Preparation of Squalene Oil-Based Emulsion Adjuvants Employing a Self-Emulsifying Drug Delivery System and Assessment of Mycoplasma hyopneumoniae-Specific Antibody Titers in BALB/c Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
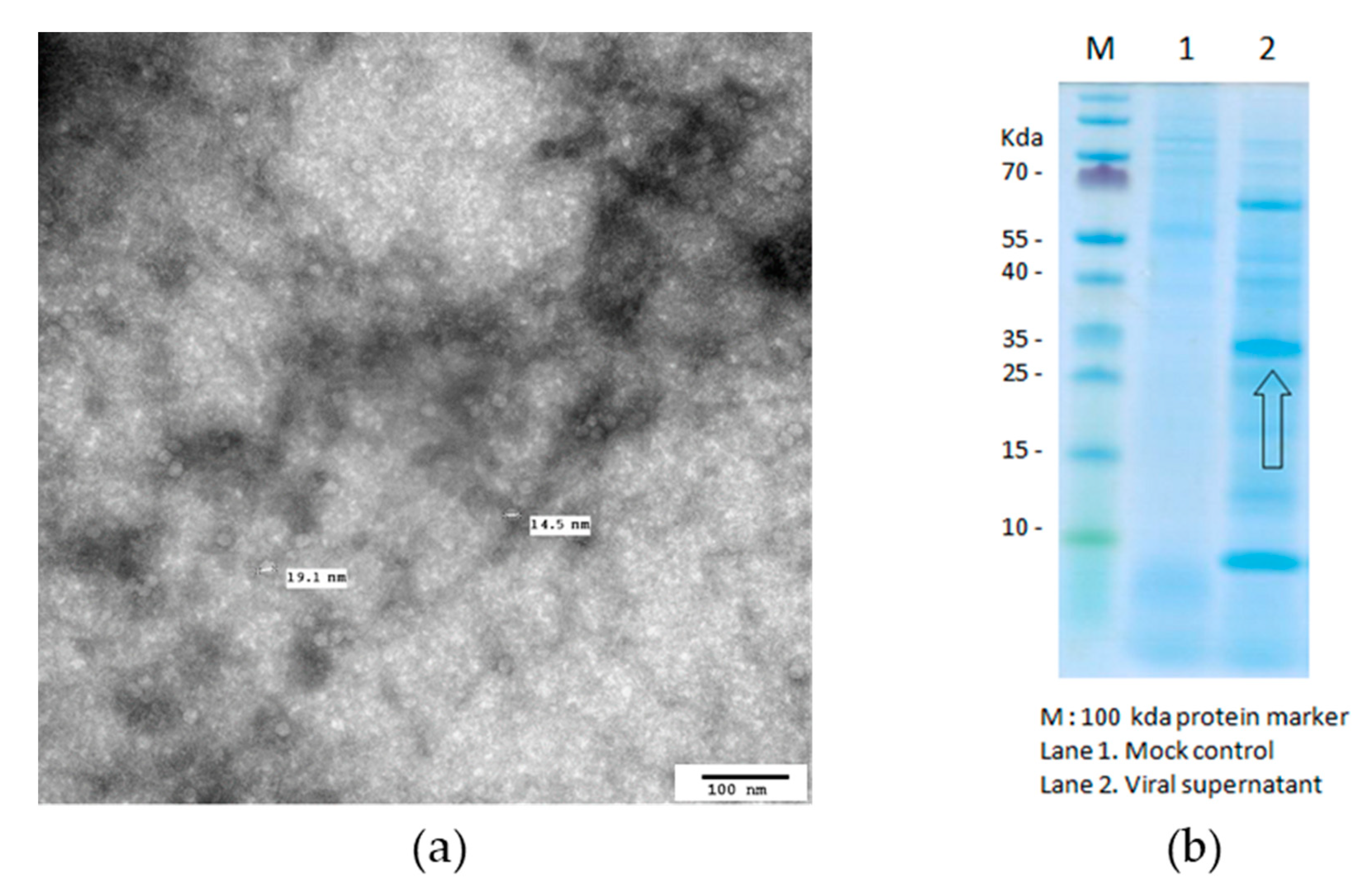
2.2. Preparation and Characterization of PCV2 Antigen
2.3. Preparation and Characterization of Mycoplasma hyopneumoniae Antigen
2.3.1. Culture Condition of Mycoplasma hyopneumoniae Antigen
2.3.2. Characterization Using Mycoplasma Selection Media
2.3.3. Identification of Mycoplasma hyopneumoniae Using Polymerase Chain Reaction (PCR)
2.4. Construction of Pseudo-Ternary Phase Diagrams
2.5. Self-Emulsification Study
2.6. Preparation of Emulsions
2.7. Particle Size and Zeta Potential of Emulsion-Based Adjuvants
2.8. Preparation of Vaccines
2.9. ELISA Analysis Method for PCV2
2.10. Toxicity Study
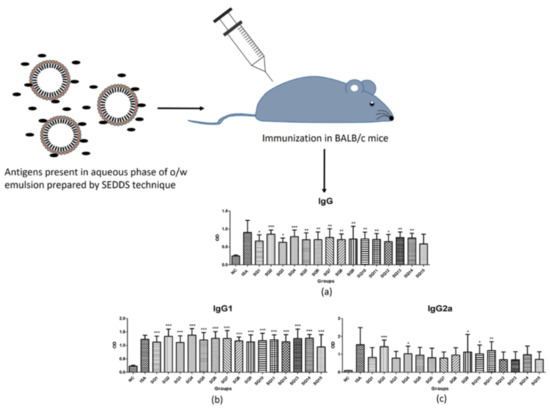
2.11. Determination of Adjuvant-Dependent Antibody Subclasses
2.12. Measurement of Mycoplasma hyopneumoniae-Specific Antibody Titers
2.13. Statistical Analysis
3. Results
3.1. Characterization of PCV2 Antigen Formation
3.2. Characterization of Mycoplasma hyopneumoniae Antigen Formation
3.2.1. Measurement of Mycoplasma hyopneumoniae Antigen Titer
3.2.2. Optical and Microscopic Observation for the Characterization of Mycoplasma hyopneumoniae
3.2.3. Identification of Mycoplasma hyopneumoniae Using PCR Method
3.3. Pseudo-Ternary Phase Diagram and Self-Emulsification Study
3.4. Preparation and Characterization of Emulsion Adjuvants
3.5. Toxicity Evaluation
3.6. Determination of Adjuvant-Dependent Antibody Subclasses
3.7. Measurement of Mycoplasma hyopneumoniae Specific Antibody Level
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lee, S.; Nguyen, M.T. Recent advances of vaccine adjuvants for infectious diseases. Immune Netw. 2015, 15, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Bastola, R.; Lee, S. Physicochemical properties of particulate vaccine adjuvants: Their pivotal role in modulating immune responses. J. Pharm. Investig. 2019, 49, 279–285. [Google Scholar] [CrossRef]
- Fan, Y.; Guo, L.; Hou, W.; Guo, C.; Zhang, W.; Ma, X.; Ma, L.; Song, X. The adjuvant activity of Epimedium polysaccharide-propolis flavone liposome on enhancing immune responses to inactivated porcine circovirus vaccine in mice. Evid.-Based Complement. Altern. Med. 2015. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.T.; Zhang, X.M.; Sun, P.F.; Sun, L.J.; Guo, X.; Tian, T.; Zhang, J.; Guo, Q.Y.; Li, X.; Guo, L.J.; et al. Intranasal immunization using mannatide as a novel adjuvant for an inactivated influenza vaccine and its adjuvant effect compared with MF59. PLoS ONE 2017, 12, e0169501. [Google Scholar] [CrossRef]
