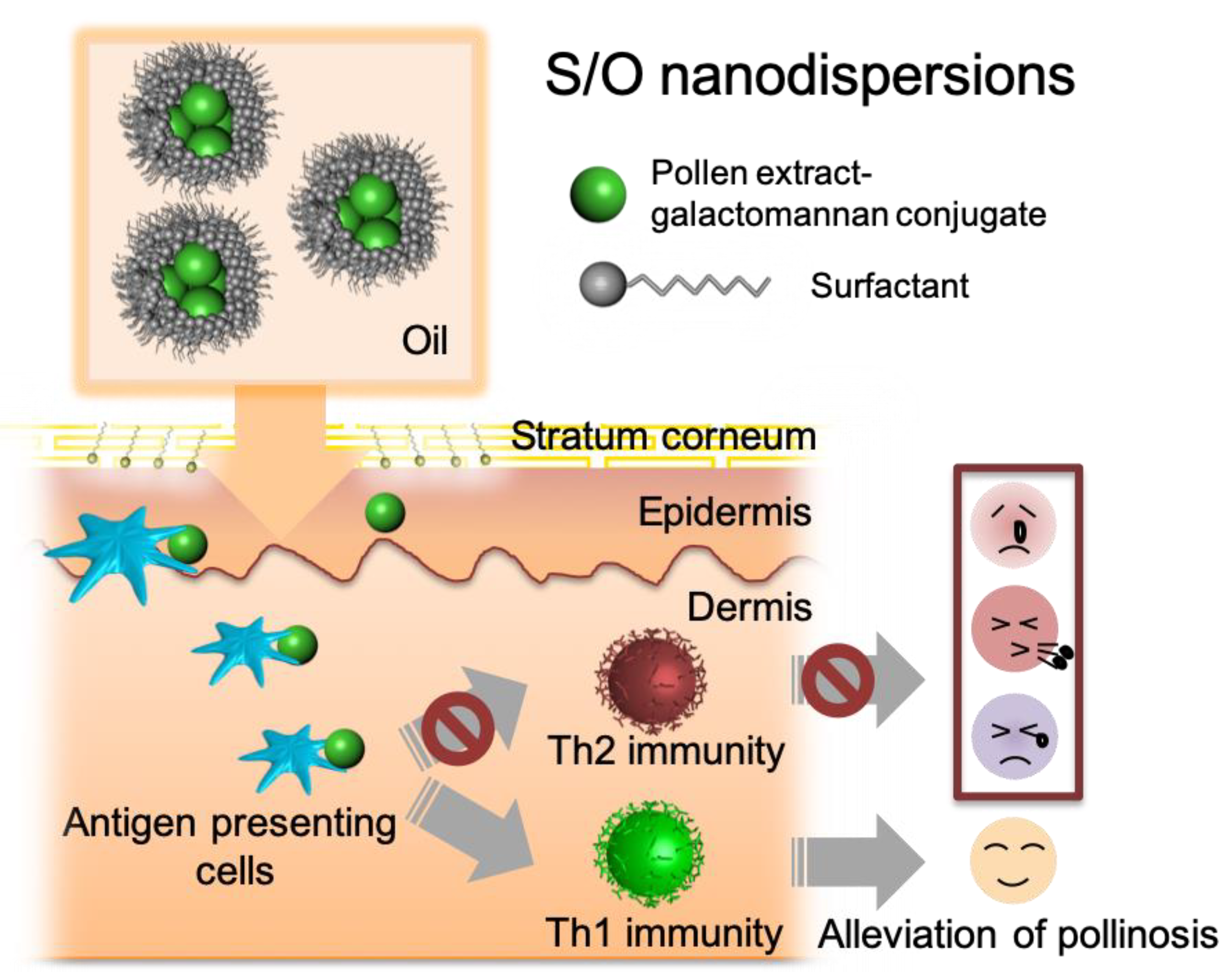
Transcutaneous Delivery of Immunomodulating Pollen Extract-Galactomannan Conjugate by Solid-in-Oil Nanodispersions for Pollinosis Immunotherapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals
2.3. Preparation of the S/O Nanodispersions
2.4. In Vitro Analysis of the Antigen Release from the S/O Nanodispersions
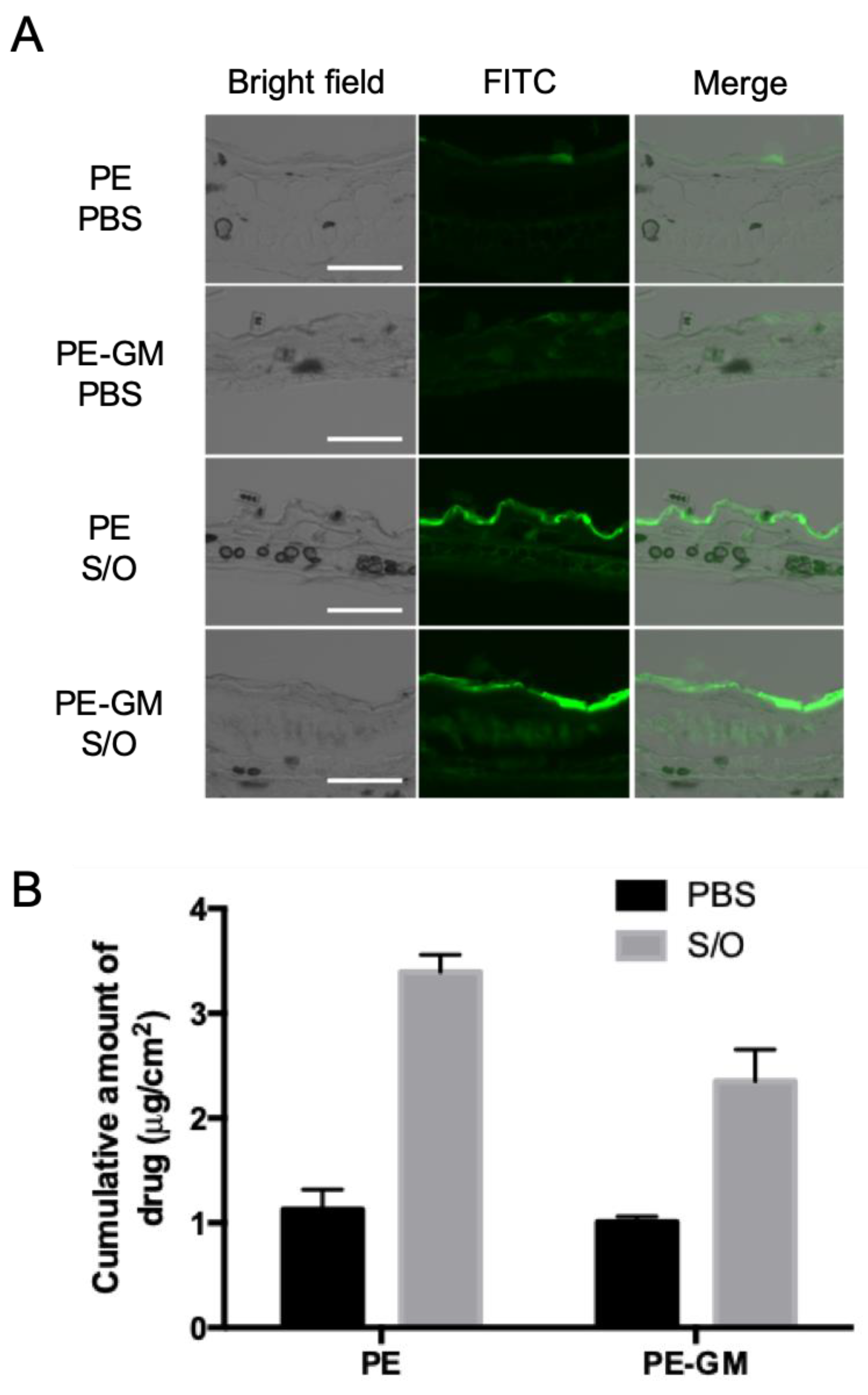
2.5. In Vitro Analysis of the Skin Permeation of the Antigen by the S/O Nanodispersions
2.6. In Vivo Analysis of the Skin Permeation of Antigen by S/O Nanodispersions
2.7. Antigen Uptake by DC2.4 Cells
2.8. Confocal Imaging of DC2.4 Cells
2.9. Sensitization
2.10. TCIT Treatment of the PE and PE-GM
2.11. TCIT Treatment of the Pollinosis Mouse Model Using PE-GM
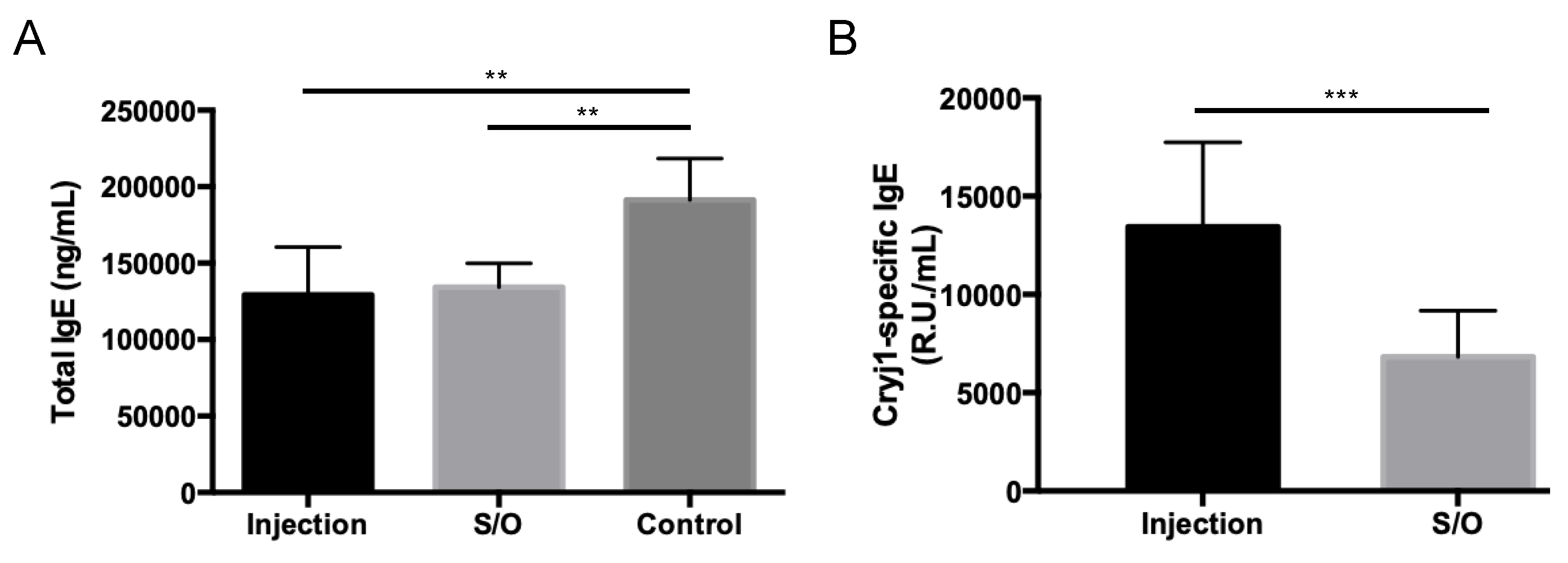
2.12. Measurement of the Serum Antibody Levels
2.13. Cytokine Measurement
2.14. Effect on Pollen-Induced Nose Rubbing
2.15. Statistical Tests
3. Results and Discussion
3.1. Preparation and Characterization of S/O Nanodispersions Carrying PE or PE-GM
3.2. Skin Permeation by S/O Nanodispersions Carrying PE or PE-GM
3.3. Antigen Uptake by DC2.4 Cells
3.4. Comparison of TCIT Using S/O Nanodispersions Carrying PE or PE-GM
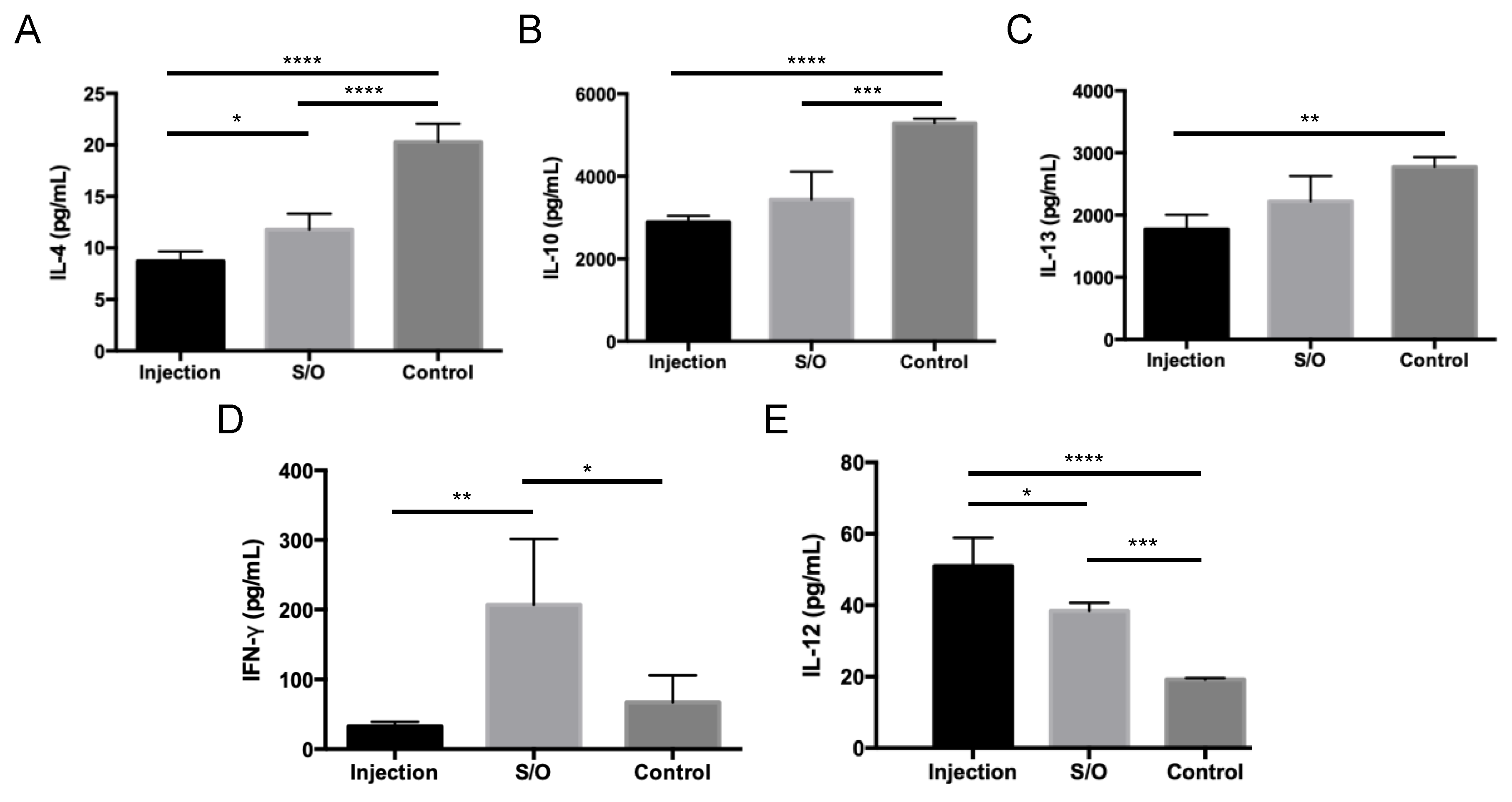
3.5. Comparison of the Differences between TCIT and SCIT Using PE-GM
