Review of Advanced Hydrogel-Based Cell Encapsulation Systems for Insulin Delivery in Type 1 Diabetes Mellitus
Abstract
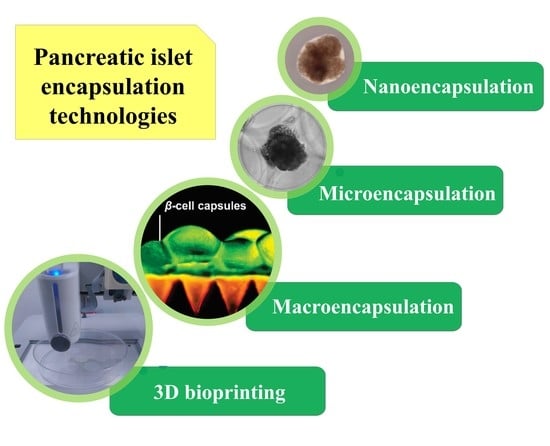
:1. Introduction
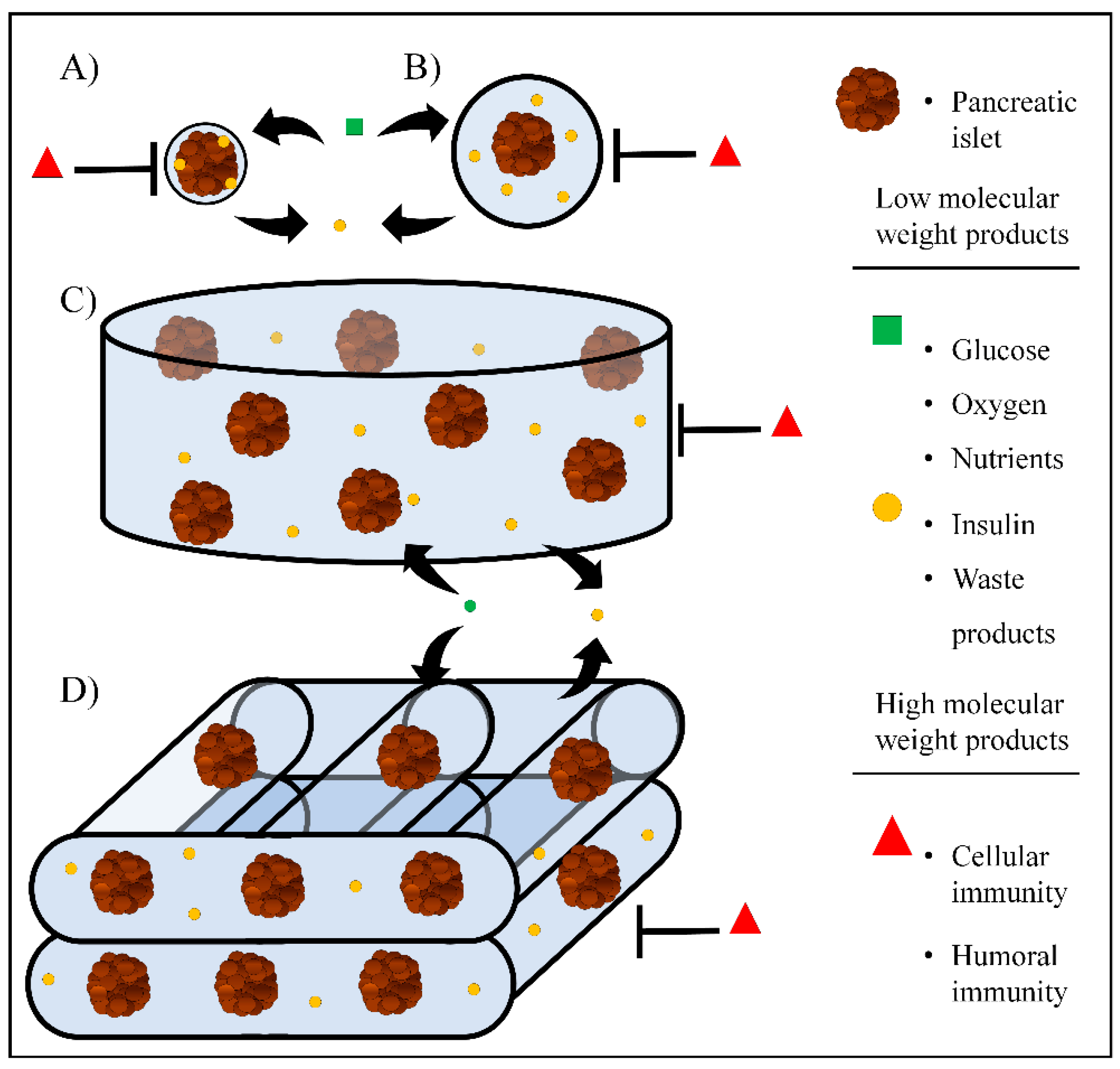
2. Nanoencapsulation
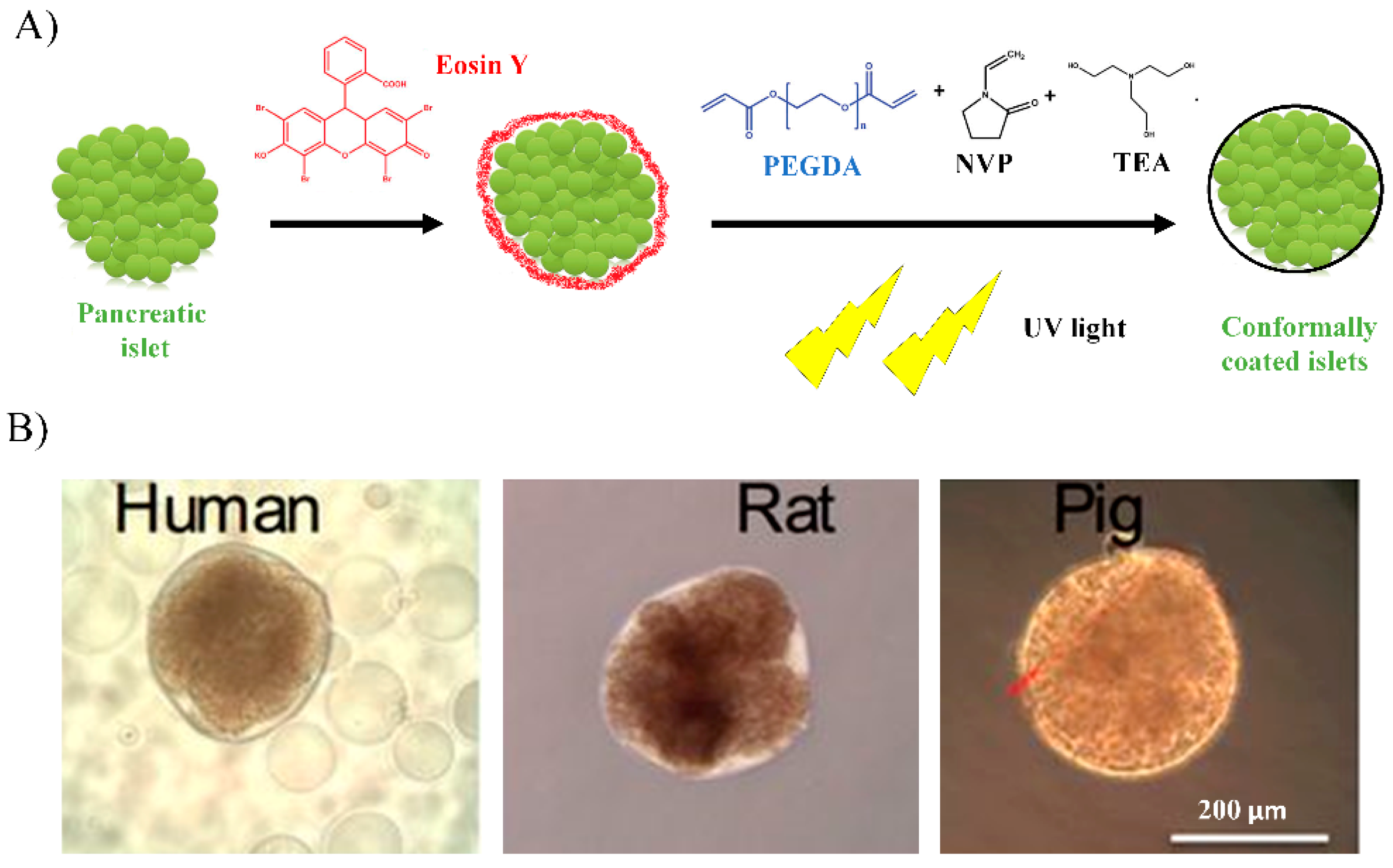
2.1. Conformal Coating
2.2. In Vivo Approaches
2.3. Clinical Trials
2.4. Advantages and Limitations of Nanoencapsulation
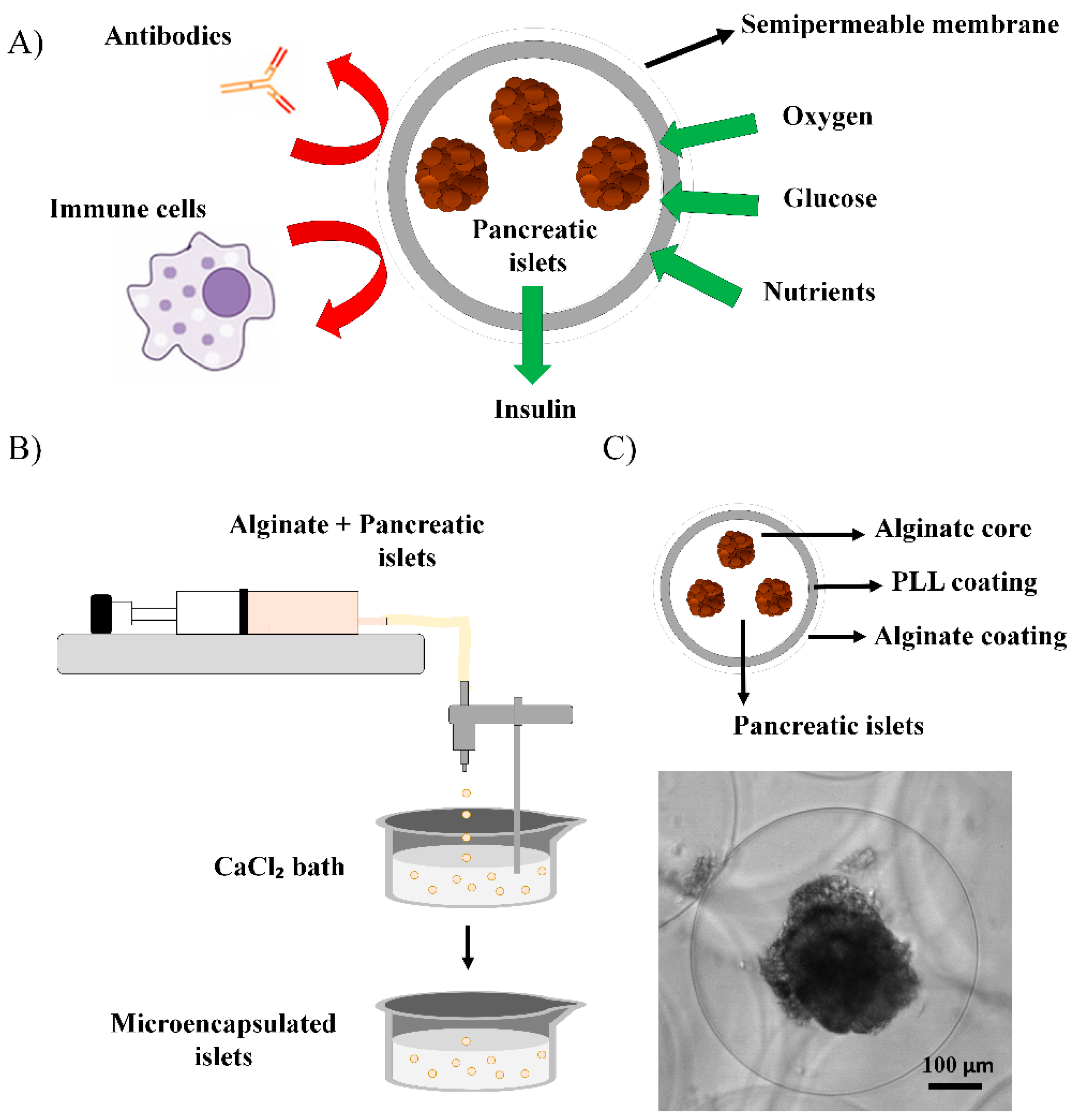
3. Microencapsulation
3.1. In Vivo Approaches
3.2. Clinical Trials
3.3. Advantages and Limitations of Microencapsulation
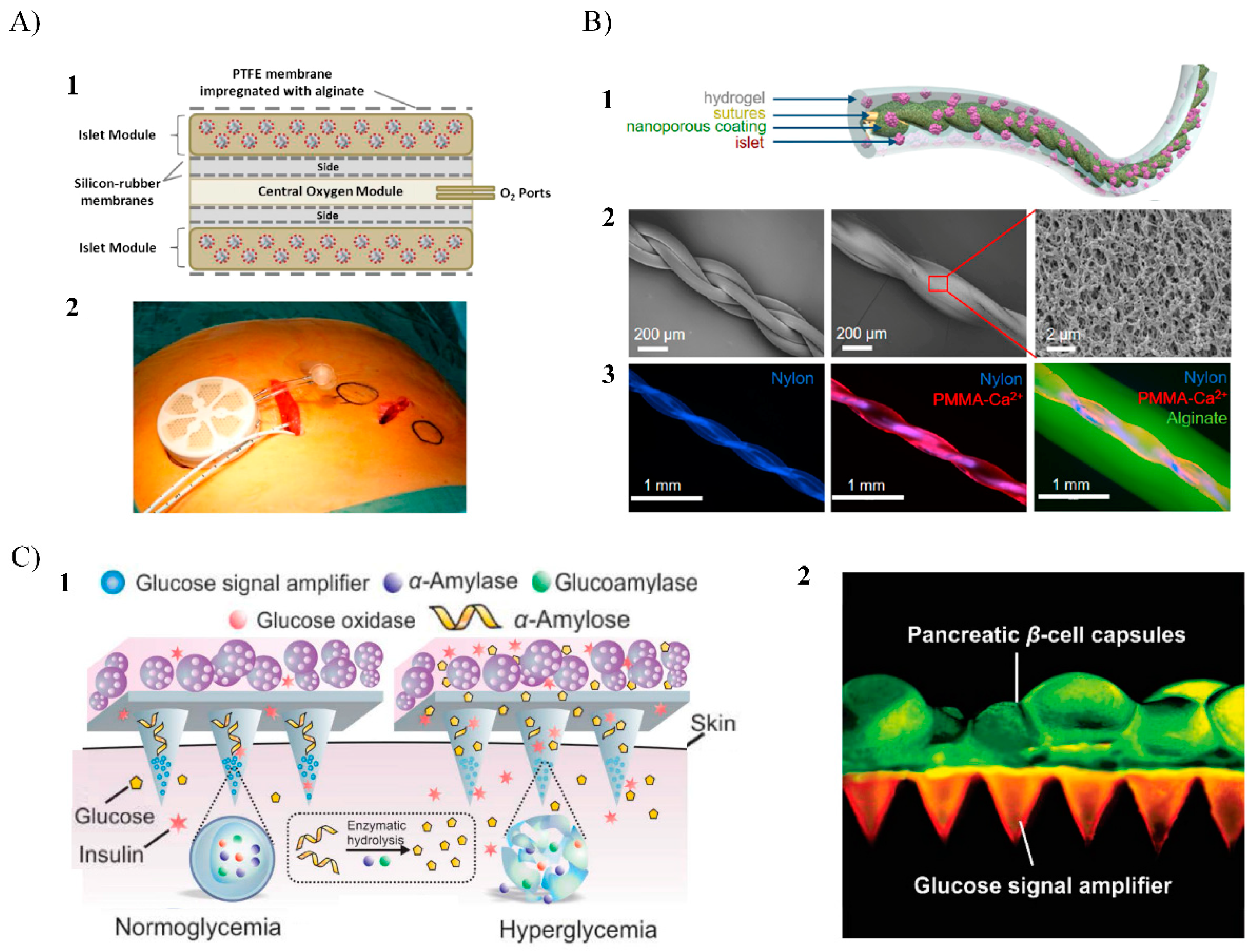
4. Macroencapsulation
4.1. In Vivo Approaches
4.2. Clinical Trials
4.3. Advantages and Limitations
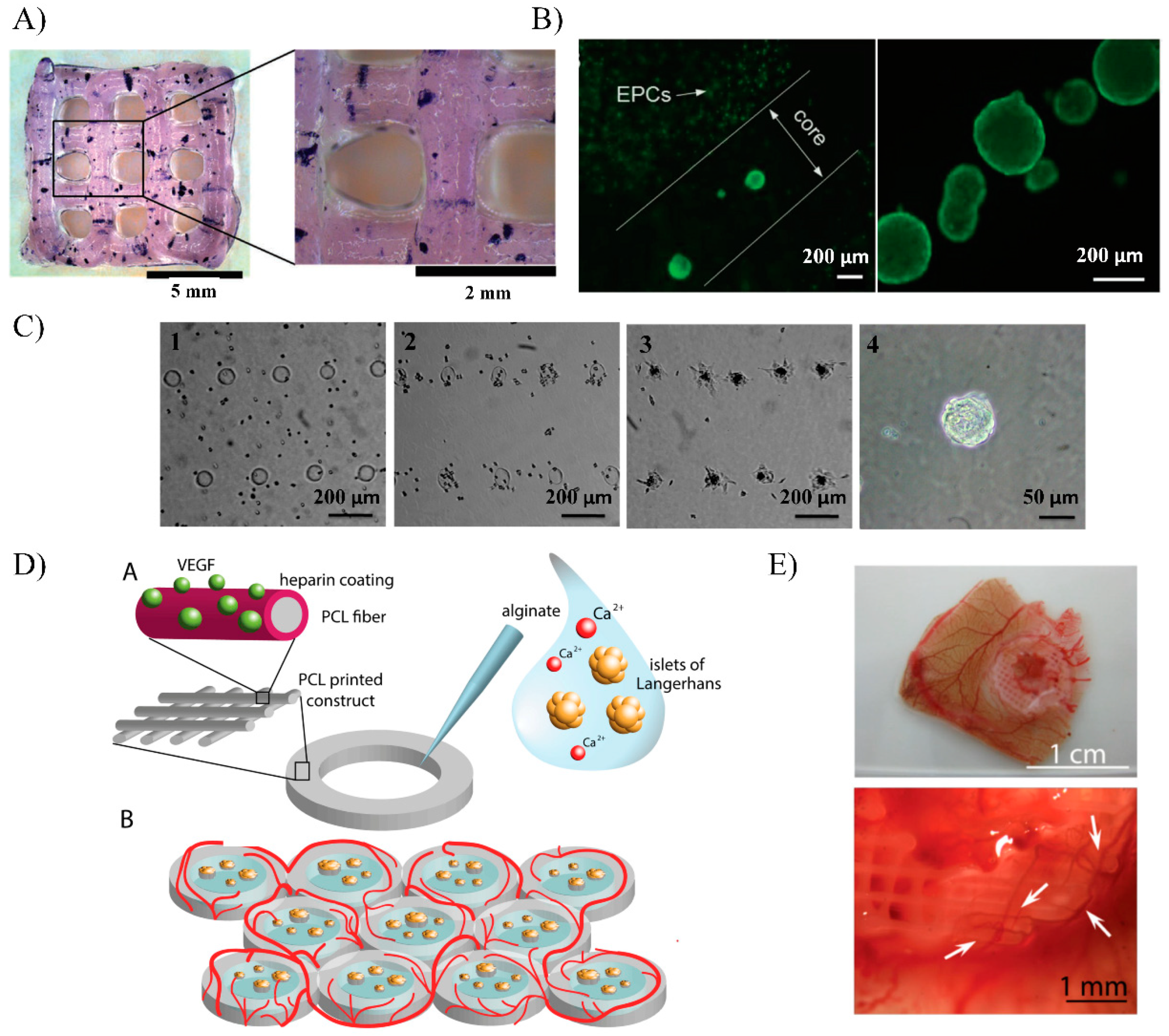
5. Bioprinting
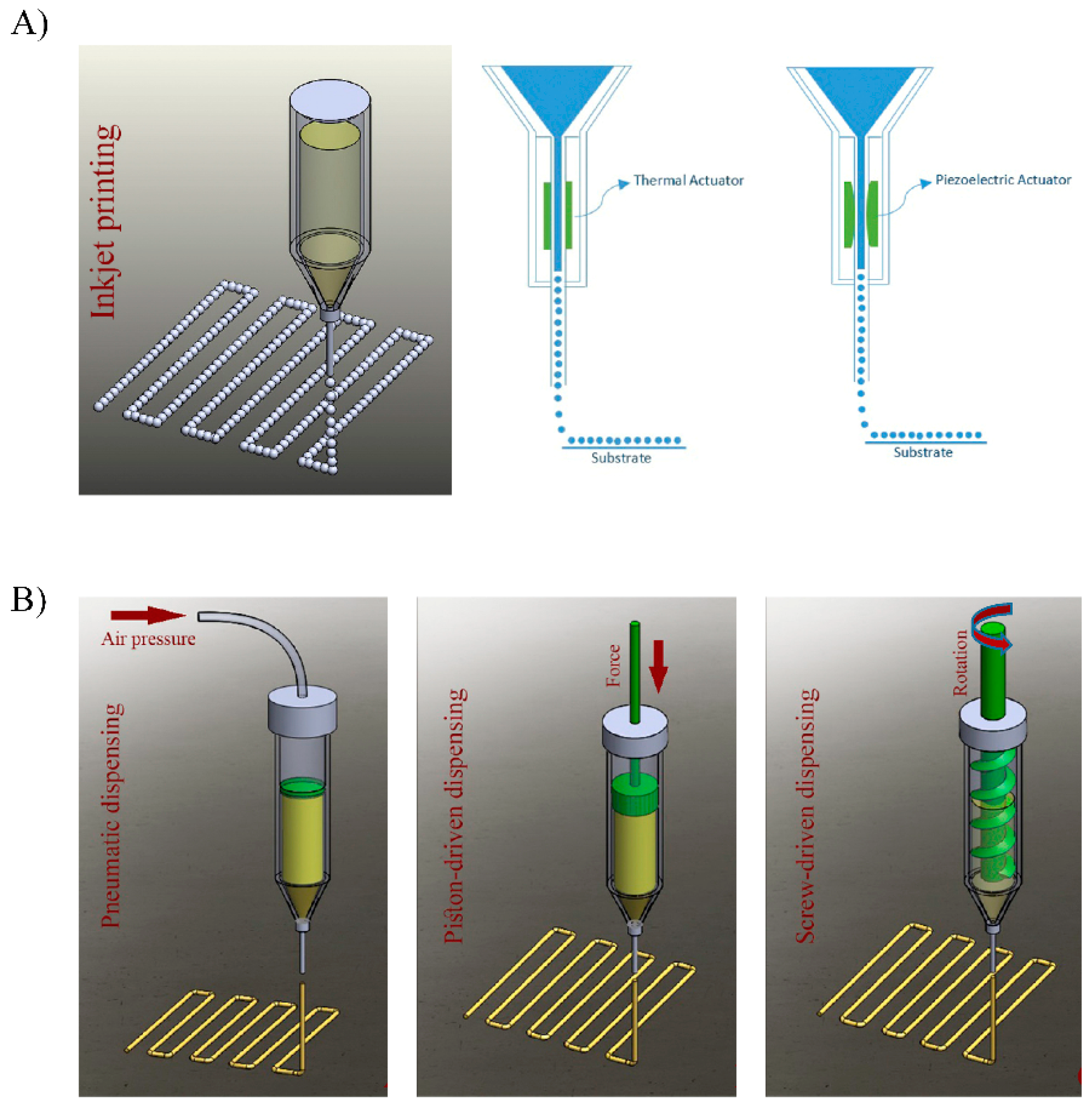
5.1. Bioprinting Technology Fundaments
5.1.1. Inkjet Bioprinting
5.1.2. Bioprinting Process Stages
5.2. Early Approaches
5.3. Advantages and Limitations
6. Conclusions and Future Perspectives
Funding
Acknowledgments
Conflicts of Interest
References
- Vieira, A.; Druelle, N.; Avolio, F.; Napolitano, T.; Navarro-Sanz, S.; Silvano, S.; Collombat, P. Beta-cell replacement strategies: The increasing need for a “beta-Cell Dogma”. Front. Genet. 2017, 8, 75. [Google Scholar] [CrossRef] [PubMed]
- Wen-Peng, Y.; Maciej, H. Type 1 diabetes prevalence increasing globally and regionally: The role of natural selection and life expectancy at birth. BMJ Open Diabetes Res. Care 2016, 4. [Google Scholar] [CrossRef]
- Montanya, E. Islet-and stem-cell-based tissue engineering in diabetes. Curr. Opin. Biotechnol. 2004, 15, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Amer, L.D.; Mahoney, M.J.; Bryant, S.J. Tissue engineering approaches to cell-based type 1 diabetes therapy. Tissue Eng. Part B Rev. 2014, 20, 455–467. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhao, R.; Liu, J.; Tian, M.; Lu, Y.; He, T.; Cheng, M.; Liang, K.; Li, X.; Wang, X.; et al. Small islets transplantation superiority to large ones: Implications from islet microcirculation and revascularization. J. Diabetes Res. 2014, 2014, 192093. [Google Scholar] [CrossRef] [PubMed]
- Skrzypek, K.; Groot Nibbelink, M.; van Lente, J.; Buitinga, M.; Engelse, M.A.; de Koning, E.J.P.; Karperien, M.; van Apeldoorn, A.; Stamatialis, D. Pancreatic islet macroencapsulation using microwell porous membranes. Sci. Rep. 2017, 9186. [Google Scholar] [CrossRef] [PubMed]
- Cooke, D.W.; Plotnick, L. Type 1 diabetes mellitus in pediatrics. Pediatr. Rev. 2008, 29, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Harlan, D.M.; Kenyon, N.S.; Korsgren, O.; Roep, B.O.; Immunology of Diabetes Society. Current advances and travails in islet transplantation. Diabetes 2009, 58, 2175–2184. [Google Scholar] [CrossRef] [PubMed]
- Gruessner, A.C.; Sutherland, D.E. Analysis of United States (US) and non-US pancreas transplants as reported to the International Pancreas Transplant Registry (IPTR) and to the United Network for Organ Sharing (UNOS). Clin. Transpl. 1998, 53–73, 12211799. [Google Scholar]
- Sutherland, D.E.; Gruessner, R.W.; Dunn, D.L.; Matas, A.J.; Humar, A.; Kandaswamy, R.; Mauer, S.M.; Kennedy, W.R.; Goetz, F.C.; Robertson, R.P.; et al. Lessons learned from more than 1000 pancreas transplants at a single institution. Ann. Surg. 2001, 233, 463–501. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, D.E.R.; Gores, P.F.; Farney, A.C.; Wahoff, D.C.; Matas, A.J.; Dunn, D.L.; Gruessner, R.W.G.; Najarian, J.S. Evolution of kidney, pancreas, and islet transplantation for patients with diabetes at the University of Minnesota. Am. J. Surg. 1993, 166, 456–491. [Google Scholar] [CrossRef]
- Beck, J.; Angus, R.; Madsen, B.; Britt, D.; Vernon, B.; Nguyen, K.T. Islet encapsulation: Strategies to enhance islet cell functions. Tissue Eng. 2007, 13, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Figliuzzi, M.; Bonandrini, B.; Silvani, S.; Remuzzi, A. Mesenchymal stem cells help pancreatic islet transplantation to control type 1 diabetes. World J. Stem Cells 2014, 6, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, A.M.; Lakey, J.R.; Ryan, E.A.; Korbutt, G.S.; Toth, E.; Warnock, G.L.; Kneteman, N.M.; Rajotte, R.V. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N. Engl. J. Med. 2000, 343, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Bottino, R.; Knoll, M.F.; Knoll, C.A.; Bertera, S.; Trucco, M.M. The future of islet transplantation is now. Front. Med. 2018, 5, 202. [Google Scholar] [CrossRef] [PubMed]
- Desai, T.; Shea, L.D. Advances in islet encapsulation technologies. Nat. Rev. Drug Discov. 2017, 16, 338–350. [Google Scholar] [CrossRef] [PubMed]
