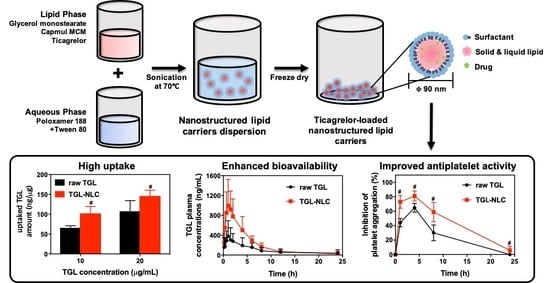
Systemic Design and Evaluation of Ticagrelor-Loaded Nanostructured Lipid Carriers for Enhancing Bioavailability and Antiplatelet Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. HPLC Analysis
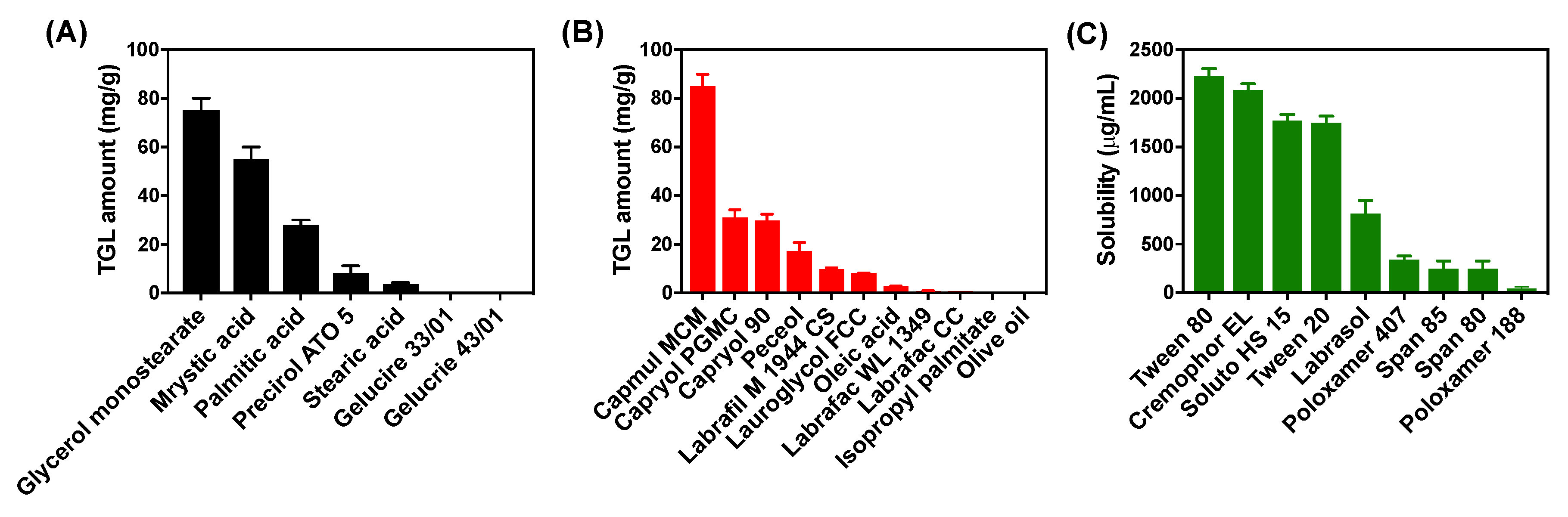
2.3. Solubility Study
2.4. Preparation of TGL-NLC
2.5. Optimization of TGL-NLC
2.5.1. Particle size (Y1) and Polydispersity Index (Y2)
2.5.2. Encapsulation Efficiency (Y3)
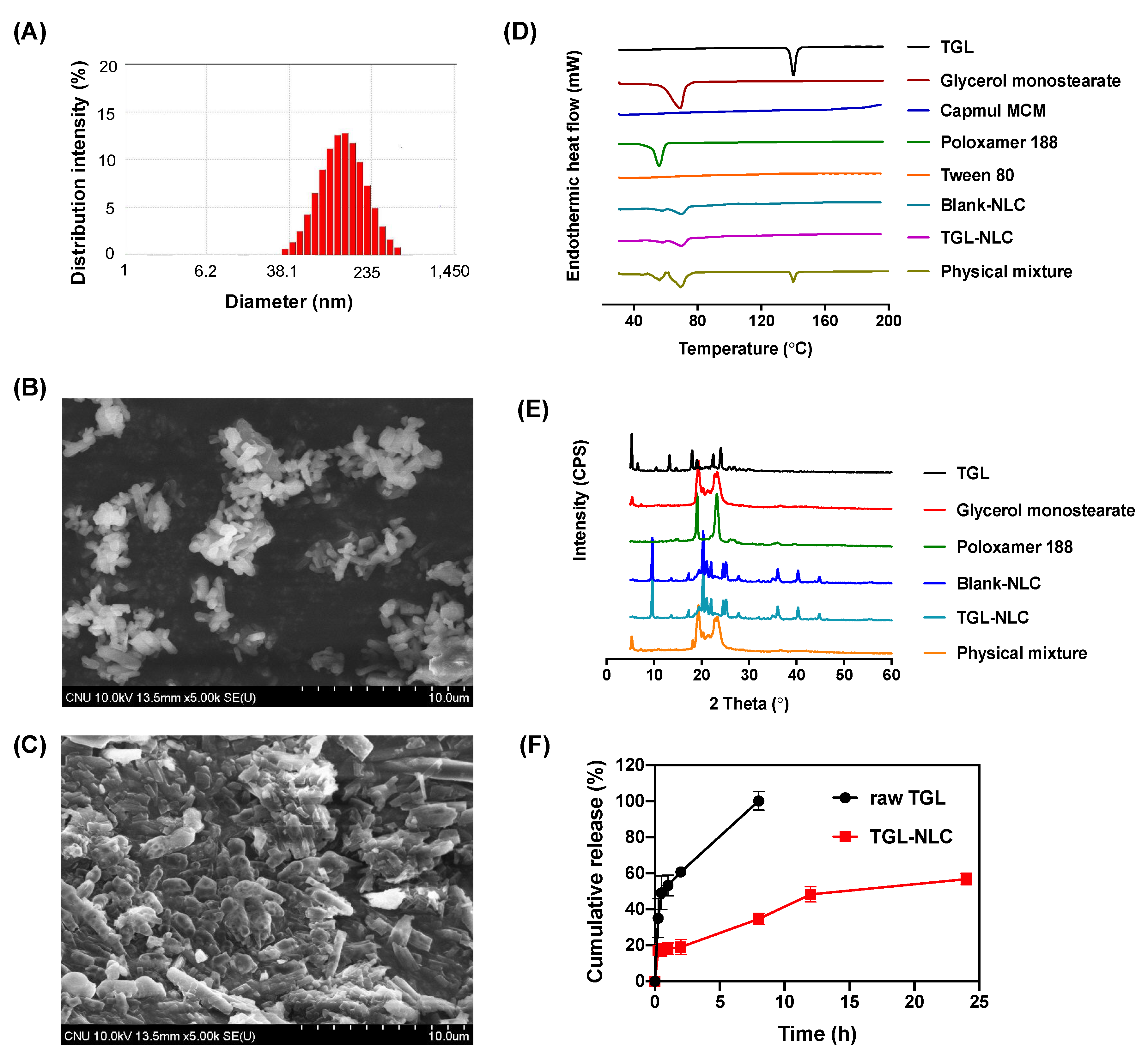
2.6. Characterization of Optimized TGL-NLC
2.7. Cell Studies
2.7.1. Cell Culture
2.7.2. Cytotoxicity Study
2.7.3. Cellular Uptake Study
2.8. Pharmacokinetic Study
2.8.1. Animal Study
2.8.2. LC-MS/MS analysis of TGL
2.8.3. Pharmacokinetic Data Analysis
2.9. Pharmacodynamic Study
2.10. Statistical Analysis
3. Results
3.1. Optimization and Characterization of TGL-NLC
3.1.1. Solubility Study for TGL-NLC
3.1.2. Optimization of TGL-NLC
3.1.3. Characterization of Optimized TGL-NLC
3.2. Cell Studies
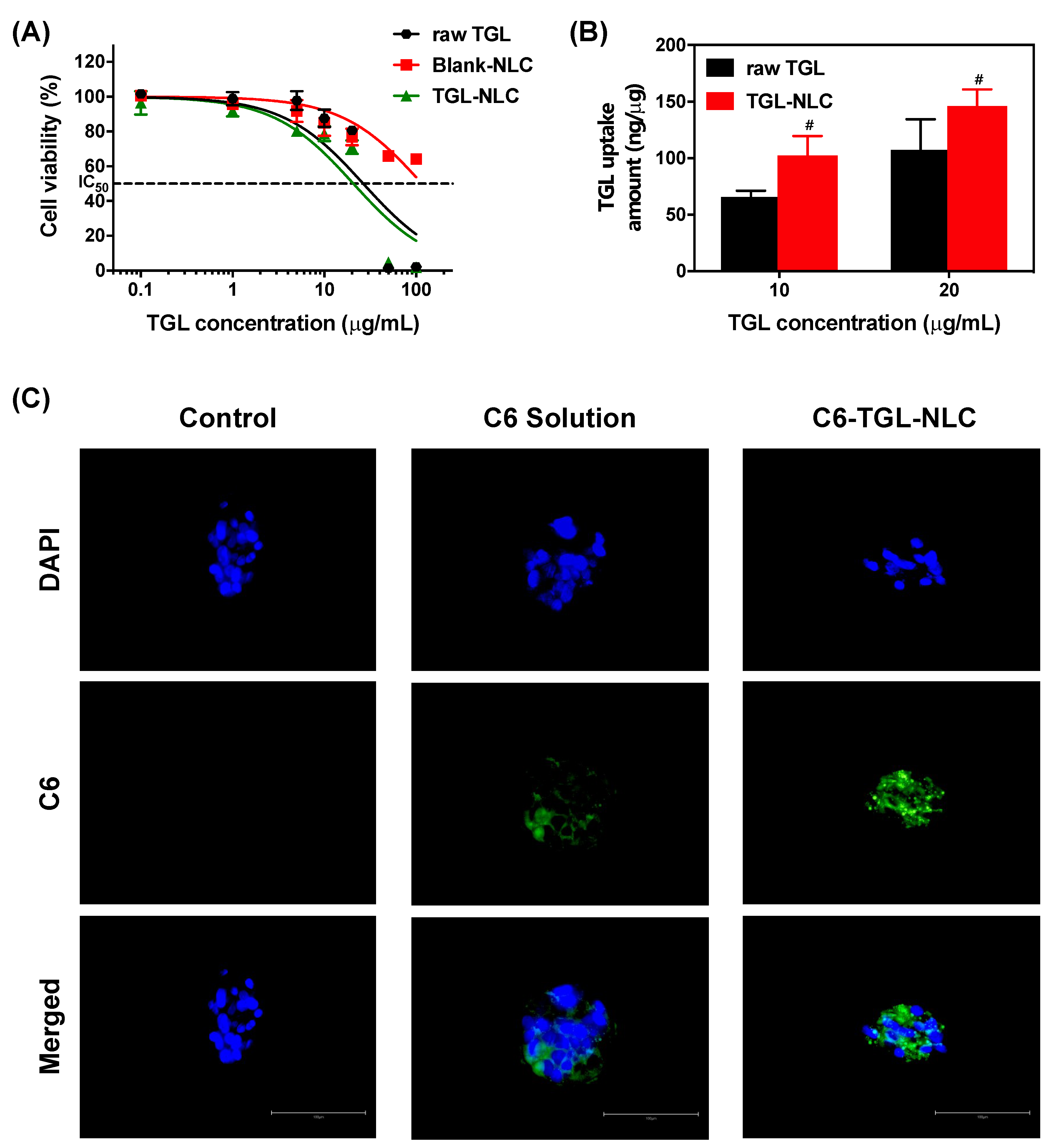
3.2.1. Cytotoxicity Study
3.2.2. Cellular Uptake Study
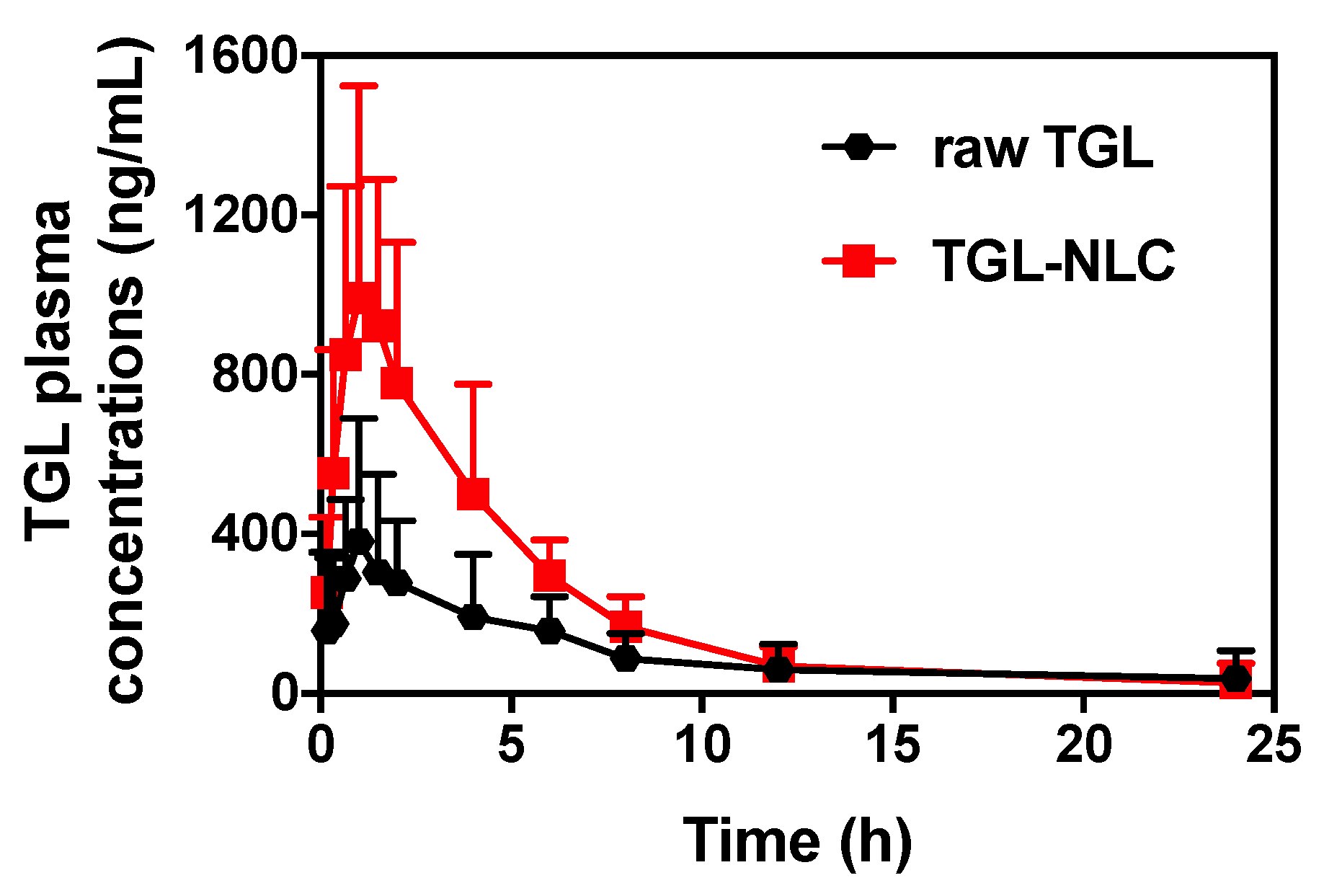
3.3. Pharmacokinetic Study
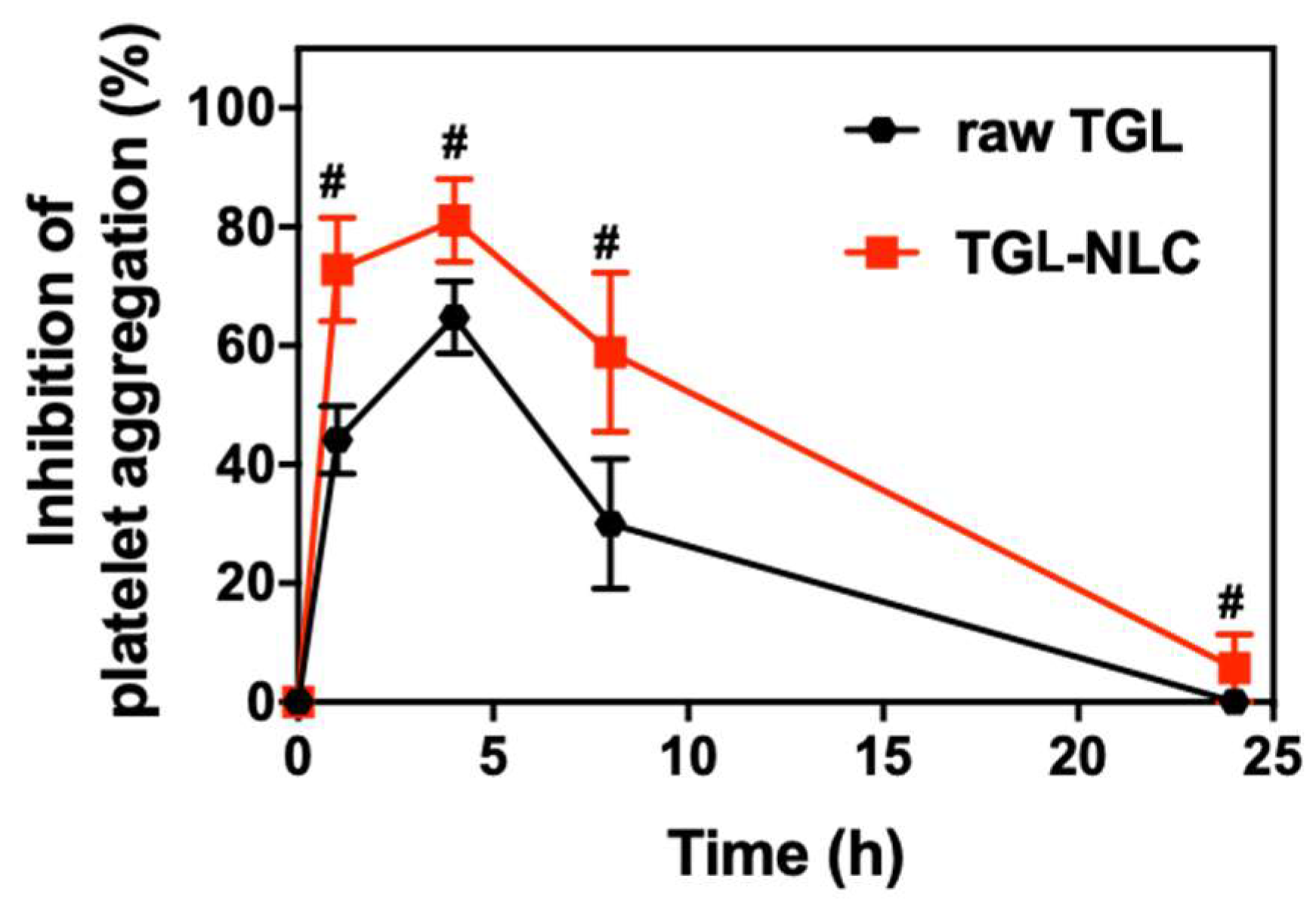
3.4. Pharmacodynamic Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Husted, S.; van Giezen, J.J. Ticagrelor: The first reversibly binding oral P2Y12 receptor antagonist. Cardiovasc. Ther. 2009, 27, 259–274. [Google Scholar] [CrossRef] [PubMed]
- Kubisa, M.J.; Jezewski, M.P.; Gasecka, A.; Siller-Matula, J.M.; Postula, M. Ticagrelor-toward more efficient platelet inhibition and beyond. Ther. Clin. Risk Manag. 2018, 14, 129–140. [Google Scholar] [CrossRef]
- Norgard, N.B.; Abu-Fadel, M. Comparison of prasugrel and clopidogrel in patients with acute coronary syndrome undergoing percutaneous coronary intervention. Vasc. Health Risk Manag. 2009, 5, 873–882. [Google Scholar] [CrossRef]
- Ghadi, R.; Dand, N. BCS class IV drugs: Highly notorious candidates for formulation development. J. Control. Release 2017, 248, 71–95. [Google Scholar] [CrossRef]