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Okubo, K.; Kurono, Y.; Ichimura, K.; Enomoto, T.; Okamoto, Y.; Kawauchi, H.; Suzaki, H.; Fujieda, S.; Masuyama, K. Japanese guidelines for allergic rhinitis 2017. Allergol. Int. 2017, 66, 205–219. [Google Scholar] [CrossRef] [PubMed]
- Pfaar, O.; Demoly, P.; Gerth Van Wijk, R.; Bonini, S.; Bousquet, J.; Canonica, G.W.; Durham, S.R.; Jacobsen, L.; Malling, H.J.; Papadopoulos, N.G.; et al. Recommendations for the standardization of clinical outcomes used in allergen immunotherapy trials for allergic rhinoconjunctivitis: An EAACI Position Paper. Allergy Eur. J. Allergy Clin. Immunol. 2014, 69, 854–867. [Google Scholar] [CrossRef] [PubMed]
- Focke, M.; Swoboda, I.; Marth, K.; Valenta, R. Developments in allergen-specific immunotherapy: From allergen extracts to allergy vaccines bypassing allergen-specific immunoglobulin e and T cell reactivity. Clin. Exp. Allergy 2010, 40, 385–397. [Google Scholar] [CrossRef] [PubMed]
- Masuyama, K.; Matsuoka, T.; Kamijo, A. Current status of sublingual immunotherapy for allergic rhinitis in Japan. Allergol. Int. 2018, 67, 320–325. [Google Scholar] [CrossRef]
- Prickett, S.R.; Rolland, J.M.; O’Hehir, R.E. Immunoregulatory T cell epitope peptides: The new frontier in allergy therapy. Clin. Exp. Allergy 2015, 45, 1015–1026. [Google Scholar] [CrossRef]
