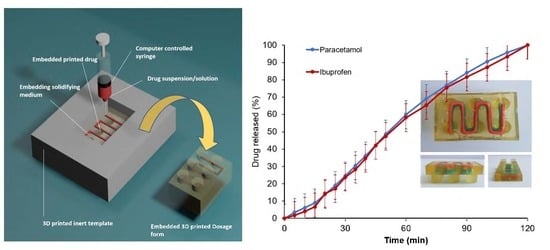
Embedded 3D Printing of Novel Bespoke Soft Dosage Form Concept for Pediatrics
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Embedding and Embedded Materials
2.2.1. Embedding Medium
2.2.2. Embedded Drug Paste
2.3. Modification of Dual FDM 3D Printer
2.4. Design of Template and Pattern
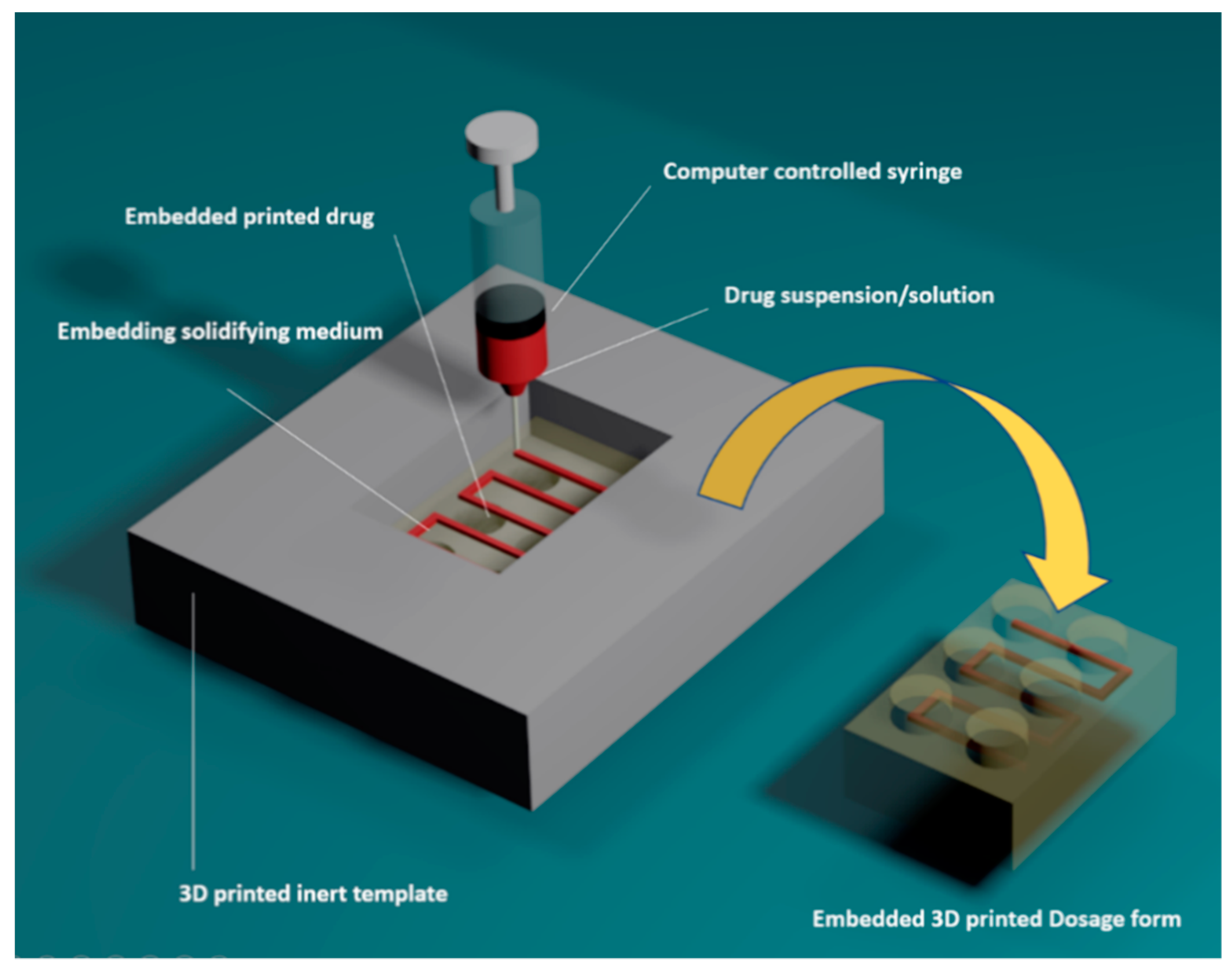
2.5. Embedded 3D Printing Process
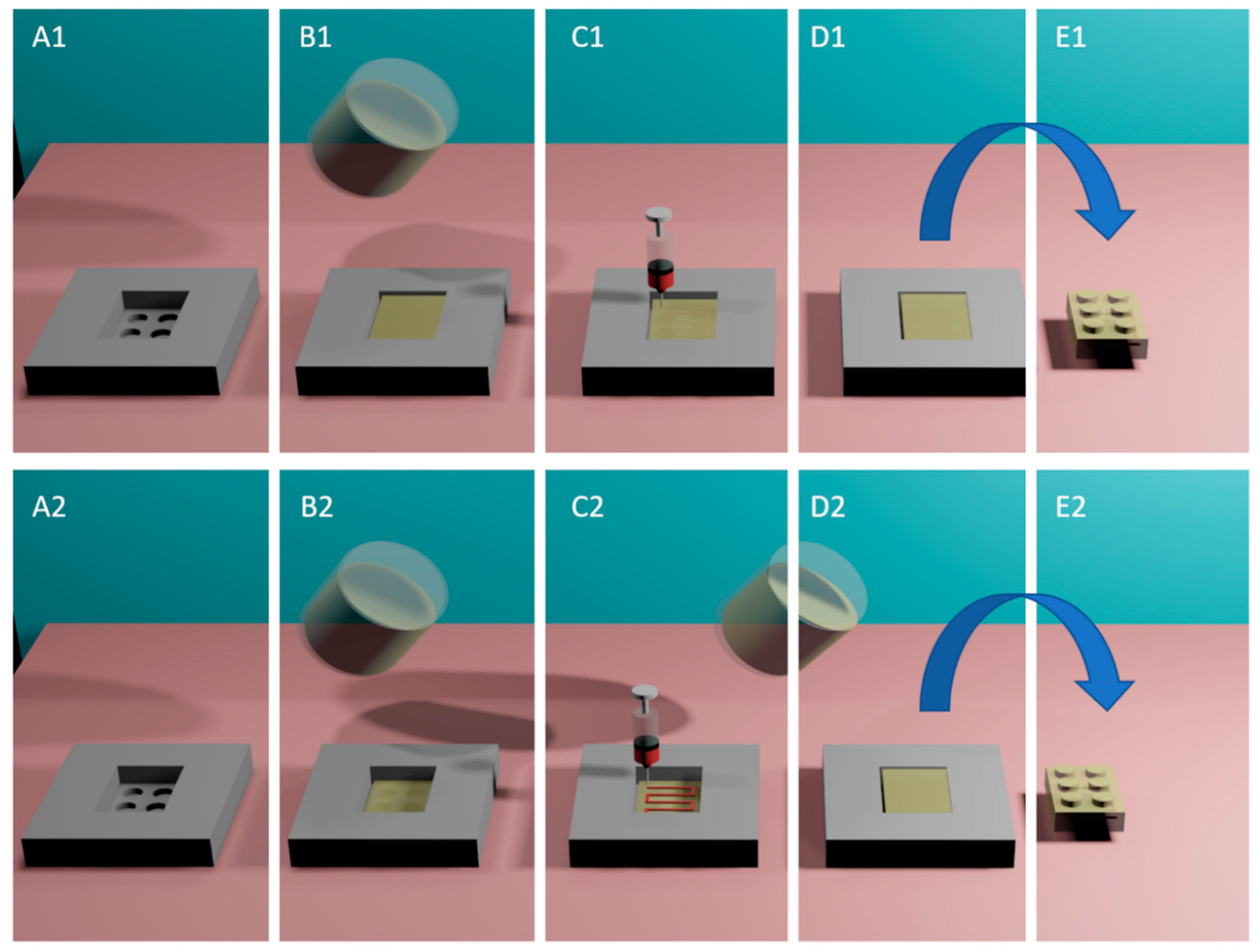
2.5.1. One-Step Embedded 3D Printing
2.5.2. Multi-Step Embedded 3D Printing
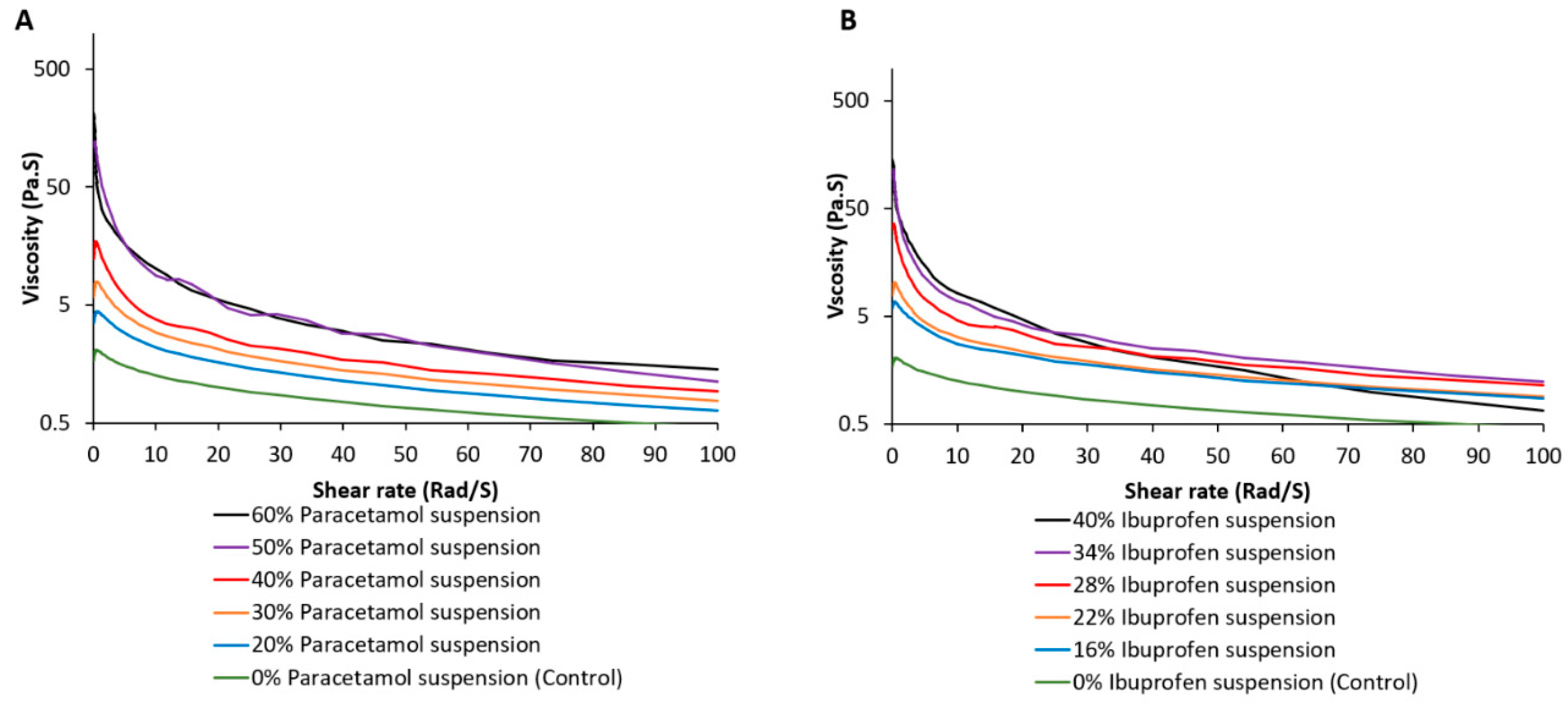
2.6. Rheological Studies of Embedded Material
2.7. Scanning Electron Microscopy (SEM)
2.8. Drug Contents Using HPLC
