R.; Harris, H.; Morton, D.A.V.; Staniforth, J.N. The influence of force control agents on the cohesive-adhesive balance in dry powder inhaler formulations. Kona 2005, 23, 109–121. [Google Scholar] [CrossRef]
- Han, X.; Ghoroi, C.; Dave, R. Dry coating of micronized API powders for improved dissolution of directly compacted tablets with high drug loading. Int. J. Pharm. 2013, 442, 74–85. [Google Scholar] [CrossRef]
- Morton, D. Dry Powder Inhaler Formulations Comprising Surface-Modified Particles with Anti-Adherent Additives. U.S. Patent 2005-GB50211, 1 June 2006. [Google Scholar]
- Aquino, R.P.; Prota, L.; Auriemma, G.; Santoro, A.; Mencherini, T.; Colombo, G.; Russo, P. Dry powder inhalers of gentamicin and leucine: Formulation parameters, aerosol performance and in vitro toxicity on CuFi1 cells. Int. J. Pharm 2012, 426, 100–107. [Google Scholar] [CrossRef]
- Muhsin, M.D.A.; George, G.; Beagley, K.; Ferro, V.; Wang, H.; Islam, N. Effects of Chemical Conjugation of L-Leucine to Chitosan on Dispersibility and Controlled Release of Drug from a Nanoparticulate Dry Powder Inhaler Formulation. Mol. Pharm. 2016, 13, 1455–1466. [Google Scholar] [CrossRef]
- Mangal, S.; Nie, H.; Xu, R.; Guo, R.; Cavallaro, A.; Zemlyanov, D.; Zhou, Q. Physico-chemical properties, aerasolization and dissolution of co-spray dried azithromycin particles with L-leucine for inhalation. Pharm. Res. 2018, 35, 1–15. [Google Scholar] [CrossRef]
- Fussell, A.L.; Grasmeijer, F.; Frijlink, H.W.; Boer, A.H.; Offerhaus, H.L. CARS microscopy as a tool for studying the distribution of micronised drugs in adhesive mixtures for inhalation. J. Raman Spectrosc. 2014, 45, 495–500. [Google Scholar] [CrossRef]
- Afrose, A.; White, E.T.; Howes, T.; George, G.; Rashid, A.; Rintoul, L.; Islam, N. Preparation of Ibuprofen Microparticles by Antisolvent Precipitation Crystallization Technique: Characterization, Formulation, and In Vitro Performance. J. Pharm. Sci. 2018, 107, 3060–3069. [Google Scholar] [CrossRef]
- Fang, W.-J.; Qi, W.; Kinzell, J.; Prestrelski, S.; Carpenter, J.F. Effects of Excipients on the Chemical and Physical Stability of Glucagon during Freeze-Drying and Storage in Dried Formulations. Pharm. Res. 2012, 29, 3278–3291. [Google Scholar] [CrossRef]
- Stigsnaes, P.; Frokjaer, S.; Bjerregaard, S.; Van de Weert, M.; Kingshott, P.; Moeller, E.H. Characterization and physical stability of PEGylated glucagon. Int. J. Pharm. 2007, 330, 89–98. [Google Scholar] [CrossRef]
- Vonhoff, S.; Condliffe, J.; Schiffter, H. Implementation of an FTIR calibration curve for fast and objective determination of changes in protein secondary structure during formulation development. J. Pharm. Biomed. Anal. 2010, 51, 39–45. [Google Scholar] [CrossRef]
- Alway, B.; Sangchantra, R.; Stewart, P.J. Modeling the dissolution of diazepam in lactose interactive mixtures. Int. J. Pharm. 1996, 130, 213–224. [Google Scholar] [CrossRef]
- Liu, J.; Stewart, P.J. Deaggregation during the Dissolution of Benzodiazepines in Interactive Mixtures. J. Pharm. Sci. 1998, 87, 1632–1638. [Google Scholar] [CrossRef]
- Crooks, M.J.; Ho, R. Ordered mixing in direct compression of tablets. Powder Technol. 1976, 14, 161–167. [Google Scholar] [CrossRef]