- Wise, S.K.; Schlosser, R.J. Subcutaneous and sublingual immunotherapy for allergic rhinitis: What is the evidence? Am. J. Rhinol. Allergy 2012, 26, 18–22. [Google Scholar] [CrossRef]
- O’Hehir, R.E.; Prickett, S.R.; Rolland, J.M. T Cell Epitope Peptide Therapy for Allergic Diseases. Curr. Allergy Asthma Rep. 2016, 16, 1–9. [Google Scholar] [CrossRef]
- Kitaoka, M.; Shin, Y.; Kamiya, N.; Kawabe, Y.; Kamihira, M.; Goto, M. Transcutaneous Peptide Immunotherapy of Japanese Cedar Pollinosis Using Solid-in-Oil Nanodispersion Technology. AAPS Pharm. Sci. Tech. 2015, 16, 1–20. [Google Scholar] [CrossRef]
- Sakaguchi, M.; Hirahara, K.; Fujimura, T.; Toda, M. Approaches to immunotherapies for Japanese cedar pollinosis. Auris Nasus Larynx 2011, 38, 431–438. [Google Scholar] [CrossRef]
- Takagi, H.; Hiroi, T.; Yang, L.; Tada, Y.; Yuki, Y.; Takamura, K.; Ishimitsu, R.; Kawauchi, H.; Kiyono, H.; Takaiwa, F. A rice-based edible vaccine expressing multiple T cell epitopes induces oral tolerance for inhibition of Th2-mediated IgE responses. Proc. Natl. Acad. Sci. USA 2005, 102, 17525–17530. [Google Scholar] [CrossRef]