2.9. Dissolution Test for Oral Doses
2.10. Statistical Analysis
3. Results and Discussion
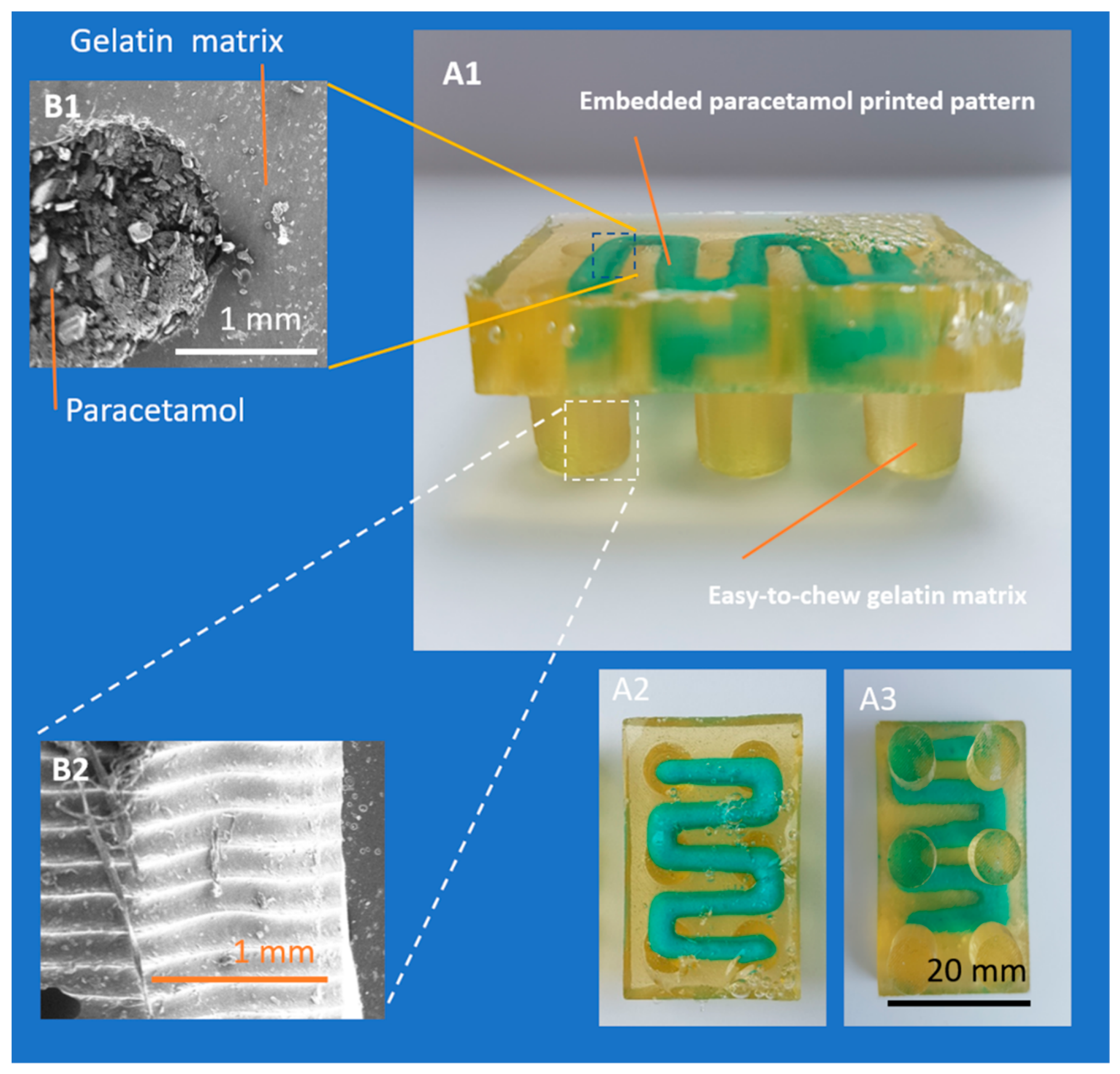
3.1. Development of Embedded Phase for Pharmaceutical e-3DP
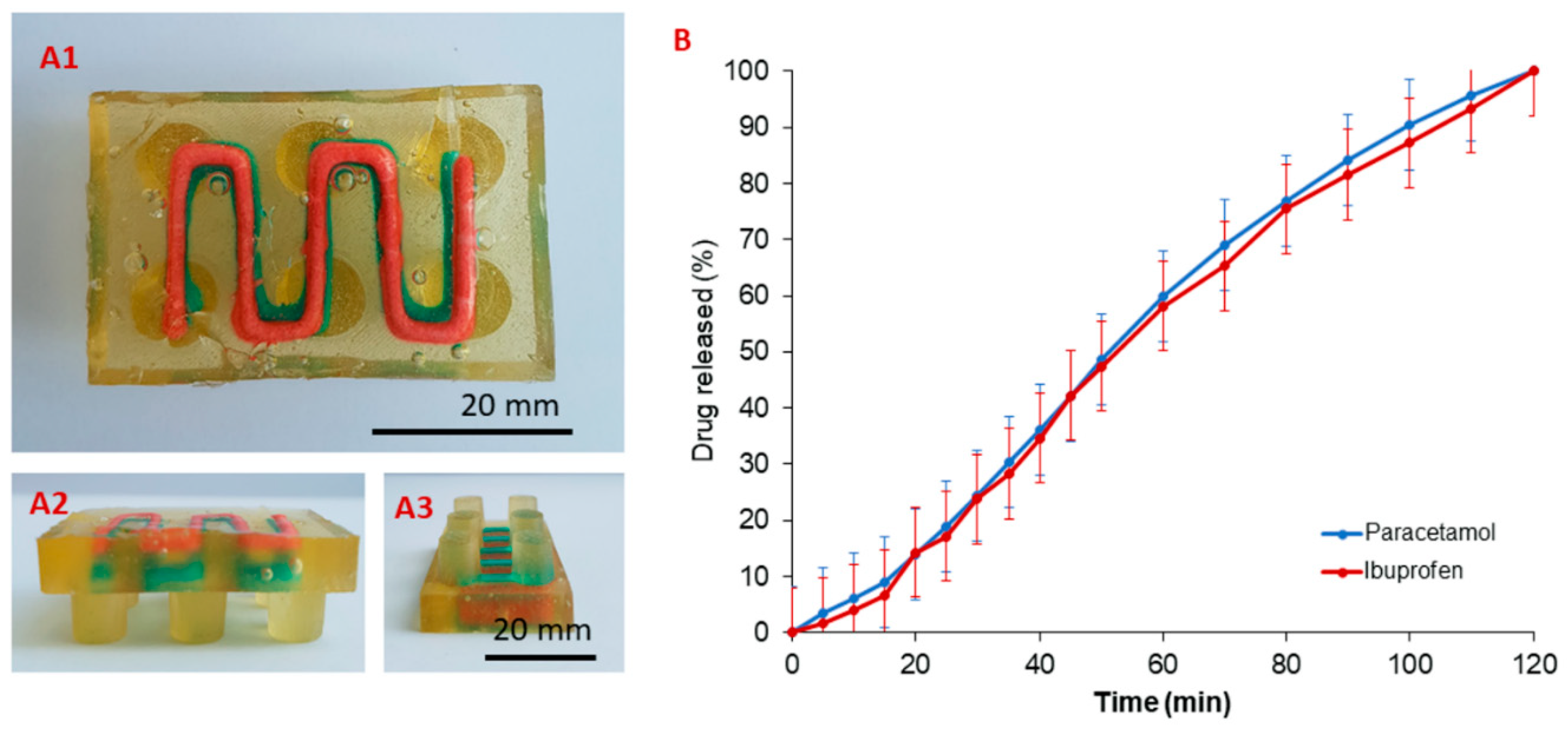
3.2. Single- and Multi-Stage e-3DP
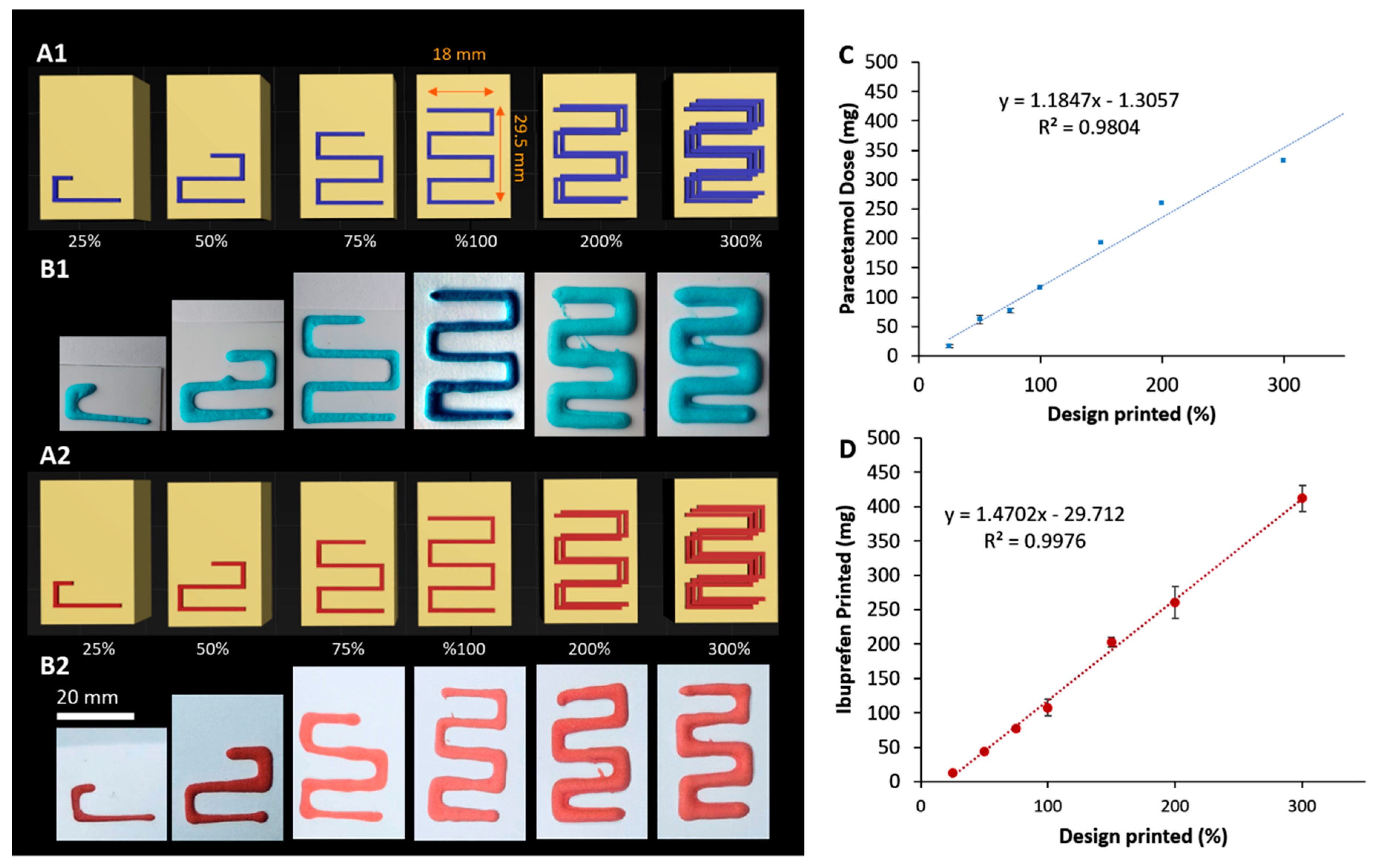
3.3. Impact of Printing Parameters on Drug Dosing Amount and Accuracy
3.4. Drug Release from 3D Printed Oral Doses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Liu, F.; Ranmal, S.; Batchelor, H.K.; Orlu-Gul, M.; Ernest, T.B.; Thomas, I.W.; Flanagan, T.; Tuleu, C. Patient-centred pharmaceutical design to improve acceptability of medicines: Similarities and differences in paediatric and geriatric populations. Drugs 2014, 74, 1871–1889. [Google Scholar] [CrossRef]
- Ranmal, S.R.; Cram, A.; Tuleu, C. Age-appropriate and acceptable paediatric dosage forms: Insights into end-user perceptions, preferences and practices from the Children’s Acceptability of Oral Formulations (CALF) Study. Int. J. Pharm. 2016, 514, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.A.; Catapano, M.; Hirschfeld, S.; Giaquinto, C.; Global Research in Paediatric. Paediatric drug development: The impact of evolving regulations. Adv. Drug Deliv. Rev. 2014, 73, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Madathilethu, J.; Roberts, M.; Peak, M.; Blair, J.; Prescott, R.; Ford, J.L. Content uniformity of quartered hydrocortisone tablets in comparison with mini-tablets for paediatric dosing. BMJ Paediatr. Open 2018, 2, e000198. [Google Scholar] [CrossRef] [PubMed]
- Saimbi, S.; Madden, V.; Stirling, H.; Yahyouche, A.; Batchelor, H. Comparison of Hydrocortisone 10 Mg Tablets: Tablet Hardness Optimised for Adult Use Has Negative Consequences for Paediatric Use. Arch. Dis. Child. 2016, 101, e2. [Google Scholar] [CrossRef] [PubMed]
- Hanawa, T.; Watanabe, A.; Tsuchiya, T.; Ikoma, R.; Hidaka, M.; Sugihara, M. New oral dosage form for elderly patients: Preparation and characterization of silk fibroin gel. Chem. Pharm. Bull. (Tokyo) 1995, 43, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Dairaku, M.; Togashi, M. Development of Air Push Jelly Formulation. Yakuzaigaku (J. Pharm. Sci. Technol. Jpn.) 2005, 65, 209–214. [Google Scholar]
- Wagner, C.L.; Shary, J.R.; Nietert, P.J.; Wahlquist, A.E.; Ebeling, M.D.; Hollis, B.W. Bioequivalence Studies of Vitamin D Gummies and Tablets in Healthy Adults: Results of a Cross-Over Study. Nutrients 2019, 11. [Google Scholar] [CrossRef]
- Adult Multivitamin Gummies Tablet, Chewable. Available online: https://www.webmd.com/drugs/2/drug-163130/adult-multivitamin-gummies-oral/details (accessed on 2 May 2019).
