Contribution of Molecular Structure to Self-Assembling and Biological Properties of Bifunctional Lipid-Like 4-(N-Alkylpyridinium)-1,4-Dihydropyridines
Abstract
1. Introduction
2. Materials and Methods
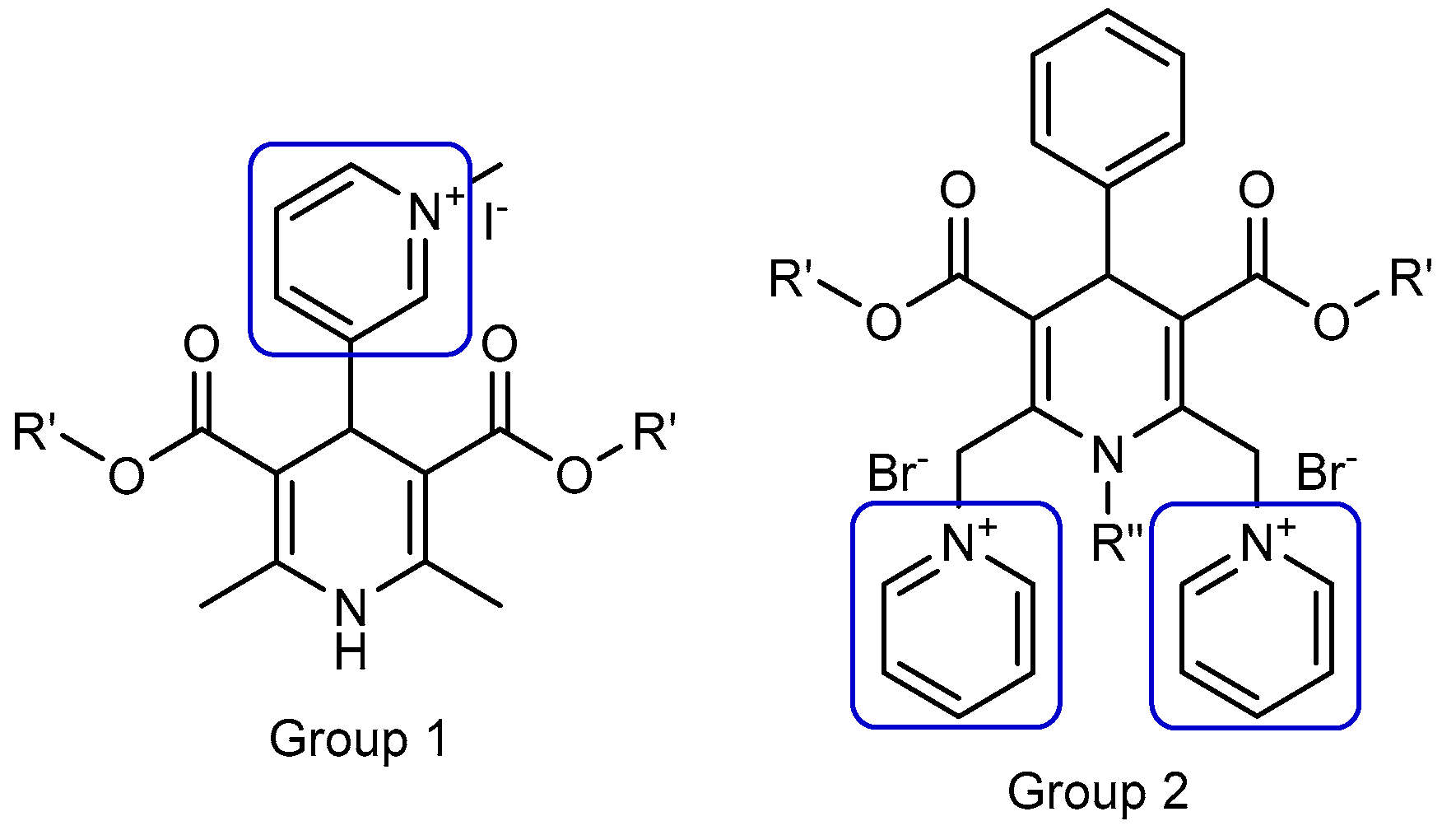
2.1. 4-(N-Alkylpyridinium)-1,4-Dihydropyridine (1,4-DHP) Derivatives
2.2. Cell Culture and Measurement of Cell Viability
2.3. Basal Cytotoxicity Test
2.4. Estimation of LD50 from IC50 Values
2.5. Toxicity Screen
2.6. Microorganism Cultivation and Toxicity Test
2.7. Phospholipid Binding Assay
2.8. Self-Assembling Properties by Dynamic Light Scattering Measurements
2.9. Determination of CAC
2.10. Transmission Electron Microscopy (TEM)
2.11. Statistical Analysis
3. Results and Discussion