- Usui, M.; Saito, A.; Taniguchi, N.; Nishijima, N.; Azakami, H.; Kato, A. Reduction of antigenicity of Cry j I, major allergen of Japanese cedar pollen, by the attachment of polysaccharides. Biosci. Biotechnol. Biochem. 2003, 67, 2425–2430. [Google Scholar] [CrossRef] [PubMed]
- Aoki, R.; Saito, A.; Azakami, H.; Kato, A. Effects of various saccharides on the masking of epitope sites and uptake in the gut of cedar allergen Cry j 1 -saccharide conjugates by a naturally occurring maillard reaction. J. Agric. Food Chem. 2010, 58, 7986–7990. [Google Scholar] [CrossRef] [PubMed]
- Murakami, D.; Sawatsubashi, M.; Omori, H.; Saito, A.; Kato, A.; Komune, S.; Nakagawa, T. Safety and efficacy of short-term oral immunotherapy with Cry j 1-galactomannan conjugate for Japanese cedar pollinosis: A randomized controlled trial. Sci. Rep. 2017, 7, 46142. [Google Scholar] [CrossRef] [PubMed]
- Anthony, J.; Frew, M.D. Sublingual immunotherapy. N. Engl. J. Med. 2008, 358, 2259–2264. [Google Scholar]
- Werfel, T. Epicutaneous allergen administration: A novel approach for allergen-specific immunotherapy? J. Allergy Clin. Immunol. 2009, 124, 1003–1004. [Google Scholar] [CrossRef] [PubMed]
- Imanaka, T.; Sato, I.; Kawasaki, Y.; Kanazawa, Y.; Kawakami, K. An analysis of factors associated with compliance and dropout of sublingual immunotherapy on Japanese cedar pollinosis patients. Int. Forum Allergy Rhinol. 2019, 9, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Mitragotri, S. Immunization without needles. Nat. Rev. Immunol. 2005, 5, 905–916. [Google Scholar] [CrossRef]
