Natural Diatom Biosilica as Microshuttles in Drug Delivery Systems
Abstract
1. Introduction
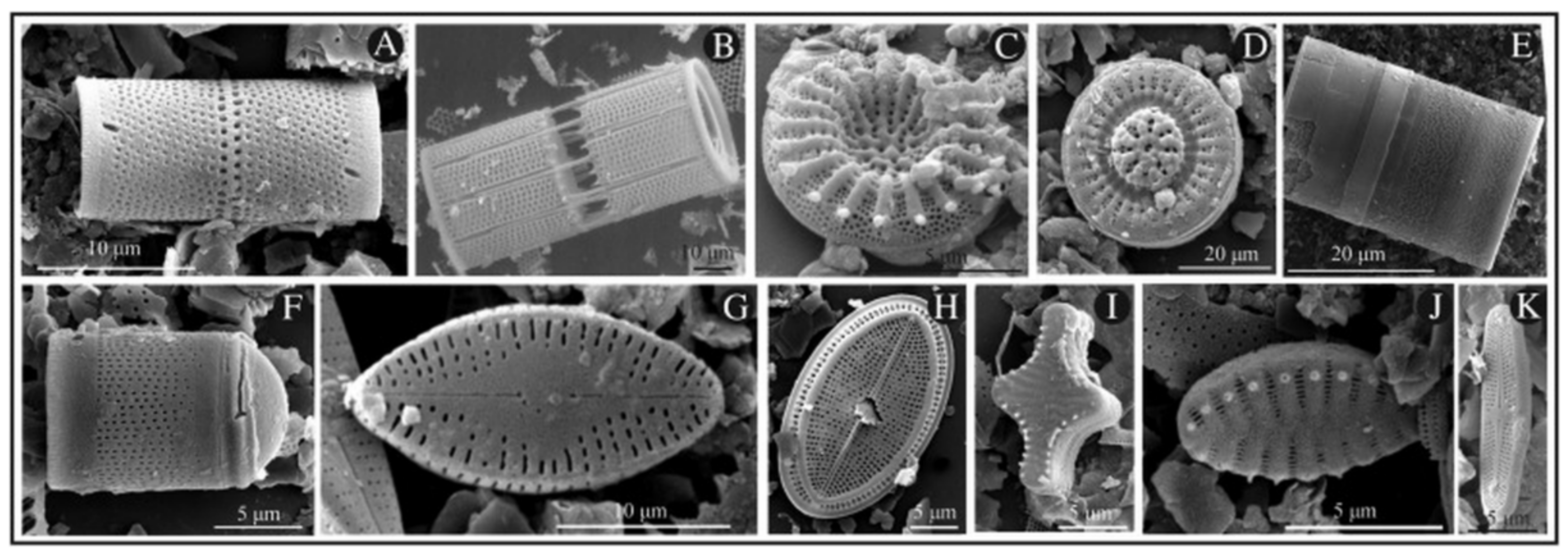
2. Diatoms as a Natural Biocompatible Material for Therapeutic Applications
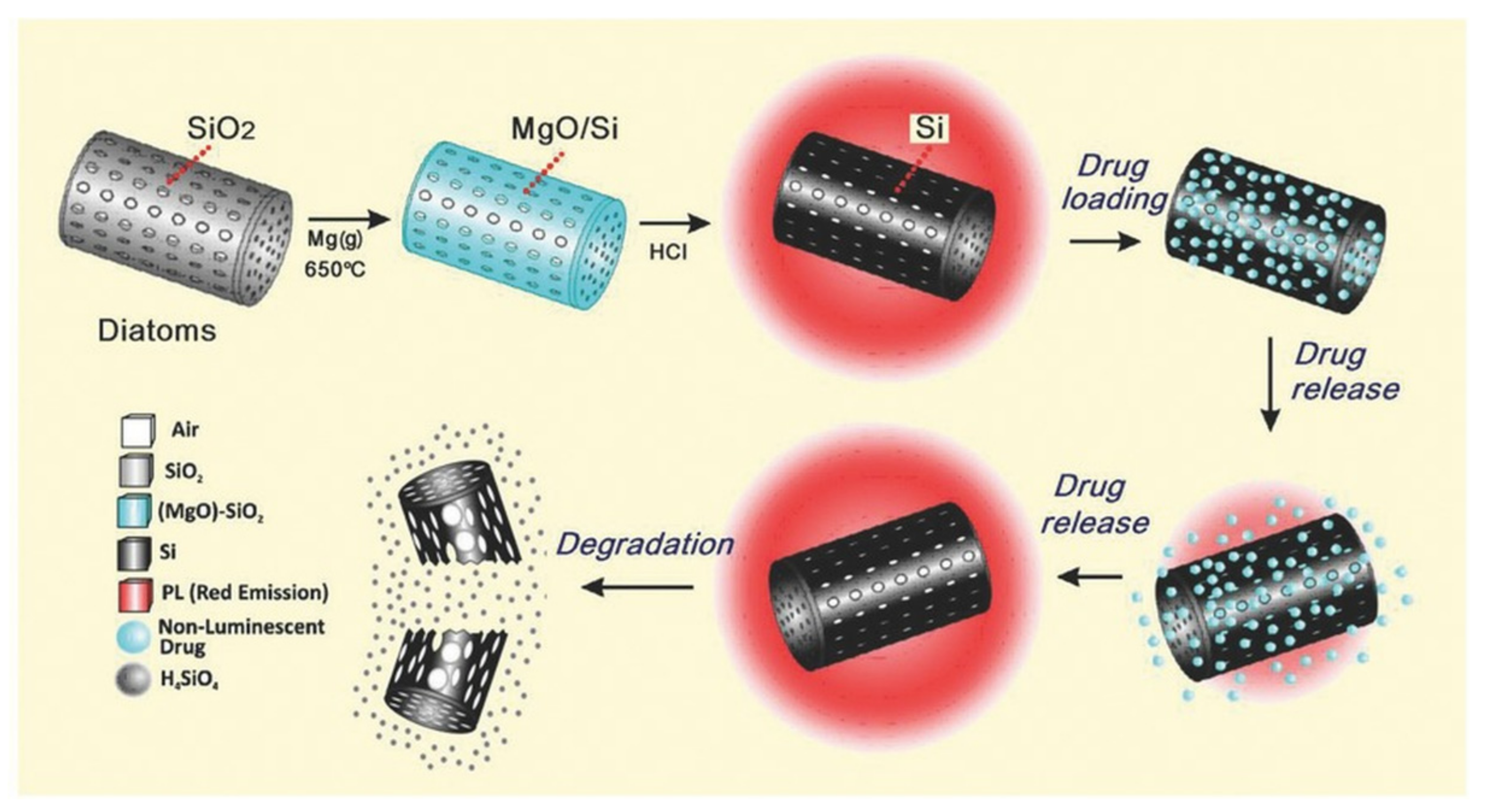
3. Diatoms as Drug Carriers
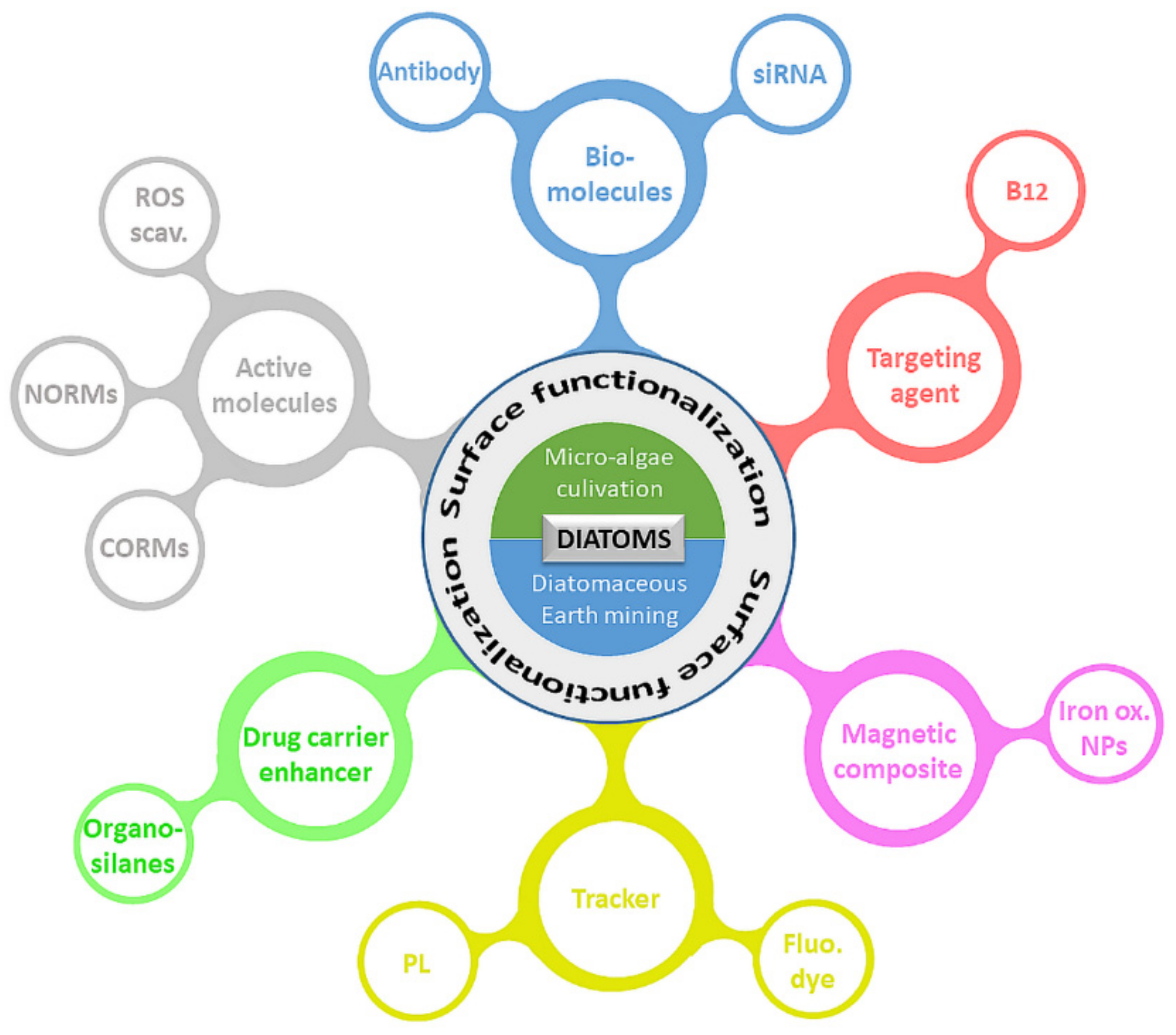
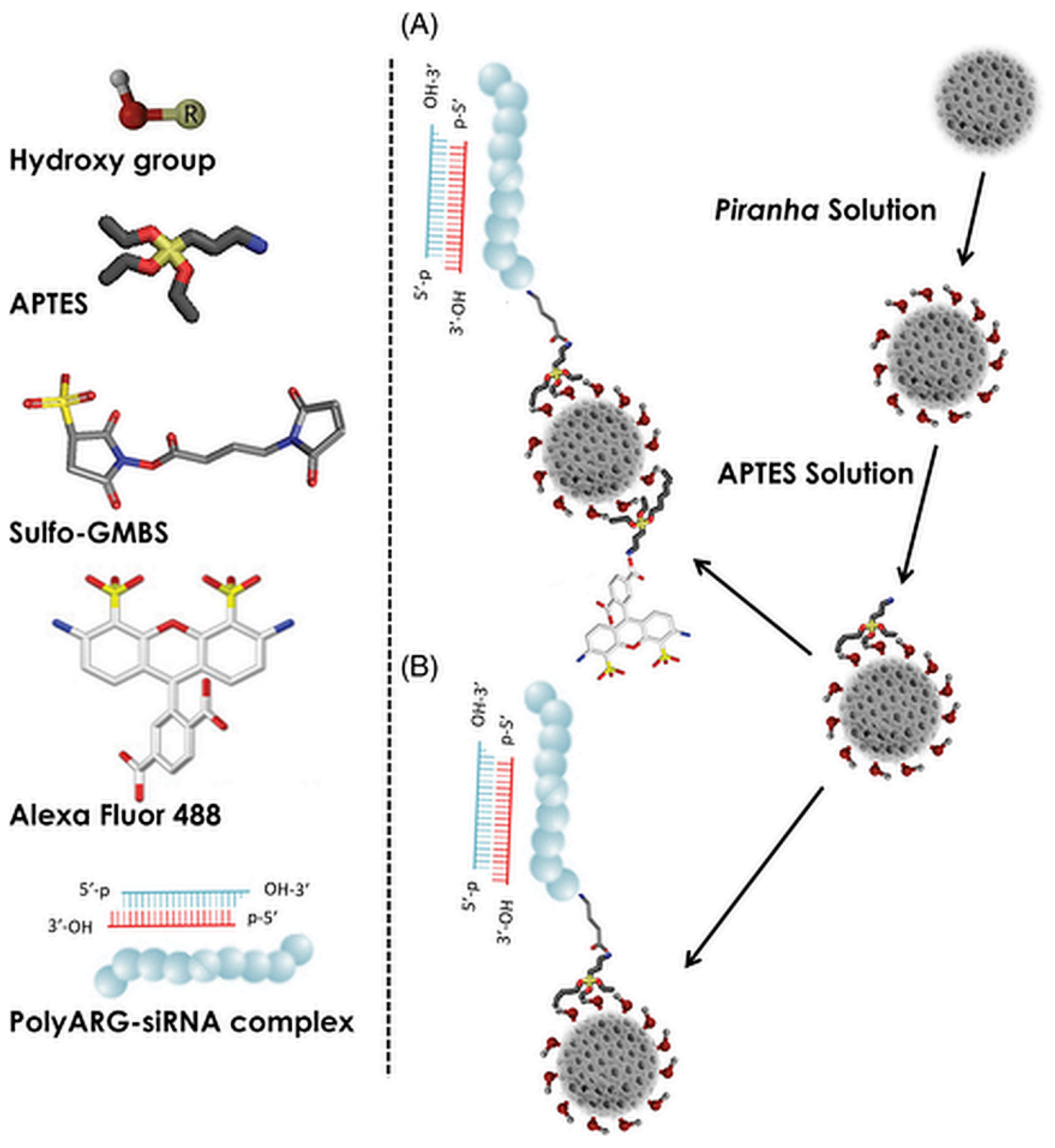
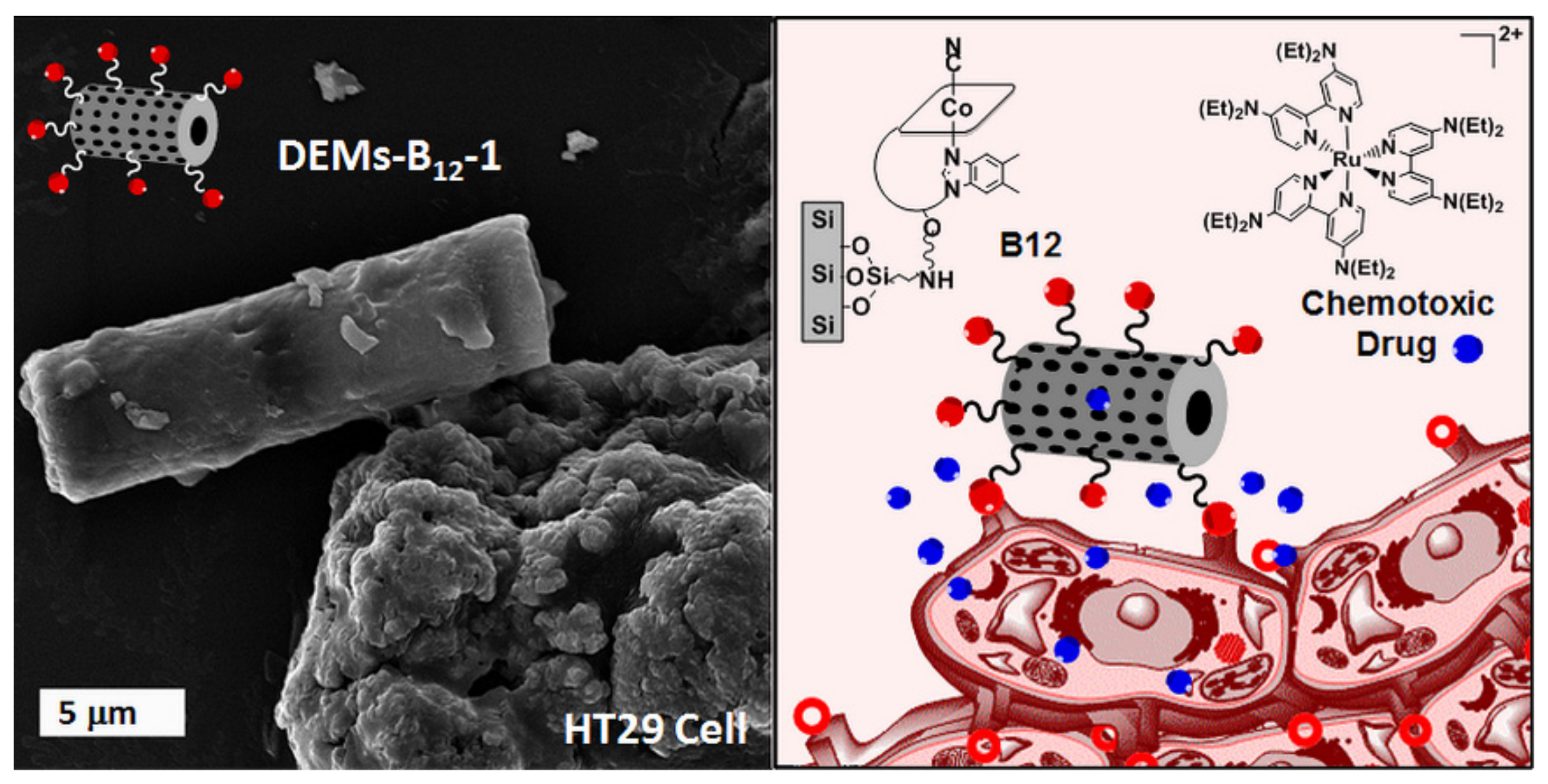
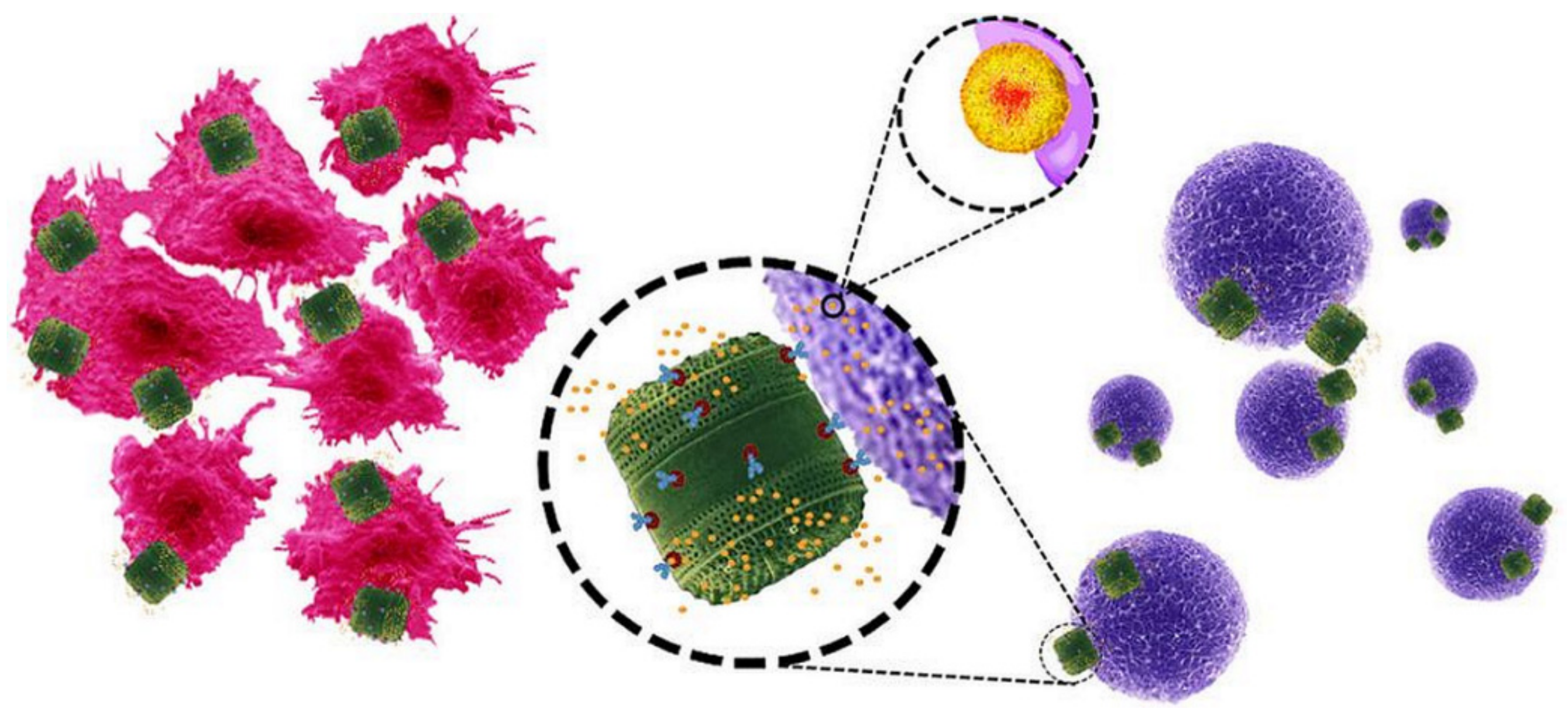
4. Functionalized Diatoms: Smart Targeting Vehicles for Drug Delivery
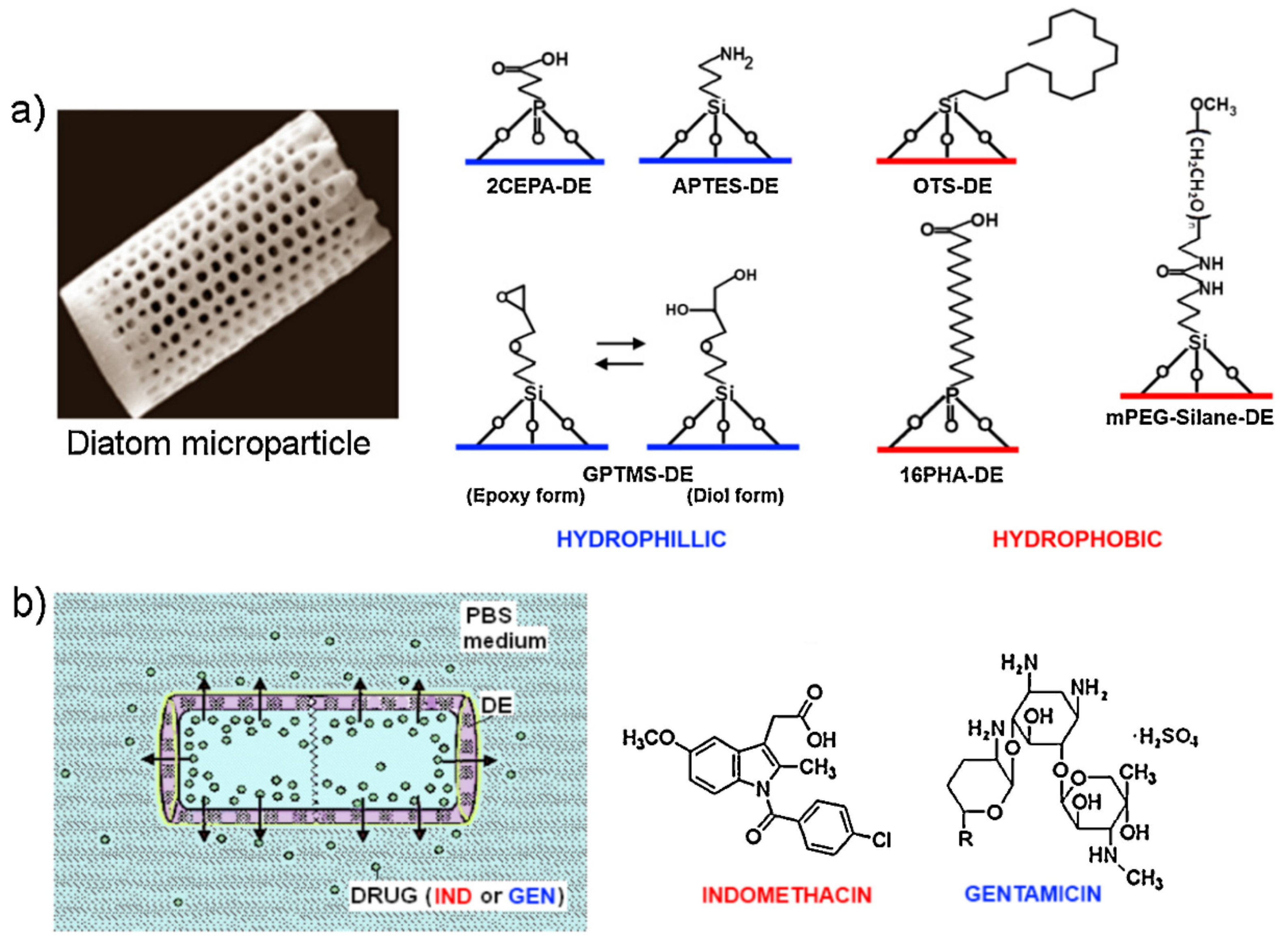
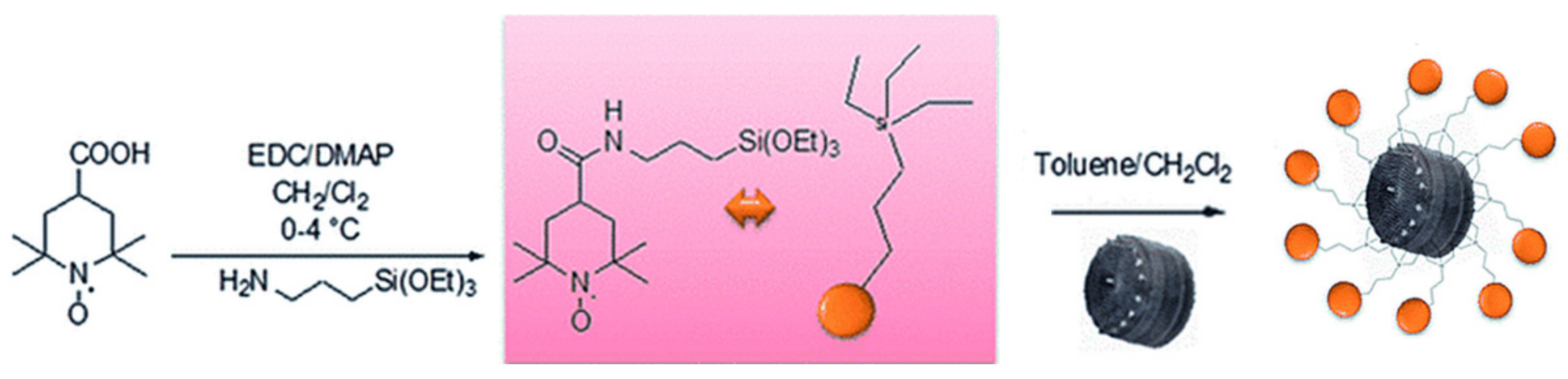
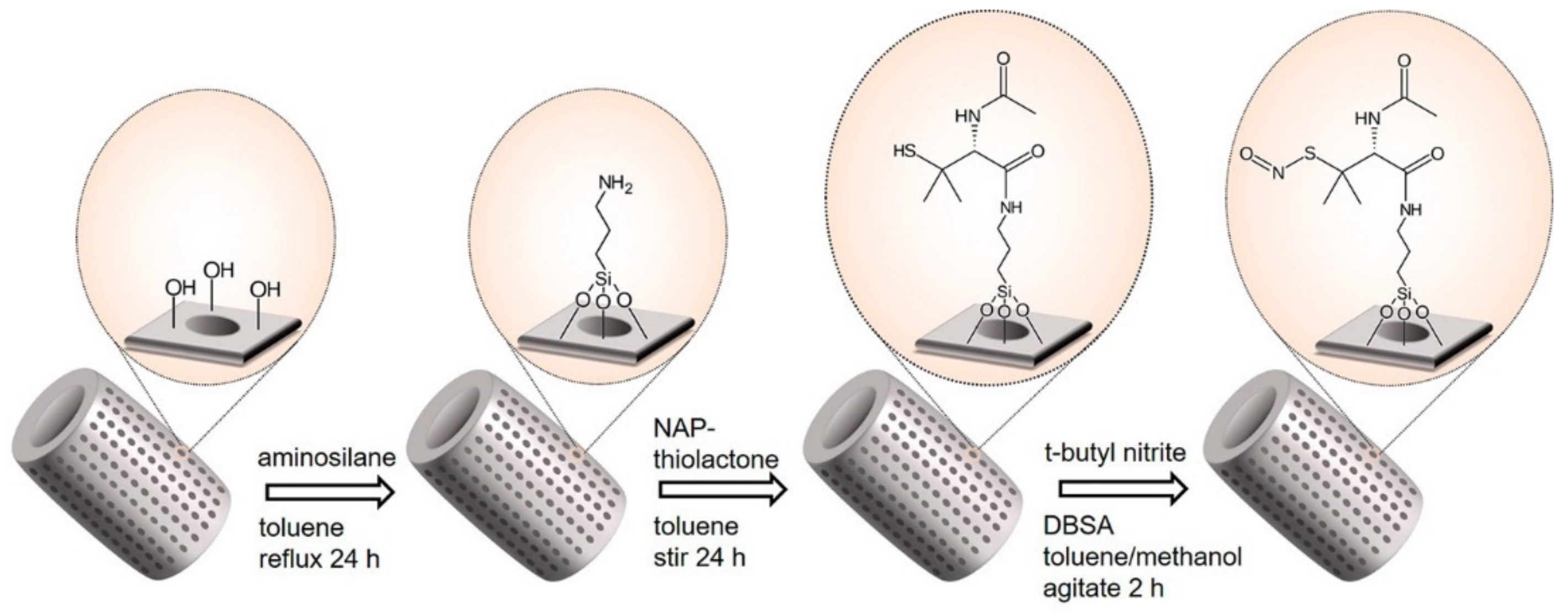
4.1. Diatoms Functionalized via Organosilane Coating
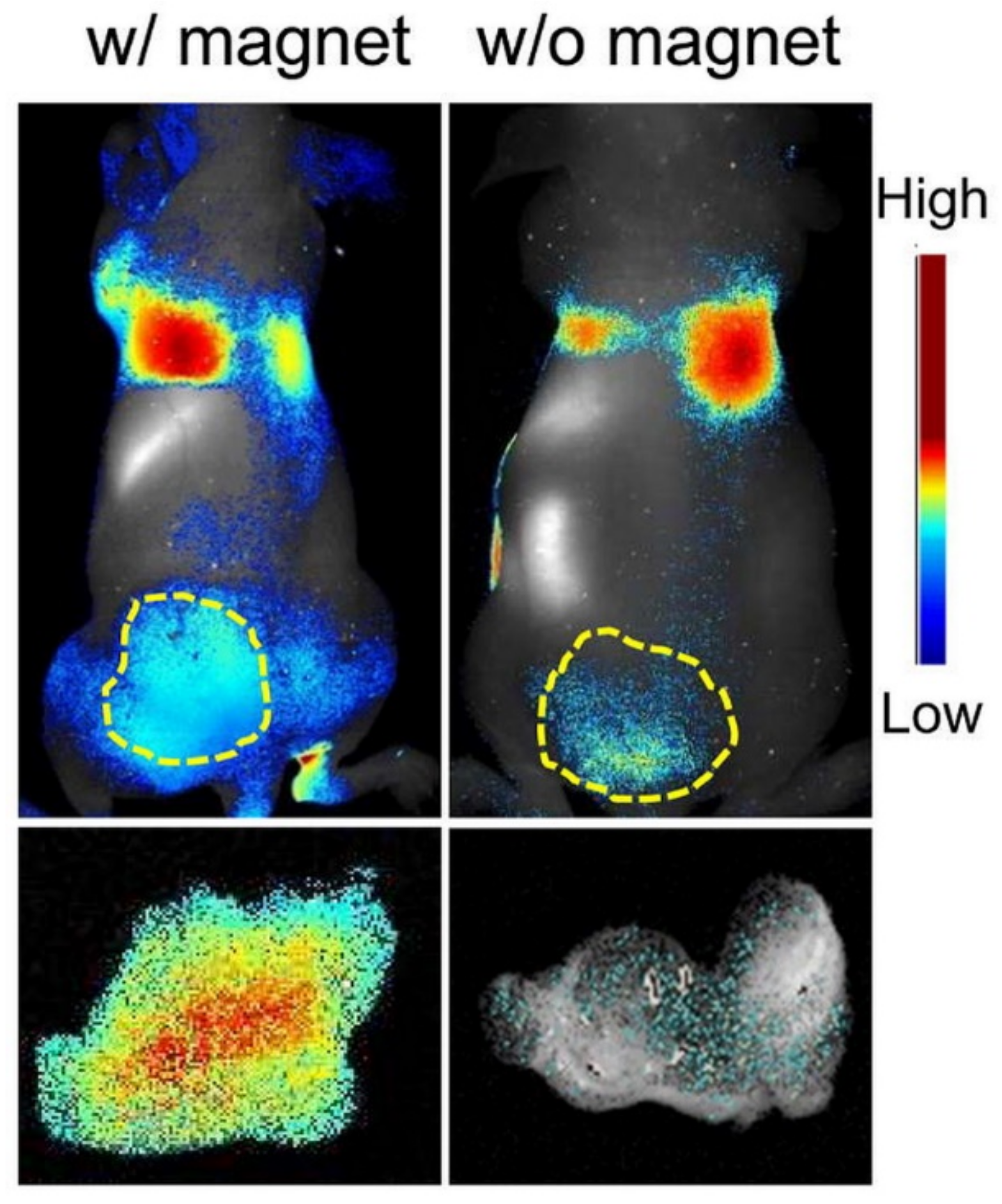
4.2. Diatoms Functionalized with Magnetic Coating and Antibodies
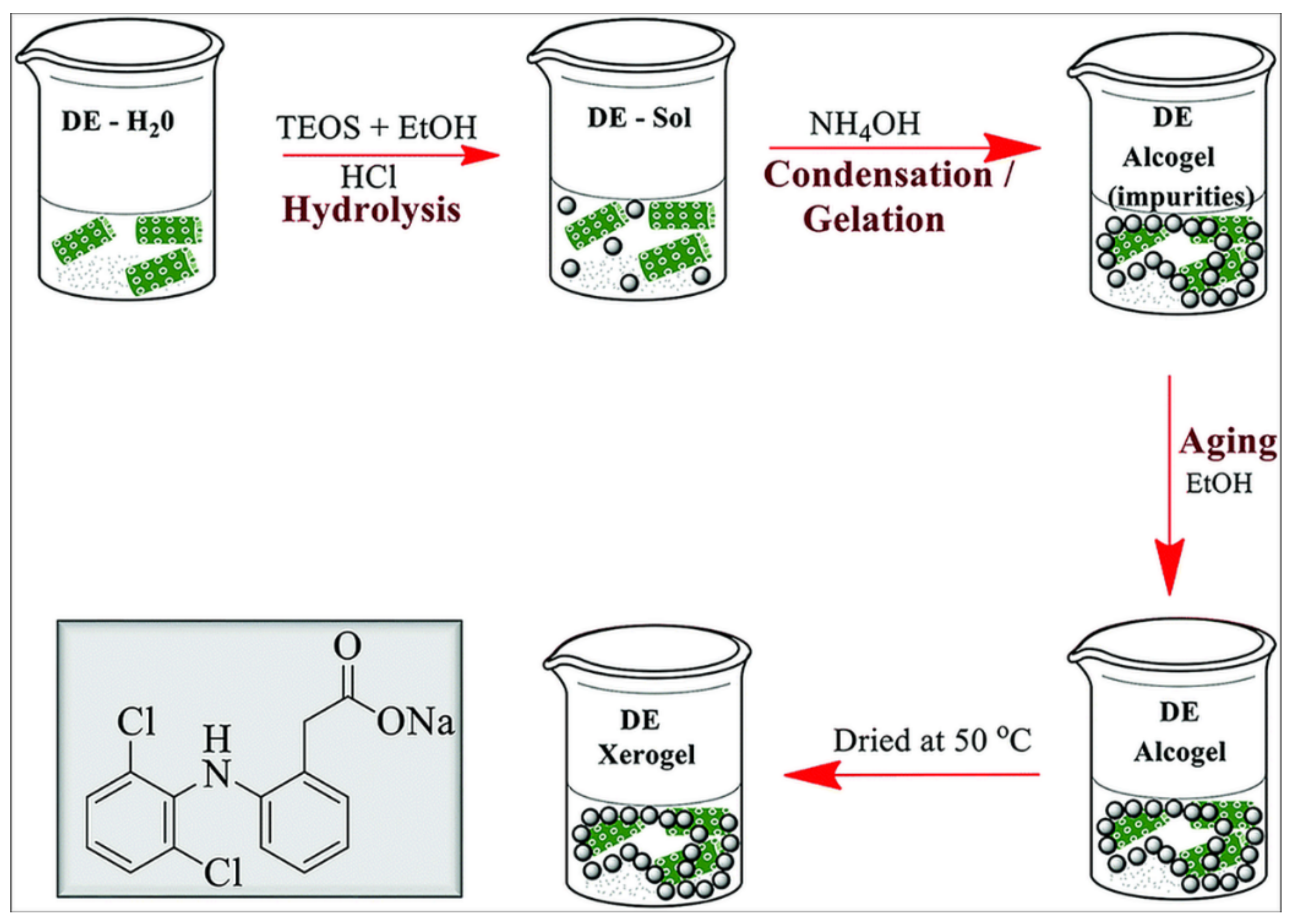
5. Diatom Composite Formulations in Drug Delivery Systems
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yun, Y.H.; Lee, B.K.; Park, K. Controlled Drug Delivery: Historical perspective for the next generation. J. Control. Release 2015, 219, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Albert, K.; Huang, X.C.; Hsu, H.Y. Bio-templated silica composites for next-generation biomedical applications. Adv. Colloid Interface Sci. 2017, 249, 272–289. [Google Scholar] [CrossRef] [PubMed]