- Koga, C.C.; Lee, S.Y.; Lee, Y. Consumer Acceptance of Bars and Gummies with Unencapsulated and Encapsulated Resveratrol. J. Food Sci. 2016, 81, S1222–S1229. [Google Scholar] [CrossRef]
- Imai, K. Alendronate sodium hydrate (oral jelly) for the treatment of osteoporosis: Review of a novel, easy to swallow formulation. Clin. Interv. Aging 2013, 8, 681–688. [Google Scholar] [CrossRef]
- Kunisaki, C.; Tanaka, Y.; Kosaka, T.; Miyamoto, H.; Sato, S.; Suematsu, H.; Yukawa, N.; Sato, K.; Izumisawa, Y.; Akiyama, H.; et al. A Comparative Study of Intravenous Injection Form and Oral Jelly Form of Alendronate Sodium Hydrate for Bone Mineral Disorder after Gastrectomy. Digestion 2017, 95, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Isreb, A.; Baj, K.; Wojsz, M.; Isreb, M.; Peak, M.; Alhnan, M.A. 3D printed oral theophylline doses with innovative ‘radiator-like’ design: Impact of polyethylene oxide (PEO) molecular weight. Int. J. Pharm. 2019, 564, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Okwuosa, T.C.; Soares, C.; Gollwitzer, V.; Habashy, R.; Timmins, P.; Alhnan, M.A. On demand manufacturing of patient-specific liquid capsules via co-ordinated 3D printing and liquid dispensing. Eur. J. Pharm. Sci. 2018, 118, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Oblom, H.; Zhang, J.; Pimparade, M.; Speer, I.; Preis, M.; Repka, M.; Sandler, N. 3D-Printed Isoniazid Tablets for the Treatment and Prevention of Tuberculosis-Personalized Dosing and Drug Release. AAPS Pharm. Sci. Tech. 2019, 20, 52. [Google Scholar] [CrossRef] [PubMed]
- Pereira, B.C.; Isreb, A.; Forbes, R.T.; Dores, F.; Habashy, R.; Petit, J.B.; Alhnan, M.A.; Oga, E.F. ‘Temporary Plasticiser’: A novel solution to fabricate 3D printed patient-centred cardiovascular ‘Polypill’ architectures. Eur. J. Pharm. Biopharm. 2019, 135, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Sadia, M.; Isreb, A.; Abbadi, I.; Isreb, M.; Aziz, D.; Selo, A.; Timmins, P.; Alhnan, M.A. From ‘fixed dose combinations’ to ‘a dynamic dose combiner’: 3D printed bi-layer antihypertensive tablets. Eur. J. Pharm. Sci. 2018, 123, 484–494. [Google Scholar] [CrossRef] [PubMed]
- Scoutaris, N.; Ross, S.A.; Douroumis, D. 3D Printed “Starmix” Drug Loaded Dosage Forms for Paediatric Applications. Pharm. Res. 2018, 35, 34. [Google Scholar] [CrossRef]
- Preis, M.; Oblom, H. 3D-Printed Drugs for Children-Are We Ready Yet? AAPS Pharm. Sci. Tech. 2017, 18, 303–308. [Google Scholar] [CrossRef]
- Awad, A.; Trenfield, S.J.; Gaisford, S.; Basit, A.W. 3D printed medicines: A new branch of digital healthcare. Int. J. Pharm. 2018, 548, 586–596. [Google Scholar] [CrossRef]
- Alhnan, M.A.; Okwuosa, T.C.; Sadia, M.; Wan, K.W.; Ahmed, W.; Arafat, B. Emergence of 3D Printed Dosage Forms: Opportunities and Challenges. Pharm. Res. 2016, 33, 1817–1832. [Google Scholar] [CrossRef]