- Sakata, N.; Tan, A.; Chan, N.; Obenaus, A.; Mace, J.; Peverini, R.; Sowers, L.; Chinnock, R.; Hathout, E. Efficacy comparison between intraportal and subcapsular islet transplants in a murine diabetic model. Trans. Proc. 2009, 41, 346–349. [Google Scholar] [CrossRef] [PubMed]
- Schuetz, C.; Anazawa, T.; Cross, S.E.; Labriola, L.; Meier, R.P.H.; Redfield, R.R.; Scholz, H.; Stock, P.G.; Zammit, N.W.; IPITA YIC Young Investigator Committee. Beta cell replacement therapy: The next 10 years. Transplantation 2018, 102, 215–229. [Google Scholar] [CrossRef] [PubMed]
- Korsgren, O. Islet encapsulation: Physiological possibilities and limitations. Diabetes 2017, 66, 1748–1754. [Google Scholar] [CrossRef] [PubMed]
- Tomei, A.A.; Villa, C.; Ricordi, C. Development of an encapsulated stem cell-based therapy for diabetes. Expert Opin. Biol. Ther. 2015, 15, 1321–1336. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Knobeloch, T.; Abadi, S.E.M.; Bruns, J.; Zustiak, S.P.; Kwon, G. Injectable polyethylene glycol hydrogel for islet encapsulation: An in vitro and in vivo characterization. Biomed. Phys. Eng. Express 2017. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, R.; Alexander, M.; Robles, L.; Foster, C.E.; Lakey, J.R. Islet and stem cell encapsulation for clinical transplantation. Rev. Diabet. Stud. 2014, 11, 84–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakata, N.; Sumi, S.; Yoshimatsu, G.; Goto, M.; Egawa, S.; Unno, M. Encapsulated islets transplantation: Past, present and future. World J. Gastrointest. Pathophysiol. 2012, 15, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Ravnic, D.J.; Leberfinger, A.N.; Ozbolat, I.T. Bioprinting and cellular therapies for type 1 diabetes. Trends Biotechnol. 2017, 35, 1025–1034. [Google Scholar] [CrossRef] [PubMed]
- Zhi, Z.L.; Khan, F.; Pickup, J.C. Multilayer nanoencapsulation: A nanomedicine technology for diabetes research and management. Diabetes Res. Clin. Pract. 2013, 100, 162–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kozlovskaya, V.; Zavgorodnya, O.; Chen, Y.; Ellis, K.; Tse, H.M.; Cui, W.; Thompson, J.A.; Kharlampieva, E. Ultrathin polymeric coatings based on hydrogen-bonded polyphenol for protection of pancreatic islet cells. Adv. Funct. Mater. 2012, 22, 3389–3398. [Google Scholar] [CrossRef] [PubMed]
- Krol, S.; del Guerra, S.; Grupillo, M.; Diaspro, A.; Gliozzi, A.; Marchetti, P. Multilayer nanoencapsulation. New approach for immune protection of human pancreatic islets. Nano Lett. 2006, 6, 1933–1939. [Google Scholar] [CrossRef] [PubMed]
- Shih, H.; Mirmira, R.G.; Lin, C.C. Visible light-initiated interfacial thiol-norbornene photopolymerization for forming islet surface conformal coating. J. Mater. Chem. B 2015, 2, 170–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scharp, D.W.; Marchetti, P. Encapsulated islets for diabetes therapy: History, current progress, and critical issues requiring solution. Adv. Drug Deliv. Rev. 2014, 67–68, 35–73. [Google Scholar] [CrossRef] [PubMed]
- Teramura, Y.; Iwata, H. Bioartificial pancreas microencapsulation and conformal coating of islet of Langerhans. Adv. Drug Deliv. Rev. 2010, 62, 827–840. [Google Scholar] [CrossRef] [PubMed]
- Tomei, A.A.; Manzoli, V.; Fraker, C.A.; Giraldo, J.; Velluto, D.; Najjar, M.; Pileggi, A.; Molano, R.D.; Ricordi, C.; Stabler, C.L.; et al. Device design and materials optimization of conformal coating for islets of Langerhans. Proc. Natl. Acad. Sci. USA 2014, 111, 10514–10519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hill, R.S.; Cruise, G.M.; Hager, S.R.; Lamberti, F.V.; Yu, X.; Garufis, C.L.; Yu, Y.; Mundwiler, K.E.; Cole, J.F.; Hubbell, J.A.; et al. Immunoisolation of adult porcine islets for the treatment of diabetes mellitus. The use of photopolymerizable polyethylene glycol in the conformal coating of mass-isolated porcine islets. Ann. N. Y. Acad. Sci. 1997, 831, 332–343. [Google Scholar] [CrossRef] [PubMed]
- Scharp, D.W. Encapsulated human islet allografts: Providing safety with efficacy. In Anonymous Cellular Transplantation; Elsevier Inc.: Amsterdam, The Netherlands, 2007; p. 135. [Google Scholar]
- Phelps, E.A.; Enemchukwu, N.O.; Fiore, V.F.; Sy, J.C.; Murthy, N.; Sulchek, T.A.; Barker, T.H.; Garcia, A.J. Maleimide cross-linked bioactive PEG hydrogel exhibits improved reaction kinetics and cross-linking for cell encapsulation and in situ delivery. Adv. Mater. 2012, 24, 64–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, J.T.; Cui, W.; Chaikof, E.L. Layer-by-layer assembly of a conformal nanothin PEG coating for intraportal islet transplantation. Nano Lett. 2008, 8, 1940–1948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meirigeng, Q. Transplantation of encapsulated pancreatic islets as a treatment for patients with type 1 diabetes mellitus. Adv. Med. 2014, 429710. [Google Scholar]