3.1. 4-(N-Alkylpyridinium)-1,4-Dihydropyridine Derivatives
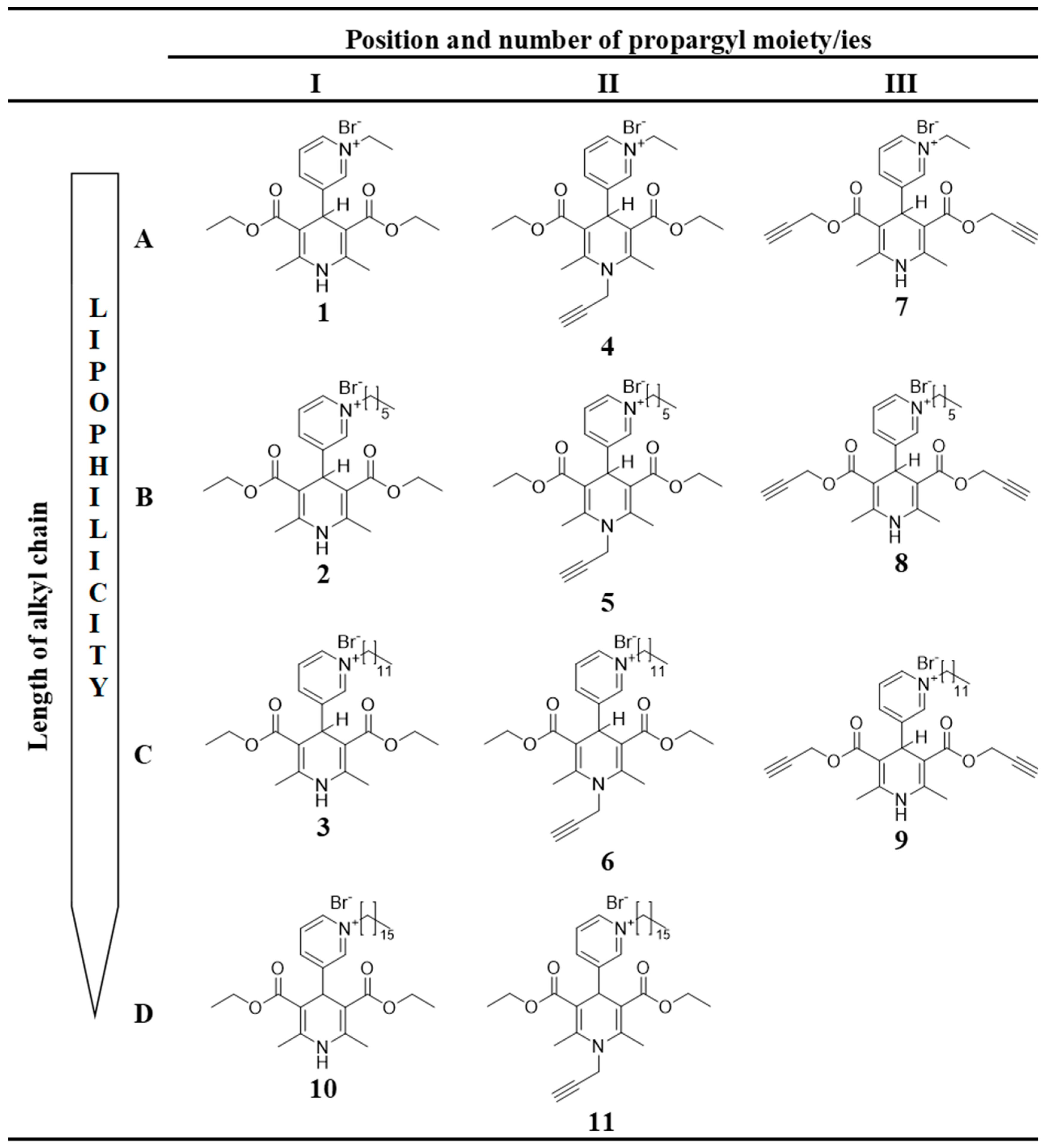
- 1,4-DHP derivatives with different lengths of alkyl moieties at the quaternised nitrogen atom at the position 4 of the 1,4-DHP ring (Figure 2, rows A–D):
- 1.1.
- 4-(N-Ethylpyridinium)-1,4-DHP derivatives (compounds 1, 4, 7);
- 1.2.
- 4-(N-Hexylpyridinium)-1,4-DHP derivatives (compounds 2, 5, 8);
- 1.3.
- 4-(N-Dodecylpyridinium)-1,4-DHP derivatives (compounds 3, 6, 9);
- 1.4.
- 4-(N-Hexadecylpyridinium)-1,4-DHP derivatives (compounds 10, 11).
- 1,4-DHP derivatives without or with shifted propargyl moiety/ies (Figure 2, columns I, II, III):
- 2.1.
- 1,4-DHP derivatives without propargyl moiety/ies (compounds 1–3 and 10);
- 2.2.