- Teng, R.; Maya, J. Absolute bioavailability and regional absorption of ticagrelor in healthy volunteers. J. Drug Assess. 2014, 3, 43–50. [Google Scholar] [PubMed]
- Mohammadi, M.R.; Nojoomi, A.; Mozafari, M.; Dubnika, A.; Inayathullah, M.; Rajadas, J. Nanomaterials engineering for drug delivery: A hybridization approach. J. Mater. Chem. B 2017, 5, 3995–4018. [Google Scholar] [CrossRef]
- Beloqui, A.; Solinis, M.A.; Rodriguez-Gascon, A.; Almeida, A.J.; Preat, V. Nanostructured lipid carriers: Promising drug delivery systems for future clinics. Nanomedicine 2016, 12, 143–161. [Google Scholar] [CrossRef] [PubMed]
- Na, Y.G.; Byeon, J.J.; Wang, M.; Huh, H.W.; Son, G.H.; Jeon, S.H.; Bang, K.H.; Kim, S.J.; Lee, H.J.; Lee, H.K.; et al. Strategic approach to developing a self-microemulsifying drug delivery system to enhance antiplatelet activity and bioavailability of ticagrelor. Int. J. Nanomed. 2019, 14, 1193–1212. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Lee, H.K.; Na, Y.G.; Bang, K.H.; Lee, H.J.; Wang, M.; Huh, H.W.; Cho, C.W. A novel composition of ticagrelor by solid dispersion technique for increasing solubility and intestinal permeability. Int. J. Pharm. 2019, 555, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Herneisey, M.; Liu, L.; Lambert, E.; Schmitz, N.; Loftus, S.; Janjic, J.M. Development of theranostic perfluorocarbon nanoemulsions as a model non-opioid pain nanomedicine using a quality by design (QbD) approach. AAPS PharmSciTech 2019, 20, 65. [Google Scholar] [CrossRef] [PubMed]
- Beg, S.; Kaur, R.; Khurana, R.K.; Rana, V.; Sharma, T.; Singh, B. QbD-based development of cationic self-nanoemulsifying drug delivery systems of paclitaxel with Improved biopharmaceutical attributes. AAPS PharmSciTech 2019, 20, 118. [Google Scholar] [CrossRef] [PubMed]