- Soon-Shiong, P.; Heintz, R.E.; Merideth, N.; Yao, Q.X.; Yao, Z.; Zheng, T.; Murphy, M.; Moloney, M.K.; Schmehl, M.; Harris, M. Insulin independence in a type 1 diabetic patient after encapsulated islet transplantation. Lancet 1994, 343, 950–951. [Google Scholar] [CrossRef]
- Calafiore, R.; Basta, G.; Luca, G.; Lemmi, A.; Montanucci, M.P.; Calabrese, G.; Racanicchi, L.; Mancuso, F.; Brunetti, P. Microencapsulated pancreatic islet allografts into nonimmunosuppressed patients with type 1 diabetes: First two cases. Diabetes Care 2006, 29, 137–138. [Google Scholar] [CrossRef] [PubMed]
- Touch, B.E.; Keogh, G.W.; Williams, L.J.; Wu, W.; Foster, J.L.; Vaithilingam, V.; Philips, R. Safety and viability of microencapsulated human islets transplanted into diabetic humans. Diabetes Care 2009, 32, 1887–1889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, P.L. Company profile: Tissue regeneration for diabetes and neurological diseases at living cell technologies. Regen. Med. 2010, 5, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Dufrane, D.; Mourad, M.; Goffin, E.; Aouassar, N.; Gianello, P.; Vandeleene, B. A simple and safe clinical procedure for human encapsulated islet transplantation in the subcutaneous tissue for diabetes treatment. Transplantation 2013, 96, 310. [Google Scholar]
- Ludwig, B.; Reichel, A.; Steffen, A.; Zimerman, B.; Schally, A.V.; Block, N.L.; Colton, C.K.; Ludwig, S.; Kersting, S.; Bonifacio, E.; et al. Transplantation of human islets without immunosuppression. Proc. Natl. Acad. Sci. USA 2013, 110, 19054–19058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dufrane, D.; Gianello, P. Macro-or microencapsulation of pig islets to cure type 1 diabetes. World J. Gastroenterol. 2012, 18, 6885–6893. [Google Scholar] [CrossRef] [PubMed]
- Allogeneic Islet Cells Transplanted Onto the Omentum. Available online: https://clinicaltrials.gov/ct2/show/NCT02213003 (accessed on 16 July 2019).
- Calafiore, R.; Basta, G.; Luca, G.; Boselli, C.; Bufalari, A.; Giustozzi, G.M.; Moggi, L.; Brunetti, P. Alginate/polyaminoacidic coherent microcapsules for pancreatic islet graft immunoisolation in diabetic recipients. Ann. N. Y. Acad. Sci. 1997, 831, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Leblond, F.A.; Simard, G.; Henley, N.; Rocheleau, B.; Huet, P.; Hallé, J. Studies on smaller (~315 μM) microcapsules: IV. feasibility and safety of intrahepatic implantations of small alginate poly-L-Lysine microcapsules. Cell Transpl. 1999, 8, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Kizilel, S.; Scavone, A.; Liu, X.; Nothias, J.M.; Ostrega, D.; Witkowski, P.; Millis, M. Encapsulation of pancreatic islets within nano-thin functional polyethylene glycol coatings for enhanced insulin secretion. Tissue Eng. Part A 2010, 16, 2217–2228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zorzi, D.; Phan, T.; Sequi, M.; Lin, Y.; Freeman, D.H.; Cicalese, L.; Rastellini, C. Impact of islet size on pancreatic islet transplantation and potential interventions to improve outcome. Cell Transplant. 2015, 24, 11–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.K.; Yoon, K.H. Current status of encapsulated islet transplantation. J. Diabetes Complicat. 2015, 29, 737–743. [Google Scholar] [CrossRef] [PubMed]
- Hwang, P.T.; Shah, D.K.; Garcia, J.A.; Bae, C.Y.; Lim, D.J.; Huiszoon, R.C.; Alexander, G.C.; Jun, H.W. Progress and challenges of the bioartificial pancreas. Nano Converg. 2016, 28, 2–4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, H.; Lu, L.; Liu, X.; Yu, L.; Lyu, Y.; Wang, B. Treatment of diabetes with encapsulated pig islets: An update on current developments. J. Zhejiang Univ. Sci. B 2015, 16, 329–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Sullivan, E.S.; Vegas, A.; Anderson, D.G.; Weir, G.C. Islets transplanted in immunoisolation devices: A review of the progress and the challenges that remain. Endocr. Rev. 2011, 32, 827–844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaithilingam, V.; Bal, S.; Tuch, B.E. Encapsulated islet transplantation: Where do we stand? Rev. Diabet. Stud. 2017, 14, 51–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Luna, V.H.; González-Reynoso, O. Encapsulation of biological agents in hydrogels for therapeutic applications. Gels 2018, 4, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, G.J.; Lee, S.J.; Atala, A. Cell-based drug delivery. In Anonymous Principles of Regenerative Medicine; Academic Press: Cambridge, MA, USA, 2008; p. 954. [Google Scholar]