- Jeffryes, C.; Agathos, S.N.; Rorrer, G. Biogenic nanomaterials from photosynthetic microorganisms. Curr. Opin. Biotech. 2015, 33, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Slowing, I.I.; Vivero-Escoto, J.L.; Wu, C.W.; Lin, V.S. Mesoporous silica nanoparticles as controlled release drug delivery and gene transfection carriers. Adv. Drug Deliv. Rev. 2008, 60, 1278–1288. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Barnes, J.C.; Bosoy, A.; Stoddart, J.F.; Zink, J.I. Mesoporous silica nanoparticles in biomedical applications. Chem. Soc. Rev. 2012, 41, 2590–2605. [Google Scholar] [CrossRef] [PubMed]
- Rosenholm, J.M.; Meinander, A.; Peuhu, E.; Niemi, R.; Eriksson, J.E.; Sahlgren, C.; Linden, M. Targeting of porous hybrid silica nanoparticles to cancer cells. ACS Nano 2009, 3, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Luo, Z.; Liu, J.; Ding, X.; Li, J.; Cai, K. Cytochrome c end-capped mesoporous silica nanoparticles as redox-responsive drug delivery vehicles for liver tumor-targeted triplex therapy in vitro and in vivo. J. Control. Release 2014, 192, 192–201. [Google Scholar] [CrossRef]
- De Oliveira, L.F.; Bouchmella, K.; Goncalves Kde, A.; Bettini, J.; Kobarg, J.; Cardoso, M.B. Functionalized Silica Nanoparticles as an Alternative Platform for Targeted Drug-Delivery of Water Insoluble Drugs. Langmuir 2016, 32, 3217–3225. [Google Scholar] [CrossRef]
- Rea, I.; Terracciano, M.; De Stefano, L. Synthetic vs Natural: Diatoms Bioderived Porous Materials for the Next Generation of Healthcare Nanodevices. Adv. Healthc. Mater. 2017, 6. [Google Scholar] [CrossRef]
- Terracciano, M.; De Stefano, L.; Rea, I. Diatoms Green Nanotechnology for Biosilica-Based Drug Delivery Systems. Pharmaceutics 2018, 10. [Google Scholar] [CrossRef]
