Functional Magnetic Core-Shell System-Based Iron Oxide Nanoparticle Coated with Biocompatible Copolymer for Anticancer Drug Delivery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
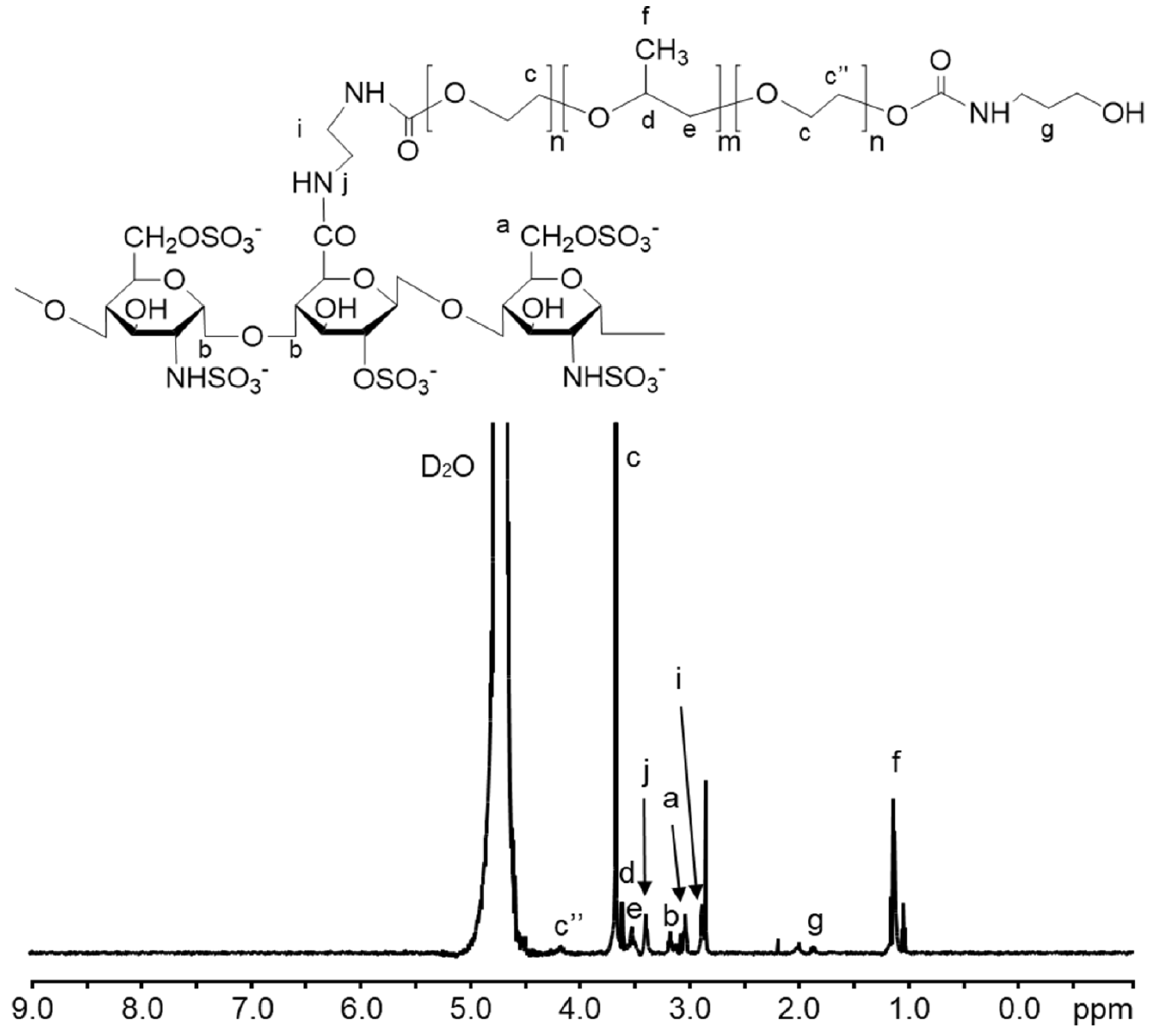
2.2. Synthesis of Heparin-Poloxamer (HP)
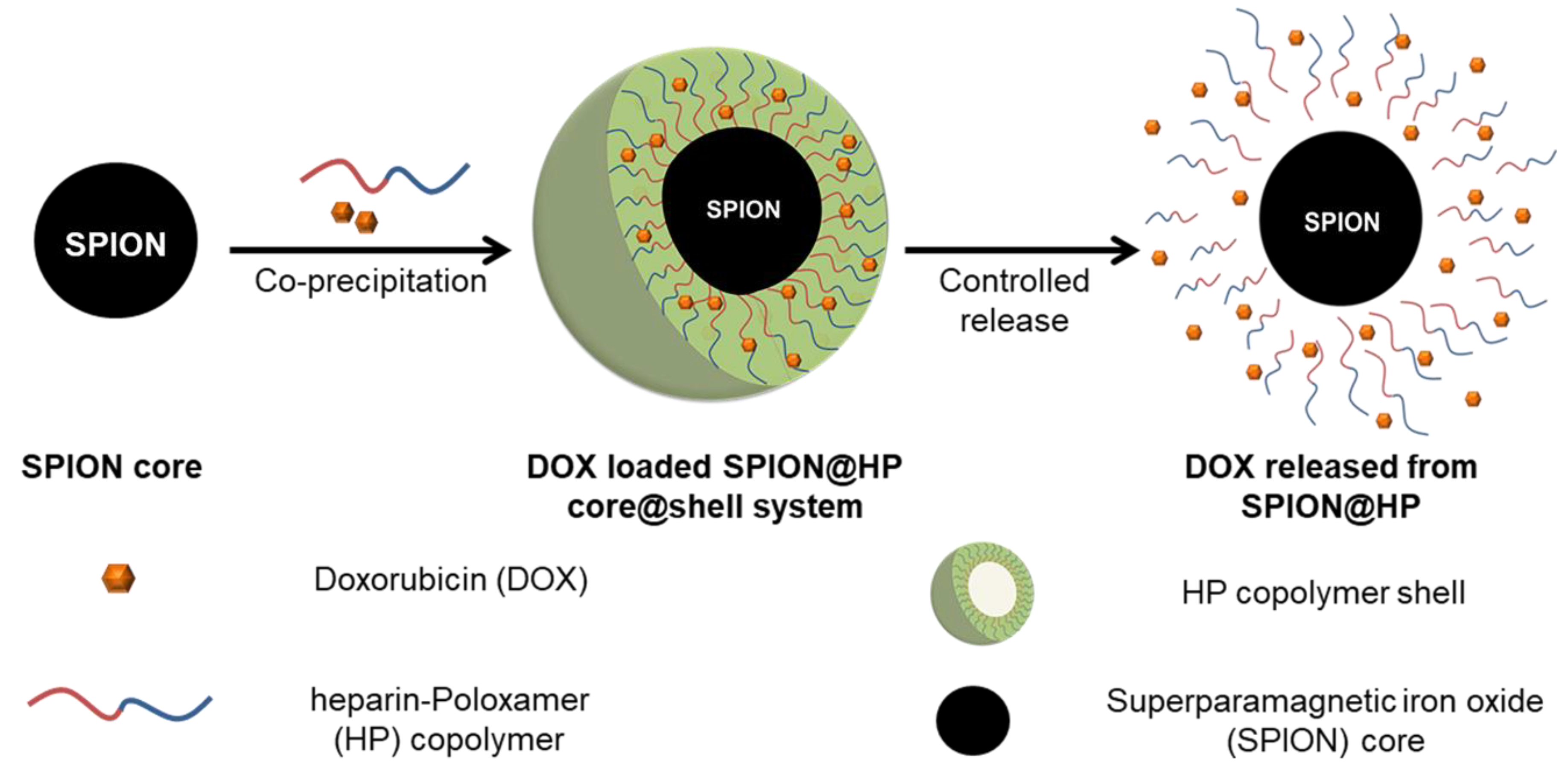
2.3. Preparation of SPIONs and SPION@HP Core-Shell System
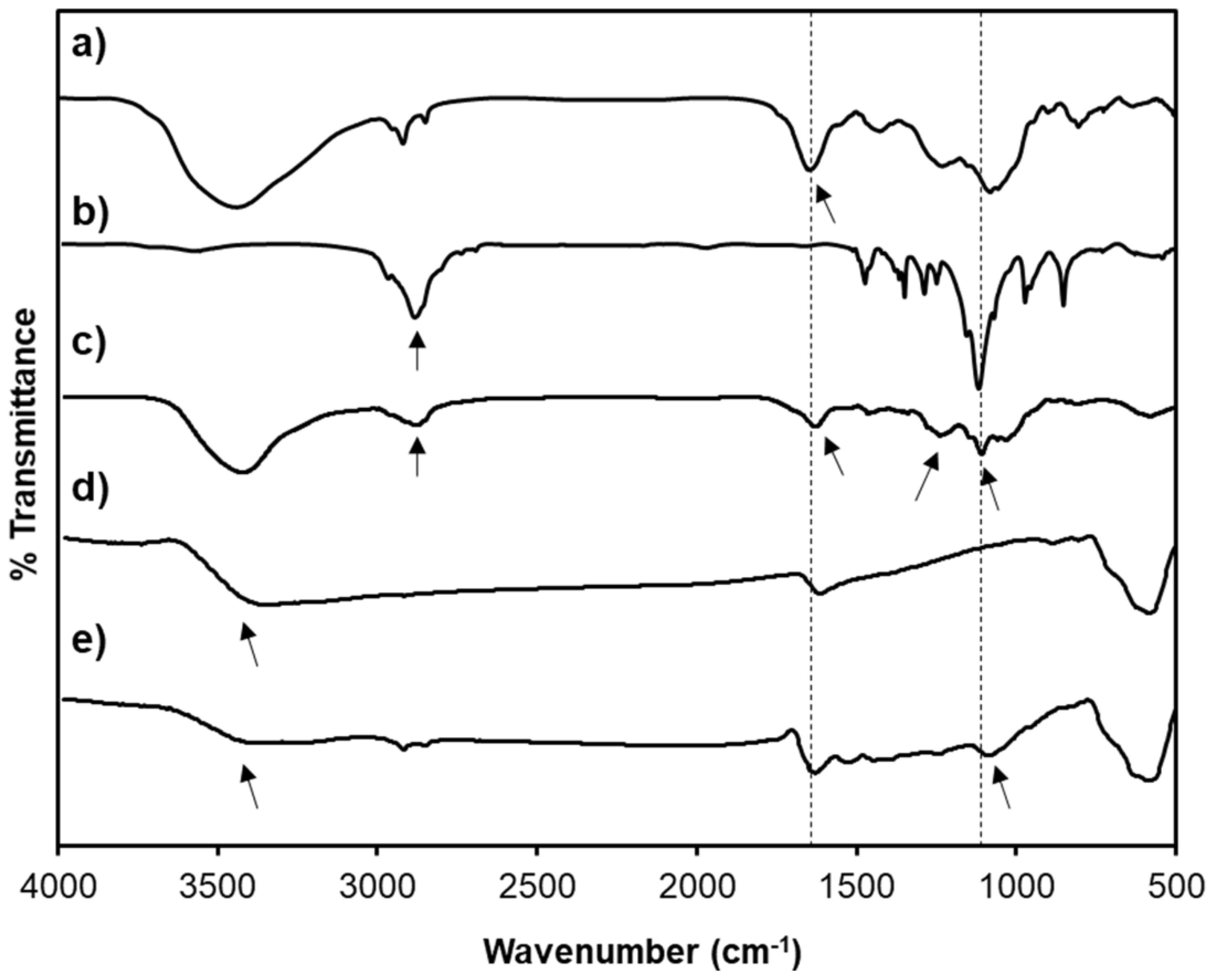
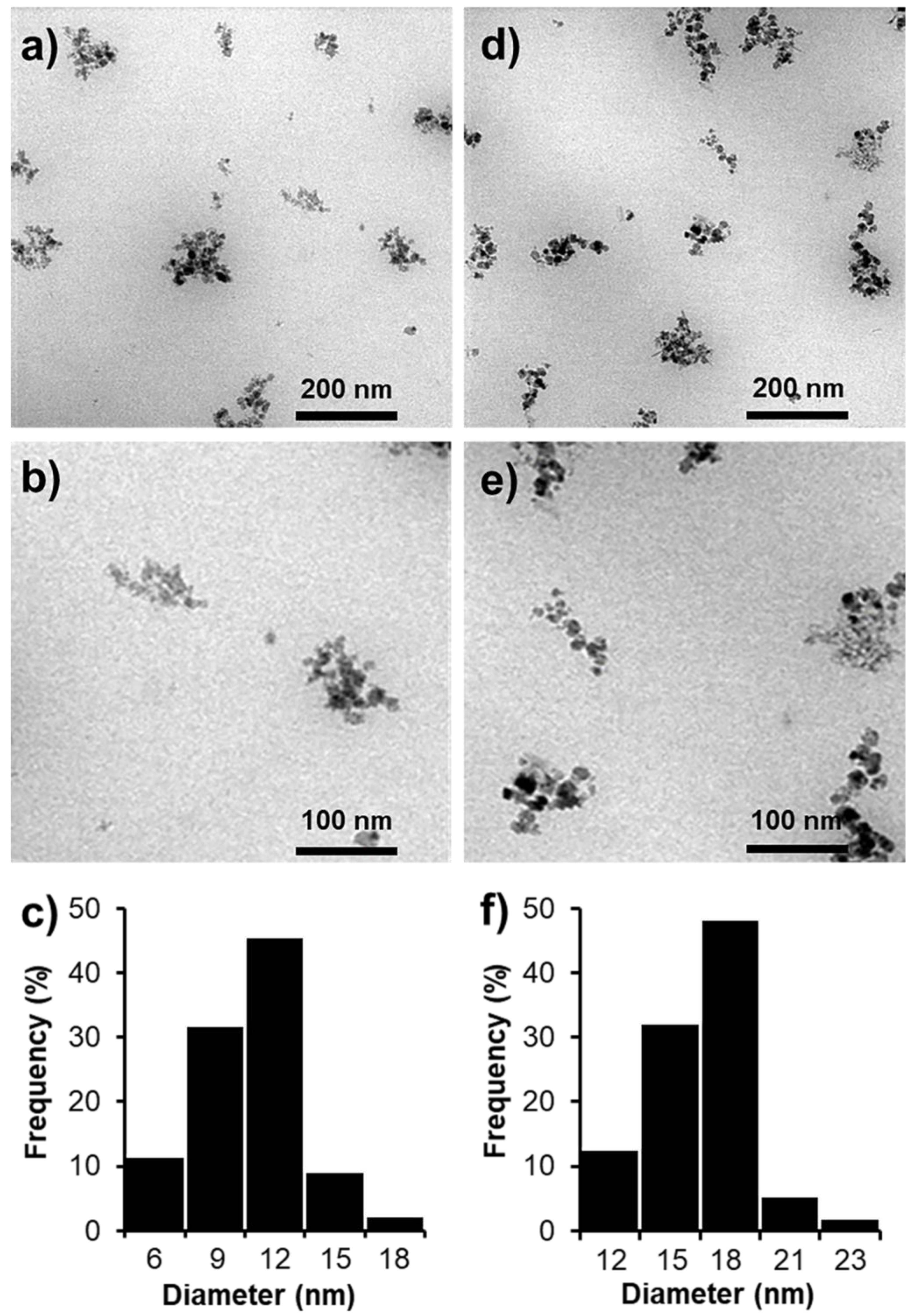
2.4. Characterizations
2.5. Drug Loading and In Vitro DOX Release Study
2.6. Cytotoxicity Study
2.7. Statistical Analysis
3. Results and Discussion
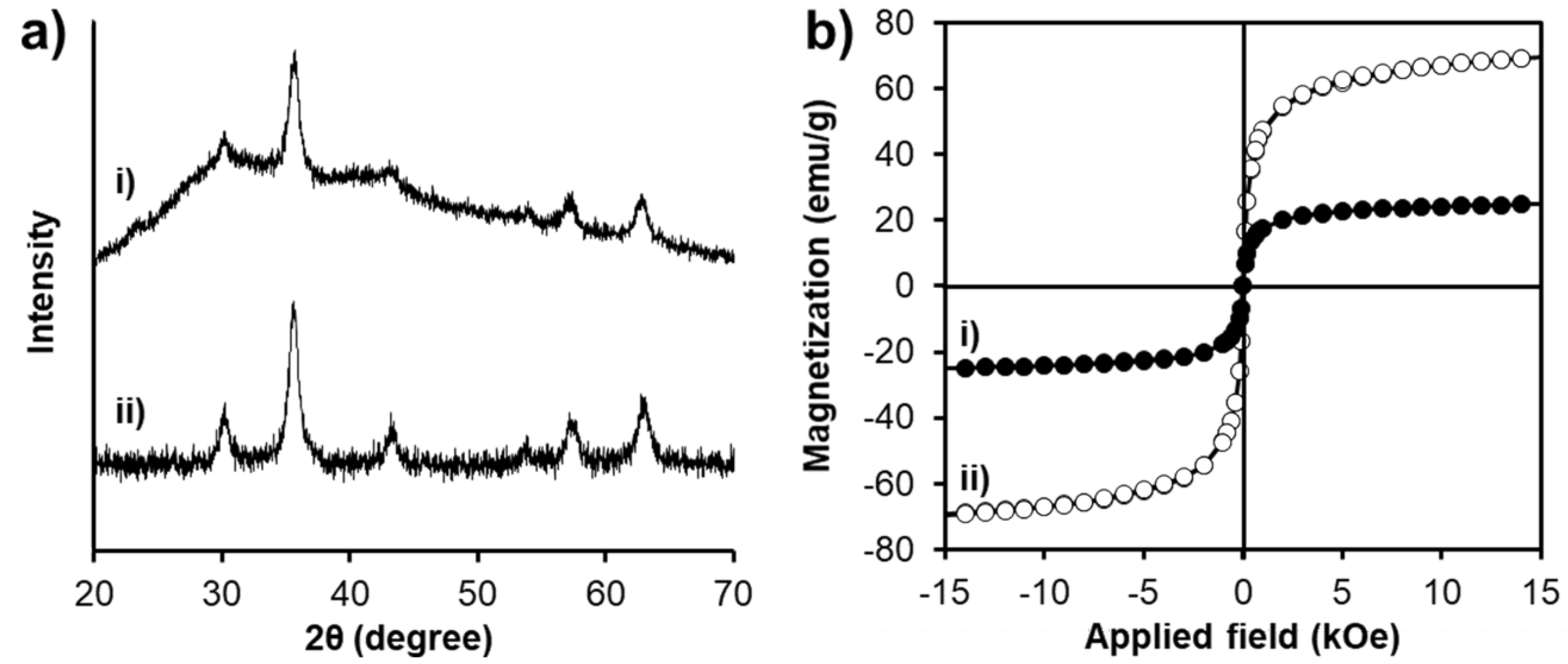
3.1. Characterizations of SPION@HP
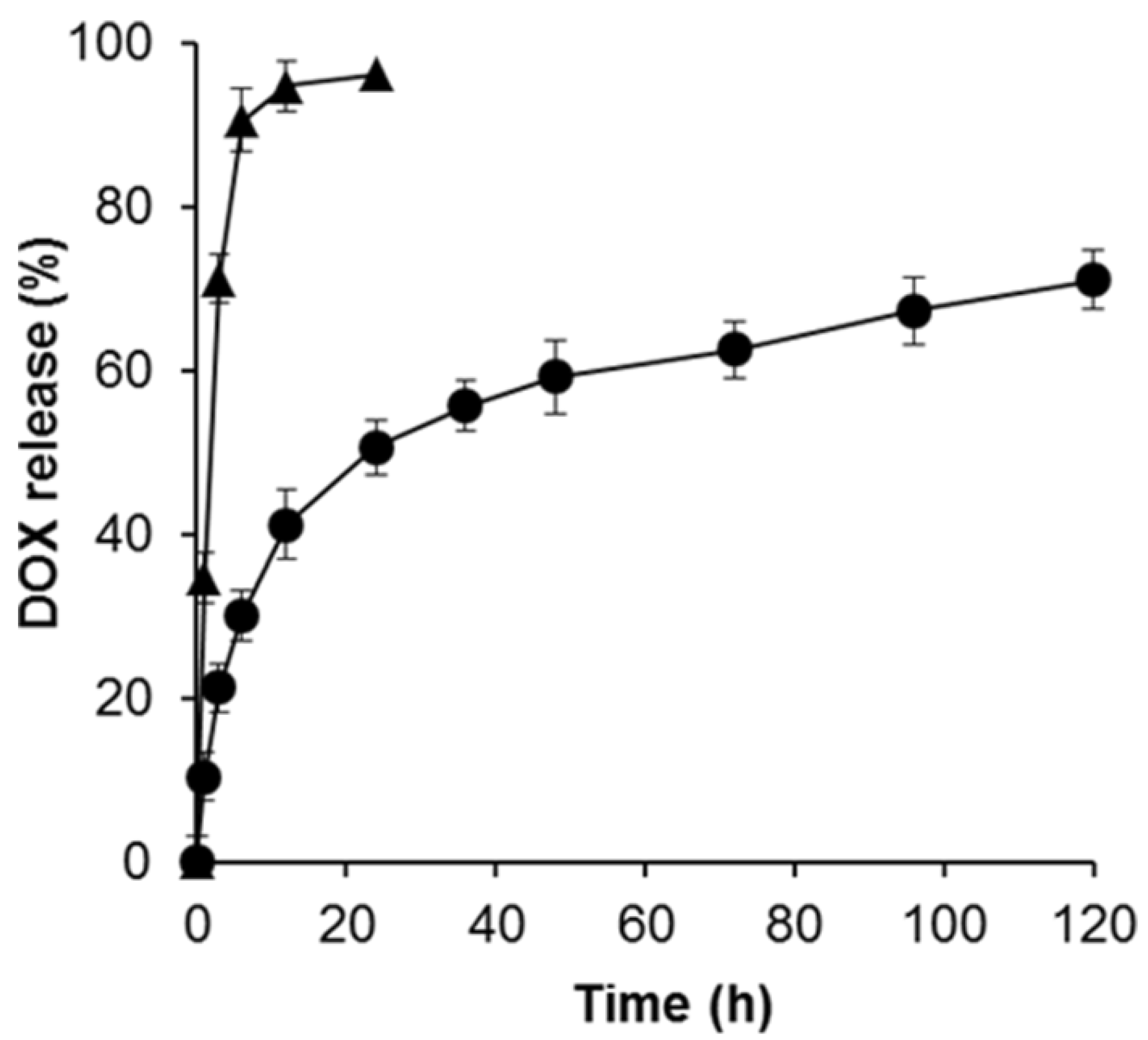
3.2. Loading Efficiency and Controlled Release of DOX
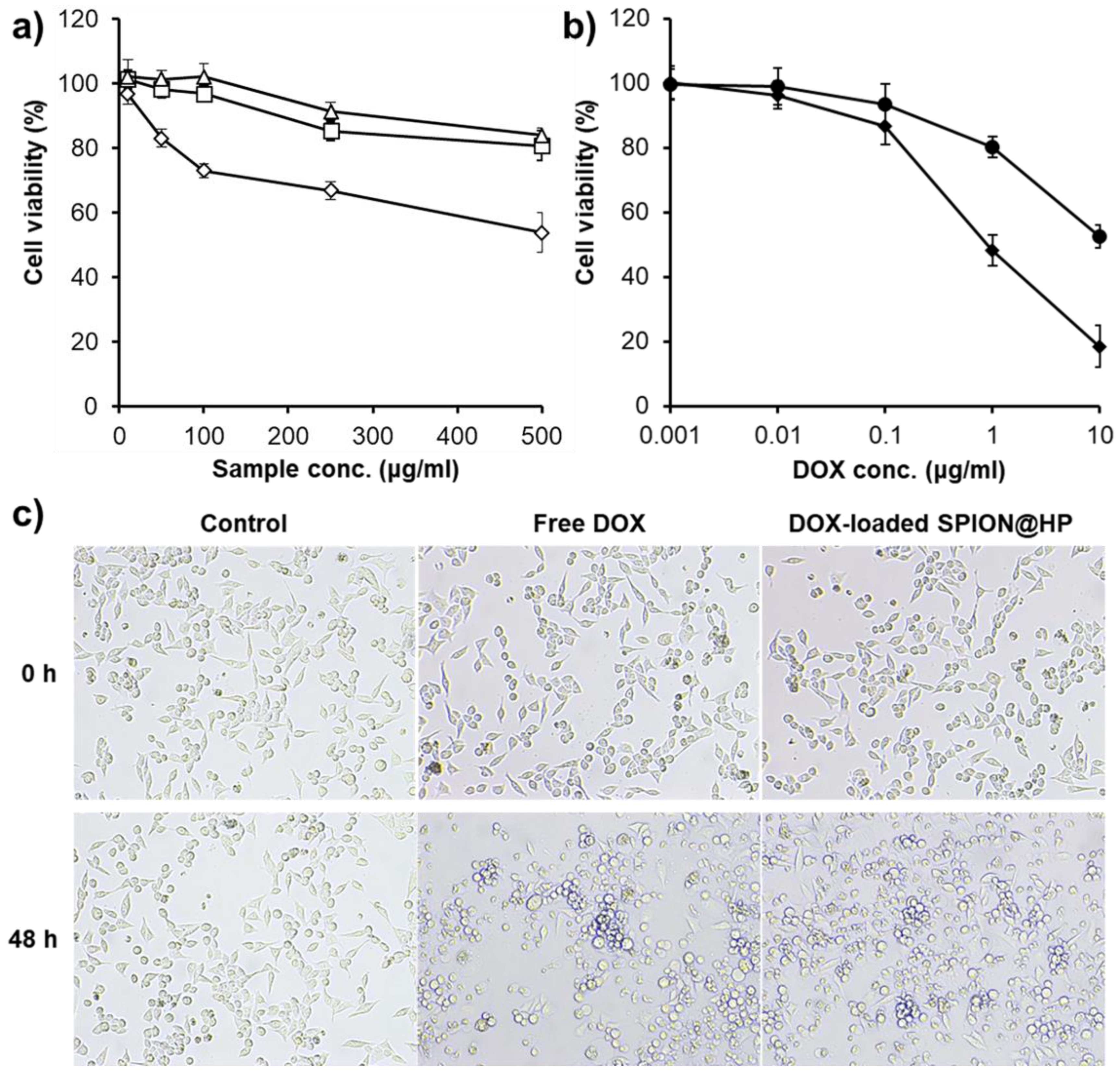
3.3. Cytotoxicity Study
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kumari, P.; Ghosh, B.; Biswas, S. Nanocarriers for cancer-targeted drug delivery. J. Drug Target. 2016, 24, 179–191. [Google Scholar] [CrossRef] [PubMed]
- ud Din, F.; Aman, W.; Ullah, I.; Qureshi, O.S.; Mustapha, O.; Shafique, S.; Zeb, A. Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. Int. J. Nanomed. 2017, 12, 7291. [Google Scholar] [CrossRef] [PubMed]
- Bao, B.Q.; Le, N.H.; Nguyen, D.H.T.; Tran, T.V.; Pham, L.P.T.; Bach, L.G.; Nguyen, D.H. Evolution and present scenario of multifunctionalized mesoporous nanosilica platform: A mini review. Mater. Sci. Eng. C 2018, 91, 912–928. [Google Scholar] [CrossRef] [PubMed]