- Vaithilingam, V.; Tuch, B.E. Islet transplantation and encapsulation: An update on recent developments. Rev. Diabetic Stud. RDS 2011, 8, 51–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zekorn, T.D.; Horcher, A.; Mellert, J.; Siebers, U.; Altug, T.; Emre, A.; Hahn, H.J.; Federlin, K. Biocompatibility and immunology in the encapsulation of islets of langerhans (bioartificial pancreas). Int. J. Artif. Organs 1996, 19, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Gasperini, L.; Mano, J.F.; Reis, R.L. Natural polymers for the microencapsulation of cells. J. R. Soc. Interface 2014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Vos, P.; Faas, M.M.; Strand, B.; Calafiore, R. Alginate-based microcapsules for immunoisolation of pancreatic islets. Biomaterials 2006, 27, 5603–5617. [Google Scholar] [CrossRef] [PubMed]
- Ertesvåg, H.; Valla, S. Biosynthesis and applications of alginates. Polym. Degrad. Stab. 1998, 59, 85–91. [Google Scholar] [CrossRef]
- Omami, M.; McGarrigle, J.J.; Reedy, M.; Isa, D.; Ghani, S.; Marchese, E.; Bochenek, M.A.; Longi, M.; Xing, Y.; Joshi, I.; et al. Islet microencapsulation: Strategies and clinical status in diabetes. Curr. Diab. Rep. 2017, 17, 47. [Google Scholar] [CrossRef] [PubMed]
- Lim, F.; Sun, A.M. Microencapsulated islets as bioartificial endocrine pancreas. Science 1980, 210, 908–910. [Google Scholar] [CrossRef] [PubMed]
- Espona-Noguera, A.; Etxebarria-Elezgarai, J.; Saenz Del Burgo, L.; Canibano-Hernandez, A.; Gurruchaga, H.; Blanco, F.J.; Orive, G.; Hernandez, R.M.; Benito-Lopez, F.; Ciriza, J.; et al. Type 1 diabetes mellitus reversal via implantation of magnetically purified microencapsulated pseudoislets. Int. J. Pharm. 2019, 560, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Strand, B.L.; Ryan, L.; Veld, P.I.; Kulseng, B.; Rokstad, A.M.; Skjåk-Bræk, G.; Espevik, T. Poly-L-Lysine induces fibrosis on alginate microcapsules via the induction of cytokines. Cell Transplant. 2001, 10, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Farina, M.; Alexander, J.F.; Thekkedath, U.; Ferrari, M.; Grattoni, A. Cell encapsulation: Overcoming barriers in cell transplantation in diabetes and beyond. Adv. Drug Deliv. Rev. 2019, 139, 92–115. [Google Scholar] [CrossRef] [PubMed]
- Kleinberger, R.M.; Burke, N.A.D.; Dalnoki-Veress, K.; Stöver, H.D.H. Systematic study of alginate-based microcapsules by micropipette aspiration and confocal fluorescence microscopy. Mater. Sci. Eng. C. 2013, 33, 4295–4304. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Ma, X.; Zhou, D.; Vacek, I.; Sun, A.M. Normalization of diabetes in spontaneously diabetic cynomolgus monkeys by xenografts of microencapsulated porcine islets without immunosuppression. J. Clin. Investig. 1996, 96, 1417–1422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duvivier-Kali, V.F.; Omer, A.; Parent, R.J.; O’Neil, J.J.; Weir, G.C. Complete protection of islets against allorejection and autoimmunity by a simple barium-alginate membrane. Diabetes 2001, 50, 1698–1705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elliott, R.B.; Escobar, L.; Tan, P.L.; Garkavenko, O.; Calafiore, R.; Basta, P.; Vasconcellos, A.V.; Emerich, D.F.; Thanos, C.; Bambra, C. Intraperitoneal alginate-encapsulated neonatal porcine islets in a placebo-controlled study with 16 diabetic cynomolgus primates. Transplant. Proc. 2005, 37, 3505–3508. [Google Scholar] [CrossRef] [PubMed]
- Morch, Y.A.; Donati, I.; Strand, B.L.; Skjak-Braek, G. Effect of Ca2+, Ba2+, and Sr2+ on alginate microbeads. Biomacromolecules 2006, 7, 1471–1480. [Google Scholar] [CrossRef] [PubMed]
- Omer, A.; Duvivier-Kali, V.F.; Trivedi, N.; Wilmot, K.; Bonner-Weir, S.; Weir, G.C. Survival and maturation of microencapsulated porcine neonatal pancreatic cell clusters transplanted into immunocompetent diabetic mice. Diabetes 2003, 52, 69–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montanucci, P.; Terenzi, S.; Santi, C.; Pennoni, I.; Bini, V.; Pescara, T.; Basta, G.; Calafiore, R. Insights in behavior of variably formulated alginate-based microcapsules for cell transplantation. Biomed. Res. Int. 2015, 2015, 965804–965811. [Google Scholar] [CrossRef] [PubMed]
- Orive, G.; Ponce, S.; Hernández, R.M.; Gascón, A.R.; Igartua, M.; Pedraz, J.L. Biocompatibility of microcapsules for cell immobilization elaborated with different type of alginates. Biomaterials 2002, 23, 3825–3831. [Google Scholar] [CrossRef]
- Lovett, M.; Lee, K.; Edwards, A.; Kaplan, D.L. Vascularization strategies for tissue engineering. Tissue Eng. Part B Rev. 2009, 15, 353–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Opara, E.C.; Kendall, W.F. Immunoisolation techniques for islet cell transplantation. Expert Opin. Biol. Ther. 2002, 2, 503–511. [Google Scholar] [PubMed]