- Byler, D.M.; Susi, H. Examination of the secondary structure of proteins by deconvolved FTIR spectra. Biopolymers 1986, 25, 469–487. [Google Scholar] [CrossRef]
- Larhrib, H.; Zeng, X.M.; Martin, G.P.; Marriott, C.; Pritchard, J. The use of different grades of lactose as a carrier for aerosolized salbutamol sulfate. Int. J. Pharm. 1999, 191, 1–14. [Google Scholar] [CrossRef]
- Corrigan, D.O.; Corrigan, O.I.; Healy, A.M. Physicochemical and in vitro deposition properties of salbutamol sulphate/ipratropium bromide and salbutamol sulphate/excipient spray dried mixtures for use in dry powder inhalers. Int. J. Pharm. 2006, 322, 22–30. [Google Scholar] [CrossRef]
- Coates Matthew, S.; Fletcher David, F.; Chan, H.-K.; Raper Judy, A. Effect of design on the performance of a dry powder inhaler using computational fluid dynamics. Part 1: Grid structure and mouthpiece length. J. Pharm. Sci. 2004, 93, 2863–2876. [Google Scholar] [CrossRef]
- Islam, N.; Tuli, R.A.; George, G.A.; Dargaville, T.R. Colloidal drug probe: Method development and validation for adhesion force measurement using Atomic Force Microscopy. Adv. Powder Technol. 2014, 25, 1240–1248. [Google Scholar] [CrossRef][Green Version]


| Samples | RD (%) | ED (%) | FPF (%) |
|---|---|---|---|
| Glucagon (2.5%) plus lactose | 95.9 (7.1) | 62.7 (2.9) | 31.7 (2.7) |
| Glucagon (2.5%) plus 5% leucine plus lactose | 100.9 (2) | 52.6 (8) | 35.5 (4.9) |
| Glucagon (2.5%) plus 5% magnesium stearate plus lactose | 101.9 (3.7) | 66.3 (3.5) | 36.3 (1.3) |
| Glucagon only | 102.8 (0.7) | 48.2 (10.9) | 6.4 (0.8) |
| Samples | Low Frequency β-Sheet | α-Helix |
|---|---|---|
| Glucagon original | - | 1649 cm−1 |
| Glucagon inhalable particles | - | 1647 cm−1 |
| Glucagon subtracted from leucine | 1612 cm−1 1625 cm−1 | 1651 cm−1 |
| Glucagon subtracted from lactose | 1652 cm−1 | |
| Glucagon subtracted from the mixture of lactose plus leucine plus glucagon | 1612 cm−1 1624 cm−1 1638 cm−1 | 1651 cm−1 |
| Glucagon subtracted from MgSt | - | 1654 cm−1 |
| Glucagon subtracted from the mixture of lactose plus MgSt plus glucagon | - | 1654 cm−1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rashid, M.A.; Elgied, A.A.; Alhamhoom, Y.; Chan, E.; Rintoul, L.; Allahham, A.; Islam, N. Excipient Interactions in Glucagon Dry Powder Inhaler Formulation for Pulmonary Delivery. Pharmaceutics 2019, 11, 207. https://doi.org/10.3390/pharmaceutics11050207
Rashid MA, Elgied AA, Alhamhoom Y, Chan E, Rintoul L, Allahham A, Islam N. Excipient Interactions in Glucagon Dry Powder Inhaler Formulation for Pulmonary Delivery. Pharmaceutics. 2019; 11(5):207. https://doi.org/10.3390/pharmaceutics11050207
Chicago/Turabian StyleRashid, Md Abdur, Amged Awad Elgied, Yahya Alhamhoom, Enoch Chan, Llew Rintoul, Ayman Allahham, and Nazrul Islam. 2019. "Excipient Interactions in Glucagon Dry Powder Inhaler Formulation for Pulmonary Delivery" Pharmaceutics 11, no. 5: 207. https://doi.org/10.3390/pharmaceutics11050207
APA StyleRashid, M. A., Elgied, A. A., Alhamhoom, Y., Chan, E., Rintoul, L., Allahham, A., & Islam, N. (2019). Excipient Interactions in Glucagon Dry Powder Inhaler Formulation for Pulmonary Delivery. Pharmaceutics, 11(5), 207. https://doi.org/10.3390/pharmaceutics11050207