- Dahmash, E.Z.; Al-Khattawi, A.; Iyire, A.; Al-Yami, H.; Dennison, T.J.; Mohammed, A.R. Quality by design (QbD) based process optimisation to develop functionalised particles with modified release properties using novel dry particle coating technique. PLoS ONE 2018, 13, e0206651. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Jhawat, V. Quality by design (QbD) approach of pharmacogenomics in drug designing and formulation development for optimization of drug delivery systems. J. Control. Release 2017, 245, 15–26. [Google Scholar] [CrossRef]
- Kim, B.S.; Na, Y.G.; Choi, J.H.; Kim, I.; Lee, E.; Kim, S.Y.; Lee, J.Y.; Cho, C.W. The improvement of skin whitening of phenylethyl resorcinol by nanostructured lipid carriers. Nanomaterials 2017, 7, 241. [Google Scholar] [CrossRef]
- Baek, J.S.; Na, Y.G.; Cho, C.W. Sustained cytotoxicity of wogonin on breast cancer cells by encapsulation in solid lipid nanoparticles. Nanomaterials 2018, 8, 159. [Google Scholar] [CrossRef]
- Yu, S.H.; Tan, G.X.; Liu, D.D.; Yang, X.G.; Pan, W.S. Nanostructured lipid carrier (NLC)-based novel hydrogels as potential carriers for nepafenac applied after cataract surgery for the treatment of inflammation: Design, characterization and in vitro cellular inhibition and uptake studies. RSC Adv. 2017, 7, 16668–16677. [Google Scholar] [CrossRef]
- Guo, X.; Zhao, Z.; Chen, D.; Qiao, M.; Wan, F.; Cun, D.; Sun, Y.; Yang, M. Co-delivery of resveratrol and docetaxel via polymeric micelles to improve the treatment of drug-resistant tumors. Asian J. Pharm. Sci. 2019, 14, 78–85. [Google Scholar] [CrossRef]
- Hvas, A.M.; Favaloro, E.J. Platelet function analyzed by light transmission aggregometry. Methods Mol. Biol. 2017, 1646, 321–331. [Google Scholar]
- Uner, M. Preparation, characterization and physico-chemical properties of solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC): Their benefits as colloidal drug carrier systems. Pharmazie 2006, 61, 375–386. [Google Scholar] [PubMed]
- Poovi, G.; Damodharan, N. Lipid nanoparticles: A challenging approach for oral delivery of BCS Class-II drugs. Future J. Pharm. Sci. 2018, 4, 191–205. [Google Scholar] [CrossRef]