- Propargyl moiety at the position 1 of the 1,4-DHP ring (1-propargyl-4-(N-alkylpyridyl)-1,4-DHP; compounds 4–6, 11);
- 2.3.
- Propargyl moieties at the positions 3 and 5 of the 1,4-DHP ring (bispropargyl 4-(N-alkylpyridinium)-1,4-DHP 3,5-dicarboxylates; compounds 7–9).
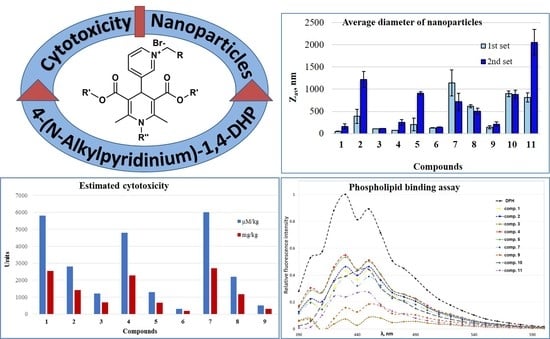
3.2. Estimation of LD50 from IC50 Values
3.3. Toxicity Test
3.4. Phospholipid Binding Assay
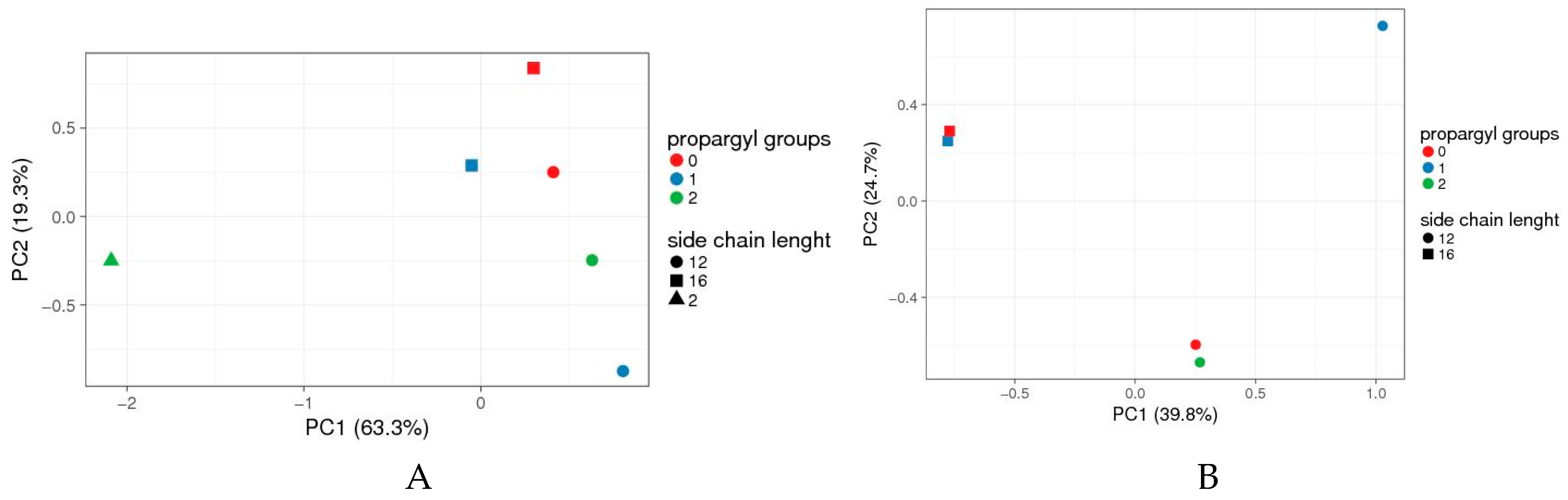
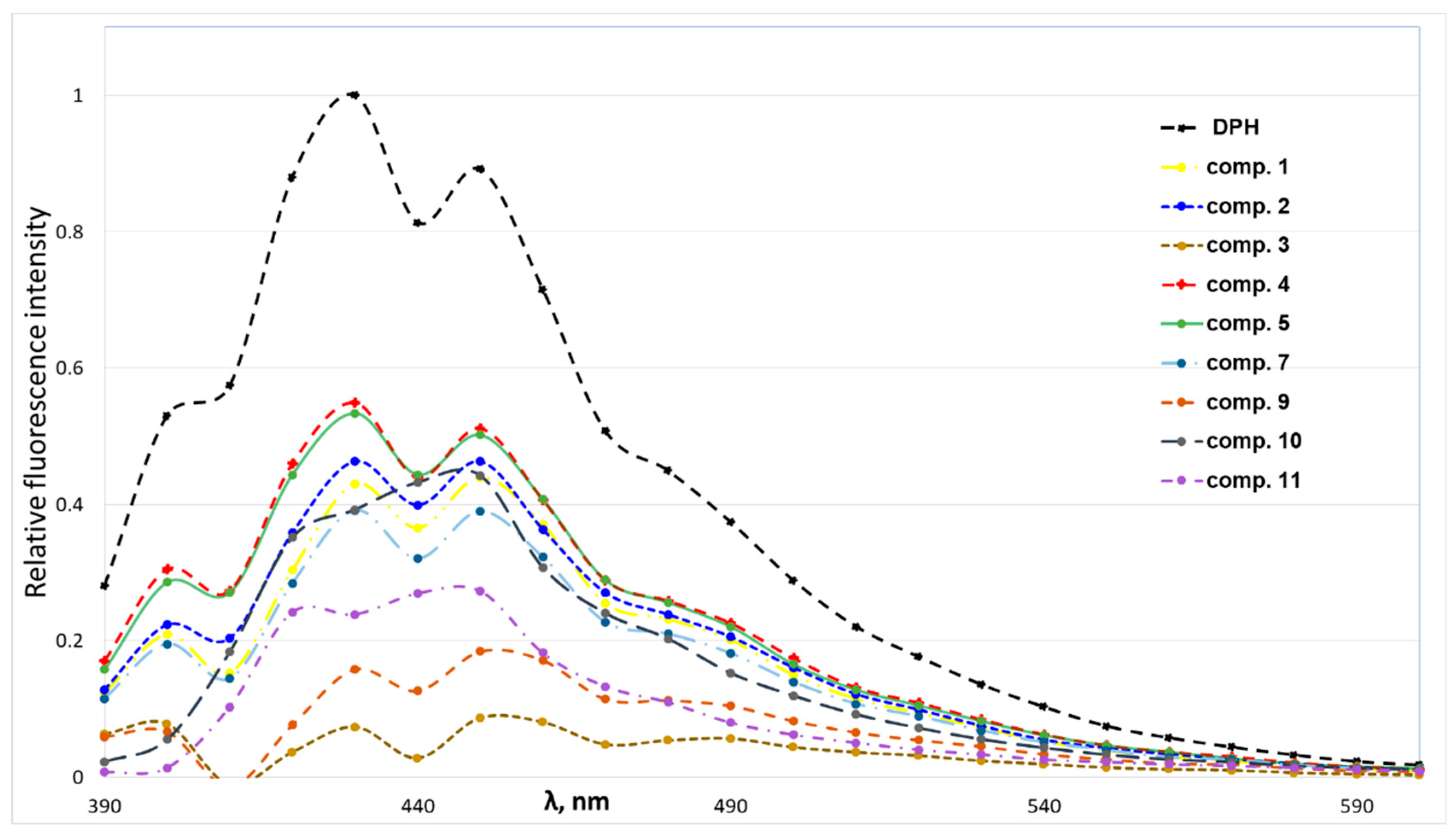
3.5. Self-Assembling Properties
3.6. Transmission Electron Microscopy (TEM)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Deshpande, P.P.; Biswas, S.; Torchilin, V.P. Current trends in the use of liposomes for tumor targeting. Nanomedicine 2013, 8, 1509–1528. [Google Scholar] [CrossRef] [PubMed]
- Felgner, P.L.; Gadek, T.R.; Holm, M.; Roman, R.; Chan, H.W.; Wenz, M.; Northrop, J.P.; Ringold, G.M.; Danielsen, M. Lipofection: A highly efficient, lipid-mediated DNA-transfection procedure. Proc. Natl. Acad. Sci. USA 1987, 84, 7413–7417. [Google Scholar] [CrossRef] [PubMed]
- De Smedt, S.C.; Demeester, J.; Hennink, W.E. Cationic polymer based gene delivery systems. Pharma Res. 2000, 17, 113–126. [Google Scholar] [CrossRef]
- Ibraheem, D.; Elaissari, A.; Fessi, H. Gene therapy and DNA delivery systems. Int. J. Pharm. 2014, 459, 70–83. [Google Scholar] [CrossRef] [PubMed]
- Idowu, T.; Samadder, P.; Arthur, G.; Schweizer, F. Design, synthesis and antitumor properties of glycosylated antitumor ether lipid (GAEL)-chlorambucil-hybrids. Chem. Phys. Lipids 2016, 194, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Andey, T.; Sudhakar, G.; Marepally, S.; Patel, A.; Banerjee, R.; Singh, M. Lipid nanocarriers of a lipid-conjugated estrogenic derivative inhibit tumor growth and enhance cisplatin activity against triple-negative breast cancer: Pharmacokinetic and efficacy evaluation. Mol. Pharm. 2015, 12, 1105–1120. [Google Scholar] [CrossRef]
- Cardoso, A.M.; Morals, C.M.; Cruz, A.R.; Silva, S.G.; do Vale, M.L.; Marques, E.F.; de Lima, M.C.P.; Jurado, A.S. New serine-derived gemini surfactants as gene delivery systems. Eur. J. Pharm. Biopharm. 2015, 89, 347–356. [Google Scholar] [CrossRef]
- Pattni, B.S.; Chupin, V.V.; Torchilin, V.P. New Developments in Liposomal Drug Delivery. Chem. Rev. 2015, 115, 10938–10966. [Google Scholar] [CrossRef]
- Lv, H.; Zhang, S.; Wang, B.; Cui, S.; Yan, J. Toxicity of cationic lipids and cationic polymers in gene delivery. J. Control. Release 2006, 114, 100–109. [Google Scholar] [CrossRef]
- Alvarez-Paino, M.; Munoz-Bonilla, A.; Fernandez-Garcia, M. Antimicrobial Polymers in the Nano-World. Nanomaterials 2017, 7, 48. [Google Scholar] [CrossRef]