- Laurent, S.; Saei, A.A.; Behzadi, S.; Panahifar, A.; Mahmoudi, M. Superparamagnetic iron oxide nanoparticles for delivery of therapeutic agents: Opportunities and challenges. Expert Opin. Drug Deliv. 2014, 11, 1449–1470. [Google Scholar] [CrossRef] [PubMed]
- Yalcin, S.; Unsoy, G.; Mutlu, P.; Khodadust, R.; Gunduz, U. Polyhydroxybutyrate-Coated Magnetic Nanoparticles for Doxorubicin Delivery: Cytotoxic Effect Against Doxorubicin-Resistant Breast Cancer Cell Line. Am. J. Ther. 2014, 21, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Fazilati, M. Anti-neoplastic Applications of Heparin Coated Magnetic Nanoparticles Against Human Ovarian Cancer. J. Inorg. Organomet. Polym. Mater. 2014, 24, 551–559. [Google Scholar] [CrossRef]
- Reyes-Ortega, F.; Delgado, Á.V.; Schneider, E.K.; Checa Fernández, B.L.; Iglesias, G.R. Magnetic Nanoparticles Coated with a Thermosensitive Polymer with Hyperthermia Properties. Polymers 2017, 10, 10. [Google Scholar] [CrossRef]
- Nguyen, D.H.; Lee, J.S.; Choi, J.H.; Park, K.M.; Lee, Y.; Park, K.D. Hierarchical self-assembly of magnetic nanoclusters for theranostics: Tunable size, enhanced magnetic resonance imagability, and controlled and targeted drug delivery. Acta Biomater. 