- Singh, D.; Jayashankar, B.; Mishra, K.P.; Tanwar, H.; Madhusudana, S.N.; Belludi, A.Y.; Tulsawani, R.; Singh, S.B.; Ganju, L. Adjuvant activity of ethanol extract of Hippophae rhamnoides leaves with inactivated rabies virus antigen. Pharm. Biol. 2018, 56, 25–31. [Google Scholar] [CrossRef]
- Bastola, R.; Noh, G.; Keum, T.; Bashyal, S.; Seo, J.E.; Choi, J.; Oh, Y.; Cho, Y.S.; Lee, S. Vaccine adjuvants: Smart components to boost the immune system. Arch. Pharmacal Res. 2017, 40, 1238–1248. [Google Scholar] [CrossRef]
- Bobbala, S.; Hook, S. Is there an optimal formulation and delivery strategy for subunit vaccines? Pharm. Res. 2016, 33, 2078–2097. [Google Scholar] [CrossRef]
- Aucouturier, J.; Dupuis, L.; Ganne, V. Adjuvants designed for veterinary and human vaccines. Vaccines 2001, 19, 2666–2672. [Google Scholar] [CrossRef]
- Patel, M.R.; Hirani, S.N.; Patel, R.B. Microemulsion for nasal delivery of Asenapine maleate in treatment of schizophrenia: Formulation considerations. J. Pharm. Investig. 2018, 48, 301–312. [Google Scholar] [CrossRef]
- Hajjar, B.; Zier, K.I.; Khalid, N.; Azarmi, S.; Lobenberg, R. Evaluation of a microemulsion-based gel formulation for topical drug delivery of diclofenac sodium. J. Pharm. Investig. 2018, 48, 351–362. [Google Scholar] [CrossRef]
- Shah, R.R.; Brito, L.A.; O′Hagan, D.T.; Amiji, M.M. Emulsions as vaccine adjuvants. In Subunit Vaccine Delivery; Foged, C., Rades, T., Perrie, Y., Hook, S., Eds.; Springer: New York, NY, USA, 2015; pp. 59–76. [Google Scholar]
- Iyer, V.; Cayatte, C.; Marshall, J.D.; Sun, J.; Schneider-Ohrum, K.; Maynard, S.K.; Rajani, G.M.; Bennett, A.S.; Remmele, R.L., Jr.; Bishop, S.M.; et al. Feasibility of freeze-drying oil-in-water emulsion adjuvants and subunit proteins to enable single-vial vaccine drug products. J. Pharm. Sci. 2017, 106, 1490–1498. [Google Scholar] [CrossRef] [PubMed]
- Brito, L.A.; Malyala, P.; O’Hagan, D.T. Vaccine adjuvant formulations: A pharmaceutical perspective. Semin. Immunol. 2013, 25, 130–145. [Google Scholar] [CrossRef] [PubMed]
- Thapa, R.K.; Choi, H.G.; Kim, J.O.; Yong, C.S. Analysis and optimization of drug solubility to improve pharmacokinetics. J. Pharm. Investig. 2017, 47, 95–110. [Google Scholar] [CrossRef]