- Sharma, N.; Madan, P.; Lin, S.S. Effect of process and formulation variables on the preparation of parenteral paclitaxel-loaded biodegradable polymeric nanoparticles: A co-surfactant study. Asian J. Pharm. Sci. 2016, 11, 404–416. [Google Scholar] [CrossRef]
- Souto, E.B.; Mehnert, W.; Muller, R.H. Polymorphic behaviour of Compritol (R) 888 ATO as bulk lipid and as SLN and NLC. J. Microencapsul. 2006, 23, 417–433. [Google Scholar] [CrossRef] [PubMed]
- Schmidts, T.; Dobler, D.; Nissing, C.; Runkel, F. Influence of hydrophilic surfactants on the properties of multiple W/O/W emulsions. J. Colloid Interface Sci. 2009, 338, 184–192. [Google Scholar] [CrossRef]
- Patel, K.; Padhye, S.; Nagarsenker, M. Duloxetine HCl lipid nanoparticles: Preparation, characterization, and dosage form design. AAPS PharmSciTech 2012, 13, 125–133. [Google Scholar] [CrossRef]
- Liu, S.S.; Ho, P.C. Formulation optimization of scutellarin-loaded HP-beta-CD/chitosan nanoparticles using response surface methodology with Box–Behnken design. Asian J. Pharm. Sci. 2017, 12, 378–385. [Google Scholar] [CrossRef]
- Shahbazi, M.A.; Santos, H.A. Improving oral absorption via drug-loaded nanocarriers: Absorption mechanisms, intestinal models and rational fabrication. Curr. Drug Metab. 2013, 14, 28–56. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.S.; Cho, C.W. Surface modification of solid lipid nanoparticles for oral delivery of curcumin: Improvement of bioavailability through enhanced cellular uptake, and lymphatic uptake. Eur. J. Pharm. Biopharm. 2017, 117, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Son, G.H.; Lee, H.J.; Na, Y.G.; Lee, H.K.; Kim, S.J.; Huh, H.W.; Kim, K.T.; Kang, J.S.; Kim, Y.H.; Myung, C.S.; et al. Formulation and statistical analysis of an herbal medicine tablet containing Morus alba leaf extracts. J. Pharm. Investig. 2018, 1–10. [Google Scholar] [CrossRef]
- Festing, M.F.; Altman, D.G. Guidelines for the design and statistical analysis of experiments using laboratory animals. ILAR J. 2002, 43, 244–258. [Google Scholar] [CrossRef]