- Maher, S.; Kumeria, T.; Aw, M.S.; Losic, D. Diatom Silica for Biomedical Applications: Recent Progress and Advances. Adv. Healthc. Mater. 2018, 7, e1800552. [Google Scholar] [CrossRef] [PubMed]
- Simovic, S.; Ghouchi-Eskandar, N.; Sinn, A.M.; Losic, D.; Prestidge, C.A. Silica materials in drug delivery applications. Curr. Drug Discov. Technol. 2011, 8, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Chao, J.T.; Biggs, M.J.P.; Pandit, A.S. Diatoms: A biotemplating approach to fabricating drug delivery reservoirs. Expert Opin. Drug. Del. 2014, 11, 1687–1695. [Google Scholar] [CrossRef] [PubMed]
- Morse, D.E. Silicon biotechnology: Harnessing biological silica production to construct new materials. Trends Biotechnol. 1999, 17, 230–232. [Google Scholar] [CrossRef]
- Mann, D.G.; Vanormelingen, P. An inordinate fondness? The number, distributions, and origins of diatom species. J. Eukaryot. Microbiol. 2013, 60, 414–420. [Google Scholar] [CrossRef]
- Jaramillo, D.; Vallejo, D.F.; Velez, M.I.; Restrepo-Moreno, S.; Pardo-Trujillo, A.; Trejos-Tamayo, R.; Murcia, H.; Kyoungwon, M.; Barbosa-Espitia, A. Middle Pleistocene palaeolimnology of a dammed tropical river: The Zarzal Formation, Cauca Valley, Colombia. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2017, 487, 194–203. [Google Scholar] [CrossRef]
- Losic, D.; Mitchell, J.G.; Voelcker, N.H. Diatomaceous Lessons in Nanotechnology and Advanced Materials. Adv. Mater. 2009, 21, 2947–2958. [Google Scholar] [CrossRef]