- Hemeg, H.A. Nanomaterials for alternative antibacterial therapy. Int. J. Nanomed. 2017, 12, 8211–8225. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Miao, L.; Zhao, Y.; Musetti, S.; Wang, Y.H.; Shi, K.; Huang, L. A novel cationic lipid with intrinsic antitumor activity to facilitate gene therapy of TRAIL DNA. Biomaterials 2016, 102, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Duhem, N.; Danhier, F.; Preat, V. Vitamin E-based nanomedicines for anti-cancer drug delivery. J. Control. Release 2014, 182, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Koudelka, S.; Turanek Knotigova, P.; Masek, J.; Prochazka, L.; Lukac, R.; Miller, A.D.; Neuzil, J.; Turanek, J. Liposomal delivery systems for anti-cancer analogues of vitamin E. J. Control. Release 2015, 207, 59–69. [Google Scholar] [CrossRef]
- Neophytou, C.M.; Constantinou, A.I. Drug Delivery Innovations for Enhancing the Anticancer Potential of Vitamin E Isoforms and Their Derivatives. BioMed Res. Int. 2015, 2015, 584862. [Google Scholar] [CrossRef] [PubMed]
- Kievit, F.M.; Zhang, M.Q. Cancer Nanotheranostics: Improving Imaging and Therapy by Targeted Delivery Across Biological Barriers. Adv. Mater. 2011, 23, H217–H247. [Google Scholar] [CrossRef] [PubMed]
- Gupta, I.; Sehgal, R.; Kanwar, R.K.; Punj, V.; Kanwar, J.R. Nanocapsules loaded with iron-saturated bovine lactoferrin have antimicrobial therapeutic potential and maintain calcium, zinc and iron metabolism. Nanomedicine 2015, 10, 1289–1314. [Google Scholar] [CrossRef]
- Hyvonen, Z.; Plotniece, A.; Reine, I.; Chekavichus, B.; Duburs, G.; Urtti, A. Novel cationic amphiphilic 1,4-dihydropyridine derivatives for DNA delivery. Biochim. Biophys. Acta Biomembr. 2000, 1509, 451–466. [Google Scholar] [CrossRef]
- Pajuste, K.; Hyvonen, Z.; Petrichenko, O.; Kaldre, D.; Rucins, M.; Cekavicus, B.; Ose, V.; Skrivele, B.; Gosteva, M.; Morin-Picardat, E.; et al. Gene delivery agents possessing antiradical activity: Self-assembling cationic amphiphilic 1,4-dihydropyridine derivatives. New J. Chem. 2013, 37, 3062–3075. [Google Scholar] [CrossRef]
- Petrichenko, O.; Rucins, M.; Vezane, A.; Timofejeva, I.; Sobolev, A.; Cekavicus, B.; Pajuste, K.; Plotniece, M.; Gosteva, M.; Kozlovska, T.; et al. Studies of the physicochemical and structural properties of self-assembling cationic pyridine derivatives as gene delivery agents. Chem. Phys. Lipids 2015, 191, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Cindric, M.; Cipak, A.; Serly, J.; Plotniece, A.; Jaganjac, M.; Mrakovcic, L.; Lovakovic, T.; Dedic, A.; Soldo, I.; Duburs, G.; et al. Reversal of multidrug resistance in murine lymphoma cells by amphiphilic dihydropyridine antioxidant derivative. Anticancer Res. 2010, 30, 4063–4069. [Google Scholar] [PubMed]