- Mourabet, M.; El Rhilassi, A.; El Boujaady, H.; Bennani-Ziatni, M.; Taitai, A. Use of response surface methodology for optimization of fluoride adsorption in an aqueous solution by Brushite. Arab. J. Chem. 2017, 10, S3292–S3302. [Google Scholar] [CrossRef]
- Schneider, A.; Hommel, G.; Blettner, M. Linear regression analysis: Part 14 of a series on evaluation of scientific publications. Dtsch. Arztebl. Int. 2010, 107, 776–782. [Google Scholar]
- Bewick, V.; Cheek, L.; Ball, J. Statistics review 7: Correlation and regression. Crit. Care 2003, 7, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo Filho, D.B.; Júnior, J.A.S.; Rocha, E.C. What is R2 all about? Leviathan (São Paulo) 2011, 3, 60–68. [Google Scholar] [CrossRef]
- Mahmood, S.; Taher, M.; Mandal, U.K. Experimental design and optimization of raloxifene hydrochloride loaded nanotransfersomes for transdermal application. Int. J. Nanomed. 2014, 9, 4331–4346. [Google Scholar]
- Yeom, D.W.; Song, Y.S.; Kim, S.R.; Lee, S.G.; Kang, M.H.; Lee, S.; Choi, Y.W. Development and optimization of a self-microemulsifying drug delivery system for atorvastatin calcium by using D-optimal mixture design. Int. J. Nanomed. 2015, 10, 3865–3878. [Google Scholar]
- Gan, L.; Zhang, C.; Wu, F.J.; Li, H.; Zhang, W.P.; Zhang, Q.J. Microencapsulated nanostructured lipid carriers as delivery system for rutin. Mater. Technol. 2018, 33, 357–363. [Google Scholar] [CrossRef]
- Bhaskar, K.; Anbu, J.; Ravichandiran, V.; Venkateswarlu, V.; Rao, Y.M. Lipid nanoparticles for transdermal delivery of flurbiprofen: Formulation, in vitro, ex vivo and in vivo studies. Lipids Health Dis. 2009, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Seta, Y.; Higuchi, F.; Kawahara, Y.; Nishimura, K.; Okada, R. Design and preparation of captopril sustained-release dosage forms and their biopharmaceutical properties. Int. J. Pharm. 1988, 41, 245–254. [Google Scholar] [CrossRef]