- Sprynskyy, M.; Pomastowski, P.; Hornowska, M.; Krol, A.; Rafinska, K.; Buszewski, B. Naturally organic functionalized 3D biosilica from diatom microalgae. Mater. Des. 2017, 132, 22–29. [Google Scholar] [CrossRef]
- Sumper, M.; Brunner, E. Learning from diatoms: Nature’s tools for the production of nanostructured silica. Adv. Funct. Mater. 2006, 16, 17–26. [Google Scholar] [CrossRef]
- Medarevic, D.P.; Losic, D.; Ibric, S.R. Diatoms—Nature materials with great potential for bioapplications. Hem. Ind. 2016, 70, 613–627. [Google Scholar] [CrossRef]
- Aw, M.S.; Simovic, S.; Addai-Mensah, J.; Losic, D. Silica microcapsules from diatoms as new carrier for delivery of therapeutics. Nanomedicine 2011, 6, 1159–1173. [Google Scholar] [CrossRef] [PubMed]
- Diab, R.; Canilho, N.; Pavel, I.A.; Haffner, F.B.; Girardon, M.; Pasc, A. Silica-based systems for oral delivery of drugs, macromolecules and cells. Adv. Colloid Interface Sci. 2017, 249, 346–362. [Google Scholar] [CrossRef] [PubMed]
- Dolatabadi, J.E.N.; De la Guardia, M. Applications of diatoms and silica nanotechnology in biosensing, drug and gene delivery, and formation of complex metal nanostructures. Trends Anal. Chem. 2011, 30, 1538–1548. [Google Scholar] [CrossRef]
- Wee, K.M.; Rogers, T.N.; Altan, B.S.; Hackney, S.A.; Hamm, C. Engineering and medical applications of diatoms. J. Nanosci. Nanotechnol. 2005, 5, 88–91. [Google Scholar] [CrossRef] [PubMed]
- Vrieling, E.G.; Sun, Q.; Beelen, T.P.; Hazelaar, S.; Gieskes, W.W.; Van Santen, R.A.; Sommerdijk, N.A. Controlled silica synthesis inspired by diatom silicon biomineralization. J. Nanosci. Nanotechnol. 2005, 5, 68–78. [Google Scholar] [CrossRef]