- Rucins, M.; Kaldre, D.; Pajuste, K.; Fernandes, M.A.S.; Vicente, J.A.F.; Klimaviciusa, L.; Jaschenko, E.; Kanepe-Lapsa, I.; Shestakova, I.; Plotniece, M.; et al. Synthesis and studies of calcium channel blocking and antioxidant activities of novel 4-pyridinium and/or N-propargyl substituted 1,4-dihydropyridine derivatives. Comptes Rendus Chim. 2014, 17, 69–80. [Google Scholar] [CrossRef]
- Jansone, B.; Kadish, I.; van Groen, T.; Beitnere, U.; Plotniece, A.; Pajuste, K.; Klusa, V. Memory-enhancing and brain protein expression-stimulating effects of novel calcium antagonist in Alzheimer’s disease transgenic female mice. Pharm. Res 2016, 113, 781–787. [Google Scholar] [CrossRef] [PubMed]
- Jansone, B.; Kadish, I.; van Groen, T.; Beitnere, U.; Moore, D.R.; Plotniece, A.; Pajuste, K.; Klusa, V. A Novel 1,4-Dihydropyridine Derivative Improves Spatial Learning and Memory and Modifies Brain Protein Expression in Wild Type and Transgenic APP(SweDI) Mice. PLoS ONE 2015, 10, e0127686. [Google Scholar] [CrossRef] [PubMed]
- Tirzite, D.; Koronova, J.; Plotniece, A. Influence of some quaternised 1,4-dihydropyridine derivatives on liposomes and erythrocyte membranes. Biochem. Mol. Biol. Int. 1998, 45, 849–856. [Google Scholar] [PubMed]
- Rucins, M.; Gosteva, M.; Domracheva, I.; Kanepe-Lapsa, I.; Belyakov, S.; Plotniece, M.; Pajuste, K.; Cekavicus, B.; Jekabsone, M.; Sobolev, A.; et al. Synthesis and Evaluation of Reducing Capacity and Calcium Channel Blocking Activity of Novel 3,5-Dipropargylcarbonyl-Substituted 1,4-Dihydropyridines. Chem. Heterocycl. Compd. 2015, 50, 1432–1443. [Google Scholar] [CrossRef]
- Stokes, W.S.; Casati, S.; Strickland, J.; Paris, M. Neutral red uptake cytotoxicity tests for estimating starting doses for acute oral toxicity tests. Curr. Protoc. Toxicol. 2008, 36, 24. [Google Scholar]
- ICCVAM. NIH Publication No 07-4519. Research Triangle Park, NC, USA; 2006. Available online: http://iccvamniehsnihgov/S (accessed on 20 January 2007).
- ICCVAM. Background Review Document: In Vitro Basal Cytotoxicity Test Methods for Estimating Acute Oral Systemic Toxicity; National Institute for Environmental Health Sciences: Research Triangle Park, NC, USA, 2006. Available online: http://iccvam.niehs.nih.gov/methods/acutetox/inv_nru_brd.htm (accessed on 28 January 2019).
- Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on classification, labelling and packaging of substances and mixtures, amending and repealing Directives 67/548/EEC and 1999/45/EC, and amending Regulation (EC) No 1907/2006 (Text with EEA relevance). Available online: http://data.europa.eu/eli/reg/2008/1272/2017-01-01 (accessed on 28 January 2019).