- Khan, S.; Baboota, S.; Ali, J.; Khan, S.; Narang, R.S.; Narang, J.K. Nanostructured lipid carriers: An emerging platform for improving oral bioavailability of lipophilic drugs. Int. J. Pharm. Investig. 2015, 5, 182–191. [Google Scholar]
- Sambuy, Y.; Angelis, I.; Ranaldi, G.; Scarino, M.L.; Stammati, A.; Zucco, F. The Caco-2 cell line as a model of the intestinal barrier: Influence of cell and culture-related factors on Caco-2 cell functional characteristics. Cell Biol. Toxicol. 2005, 21, 1–26. [Google Scholar] [CrossRef]
- Doktorovova, S.; Souto, E.B.; Silva, A.M. Nanotoxicology applied to solid lipid nanoparticles and nanostructured lipid carriers - a systematic review of in vitro data. Eur. J. Pharm. Biopharm. 2014, 87, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Tabata, Y.; Ikada, Y. Phagocytosis of Polymer Microspheres by Macrophages. Adv. Polym. Sci. 1990, 94, 107–141. [Google Scholar]
- Ye, H.L.; Shen, Z.Q.; Yu, L.; Wei, M.; Li, Y. Manipulating nanoparticle transport within blood flow through external forces: An exemplar of mechanics in nanomedicine. Proc. Math. Phys. Eng. Sci. 2018, 474, 20170845. [Google Scholar] [CrossRef]
- Hathout, R.M.; Mansour, S.; Mortada, N.D.; Geneidi, A.S.; Guy, R.H. Uptake of microemulsion components into the stratum corneum and their molecular effects on skin barrier function. Mol. Pharm. 2010, 7, 1266–1273. [Google Scholar] [CrossRef]
- Tian, C.; Asghar, S.; Wu, Y.; Chen, Z.; Jin, X.; Yin, L.; Huang, L.; Ping, Q.; Xiao, Y. Improving intestinal absorption and oral bioavailability of curcumin via taurocholic acid-modified nanostructured lipid carriers. Int. J. Nanomed. 2017, 12, 7897–7911. [Google Scholar] [CrossRef] [PubMed]
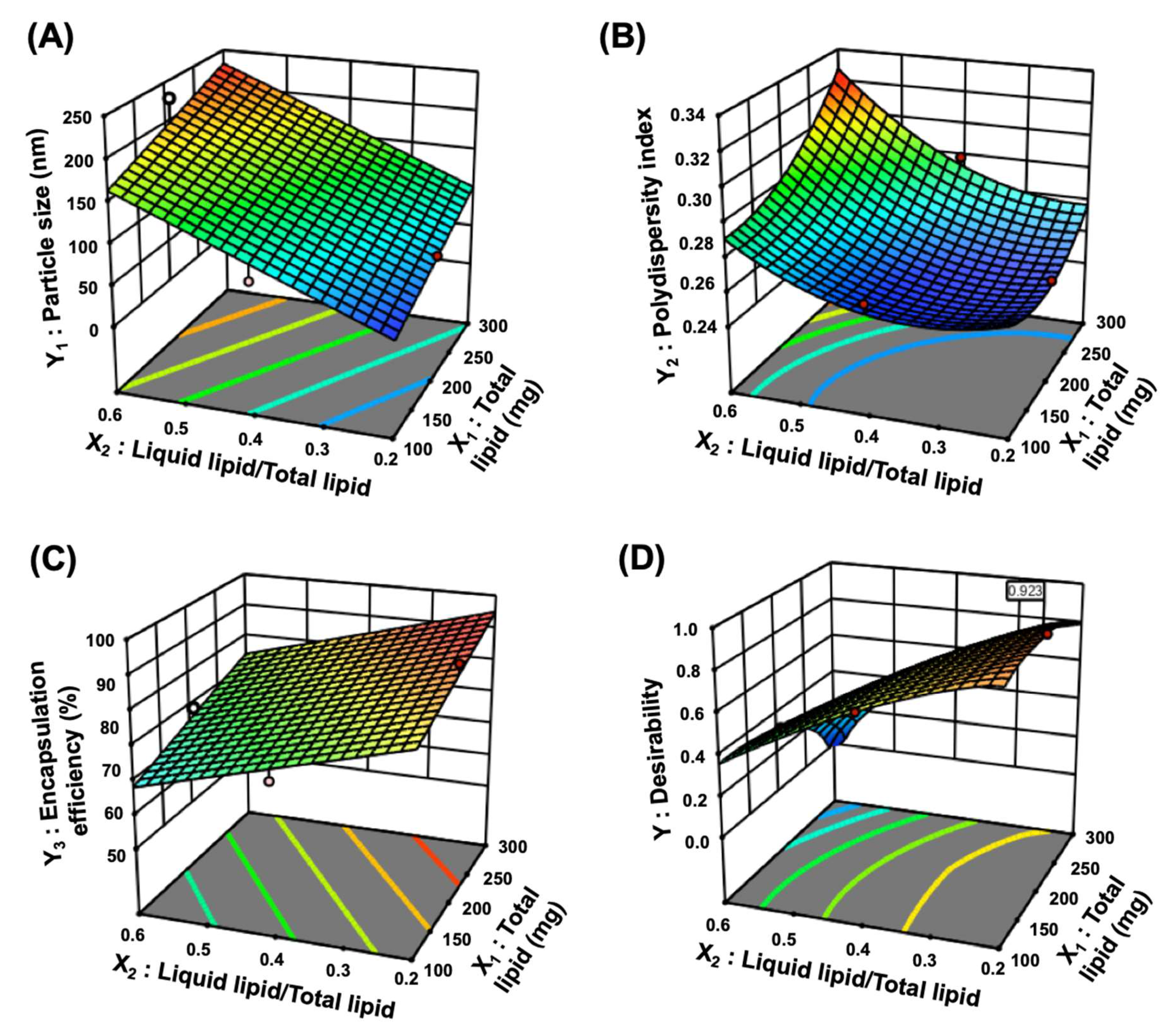
| Factors | Range | |
| Low Limit (mg) | High Limit (mg) | |
| X1: Total lipid amount | 100 | 300 |
| X2: Ratio of liquid lipid/total lipid | 0.2 | 0.6 |
| X3: Percentage of surfactant | 1 | 3 |
| Responses | Goal | |
| Y1: Particle size (nm) | Minimize | |
| Y2: Polydispersity index | Minimize | |
| Y3: Encapsulation efficiency (%) | Maximize | |