2016, 35, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Chee, H.L.; Gan, C.R.R.; Ng, M.; Low, L.; Fernig, D.G.; Bhakoo, K.K.; Paramelle, D. Biocompatible peptide-coated ultrasmall superparamagnetic iron oxide nanoparticles for in vivo contrast-enhanced magnetic resonance imaging. ACS Nano 2018, 12, 6480–6491. [Google Scholar] [CrossRef] [PubMed]
- Sakhtianchi, R.; Minchin, R.F.; Lee, K.-B.; Alkilany, A.M.; Serpooshan, V.; Mahmoudi, M. Exocytosis of nanoparticles from cells: Role in cellular retention and toxicity. Adv. Colloid Interface Sci. 2013, 201–202, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Khalkhali, M.; Rostamizadeh, K.; Sadighian, S.; Khoeini, F.; Naghibi, M.; Hamidi, M. The impact of polymer coatings on magnetite nanoparticles performance as MRI contrast agents: A comparative study. DARU J. Pharm. Sci. 2015, 23, 45. [Google Scholar] [CrossRef] [PubMed]
- Javid, A.; Ahmadian, S.; Saboury, A.; Rezaei-Zarchi, S. Anticancer effect of doxorubicin loaded heparin based super-paramagnetic iron oxide nanoparticles against the human ovarian cancer cells. World Acad. Sci. Eng. Tech. 2011, 50, 41–45. [Google Scholar]
- Cotin, G.; Piant, S.; Mertz, D.; Felder-Flesch, D.; Begin-Colin, S. Iron Oxide Nanoparticles for Biomedical Application. In Iron Oxide Nanoparticles for Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 43–88. [Google Scholar]