- Buder, B.; Alexander, M.; Krishnan, R.; Chapman, D.W.; Lakey, J.R. Encapsulated islet transplantation: Strategies and clinical trials. Immune Netw. 2013, 13, 235–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schweicher, J.; Nyitray, C.; Desai, T.A. Membranes to achieve immunoprotection of transplanted islets. Front. Biosci. 2014, 19, 49–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacobs-Tulleneers-Thevissen, D.; Chintinne, M.; Ling, Z.; Gillard, P.; Schoonjans, L.; Delvaux, G.; Strand, B.; Gorus, F.; Keymeulen, B.; Pipeleers, D. Sustained function of alginate-encapsulated human islet cell implants in the peritoneal cavity of mice leading to a pilot study in a type 1 diabetic patient. Diabetologia 2013, 56, 1605–1614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weir, G.C.; Bonner-Weir, S. Scientific and political impediments to successful islet transplantation. Diabetes 1997, 46, 1247–1256. [Google Scholar] [CrossRef] [PubMed]
- Barkai, U.; Rotem, A.; de Vos, P. Survival of encapsulated islets: More than a membrane story. World J. Transplant. 2016, 6, 69–90. [Google Scholar] [CrossRef] [PubMed]
- Dimitrioglou, N.; Kanelli, M.; Papageorgiou, E.; Karatzas, T.; Hatziavramidis, D. Paving the way for successful islet encapsulation. Drug Discov. Today 2019, 24, 737–748. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.I.; de Matos, A.N.; Brons, I.G.; Mateus, M. An overview on the development of a bio-artificial pancreas as a treatment of insulin-dependent diabetes mellitus. Med. Res. Rev. 2006, 26, 181–222. [Google Scholar] [CrossRef] [PubMed]
- Storrs, R.; Dorian, R.; King, S.R.; Lakey, J.; Rilo, H. Preclinical development of the islet sheet. Ann. N. Y. Acad. Sci. 2001, 944, 252–266. [Google Scholar] [CrossRef] [PubMed]
- Dufrane, D.; Goebbels, R.; Gianello, P. Alginate macroencapsulation of pig islets allows Correction of streptozotocin-induced diabetes in primates up to 6 months without immunosuppression. Transplantation 2010, 90, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, B.; Rotem, A.; Schmid, J.; Weir, G.C.; Colton, C.K.; Brendel, M.D.; Neufeld, T.; Block, N.L.; Yavriyants, K.; Steffen, A.; et al. Improvement of islet function in a bioartificial pancreas by enhanced oxygen supply and growth hormone releasing hormone agonist. Proc. Natl. Acad. Sci. USA 2012, 109, 5022–5027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neufeld, T.; Ludwig, B.; Barkai, U.; Weir, G.C.; Colton, C.K.; Evron, Y.; Balyura, M.; Yavriyants, K.; Zimermann, B.; Azarov, D.; et al. The efficacy of an immunoisolating membrane system for islet xenotransplantation in minipigs. PLoS ONE 2013, e70150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chin, K.; Khattak, S.F.; Bhatia, S.R.; Roberts, S.C. Hydrogel-perfluorocarbon composite scaffold promotes oxygen transport to immobilized cells. Biotechnol. Prog. 2008, 24, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Gholipourmalekabadi, M.Z.; Harrison, S.; Mozafari, B.S.; Seifalian, M.; Alexander, M. Oxygen-generating biomaterials: A new, viable paradigm for tissue engineering? Trends Biotechnol. 2016, 34, 1010–1021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berman, D.M.; Molano, R.D.; Fotino, C.; Ulissi, U.; Gimeno, J.; Mendez, A.J.; Kenyon, N.M.; Kenyon, N.S.; Andrews, D.M.; Ricordi, C.; et al. Bioengineering the endocrine pancreas: Intraomental islet transplantation within a biologic resorbable scaffold. Diabetes 2016, 65, 1350–1361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- An, D.; Chiu, A.; Flanders, J.A.; Song, W.; Shou, D.; Lu, Y.C.; Grunnet, L.G.; Winkel, L.; Ingvorsen, C.; Christophersen, N.S.; et al. Designing a retrievable and scalable cell encapsulation device for potential treatment of type 1 diabetes. Proc. Natl. Acad. Sci. USA 2018, 115, E263–E272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, Y.; Yu, J.; Wang, C.; Nguyen, N.Y.; Walker, G.M.; Buse, J.B.; Gu, Z. Microneedles integrated with pancreatic cells and synthetic glucose-signal amplifiers for smart insulin delivery. Adv. Mater. 2016, 28, 3115–3121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlsson, P.O.; Espes, D.; Sedigh, A.; Rotem, A.; Zimerman, B.; Grinberg, H.; Goldman, T.; Barkai, U.; Avni, Y.; Westermark, G.T.; et al. Transplantation of macroencapsulated human islets within the bioartificial pancreas betaAir to patients with type 1 diabetes mellitus. Am. J. Transplant. 2018, 18, 1735–1744. [Google Scholar] [CrossRef] [PubMed]
- Kepsutlu, B.; Nazli, C.; Bal, T.; Kizilel, S. Design of bioartificial pancreas with functional micro/nano-based encapsulation of islets. Curr. Pharm. Biotechnol. 2014, 15, 590–608. [Google Scholar] [CrossRef] [PubMed]