| Responses | Suggested Model | Model p-Value | Lack of Fit p-Value | R2 | Adjusted R2 | Adequate Precision |
|---|---|---|---|---|---|---|
| Y1: Particle size (nm) | Linear | <0.0001 | 0.7090 | 0.8570 | 0.8241 | 17.2218 |
| Y2: Polydispersity index | Quadratic | 0.0139 | 0.4403 | 0.8740 | 0.7119 | 7.8183 |
| Y3: Encapsulation efficiency (%) | Linear | <0.0001 | 0.7622 | 0.8430 | 0.8068 | 15.0182 |
| Run | Factors | Responses | ||||
|---|---|---|---|---|---|---|
| X1 | X2 | X3 | Y1 | Y2 | Y3 | |
| Total Lipid Amount (mg) | Ratio of Liquid Lipid/Total Lipid | Percentage of Surfactant (%) | Particle Size (nm) | Polydispersity Index | Encapsulation Efficiency (%) | |
| 1 | 200 | 0.6 | 1 | 151.2 ± 6.5 | 0.311 ± 0.031 | 84.25 ± 3.15 |
| 2 | 200 | 0.4 | 2 | 104.2 ± 3.5 | 0.303 ± 0.012 | 86.14 ± 1.42 |
| 3 | 200 | 0.4 | 2 | 114.2 ± 3.4 | 0.312 ± 0.031 | 85.32 ± 2.41 |
| 4 | 200 | 0.4 | 2 | 102.3 ± 8.1 | 0.317 ± 0.021 | 83.12 ± 3.36 |
| 5 | 100 | 0.4 | 3 | 84.2 ± 3.6 | 0.331 ± 0.024 | 78.26 ± 1.48 |
| 6 | 200 | 0.6 | 3 | 124.8 ± 5.7 | 0.277 ± 0.019 | 78.24 ± 2.85 |
| 7 | 300 | 0.2 | 2 | 104.8 ± 8.1 | 0.331 ± 0.027 | 95.12 ± 3.14 |
| 8 | 200 | 0.4 | 2 | 121.1 ± 7.1 | 0.342 ± 0.018 | 90.42 ± 1.45 |
| 9 | 100 | 0.6 | 2 | 132.1 ± 8.3 | 0.347 ± 0.014 | 74.26 ± 2.85 |
| 10 | 200 | 0.2 | 3 | 80.3 ± 3.4 | 0.36 ± 0.025 | 87.36 ± 1.64 |
| 11 | 300 | 0.4 | 1 | 115.2 ± 3.2 | 0.319 ± 0.037 | 91.34 ± 1.75 |
| 12 | 200 | 0.4 | 2 | 93.5 ± 2.7 | 0.308 ± 0.021 | 83.25 ± 2.34 |
| 13 | 100 | 0.4 | 1 | 91.5 ± 1.9 | 0.285 ± 0.033 | 82.64 ± 3.15 |
| 14 | 100 | 0.2 | 2 | 76.1 ± 3.1 | 0.361 ± 0.022 | 83.14 ± 1.48 |
| 15 | 300 | 0.4 | 3 | 124.3 ± 1.4 | 0.277 ± 0.027 | 81.26 ± 2.95 |
| 16 | 300 | 0.6 | 2 | 149.2 ± 2.5 | 0.36 ± 0.024 | 82.15 ± 3.48 |
| 17 | 200 | 0.2 | 1 | 88.1 ± 3.5 | 0.279 ± 0.018 | 95.14 ± 4.52 |
| Optimized Factors | Responses | 95 % CI * Low Predicted Value | Predicted Value | 95% CI * High Predicted Value | Actual Value | Error Percentage (%) |
|---|---|---|---|---|---|---|