- Heath, W.R.; Carbone, F.R. The skin-resident and migratory immune system in steady state and memory: Innate lymphocytes, dendritic cells and T cells. Nat. Immunol. 2013, 14, 978–985. [Google Scholar] [CrossRef]
- Tay, S.S.; Roediger, B.; Tong, P.L.; Tikoo, S.; Weninger, W. The Skin-Resident Immune Network. Curr. Dermatol. Rep. 2014, 3, 13–22. [Google Scholar] [CrossRef]
- Bos, J.D.; Meinardi, M. The 500 Dalton rule for the skin penetration of chemical compounds and drugs. Exp. Dermatol. 2000, 9, 165–169. [Google Scholar] [CrossRef]
- Madison, K.C. Barrier function of the skin: “La Raison d’Être” of the epidermis. J. Invest. Dermatol. 2003, 121, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Tahara, Y.; Honda, S.; Kamiya, N.; Piao, H.; Hirata, A.; Hayakawa, E.; Fujii, T.; Goto, M. A solid-in-oil nanodispersion for transcutaneous protein delivery. J. Control. Release 2008, 131, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Kitaoka, M.; Wakabayashi, R.; Kamiya, N.; Goto, M. Solid-in-oil nanodispersions for transdermal drug delivery systems. Biotechnol. J. 2016, 11, 1375–1385. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.; Loureiro, A.; Azoia, N.G.; Silva, C.; Cavaco-Paulo, A. Protein Formulations for Emulsions and Solid-in-Oil Dispersions. Trends Biotechnol. 2016, 34, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, R.; Sakuragi, M.; Kozaka, S.; Tahara, Y.; Kamiya, N.; Goto, M. Solid-in-Oil Peptide Nanocarriers for Transcutaneous Cancer Vaccine Delivery against Melanoma. Mol. Pharm. 2018, 15, 955–961. [Google Scholar] [CrossRef]
- Wakabayashi, R.; Kono, H.; Kozaka, S.; Tahara, Y.; Kamiya, N.; Goto, M. Transcutaneous Codelivery of Tumor Antigen and Resiquimod in Solid-in-Oil Nanodispersions Promotes Antitumor Immunity. ACS Biomater. Sci. Eng. 2019, 5, 2297–2306. [Google Scholar] [CrossRef]
- Kong, Q.; Kitaoka, M.; Wakabayashi, R.; Kamiya, N.; Goto, M. Transcutaneous immunotherapy of pollinosis using solid-in-oil nanodispersions loaded with T cell epitope peptides. Int. J. Pharm. 2017, 529, 401–409. [Google Scholar] [CrossRef]
- Al-Ghouleh, A.; Johal, R.; Sharquie, I.K.; Emara, M.; Harrington, H.; Shakib, F.; Ghaemmaghami, A.M. The glycosylation pattern of common allergens: The recognition and uptake of Der p 1 by epithelial and dendritic cells is carbohydrate dependent. PLoS One 2012, 7, 1–11. [Google Scholar] [CrossRef]