- Suppi, S.; Kasemets, K.; Ivask, A.; Kunnis-Beres, K.; Sihtmae, M.; Kurvet, I.; Aruoja, V.; Kahru, A. A novel method for comparison of biocidal properties of nanomaterials to bacteria, yeasts and algae. J. Hazard. Mater. 2015, 286, 75–84. [Google Scholar] [CrossRef]
- Entian, K.-D.; Kötter, P. 23 Yeast Mutant and Plasmid Collections. In Methods in Microbiology; Brown, A.J.P., Tuite, M., Eds.; Academic Press: Cambridge, MA, USA, 1998; Volume 26, pp. 431–449. [Google Scholar]
- Metsalu, T.; Vilo, J. ClustVis: A web tool for visualizing clustering of multivariate data using Principal Component Analysis and heatmap. Nucleic Acids Res. 2015, 43, W566–W570. [Google Scholar] [CrossRef]
- Ma, J.Y.; Ma, J.K.; Weber, K.C. Fluorescence studies of the binding of amphiphilic amines with phospholipids. J. Lipid Res. 1985, 26, 735–744. [Google Scholar]
- Joondan, N.; Jhaumeer-Laulloo, S.; Caumul, P. A study of the antibacterial activity of l-Phenylalanine and l-Tyrosine esters in relation to their CMCs and their interactions with 1,2-dipalmitoyl-sn-glycero-3-phosphocholine, DPPC as model membrane. Microbiol. Res. 2014, 169, 675–685. [Google Scholar] [CrossRef]
- Topel, Ö.; Çakır, B.A.; Budama, L.; Hoda, N. Determination of critical micelle concentration of polybutadiene-block-poly(ethyleneoxide) diblock copolymer by fluorescence spectroscopy and dynamic light scattering. J. Mol. Liq. 2013, 177, 40–43. [Google Scholar] [CrossRef]
- Hantzsch, A. Ueber die Synthese pyridinartiger Verbindungen aus Acetessigäther und Aldehydammoniak. Justus Liebigs Ann. Chem. 1882, 215, 1–82. [Google Scholar] [CrossRef]
- Makarova, N.V.; Koronova, Z.V.; Plotnietse, A.V.; Tirzite, D.Y.; Tirzit, G.D.; Duburs, G.Y. Synthesis of 1,4-dihydropyridines having an N-alkylpyridinium substituent at the 4-position and their affinity towards liposomal membranes. Chem. Heterocycl. Compd. 1995, 31, 969–973. [Google Scholar] [CrossRef]
- Makarova, N.V.; Plotnietse, A.; Tirzitis, G.; Turovskii, I.; Dubur, G. Some transformations of N-ethoxycarbonyl-methylpyridinium bromides with a pyridyl or 1,4-dihydropyridyl substituent at position 3. Chem. Heterocycl. Compd. 1997, 33, 175–183. [Google Scholar] [CrossRef]
- Spielmann, H.; Grune, B.; Liebsch, M.; Seiler, A.; Vogel, R. Successful validation of in vitro methods in toxicology by ZEBET, the National Centre for Alternatives in Germany at the BfR (Federal Institute for Risk Assessment). Exp. Toxicol. Pathol. 2008, 60, 225–233. [Google Scholar] [CrossRef]
- Jolliffe, I.T.; Cadima, J. Principal component analysis: A review and recent developments. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2016, 374, 20150202. [Google Scholar] [CrossRef]
- Bro, R.; Smilde, A.K. Principal component analysis. Anal. Methods 2014, 6, 2812–2831. [Google Scholar] [CrossRef]