- Baidal, D.A.; Ricordi, C.; Berman, D.M.; Alvarez, A.; Padilla, N.; Ciancio, G.; Linetsky, E.; Pileggi, A.; Alejandro, R. Bioengineering of an intraabdominal endocrine pancreas. N. Engl. J. Med. 2017, 376, 1887–1889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dionne, K.E.; Colton, C.K.; Lyarmush, M. Effect of hypoxia on insulin secretion by isolated rat and canine islets of langerhans. Diabetes 1993, 42, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Endo, H.; Okuyama, H.; Takeda, T.; Iwahashi, H.; Imagawa, A.; Yamagata, K.; Shimomura, I.; Inoue, M. Cellular hypoxia of pancreatic beta-cells due to high levels of oxygen consumption for insulin secretion in vitro. J. Biol. Chem. 2011, 286, 12524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weaver, J.D.; Headen, D.M.; Aquart, J.; Johnson, C.T.; Shea, L.D.; Shirwan, H.; Garcia, A.J. Vasculogenic hydrogel enhances islet survival, engraftment, and function in leading extrahepatic sites. Sci. Adv. 2017, 3, e1700184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trivedi, N.; Steil, G.M.; Colton, C.K.; Bonner-Weir, S.; Weir, G.C. Improved vascularization of planar membrane diffusion devices following continuous infusion of vascular endothelial growth factor. Cell Transplant. 2000, 9, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Weaver, J.D.; Headen, D.M.; Hunckler, M.D.; Coronel, M.M.; Stabler, C.L.; Garcia, A.J. Design of a vascularized synthetic poly(ethylene glycol) macroencapsulation device for islet transplantation. Biomaterials 2018, 172, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Chandorkar, Y.; K, R.; Basu, B. The foreign body response demystified. ACS Biomater. Sci. Eng. 2019, 5, 19–44. [Google Scholar] [CrossRef]
- Tang, L.; Thevenot, P.; Hu, W. Surface chemistry influences implant biocompatibility. Curr. Top. Med. Chem. 2008, 8, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Kačarević, Ž.P.; Rider, P.M.; Alkildani, S.; Retnasingh, S.; Smeets, R.; Jung, O.; Ivanišević, Z.; Barbeck, M. An introduction to 3D bioprinting: Possibilities, challenges and future aspects. Materials 2018, 11, 2199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rider, P.; Kačarević, Ž.P.; Alkildani, S.; Retnasingh, S.; Barbeck, M. Bioprinting of tissue engineering scaffolds. J. Tissue Eng. 2018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boyd-Moss, M.; Fox, K.; Brandt, M.; Nisbet, D.; Williams, R. Bioprinting and biofabrication with peptide and protein biomaterials. Adv. Exp. Med. Biol. 2017, 1030, 95. [Google Scholar] [PubMed]
- Cui, H.; Nowicki, M.; Fisher, J.P.; Zhang, L.G. 3D Bioprinting for organ regeneration. Adv. Healthc. Mater. 2017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gopinathan, J.; Noh, I. Recent trends in bioinks for 3D printing. Biomater. Res. 2018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petta, D.; Armiento, A.R.; Grijpma, D.; Alini, M.; Eglin, D.; D’Este, M. 3D bioprinting of a hyaluronan bioink through enzymatic-and visible light-crosslinking. Biofabrication 2018, 10, 044104. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Yeon, Y.K.; Lee, J.M.; Chao, J.R.; Lee, Y.J.; Seo, Y.B.; Sultan, M.T.; Lee, O.J.; Lee, J.S.; Yoon, S.I.; et al. Precisely printable and biocompatible silk fibroin bioink for digital light processing 3D printing. Nat. Commun. 2018, 1620. [Google Scholar] [CrossRef] [PubMed]
- Rutz, A.L.; Hyland, K.E.; Jakus, A.E.; Burghardt, W.R.; Shah, R.N. A multimaterial bioink method for 3D printing tunable, cell-compatible hydrogels. Adv. Mater. 2015, 27, 1607–1614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yue, Z.; Liu, X.; Coates, P.T.; Wallace, G.G. Advances in printing biomaterials and living cells: Implications for islet cell transplantation. Curr. Opin. Organ Transplant. 2016, 21, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Derakhshanfar, S.; Mbeleck, R.; Xu, K.; Zhang, X.; Zhong, W.; Xing, M. 3D bioprinting for biomedical devices and tissue engineering: A review of recent trends and advances. Bioact. Mater. 2018, 3, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Hölzl, K.; Lin, S.; Tytgat, L.; Van Vlierberghe, S.; Gu, L.; Ovsianikov, A. Bioink properties before, during and after 3D bioprinting. Biofabrication 2016, 8, 032002. [Google Scholar] [CrossRef] [PubMed]
- Ozbolat, I.T.; Yin, Y.u. Bioprinting toward organ fabrication: Challenges and future trends. TBME 2013, 60, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Bishop, E.S.; Mostafa, S.; Pakvasa, M.; Luu, H.H.; Lee, M.J.; Wolf, J.M.; Ameer, G.A.; He, T.; Reid, R.R. 3-D bioprinting technologies in tissue engineering and regenerative medicine: Current and future trends. Genes Dis. 2017, 4, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Kirchmajer, D.M.; Gorkin, I.I.I.R.; in het Panhuis, M. An overview of the suitability of hydrogel-forming polymers for extrusion-based 3D-printing. J. Mater. Chem. B 2015, 3, 4105–4117. [Google Scholar]