- Larsen, E.K.U.; Nielsen, T.; Wittenborn, T.; Rydtoft, L.M.; Lokanathan, A.R.; Hansen, L.; Østergaard, L.; Kingshott, P.; Howard, K.A.; Besenbacher, F.; et al. Accumulation of magnetic iron oxide nanoparticles coated with variably sized polyethylene glycol in murine tumors. Nanoscale 2012, 4, 2352. [Google Scholar] [CrossRef] [PubMed]
- Yen, S.K.; Padmanabhan, P.; Selvan, S.T. Multifunctional Iron Oxide Nanoparticles for Diagnostics, Therapy and Macromolecule Delivery. Theranostics 2013, 3, 986–1003. [Google Scholar] [CrossRef] [PubMed]
- Maia, J.L.; Santana, M.H.A.; Ré, M.I. The effect of some processing conditions on the characteristics of biodegradable microspheres obtained by an emulsion solvent evaporation process. Braz. J. Chem. Eng. 2004, 21, 1–12. [Google Scholar] [CrossRef]
- Park, S.; Kim, H.S.; Kim, W.J.; Yoo, H.S. Pluronic@Fe3O4 nanoparticles with robust incorporation of doxorubicin by thermo-responsiveness. Int. J. Pharm. 2012, 424, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Yallapu, M.M.; Foy, S.P.; Jain, T.K.; Labhasetwar, V. PEG-Functionalized Magnetic Nanoparticles for Drug Delivery and Magnetic Resonance Imaging Applications. Pharm. Res. 2010, 27, 2283–2295. [Google Scholar] [CrossRef] [PubMed]
- Kayal, S.; Ramanujan, R.V. Doxorubicin loaded PVA coated iron oxide nanoparticles for targeted drug delivery. Mater. Sci. Eng. C 2010, 30, 484–490. [Google Scholar] [CrossRef]