- Veliz, D.S.; Alam, C.; Nietzel, T.; Wyborski, R.; Rivero-Muller, A.; Alam, P. Diatom-inspired skeletonisation of insulin—Mechanistic insights into crystallisation and extracellular bioactivity. Colloids Surf. B 2015, 133, 140–147. [Google Scholar] [CrossRef]
- Szewczyk, A.; Prokopowicz, M.; Sawicki, W.; Majda, D.; Walker, G. Aminopropyl-functionalized mesoporous silica SBA-15 as drug carrier for cefazolin: Adsorption profiles, release studies, and mineralization potential. Micropor. Mesopor. Mater. 2019, 274, 113–126. [Google Scholar] [CrossRef]
- Izquierdo-Barba, I.; Ruiz-Gonzalez, L.; Doadrio, J.C.; Gonzalez-Calbet, J.M.; Vallet-Regi, M. Tissue regeneration: A new property of mesoporous materials. Solid State Sci. 2005, 7, 983–989. [Google Scholar] [CrossRef]
- Jo, Y.K.; Choi, B.H.; Kim, C.S.; Cha, H.J. Diatom-Inspired Silica Nanostructure Coatings with Controllable Microroughness Using an Engineered Mussel Protein Glue to Accelerate Bone Growth on Titanium-Based Implants. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef]
- Belegratis, M.R.; Schmidt, V.; Nees, D.; Stadlober, B.; Hartmann, P. Diatom-inspired templates for 3D replication: Natural diatoms versus laser written artificial diatoms. Bioinspir. Biomim. 2014, 9, 016004. [Google Scholar] [CrossRef]
- Hundertmark, C.; Tinter, R.; Ortelt, M.; Hauser, M.J.B. Diatom-inspired Plastic Deformation Elements for Energy Absorption in Automobiles. J. Bionic Eng. 2015, 12, 613–623. [Google Scholar] [CrossRef]