- Suzuki, Y.; Kassai, M.; Hirose, T.; Katayama, S.; Nakamura, K.; Akiyama, H.; Teshima, R.; Nakamura, S. Modulation of Immunoresponse in BALB/c Mice by Oral Administration of Fag e 1−Glucomannan Conjugate. J. Agric. Food Chem. 2009, 57, 9787–9792. [Google Scholar] [CrossRef]
- Rupa, P.; Nakamura, S.; Katayama, S.; Mine, Y. Effects of ovalbumin glycoconjugates on alleviation of orally induced egg allergy in mice via dendritic-cell maturation and T-cell activation. Mol. Nutr. Food Res. 2014, 58, 405–417. [Google Scholar] [CrossRef]
- Kiel, M.A.; Röder, E.; Gerth Van Wijk, R.; Al, M.J.; Hop, W.C.J.; Rutten-Van Mölken, M.P.M.H. Real-life compliance and persistence among users of subcutaneous and sublingual allergen immunotherapy. J. Allergy Clin. Immunol. 2013, 132, 353–360.e2. [Google Scholar] [CrossRef] [PubMed]
- Kitaoka, M.; Naritomi, A.; Kawabe, Y.; Kamihira, M.; Kamiya, N.; Goto, M. Transcutaneous pollinosis immunotherapy using a solid-in-oil nanodispersion system carrying T cell epitope peptide and R848. Bioeng. Transl. Med. 2017, 2, 102–108. [Google Scholar] [CrossRef] [PubMed]
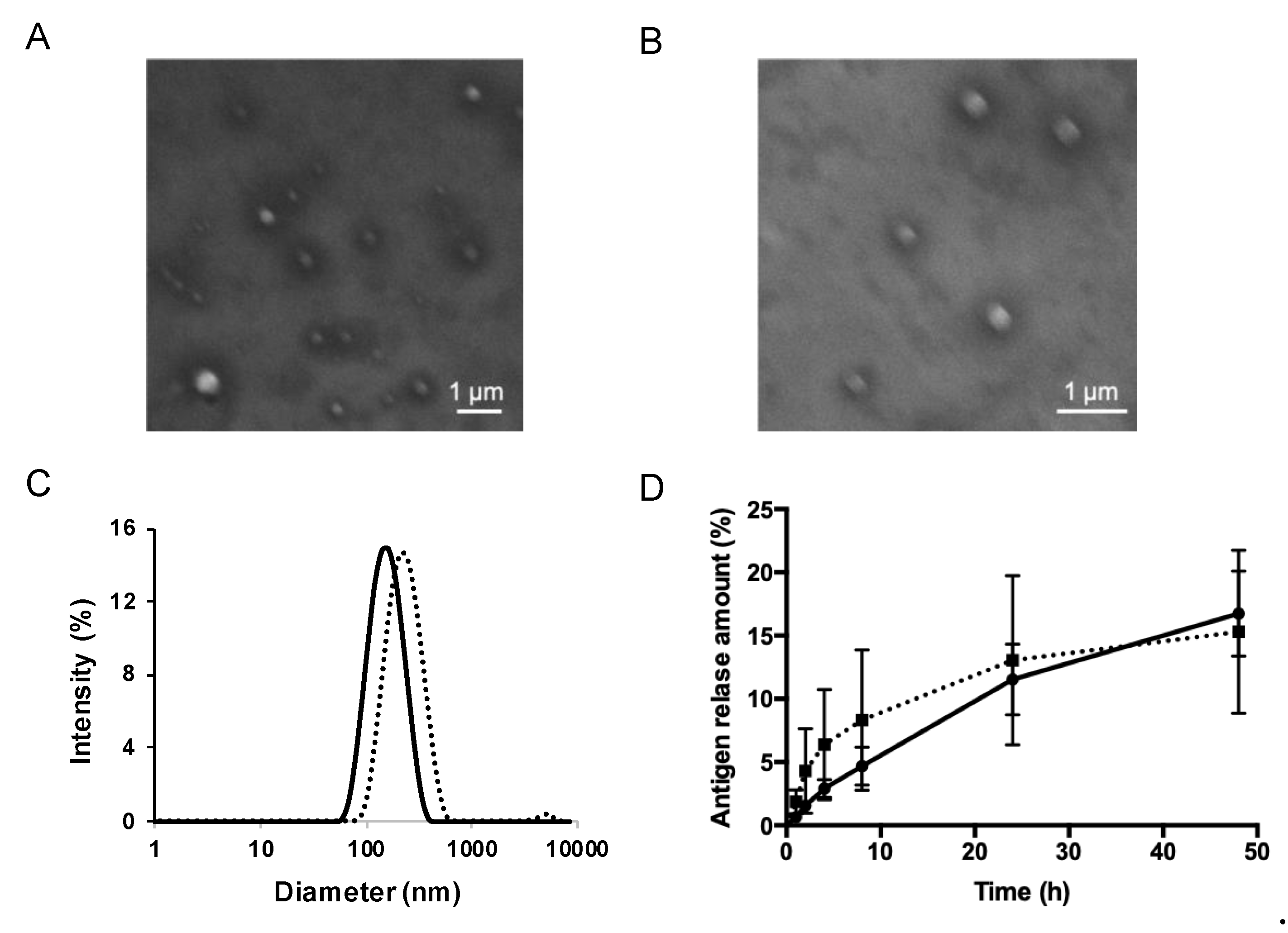


| Sample | Diameter (nm) | PDI |
|---|---|---|
| PE-S/O | 163.9 ± 0.4 | 0.181–0.196 |