- Martinez, P.R.; Goyanes, A.; Basit, A.W.; Gaisford, S. Fabrication of drug-loaded hydrogels with stereolithographic 3D printing. Int. J. Pharm. 2017, 532, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Ali, S.; Bermak, A. Recent Developments in Printing Flexible and Wearable Sensing Electronics for Healthcare Applications. Sensors (Basel) 2019, 19. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Ran, M.; Luo, Q.; Shu, M.; Guo, Q.; Zhu, Y.; Xie, X.; Zhang, C.; Wan, C. Alternating Acetaminophen and Ibuprofen versus Monotherapies in Improvements of Distress and Reducing Refractory Fever in Febrile Children: A Randomized Controlled Trial. Paediatr. Drugs 2017, 19, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Paul, I.M.; Sturgis, S.A.; Yang, C.; Engle, L.; Watts, H.; Berlin, C.M., Jr. Efficacy of standard doses of Ibuprofen alone, alternating, and combined with acetaminophen for the treatment of febrile children. Clin. Ther. 2010, 32, 2433–2440. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.; Stang, A.S.; Ganshorn, H.; Hartling, L.; Maconochie, I.K.; Thomsen, A.M.; Johnson, D.W. Combined and alternating paracetamol and ibuprofen therapy for febrile children. Evid. Based Child Health 2014, 9, 675–729. [Google Scholar] [CrossRef]
- Convention, U. United States Pharmacopia 31-NF; Unider States Pharmacopial Convention: Rockville, MD, USA, 2007. [Google Scholar]
- Grosskopf, A.K.; Truby, R.L.; Kim, H.; Perazzo, A.; Lewis, J.A.; Stone, H.A. Viscoplastic Matrix Materials for Embedded 3D Printing. ACS Appl. Mater. Interfaces 2018, 10, 23353–23361. [Google Scholar] [CrossRef]
- Muth, J.T.; Vogt, D.M.; Truby, R.L.; Menguc, Y.; Kolesky, D.B.; Wood, R.J.; Lewis, J.A. Embedded 3D printing of strain sensors within highly stretchable elastomers. Adv. Mater. 2014, 26, 6307–6312. [Google Scholar] [CrossRef]
- Truby, R.L.; Wehner, M.; Grosskopf, A.K.; Vogt, D.M.; Uzel, S.G.M.; Wood, R.J.; Lewis, J.A. Soft Somatosensitive Actuators via Embedded 3D Printing. Adv. Mater. 2018, 30, e1706383. [Google Scholar] [CrossRef]
- Foox, M.; Zilberman, M. Drug delivery from gelatin-based systems. Expert. Opin. Drug Deliv. 2015, 12, 1547–1563. [Google Scholar] [CrossRef]
- Young, S.; Wong, M.; Tabata, Y.; Mikos, A.G. Gelatin as a delivery vehicle for the controlled release of bioactive molecules. J. Control. Release 2005, 109, 256–274. [Google Scholar] [CrossRef]
- Kennedy, J.R.; Kent, K.E.; Brown, J.R. Rheology of dispersions of xanthan gum, locust bean gum and mixed biopolymer gel with silicon dioxide nanoparticles. Mater. Sci. Eng. C. Mater. Biol. Appl. 2015, 48, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Alves, M.M.; Antonov, Y.A.; Goncalves, M.P. Phase equilibria and mechanical properties of gel-like water-gelatin-locust bean gum systems. Int. J. Biol. Macromol. 2000, 27, 41–47. [Google Scholar] [CrossRef]
- Kokki, H. Nonsteroidal anti-inflammatory drugs for postoperative pain: A focus on children. Paediatr. Drugs 2003, 5, 103–123. [Google Scholar] [CrossRef] [PubMed]
- Malya, R.R. Does combination treatment with ibuprofen and acetaminophen improve fever control? Ann. Emerg. Med. 2013, 61, 569–570. [Google Scholar] [CrossRef]
- Sjoukes, A.; Venekamp, R.P.; van de Pol, A.C.; Hay, A.D.; Little, P.; Schilder, A.G.; Damoiseaux, R.A. Paracetamol (acetaminophen) or non-steroidal anti-inflammatory drugs, alone or combined, for pain relief in acute otitis media in children. Cochrane Database Syst. Rev. 2016, 12, CD011534. [Google Scholar] [CrossRef]
- Wong, T.; Stang, A.S.; Ganshorn, H.; Hartling, L.; Maconochie, I.K.; Thomsen, A.M.; Johnson, D.W. Cochrane in context: Combined and alternating paracetamol and ibuprofen therapy for febrile children. Evid. Based Child Health 2014, 9, 730–732. [Google Scholar] [CrossRef]