- Egorova, K.S.; Ananikov, V.P. Toxicity of ionic liquids: Eco(cyto)activity as complicated, but unavoidable parameter for task-specific optimization. ChemSusChem 2014, 7, 336–360. [Google Scholar] [CrossRef]
- Sharma, V.K.; Mukhopadhyay, R. Deciphering interactions of ionic liquids with biomembrane. Biophys. Rev. 2018, 10, 721–734. [Google Scholar] [CrossRef]
- Cortesi, R.; Esposito, E.; Menegatti, E.; Gambari, R.; Nastruzzi, C. Effect of cationic liposome composition on in vitro cytotoxicity and protective effect on carried DNA. Int. J. Pharm. 1996, 139, 69–78. [Google Scholar] [CrossRef]
- Singh, R.P.; Gangadharappa, H.V.; Mruthunjaya, K. Phospholipids: Unique carriers for drug delivery systems. J. Drug Deliv. Sci. Technol. 2017, 39, 166–179. [Google Scholar] [CrossRef]
- Joondan, N.; Caumul, P.; Akerman, M.; Jhaumeer-Laulloo, S. Synthesis, micellisation and interaction of novel quaternary ammonium compounds derived from l-Phenylalanine with 1,2-dipalmitoyl-sn-glycero-3-phosphocholine as model membrane in relation to their antibacterial activity, and their selectivity over human red blood cells. Bioorg. Chem. 2015, 58, 117–129. [Google Scholar]
- Castillo, J.A.; Pinazo, A.; Carilla, J.; Infante, M.R.; Alsina, M.A.; Haro, I.; Clapés, P. Interaction of Antimicrobial Arginine-Based Cationic Surfactants with Liposomes and Lipid Monolayers. Langmuir 2004, 20, 3379–3387. [Google Scholar] [CrossRef]
- Matyszewska, D.; Tappura, K.; Orädd, G.; Bilewicz, R. Influence of Perfluorinated Compounds on the Properties of Model Lipid Membranes. J. Phys. Chem. B 2007, 111, 9908–9918. [Google Scholar] [CrossRef]
- Chibowski, E.; Szcześ, A. Zeta potential and surface charge of DPPC and DOPC liposomes in the presence of PLC enzyme. Adsorption 2016, 22, 755–765. [Google Scholar] [CrossRef]
- Muhamadejev, R.; Petrova, M.; Smits, R.; Plotniece, A.; Pajuste, K.; Duburs, G.; Liepinsh, E. Study of interactions of mononucleotides with 1,4-dihydropyridine vesicles using NMR and ITC techniques. New J. Chem. 2018. [Google Scholar] [CrossRef]

| Comp. | Cytotoxicity IC50, μM | Basal Cytotoxicity Estimated a LD50 | ||
|---|---|---|---|---|
| HT-1080 | MH-22A | mM/kg | mg/kg | |
| 1 | ne | ne | 5.8 ± 0.3 | 2548 |
| 2 | 26 ± 2 | 67 ± 4 | 2.8 ± 0.3 | 1404 |
| 3 | 37 ± 2 | 15 ± 1 | 1.2 ± 0.4 | 692 |
| 4 | ne | ne | 4.8 ± 0.1 | 2280 |
| 5 | 5 ± 0.3 | 16 ± 1 | 1.3 ± 0.4 | 669 |
| 6 | 4 ± 0.4 | 1 ± 0.2 | 0.3 ± 0.1 | 183 |
| 7 | ne | ne | >6 | >2710 |
| 8 | 76 ± 8 | 80 ± 5 | 2.2 ± 0.4 | 1165 |
| 9 | 2 ± 0.2 | 1 ± 0.2 | 0.5 ± 0.05 | 300 |
| Comp. | Mw a | CAC, µM | Zeta-Pot b. ± SD, mV | PDI ± SD | Dmean ± SD, nm (%) | ||
|---|---|---|---|---|---|---|---|
| 1 Set c | 2 Set d | 1 Set c | 2 Set d | ||||