- Shah, R.R.; Dodd, S.; Schaefer, M.; Ugozzoli, M.; Singh, M.; Otten, G.R.; Amiji, M.M.; O′Hagan, D.T.; Brito, L.A. The development of self-emulsifying oil-in-water emulsion adjuvant and an evaluation of the impact of droplet size on performance. J. Pharm. Sci. 2015, 104, 1352–1361. [Google Scholar] [CrossRef]
- Caron, J.; Ouardani, M.; Dea, S. Diagnosis and differentiation of Mycoplasma hyopneumoniae and Mycoplasma hyorhinis infections in pigs by PCR amplification of the p36 and p46 genes. J. Clin. Microbiol. 2000, 38, 1390–1396. [Google Scholar]
- Wang, Z.; Pal, R. Enlargement of nanoemulsion region in pseudo-ternary mixing diagrams for a drug delivery system. J. Surfactants Deterg. 2014, 17, 49–58. [Google Scholar] [CrossRef]
- Lee, S.; Lee, J.; Choi, Y.W. Design and evaluation of prostaglandin E1 (PGE1) intraurethral liquid formulation employing self-microemulsifying drug delivery system (SMEDDS) for erectile dysfunction treatment. Biol. Pharm. Bull. 2008, 31, 668–672. [Google Scholar] [CrossRef][Green Version]
- Stipkovits, L.; Czifra, G.; Sundquist, B. Indirect ELISA for the detection of a specific antibody response against Mycoplasma gallisepticum. Avian Pathol. 1993, 22, 481–494. [Google Scholar] [CrossRef]
- Hou, W.; Papadopoulos, K.D. W1/O/W2 and O1/W/O2 globules stabilized with Span 80 and Tween 80. Colloids Surf. A: Physicochem. Eng. Asp. 1997, 125, 181–187. [Google Scholar] [CrossRef]
- Nepal, P.R.; Han, H.K.; Choi, H.K. Preparation and in vitro–in vivo evaluation of Witepsol® H35 based self-nanoemulsifying drug delivery systems (SNEDDS) of coenzyme Q10. Eur. J. Pharm. Sci. 2010, 39, 224–232. [Google Scholar] [CrossRef]
- Lai, R.P.J.; Seaman, M.S.; Tonks, P.; Wegmann, F.; Seilly, D.J.; Frost, S.D.W.; LaBranche, C.C.; Montefiori, D.C.; Dey, A.K.; Shrivastava, I.K.; et al. Mixed adjuvant formulations reveal a new combination that elicit antibody response comparable to Freund′s adjuvants. PLoS ONE 2012, 7, e35083. [Google Scholar] [CrossRef] [PubMed]
- Mair, K.H.; Koinig, H.; Gerner, W.; Hohne, A.; Bretthauer, J.; Kroll, J.J.; Roof, M.B.; Saalmuller, A.; Stadler, K.; Libanova, R. Carbopol improves the early cellular immune responses induced by the modified-life vaccine Ingelvac PRRS® MLV. Vet. Microbiol. 2015, 176, 352–357. [Google Scholar] [CrossRef] [PubMed]
| Ingredients | SQ1 | SQ2 | SQ3 | SQ4 | SQ5 | SQ6 | SQ7 | SQ8 | SQ9 | SQ10 | SQ11 | SQ12 | SQ13 | SQ14 | SQ15 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Span 80 | 2.4 | 4.1 | 8.2 | 2.4 | 4.1 | 8.2 | 2.4 | 4.1 | 8.2 | 2.4 | 4.1 | 8.2 | 2.4 | 4.1 | 8.2 |