- Nguyen, D.H.; Bae, J.W.; Choi, J.H.; Lee, J.S.; Park, K.D. Bioreducible cross-linked Pluronic micelles: pH-triggered release of doxorubicin and folate-mediated cellular uptake. J. Bioact. Compat. Polym. 2013, 28, 341–354. [Google Scholar] [CrossRef]
- Tong, N.-A.N.; Nguyen, T.H.; Nguyen, D.H.; Nguyen, C.K.; Tran, N.Q. Preparation of the Cationic Dendrimer-Based Hydrogels for Controlled Heparin Release. J. Macromol. Sci. Part A 2015, 52, 830–837. [Google Scholar] [CrossRef]
- Chiappetta, D.A.; Hocht, C.; Taira, C.; Sosnik, A. Efavirenz-loaded polymeric micelles for pediatric anti-HIV pharmacotherapy with significantly higher oral bioavailability. Nanomedicine 2010, 5, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Yallapu, M.M.; Othman, S.F.; Curtis, E.T.; Gupta, B.K.; Jaggi, M.; Chauhan, S.C. Multi-functional magnetic nanoparticles for magnetic resonance imaging and cancer therapy. Biomaterials 2011, 32, 1890–1905. [Google Scholar] [CrossRef] [PubMed]
- Ternent, L.; Mayoh, D.A.; Lees, M.R.; Davies, G.L. Heparin-stabilised iron oxide for MR applications: A relaxometric study. J. Mater. Chem. B 2016, 4, 3065–3074. [Google Scholar] [CrossRef]
- Wang, Y.X.; Xuan, S.; Port, M.; Idee, J.M. Recent advances in superparamagnetic iron oxide nanoparticles for cellular imaging and targeted therapy research. Curr. Pharm. Des. 2015, 19, 6575–6593. [Google Scholar] [CrossRef]