| X1: 189.3 mg | Y1: Particle size (nm) | 74.4 | 85.8 | 97.3 | 87.6 ± 6.6 | 2.1 |
| X2: 0.2 | Y2: Polydispersity index | 0.244 | 0.276 | 0.308 | 0.259 ± 0.013 | 6.2 |
| X3: 1.0% | Y3: Encapsulation efficiency (%) | 90.1 | 93.1 | 96.2 | 92.1 ± 3.1 | 1.1 |
| Pharmacokinetic Parameters | Samples | |
|---|---|---|
| Raw TGL | TGL-NLC | |
| Tmax (h) | 2.65 ± 0.82 | 1.20 ± 0.12 |
| Cmax (ng/mL) | 461.75 ± 88.77 | 1050.44 ± 170.14 # |
| AUC0–∞ (ng·h/mL) | 2103.01 ± 283.36 | 5362.43 ± 808.51 # |
| T1/2 (h) | 3.46 ± 0.56 | 4.80 ± 1.21 |
| RBA (%) vs. raw TGL | 254.99 | |
| Parameter | Samples | |
|---|---|---|
| Raw TGL | TGL-NLC | |
| AUIC0–24 (%⋅h) | 615.0 ± 91.9 | 1064.2 ± 121.5 # |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Son, G.-H.; Na, Y.-G.; Huh, H.W.; Wang, M.; Kim, M.-K.; Han, M.-G.; Byeon, J.-J.; Lee, H.-K.; Cho, C.-W. Systemic Design and Evaluation of Ticagrelor-Loaded Nanostructured Lipid Carriers for Enhancing Bioavailability and Antiplatelet Activity. Pharmaceutics 2019, 11, 222. https://doi.org/10.3390/pharmaceutics11050222
Son G-H, Na Y-G, Huh HW, Wang M, Kim M-K, Han M-G, Byeon J-J, Lee H-K, Cho C-W. Systemic Design and Evaluation of Ticagrelor-Loaded Nanostructured Lipid Carriers for Enhancing Bioavailability and Antiplatelet Activity. Pharmaceutics. 2019; 11(5):222. https://doi.org/10.3390/pharmaceutics11050222
Chicago/Turabian StyleSon, Gi-Ho, Young-Guk Na, Hyun Wook Huh, Miao Wang, Min-Ki Kim, Min-Gu Han, Jin-Ju Byeon, Hong-Ki Lee, and Cheong-Weon Cho. 2019. "Systemic Design and Evaluation of Ticagrelor-Loaded Nanostructured Lipid Carriers for Enhancing Bioavailability and Antiplatelet Activity" Pharmaceutics 11, no. 5: 222. https://doi.org/10.3390/pharmaceutics11050222
APA StyleSon, G.-H., Na, Y.-G., Huh, H. W., Wang, M., Kim, M.-K., Han, M.-G., Byeon, J.-J., Lee, H.-K., & Cho, C.-W. (2019). Systemic Design and Evaluation of Ticagrelor-Loaded Nanostructured Lipid Carriers for Enhancing Bioavailability and Antiplatelet Activity. Pharmaceutics, 11(5), 222. https://doi.org/10.3390/pharmaceutics11050222