| Cremophor ELP | 4.6 | 7.6 | 15.1 | 4.6 | 7.6 | 15.1 | 4.6 | 7.6 | 15.1 | 4.6 | 7.6 | 15.1 | 4.6 | 7.6 | 15.1 |
| Squalene oil | 3.0 | 5.0 | 10.0 | 3.0 | 5.0 | 10.0 | 3.0 | 5.0 | 10.0 | 3.0 | 5.0 | 10.0 | 3.0 | 5.0 | 10.0 |
| Water | 90.0 | 83.3 | 66.7 | ||||||||||||
| Carbopol®C-971P NF sol’n (0.01% w/v) | 90.0 | 83.3 | 66.7 | ||||||||||||
| Carbopol®971PNF sol’n (0.02% w/v) | 90.0 | 83.3 | 66.7 | ||||||||||||
| Carbopol®940 sol’n (0.01% w/v) | 90.0 | 83.3 | 66.7 | ||||||||||||
| Carbopol®940 sol’n (0.02% w/v) | 90.0 | 83.3 | 66.7 | ||||||||||||
| Total | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
| Negative Control | Mycoplasma hyopneumoniae | Positive Control (Mycoplasma gallisepticum) | |
|---|---|---|---|
| Mycoplasma broth |  |  |  |
| Mycoplasma agar |  |  |  |
| Sample Code | Percentage Composition of SEDDS | Formulations Used for Self-Emulsification Test | Visual Grade | |
|---|---|---|---|---|
| Smix | Squalene Oil | |||
| SQ1-SQ3 | 70% | 30% | Water | A |
| SQ4-SQ6 | 70% | 30% | C-971P NF solution (0.01% w/v) | B |
| SQ7-SQ9 | 70% | 30% | C-971P NF solution (0.02% w/v) | B |
| SQ10-SQ12 | 70% | 30% | C-940 solution (0.01% w/v) | B |
| SQ13-SQ15 | 70% | 30% | C-940 solution (0.02% w/v) | B |
| Sample Code | Particle Size (nm) | Zeta Potential (mV) | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 Month | 1 Month | 3 Months | 6 Months | 0 Month | 1 Month | 3 Months | 6 Months | |
| SQ1 | 1589.9 ± 211.3 | 911.5 ± 148.5 | 1350.8 ± 74.8 | 649.6 ± 54.4 | −43.58 ± 1.44 | 0.19 ± 6.39 | 17.63 ± 13.50 | 1.47 ± 10.11 |
| SQ2 | 1211.4 ± 52.0 | 900.6 ± 56.5 | 1082.0 ± 22.7 | 750.9 ± 52.5 | −25.24 ± 0.83 | −18.23 ± 2.98 | −10.47 ± 0.35 | −6.41 ± 5.36 |
| SQ3 | 690.7 ± 36.6 | 427.5 ± 17.4 | 557.1 ± 20.8 | 457.1 ± 19.6 | −29.08 ± 3.50 | −8.44 ± 5.64 | −6.39 ± 8.33 | 11.11 ± 18.60 |
| SQ4 | 100.1 ± 15.2 | 80.7 ± 3.1 | 228.1 ± 48.2 | 402.7 ± 50.4 | −10.86 ± 1.75 | −15.71 ± 2.65 | 11.85 ± 7.72 | −10.81 ± 2.21 |
| SQ5 | 371.4 ± 179.0 | 228.3 ± 62.4 | 455.6 ± 112.3 | 410.4 ± 65.6 | −25.11 ± 0.65 | −18.86 ± 1.17 | −4.81 ± 7.72 | −7.94 ± 1.62 |
| SQ6 | 1438.4 ± 75.1 | 570.6 ± 19.3 | 798.6 ± 18.0 | 570.9 ± 28.8 | −31.86 ± 2.16 | −13.22 ± 3.94 | −10.29 ± 11.92 | −0.61 ± 13.35 |
| SQ7 | 45.2 ± 0.5 | 49.6 ± 0.6 | 70.5 ± 2.1 | 90.2 ± 3.3 | −0.59 ± 8.16 | −16.96 ± 1.46 | 15.82 ± 4.63 | 6.24 ± 9.56 |
| SQ8 | 1284.1 ± 31.7 | 567.2 ± 32.2 | 625.1 ± 59.4 | 591.1 ± 60.7 | 2.84 ± 11.48 | −13.84 ± 0.97 | −10.79 ± 10.38 | −9.18 ± 1.93 |