- Sarker, M.D.; Naghieh, S.; Sharma, N.K.; Chen, X. 3D biofabrication of vascular networks for tissue regeneration: A report on recent advances. J. Pharm. Anal. 2018, 8, 277–296. [Google Scholar] [CrossRef] [PubMed]
- Mandrycky, C.; Wang, Z.; Kim, K.; Kim, D.H. 3D bioprinting for engineering complex tissues. Biotechnol. Adv. 2016, 34, 422–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Datta, P.; Barui, A.; Wu, Y.; Ozbolat, I.T.; Ozbolat, V.; Moncal, K.K. Essential steps in bioprinting: From pre-to post-bioprinting. Biotechnol. Adv. 2018, 36, 1481–1504. [Google Scholar] [CrossRef] [PubMed]
- Ballyns, J.J.; Bonassar, L.J. Image-guided tissue engineering. J. Cell. Mol. Med. 2009, 13, 1428–1436. [Google Scholar] [CrossRef] [PubMed]
- Sodupe-Ortega, E.; Sanz-Garcia, A.; Pernia-Espinoza, A.; Escobedo-Lucea, C. Accurate calibration in multi-material 3D bioprinting for tissue engineering. Materials 2018, 11, 1402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McElheny, C.; Hayes, D.; Devireddy, R. Design and fabrication of a low-cost three-dimensional bioprinter. J. Med. Devices 2017, 11, 041001. [Google Scholar] [CrossRef] [PubMed]
- Berg, J.; Hiller, T.; Kissner, M.S.; Qazi, T.H.; Duda, G.N.; Hocke, A.C.; Hippenstiel, S.; Elomaa, L.; Weinhart, M.; Fahrenson, C.; et al. Optimization of cell-laden bioinks for 3D bioprinting and efficient infection with influenza A virus. Sci. Rep. 2018, 13877, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Varkey, M.; Visscher, D.O.; van Zuijlen, P.P.; Atala, M.A.; Yoo, J.J. Skin bioprinting: The future of burn wound reconstruction? Burn. Trauma 2019, 4, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozbolat, I.T. 3D Bioprinting: Fundamentals, Principles, and Applications; Elsevier Science: San Diego, CA, USA, 2016; pp. 11–21. [Google Scholar]
- Li, Z.; Huang, S.; Liu, Y.; Yao, B.; Hu, T.; Shi, H.; Xie, J.; Fu, X. Tuning alginate-gelatin bioink properties by varying solvent and their impact on stem cell behavior. Sci. Rep. 2018, 8020–8028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blaeser, A.; Duarte Campos, D.F.; Puster, U.; Richtering, W.; Stevens, M.M.; Fischer, H. Controlling shear stress in 3D bioprinting is a key factor to balance printing resolution and stem cell integrity. Adv. Healthc. Mater. 2016, 5, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Yeong, W.Y. Design and printing strategies in 3D bioprinting of cell-hydrogels: A review. Adv. Healthc. Mater. 2016, 5, 2856–2865. [Google Scholar] [CrossRef] [PubMed]
- Duin, S.; Schütz, K.; Ahlfeld, T.; Lehmann, S.; Lode, A.; Ludwig, B.; Gelinsky, M. 3D Bioprinting of functional islets of langerhans in an alginate/methylcellulose hydrogel blend. Adv. Healthc. Mater. 2019, 8, e180163. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Carter, S.D.; Renes, M.J.; Kim, J.; Rojas-Canales, D.M.; Penko, D.; Angus, C.; Beirne, S.; Drogemuller, C.J.; Yue, Z.; et al. Development of a coaxial 3D printing platform for biofabrication of implantable islet-containing constructs. Adv. Healthc. Mater. 2019, e1801181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Zhou, F.; Xing, R.; Lin, Y.; Han, Y.; Teng, C.; Wang, Q. Development of large-scale size-controlled adult pancreatic progenitor cell clusters by an inkjet-printing technique. ACS Appl. Mater. Interfaces 2015, 8, 11624–11630. [Google Scholar] [CrossRef] [PubMed]
- Marchioli, G.; Di Luca, A.; de Koning, E.; Engelse, M.; Van Blitterswijk, C.A.; Karperien, M.; Van Apeldoorn, A.A.; Moroni, L. Hybrid polycaprolactone/alginate scaffolds functionalized with VEGF to promote de novo vessel formation for the transplantation of islets of langerhans. Adv. Healthc. Mater. 2016, 5, 1606–1616. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Guvendiren, M. Recent advances in bioink design for 3D bioprinting of tissues and organs. Front. Bioeng. Biotechnol. 2017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chia, H.N.; Wu, B.M. Recent advances in 3D printing of biomaterials. J. Biol. Eng. 2015, 9, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tappa, K.; Jammalamadaka, U. Novel biomaterials used in medical 3D printing techniques. J. Funct. Biomater. 2018, 9, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolan, K.; Liu, Y.; Baldridge, J.; Murphy, C.; Semon, J.; Day, D.; Leu, M. Solvent based 3D printing of biopolymer/bioactive glass composite and hydrogel for tissue engineering applications. Procedia CIRP 2017, 65, 38–43. [Google Scholar] [CrossRef]