- Li, A.B.; Zhao, X.G.; Duan, G.W.; Anderson, S.; Zhang, X. Diatom Frustule-Inspired Metamaterial Absorbers: The Effect of Hierarchical Pattern Arrays. Adv. Funct. Mater. 2019, 29. [Google Scholar] [CrossRef]
- Jiang, W.K.; Luo, S.P.; Liu, P.W.; Deng, X.Y.; Jing, Y.; Bai, C.Y.; Li, J.B. Purification of biosilica from living diatoms by a two-step acid cleaning and baking method. J. Appl. Phycol. 2014, 26, 1511–1518. [Google Scholar] [CrossRef]
- Wang, Y.; Cai, J.; Jiang, Y.G.; Jiang, X.G.; Zhang, D.Y. Preparation of biosilica structures from frustules of diatoms and their applications: Current state and perspectives. Appl. Microbiol. Biotechnol. 2013, 97, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Shahbazi, M.A.; Hamidi, M.; Makila, E.M.; Zhang, H.; Almeida, P.V.; Kaasalainen, M.; Salonen, J.J.; Hirvonen, J.T.; Santos, H.A. The mechanisms of surface chemistry effects of mesoporous silicon nanoparticles on immunotoxicity and biocompatibility. Biomaterials 2013, 34, 7776–7789. [Google Scholar] [CrossRef] [PubMed]
- Rea, I.; Martucci, N.M.; De Stefano, L.; Ruggiero, I.; Terracciano, M.; Dardano, P.; Migliaccio, N.; Arcari, P.; Tate, R.; Rendina, I.; et al. Diatomite biosilica nanocarriers for siRNA transport inside cancer cells. Biochim. Biophys. Acta 2014, 1840, 3393–3403. [Google Scholar] [CrossRef]
- Terracciano, M.; Shahbazi, M.A.; Correia, A.; Rea, I.; Lamberti, A.; De Stefano, L.; Santos, H.A. Surface bioengineering of diatomite based nanovectors for efficient intracellular uptake and drug delivery. Nanoscale 2015, 7, 20063–20074. [Google Scholar] [CrossRef]