| SQ9 | 862.4 ± 35.2 | 457.5 ± 4.7 | 398.6 ± 8.6 | 468.7 ± 34.3 | −23.13 ± 5.12 | −12.86 ± 1.88 | −6.15 ± 4.57 | −4.08 ± 11.54 |
| SQ10 | 2483.4 ± 200.9 | 1951.5 ± 172.0 | 1722.7 ± 62.6 | 700.3 ± 97.5 | 6.48 ± 8.24 | 1.21 ± 11.07 | 1.78 ± 10.73 | −11.61 ± 0.50 |
| SQ11 | 335.8 ± 59.5 | 765.3 ± 54.1 | 721.2 ± 64.7 | 483.9 ± 22.2 | −15.98 ± 1.67 | 3.95 ± 6.57 | −4.47 ± 10.36 | −9.04 ± 1.29 |
| SQ12 | 920.4 ± 45.6 | 622.5 ± 32.2 | 739.1 ± 37.4 | 537.4 ± 15.1 | −24.44 ± 1.74 | −10.79 ± 7.83 | −4.05 ± 7.56 | 7.55 ± 1.76 |
| SQ13 | 697.4 ± 91.3 | 831.7 ± 82.6 | 856.1 ± 38.7 | 621.4 ± 23.3 | 11.55 ± 1.59 | 10.18 ± 4.00 | −4.59 ± 12.61 | −12.98 ± 2.52 |
| SQ14 | 217.9 ± 21.9 | 353.4 ± 70.4 | 1086.5 ± 45.8 | 683.0 ± 44.2 | −9.18 ± 12.24 | 0.59 ± 13.56 | 1.89 ± 18.59 | −11.31 ± 3.22 |
| SQ15 | 1211.4 ± 90.8 | 968.0 ± 10.8 | 899.5 ± 54.4 | 582.1 ± 28.5 | −34.17 ± 2.56 | −14.65 ± 4.33 | 2.14 ± 9.13 | −7.56 ± 10.96 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bastola, R.; Seo, J.-E.; Keum, T.; Noh, G.; Choi, J.W.; Shin, J.I.; Kim, J.H.; Lee, S. Preparation of Squalene Oil-Based Emulsion Adjuvants Employing a Self-Emulsifying Drug Delivery System and Assessment of Mycoplasma hyopneumoniae-Specific Antibody Titers in BALB/c Mice. Pharmaceutics 2019, 11, 667. https://doi.org/10.3390/pharmaceutics11120667
Bastola R, Seo J-E, Keum T, Noh G, Choi JW, Shin JI, Kim JH, Lee S. Preparation of Squalene Oil-Based Emulsion Adjuvants Employing a Self-Emulsifying Drug Delivery System and Assessment of Mycoplasma hyopneumoniae-Specific Antibody Titers in BALB/c Mice. Pharmaceutics. 2019; 11(12):667. https://doi.org/10.3390/pharmaceutics11120667
Chicago/Turabian StyleBastola, Rakesh, Jo-Eun Seo, Taekwang Keum, Gyubin Noh, Jae Woong Choi, Jong Il Shin, Ju Hun Kim, and Sangkil Lee. 2019. "Preparation of Squalene Oil-Based Emulsion Adjuvants Employing a Self-Emulsifying Drug Delivery System and Assessment of Mycoplasma hyopneumoniae-Specific Antibody Titers in BALB/c Mice" Pharmaceutics 11, no. 12: 667. https://doi.org/10.3390/pharmaceutics11120667
APA StyleBastola, R., Seo, J.-E., Keum, T., Noh, G., Choi, J. W., Shin, J. I., Kim, J. H., & Lee, S. (2019). Preparation of Squalene Oil-Based Emulsion Adjuvants Employing a Self-Emulsifying Drug Delivery System and Assessment of Mycoplasma hyopneumoniae-Specific Antibody Titers in BALB/c Mice. Pharmaceutics, 11(12), 667. https://doi.org/10.3390/pharmaceutics11120667