| 1 | 359 | - | −8.43 ± 2.14 | 0.18 ± 0.01 | 0.40 ± 0.16 | 58 ± 24 (98) | 195 ± 75 (59) 45 ± 13 (41) |
| 2 | 415 | - | −11.39 ± 1.85 | 0.50 ± 0.10 | 0.91 ± 0.10 | 142 ± 24 (95) | 225 ± 25 (100) |
| 3 | 499 | 9.3 | +57.73 ± 2.30 | 0.14 ± 0.01 | 0.17 ± 0.02 | 124 ± 51 (100) | 140 ± 64 (100) |
| 4 | 397 | - | −5.47 ± 1.97 | 0.16 ± 0.02 | 0.41 ± 0.07 | 79 ± 29 (99) | 238 ± 76 (78) 55 ± 19 (22) |
| 5 | 453 | - | −9.96 ± 0.72 | 0.72 ± 0.32 | 0.59 ± 0.01 | 513 ± 55 (78) | 503 ± 66 (100) |
| 6 | 537 | 43.3 | +26.07 ± 0.78 | 0.15 ± 0.02 | 0.20 ± 0.01 | 154 ± 64 (100) | 184 ± 97 (100) |
| 7 | 379 | - | −0.78 ± 0.26 | 0.90 ± 0.03 | 0.73 ± 0.09 | 258 ± 33 (100) | 185 ± 24 (100) |
| 8 | 435 | - | - | 0.60 ± 0.06 | 0.45 ± 0.05 | 325 ± 50 (100) | 330 ± 49 (100) |
| 9 | 519 | 9.3 | +32.00 ± 4.81 | 0.36 ± 0.08 | 0.57 ± 0.05 | 65 ± 21 (73) 297 ± 78 (26) | 415 ± 88 (98) 58 ± 7 (2) |
| 10 | 555 | 28.2 | +49.70 ± 7.94 | 0.10 ± 0.06 | 0.25 ± 0.05 | 221 ± 15 (100) | 199 ± 73 (100) |
| 11 | 593 | 7.6 | +62.80 ± 0.78 | 0.37 ± 0.03 | 0.33 ± 0.03 | 145 ± 7 (91) 51 ± 5 (9) | 143 ± 43 (96) 41 ± 9 (4) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rucins, M.; Dimitrijevs, P.; Pajuste, K.; Petrichenko, O.; Jackevica, L.; Gulbe, A.; Kibilda, S.; Smits, K.; Plotniece, M.; Tirzite, D.; et al. Contribution of Molecular Structure to Self-Assembling and Biological Properties of Bifunctional Lipid-Like 4-(N-Alkylpyridinium)-1,4-Dihydropyridines. Pharmaceutics 2019, 11, 115. https://doi.org/10.3390/pharmaceutics11030115
Rucins M, Dimitrijevs P, Pajuste K, Petrichenko O, Jackevica L, Gulbe A, Kibilda S, Smits K, Plotniece M, Tirzite D, et al. Contribution of Molecular Structure to Self-Assembling and Biological Properties of Bifunctional Lipid-Like 4-(N-Alkylpyridinium)-1,4-Dihydropyridines. Pharmaceutics. 2019; 11(3):115. https://doi.org/10.3390/pharmaceutics11030115
Chicago/Turabian StyleRucins, Martins, Pavels Dimitrijevs, Klavs Pajuste, Oksana Petrichenko, Ludmila Jackevica, Anita Gulbe, Signe Kibilda, Krisjanis Smits, Mara Plotniece, Dace Tirzite, and et al. 2019. "Contribution of Molecular Structure to Self-Assembling and Biological Properties of Bifunctional Lipid-Like 4-(N-Alkylpyridinium)-1,4-Dihydropyridines" Pharmaceutics 11, no. 3: 115. https://doi.org/10.3390/pharmaceutics11030115
APA StyleRucins, M., Dimitrijevs, P., Pajuste, K., Petrichenko, O., Jackevica, L., Gulbe, A., Kibilda, S., Smits, K., Plotniece, M., Tirzite, D., Pajuste, K., Sobolev, A., Liepins, J., Domracheva, I., & Plotniece, A. (2019). Contribution of Molecular Structure to Self-Assembling and Biological Properties of Bifunctional Lipid-Like 4-(N-Alkylpyridinium)-1,4-Dihydropyridines. Pharmaceutics, 11(3), 115. https://doi.org/10.3390/pharmaceutics11030115