- Martucci, N.M.; Migliaccio, N.; Ruggiero, I.; Albano, F.; Cali, G.; Romano, S.; Terracciano, M.; Rea, I.; Arcari, P.; Lamberti, A. Nanoparticle-based strategy for personalized B-cell lymphoma therapy. Int. J. Nanomed. 2016, 11, 6089–6101. [Google Scholar] [CrossRef]
- Terracciano, M.; Napolitano, M.; De Stefano, L.; De Luca, A.C.; Rea, I. Gold decorated porous biosilica nanodevices for advanced medicine. Nanotechnology 2018, 29, 235601. [Google Scholar] [CrossRef]
- Zhang, H.; Shahbazi, M.A.; Makila, E.M.; Da Silva, T.H.; Reis, R.L.; Salonen, J.J.; Hirvonen, J.T.; Santos, H.A. Diatom silica microparticles for sustained release and permeation enhancement following oral delivery of prednisone and mesalamine. Biomaterials 2013, 34, 9210–9219. [Google Scholar] [CrossRef]
- Cicco, S.R.; Vona, D.; Gristina, R.; Sardella, E.; Ragni, R.; Lo Presti, M.; Farinola, G.M. Biosilica from Living Diatoms: Investigations on Biocompatibility of Bare and Chemically Modified Thalassiosira weissflogii Silica Shells. Bioengineering 2016, 3. [Google Scholar] [CrossRef] [PubMed]
- Frohlich, E. The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. Int. J. Nanomed. 2012, 7, 5577–5591. [Google Scholar] [CrossRef] [PubMed]
- Witecka, A.; Yamamoto, A.; Dybiec, H.; Swieszkowski, W. Surface characterization and cytocompatibility evaluation of silanized magnesium alloy AZ91 for biomedical applications. Sci. Technol. Adv. Mater. 2012, 13, 064214. [Google Scholar] [CrossRef] [PubMed]