- Baliga, S.; Muglikar, S.; Kale, R. Salivary pH: A diagnostic biomarker. J. Indian Soc. Periodontol. 2013, 17, 461–465. [Google Scholar] [CrossRef]
- Rivera-Leyva, J.C.; Garcia-Flores, M.; Valladares-Mendez, A.; Orozco-Castellanos, L.M.; Martinez-Alfaro, M. Comparative Studies on the Dissolution Profiles of Oral Ibuprofen Suspension and Commercial Tablets using Biopharmaceutical Classification System Criteria. Indian J. Pharm. Sci. 2012, 74, 312–318. [Google Scholar] [CrossRef]
- Shaw, L.R.; Irwin, W.J.; Grattan, T.J.; Conway, B.R. The effect of selected water-soluble excipients on the dissolution of paracetamol and Ibuprofen. Drug Dev. Ind. Pharm. 2005, 31, 515–525. [Google Scholar] [CrossRef]
- Parvathy, K.S.; Susheelamma, N.S.; Tharanathan, R.N.; Gaonkar, A.K. A simple non-aqueous method for carboxymethylation of galactomannans. Carbohydr. Polym. 2005, 62, 137–141. [Google Scholar] [CrossRef]
- Sierakowski, M.R.; Milas, M.; Desbrieres, J.; Rinaudo, M. Specific modifications of galactomannans. Carbohydr. Polym. 2000, 42, 51–57. [Google Scholar] [CrossRef]
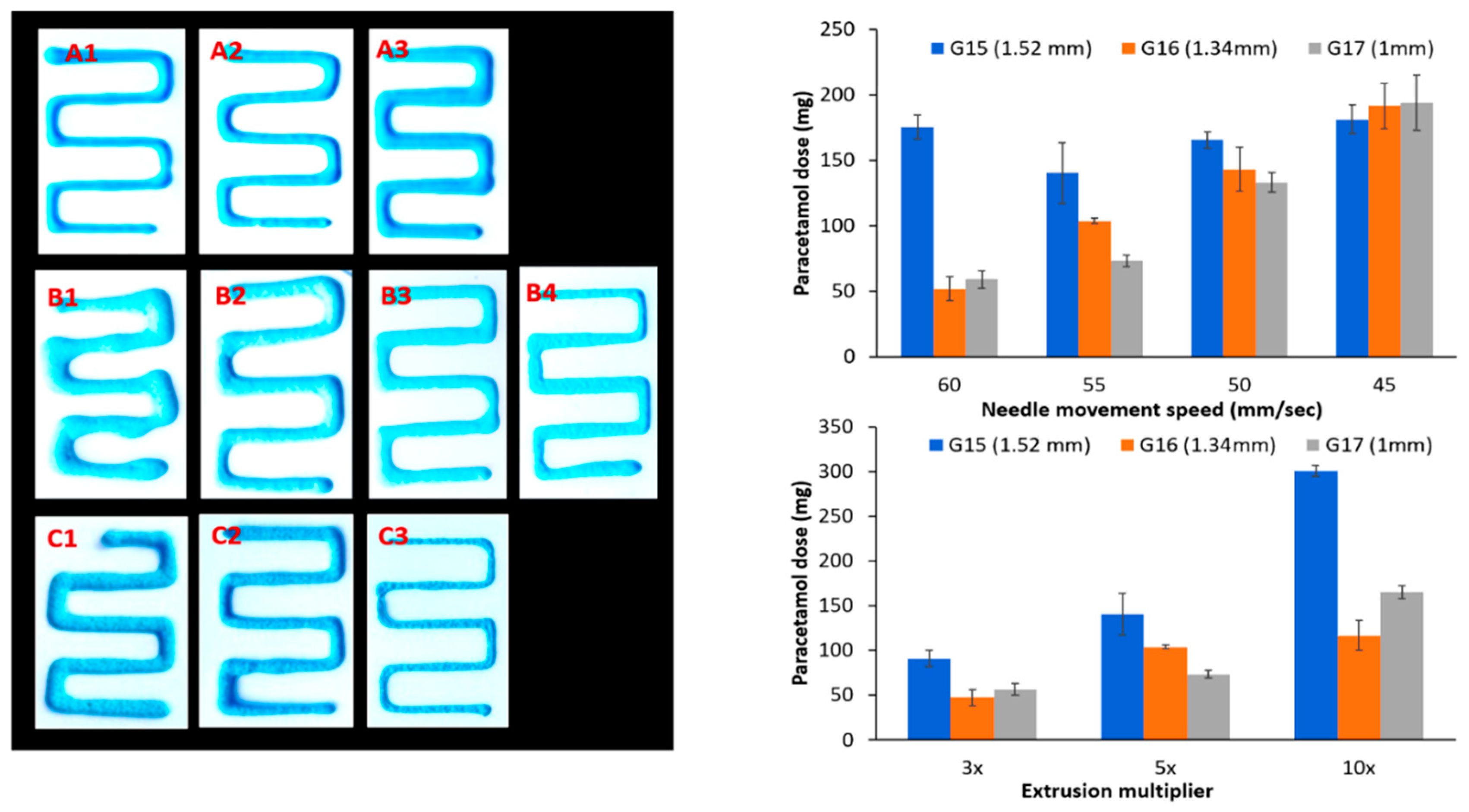
| Extrusion Multiplier * | Needle Size G15 (1.52 mm) ±SD | SD% | Needle Size G16 (1.34 mm) ±SD | SD% | Needle Size G17 (1 mm) ±SD | SD% |
|---|---|---|---|---|---|---|
| 3× | 90.8 ± 4.3 | 4.7% | 47.4 ± 4.2 | 5.1% | 56.5 ± 6.9 | 12.2% |
| 5× | 140.3 ± 23.3 | 16.6% | 103.6 ± 2.1 | 2% | 73.3 ± 4.4 | 6% |
| 10× | 300.8 ± 9.4 | 3.12% | 116.6 ± 7.2 | 6.2% | 165.2 ± 22.4 | 13.6% |
| Speed (mm/sec) ** | ||||||
| 60 | 175.5 ± 9.0 | 5.1% | 51.8 ± 9.1 | 17.6% | 59.2 ± 6.7 | 11.3% |
| 55 | 140.3 ± 23.3 | 16.6% | 103.6 ± 2.1 | 2% | 73.3 ± 4.4 | 6% |
| 50 | 165.8 ± 6.2 | 3.7% | 143.2 ± 16.9 | 11.8% | 133.0 ± 7.3 | 5.5% |
| 45 | 181.4 ± 11.0 | 6.1% | 191.5 ± 17.2 | 9% | 194.0 ± 21.3 | 10.9% |
| Printing Pattern | Paracetamol Dose (mg) ±SD | SD% | Ibuprofen Dose (mg) ± SD | SD% |
|---|---|---|---|---|
| 25% | 16.1 ± 2.7 | 16.9% | 12.1 ± 1.0 | 8.1 |
| 50% | 61.7 ± 5.1 | 8.2% | 44 ± 1.9 | 4.4 |
| 75% | 77 ± 12.2 | 15.8% | 77 ± 4.0 | 5.2 |
| 100% | 116.7 ± 7.0 | 6.0% | 107.4 ± 12.1 | 11.3 |
| 200% | 259.4 ± 16.8 | 6.5% | 260.3 ± 23.6 | 9.1 |
| 300% | 329.8 ± 6.4 | 2% | 411.9 ± 19.2 | 4. 7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rycerz, K.; Stepien, K.A.; Czapiewska, M.; Arafat, B.T.; Habashy, R.; Isreb, A.; Peak, M.; Alhnan, M.A. Embedded 3D Printing of Novel Bespoke Soft Dosage Form Concept for Pediatrics. Pharmaceutics 2019, 11, 630. https://doi.org/10.3390/pharmaceutics11120630
Rycerz K, Stepien KA, Czapiewska M, Arafat BT, Habashy R, Isreb A, Peak M, Alhnan MA. Embedded 3D Printing of Novel Bespoke Soft Dosage Form Concept for Pediatrics. Pharmaceutics. 2019; 11(12):630. https://doi.org/10.3390/pharmaceutics11120630
Chicago/Turabian StyleRycerz, Katarzyna, Krzysztof Adam Stepien, Marta Czapiewska, Basel T. Arafat, Rober Habashy, Abdullah Isreb, Matthew Peak, and Mohamed A. Alhnan. 2019. "Embedded 3D Printing of Novel Bespoke Soft Dosage Form Concept for Pediatrics" Pharmaceutics 11, no. 12: 630. https://doi.org/10.3390/pharmaceutics11120630
APA StyleRycerz, K., Stepien, K. A., Czapiewska, M., Arafat, B. T., Habashy, R., Isreb, A., Peak, M., & Alhnan, M. A. (2019). Embedded 3D Printing of Novel Bespoke Soft Dosage Form Concept for Pediatrics. Pharmaceutics, 11(12), 630. https://doi.org/10.3390/pharmaceutics11120630