- Nguyen, D.H.; Lee, J.S.; Bae, J.W.; Choi, J.H.; Lee, Y.; Son, J.Y.; Park, K.D. Targeted doxorubicin nanotherapy strongly suppressing growth of multidrug resistant tumor in mice. Int. J. Pharm. 2015, 495, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.H.; Choi, J.H.; Joung, Y.K.; Park, K.D. Disulfide-crosslinked heparin-pluronic nanogels as a redox-sensitive nanocarrier for intracellular protein delivery. J. Bioact. Compat. Polym. 2011, 26, 287–300. [Google Scholar] [CrossRef]
- Moon, H.T.; Jeon, O.C.; Byun, Y.; Kim, Y.J.; Lee, Y.-K. Evaluation of the oral absorption of heparin conjugated with sodium deoxycholate as a facilitating agent in GI tract. Macromol. Res. 2009, 17, 79–83. [Google Scholar] [CrossRef]
- Niers, T.M.H.; Klerk, C.P.W.; DiNisio, M.; Van Noorden, C.J.F.; Büller, H.R.; Reitsma, P.H.; Richel, D.J. Mechanisms of heparin induced anti-cancer activity in experimental cancer models. Crit. Rev. Oncol. Hematol. 2007, 61, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Lopera, S.A.; Plaza, R.C.; Delgado, A.V. Synthesis and characterization of spherical magnetite/biodegradable polymer composite particles. J. Colloid Interface Sci. 2001, 240, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Le Thi, T.N.; Nguyen, T.H.; Hoang, D.Q.; Tran, T.V.; Nguyen, N.T.; Nguyen, D.H. Development of new magnetic nanoparticles: Oligochitosan obtained by γ-rays and –coated Fe3O4 nanoparticles. Appl. Surf. Sci. 2017, 422, 863–868. [Google Scholar] [CrossRef]