- Song, J.; Millman, J.R. Economic 3D-printing approach for transplantation of human stem cell-derived beta-like cells. Biofabrication 2016, 015002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farina, M.; Chua, C.Y.X.; Ballerini, A.; Thekkedath, U.; Alexander, J.F.; Rhudy, J.R.; Torchio, G.; Fraga, D.; Pathak, R.R.; Villanueva, M.; et al. Transcutaneously refillable, 3D-printed biopolymeric encapsulation system for the transplantation of endocrine cells. Biomaterials 2018, 177, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Farina, M.; Ballerini, A.; Fraga, D.W.; Nicolov, E.; Hogan, M.; Demarchi, D.; Scaglione, F.; Sabek, O.M.; Horner, P.; Thekkedath, U.; et al. 3D printed vascularized device for subcutaneous transplantation of human islets. Biotechnol. J. 2017, 12, 1700169. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Lee, J.B.; Park, Y.; Lee, D.Y. 3D bioprinting for artificial pancreas organ. Adv. Exp. Med. Biol. 2018, 1064, 355. [Google Scholar] [PubMed]
- Pati, F.; Cho, D. Bioprinting of 3D tissue models using decellularized extracellular matrix bioink. Methods Mol. Biol. 2017, 1612, 381. [Google Scholar] [PubMed]
- Suntornnond, R.; Tan, E.Y.S.; An, J.; Chua, C.K. A highly printable and biocompatible hydrogel composite for direct printing of soft and perfusable vasculature-like structures. Sci. Rep. 2017, 16902. [Google Scholar] [CrossRef] [PubMed]
- Hinton, T.J.; Jallerat, Q.; Palchesko, R.N.; Park, J.H.; Grodzicki, M.S.; Shue, H.J.; Ramadan, M.H.; Hudson, A.R.; Feinberg, A.W. Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels. Sci. Adv. 2015, e1500758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Chen, M.; Fan, X.; Zhou, H. Recent advances in bioprinting techniques: Approaches, applications, and future prospects. J. Transl. Med. 2016, 271. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Investigator or Company | Type of Encapsulation | Hydrogel-like Biomaterial | Islet Source | Implantation Site | Dose | Immunossupresion | Country |
|---|---|---|---|---|---|---|---|
| Novocell, In. (Viacyte, Inc.) [37] | Nanoencapsulation | PEG | Allogeneic | Subcutaneous | Undetermined | No | USA |
| Soon-Shiong et al. [38] | Microencapsulation | Alginate-Poly-L-Lysine | Allogeneic | Peritoneum | 1040,000 islets equivalents/patient | Yes | USA |
| Calafiore et al. [39] | Microencapsulation | Alginate-Poly-L-Ornintine | Allogeneic | Peritoneum | 400,000–600,000 islets equivalents/patient | No | Italy |
| Tuch et al. [40] | Microencapsulation | Barium cross-linked alginate | Allogeneic | Peritoneum | 178,000 islets equivalents/ patient | No | Australia |
| Living Cell Technologies (LCT) [41] | Microencapsulation | Alginate-Poly-L-Ornintine | Xenogeneic-porcine insulin-producing cells | Peritoneum | 5000–10,000 islets equivalents/kg body weight | No | Australia |
| Dufrane et al. [42] | Macroencapsulation-Monolayer Cellular Device | Collagen/Alginate | Allogeneic | Subcutaneous space | Undetermined | Undetermined | Belgium |
| Beta-O2 Technologies [43] | Macroencapsulation-β-Air device | Alginate | Allogeneic | Peritoneum | 2100 islets equivalents/kg body weight | No | Germany |
| Alejandro Rodolfo et al. [44] | Macroencapsulation-BioHub | Thrombin/patient’s own plasma | Allogeneic | Omentum | 600,000 islets equivalents/patient | Yes | USA |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Espona-Noguera, A.; Ciriza, J.; Cañibano-Hernández, A.; Orive, G.; Hernández, R.M.; Saenz del Burgo, L.; Pedraz, J.L. Review of Advanced Hydrogel-Based Cell Encapsulation Systems for Insulin Delivery in Type 1 Diabetes Mellitus. Pharmaceutics 2019, 11, 597. https://doi.org/10.3390/pharmaceutics11110597
Espona-Noguera A, Ciriza J, Cañibano-Hernández A, Orive G, Hernández RM, Saenz del Burgo L, Pedraz JL. Review of Advanced Hydrogel-Based Cell Encapsulation Systems for Insulin Delivery in Type 1 Diabetes Mellitus. Pharmaceutics. 2019; 11(11):597. https://doi.org/10.3390/pharmaceutics11110597
Chicago/Turabian StyleEspona-Noguera, Albert, Jesús Ciriza, Alberto Cañibano-Hernández, Gorka Orive, Rosa María Hernández, Laura Saenz del Burgo, and Jose Luis Pedraz. 2019. "Review of Advanced Hydrogel-Based Cell Encapsulation Systems for Insulin Delivery in Type 1 Diabetes Mellitus" Pharmaceutics 11, no. 11: 597. https://doi.org/10.3390/pharmaceutics11110597
APA StyleEspona-Noguera, A., Ciriza, J., Cañibano-Hernández, A., Orive, G., Hernández, R. M., Saenz del Burgo, L., & Pedraz, J. L. (2019). Review of Advanced Hydrogel-Based Cell Encapsulation Systems for Insulin Delivery in Type 1 Diabetes Mellitus. Pharmaceutics, 11(11), 597. https://doi.org/10.3390/pharmaceutics11110597