| PE-GM-S/O | 212.8 ± 8.6 | 0.111–0.238 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, Q.; Higasijima, K.; Wakabayashi, R.; Tahara, Y.; Kitaoka, M.; Obayashi, H.; Hou, Y.; Kamiya, N.; Goto, M. Transcutaneous Delivery of Immunomodulating Pollen Extract-Galactomannan Conjugate by Solid-in-Oil Nanodispersions for Pollinosis Immunotherapy. Pharmaceutics 2019, 11, 563. https://doi.org/10.3390/pharmaceutics11110563
Kong Q, Higasijima K, Wakabayashi R, Tahara Y, Kitaoka M, Obayashi H, Hou Y, Kamiya N, Goto M. Transcutaneous Delivery of Immunomodulating Pollen Extract-Galactomannan Conjugate by Solid-in-Oil Nanodispersions for Pollinosis Immunotherapy. Pharmaceutics. 2019; 11(11):563. https://doi.org/10.3390/pharmaceutics11110563
Chicago/Turabian StyleKong, Qingliang, Kouki Higasijima, Rie Wakabayashi, Yoshiro Tahara, Momoko Kitaoka, Hiroki Obayashi, Yanting Hou, Noriho Kamiya, and Masahiro Goto. 2019. "Transcutaneous Delivery of Immunomodulating Pollen Extract-Galactomannan Conjugate by Solid-in-Oil Nanodispersions for Pollinosis Immunotherapy" Pharmaceutics 11, no. 11: 563. https://doi.org/10.3390/pharmaceutics11110563
APA StyleKong, Q., Higasijima, K., Wakabayashi, R., Tahara, Y., Kitaoka, M., Obayashi, H., Hou, Y., Kamiya, N., & Goto, M. (2019). Transcutaneous Delivery of Immunomodulating Pollen Extract-Galactomannan Conjugate by Solid-in-Oil Nanodispersions for Pollinosis Immunotherapy. Pharmaceutics, 11(11), 563. https://doi.org/10.3390/pharmaceutics11110563