- Silva, V.A.J.; Andrade, P.L.; Silva, M.P.C.; Bustamante, D.A.; De Los Santos Valladares, L.; Albino Aguiar, J. Synthesis and characterization of Fe3O4 nanoparticles coated with fucan polysaccharides. J. Magn. Magn. Mater. 2013, 343, 138–143. [Google Scholar] [CrossRef]
- Nguyen, C.K.; Tran, N.Q.; Nguyen, T.P.; Nguyen, D.H. Biocompatible nanomaterials based on dendrimers, hydrogels and hydrogel nanocomposites for use in biomedicine. Adv. Nat. Sci. Nanosci. Nanotechnol. 2017, 8, 015001. [Google Scholar] [CrossRef]
- Duncan, R. Polymer conjugates for tumour targeting and intracytoplasmic delivery. The EPR effect as a common gateway? Pharm. Sci. Technol. Today 1999, 2, 441–449. [Google Scholar] [CrossRef]
- Guo, C.; Hu, Y.; Qian, H.; Ning, J.; Xu, S. Magnetite (Fe3O4) tetrakaidecahedral microcrystals: Synthesis, characterization, and micro-Raman study. Mater. Charact. 2011, 62, 148–151. [Google Scholar] [CrossRef]
- Li, F.; Sun, J.; Zhu, H.; Wen, X.; Lin, C.; Shi, D. Preparation and characterization novel polymer-coated magnetic nanoparticles as carriers for doxorubicin. Colloids Surf. B Biointerfaces 2011, 88, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Wang, T.; Perche, F.; Taigind, A.; Torchilin, V.P. Enhanced anticancer activity of nanopreparation containing an MMP2-sensitive PEG-drug conjugate and cell-penetrating moiety. Proc. Natl. Acad. Sci. USA 2013, 110, 17047–17052. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoang Thi, T.T.; Nguyen Tran, D.-H.; Bach, L.G.; Vu-Quang, H.; Nguyen, D.C.; Park, K.D.; Nguyen, D.H. Functional Magnetic Core-Shell System-Based Iron Oxide Nanoparticle Coated with Biocompatible Copolymer for Anticancer Drug Delivery. Pharmaceutics 2019, 11, 120. https://doi.org/10.3390/pharmaceutics11030120
Hoang Thi TT, Nguyen Tran D-H, Bach LG, Vu-Quang H, Nguyen DC, Park KD, Nguyen DH. Functional Magnetic Core-Shell System-Based Iron Oxide Nanoparticle Coated with Biocompatible Copolymer for Anticancer Drug Delivery. Pharmaceutics. 2019; 11(3):120. https://doi.org/10.3390/pharmaceutics11030120
Chicago/Turabian StyleHoang Thi, Thai Thanh, Diem-Huong Nguyen Tran, Long Giang Bach, Hieu Vu-Quang, Duy Chinh Nguyen, Ki Dong Park, and Dai Hai Nguyen. 2019. "Functional Magnetic Core-Shell System-Based Iron Oxide Nanoparticle Coated with Biocompatible Copolymer for Anticancer Drug Delivery" Pharmaceutics 11, no. 3: 120. https://doi.org/10.3390/pharmaceutics11030120
APA StyleHoang Thi, T. T., Nguyen Tran, D.-H., Bach, L. G., Vu-Quang, H., Nguyen, D. C., Park, K. D., & Nguyen, D. H. (2019). Functional Magnetic Core-Shell System-Based Iron Oxide Nanoparticle Coated with Biocompatible Copolymer for Anticancer Drug Delivery. Pharmaceutics, 11(3), 120. https://doi.org/10.3390/pharmaceutics11030120