- Filippini, P.; Rainaldi, G.; Ferrante, A.; Mecheri, B.; Gabrielli, G.; Bombace, M.; Indovina, P.L.; Santini, M.T. Modulation of osteosarcoma cell growth and differentiation by silane-modified surfaces. J. Biomed. Mater. Res. 2001, 55, 338–349. [Google Scholar] [CrossRef]
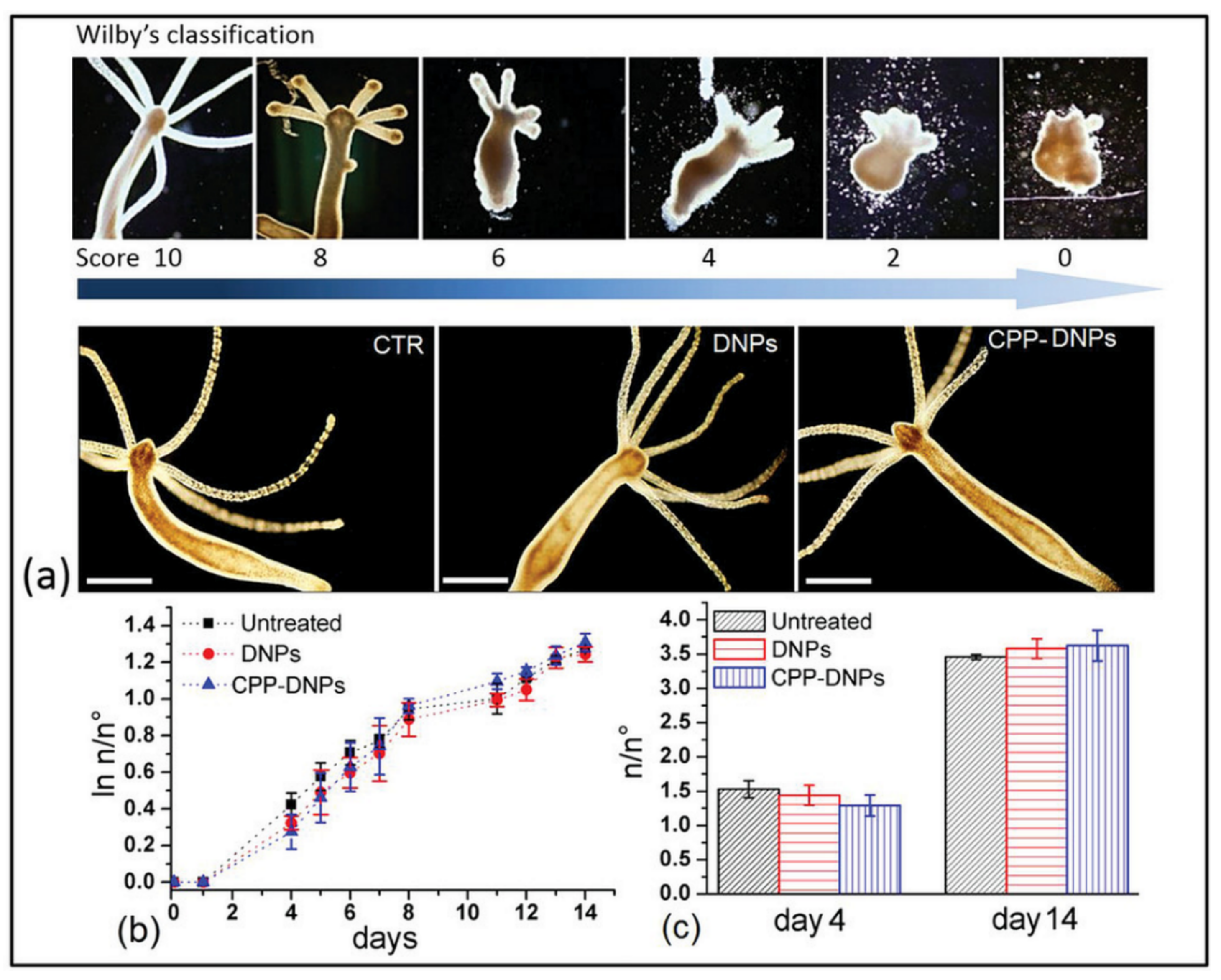
- Terracciano, M.; De Stefano, L.; Tortiglione, C.; Tino, A.; Rea, I. In Vivo Toxicity Assessment of Hybrid Diatomite Nanovectors Using Hydra vulgaris as a Model System. Adv. Biosyst. 2019, 3. [Google Scholar] [CrossRef]
- Karntanut, W.; Pascoe, D. A comparison of methods for measuring acute toxicity to Hydra vulgaris. Chemosphere 2000, 41, 1543–1548. [Google Scholar] [CrossRef]
- Wilby, O.K.; Tesh, J.M. The Hydra assay as an early screen for teratogenic potential. Toxicol. In Vitro 1990, 4, 582–583. [Google Scholar] [CrossRef]
- Ahire, E.; Thakkar, S.; Darshanwad, M.; Misra, M. Parenteral nanosuspensions: A brief review from solubility enhancement to more novel and specific applications. Acta Pharm. Sin. B 2018, 8, 733–755. [Google Scholar] [CrossRef]
- Tian, X.; Li, H.; Zhang, D.; Liu, G.; Jia, L.; Zheng, D.; Shen, J.; Shen, Y.; Zhang, Q. Nanosuspension for parenteral delivery of a p-terphenyl derivative: Preparation, characteristics and pharmacokinetic studies. Colloids Surf. B 2013, 108, 29–33. [Google Scholar] [CrossRef]
- Sun, B.; Yeo, Y. Nanocrystals for the parenteral delivery of poorly water-soluble drugs. Curr. Opin. Solid State Mater. Sci. 2012, 16, 295–301. [Google Scholar] [CrossRef]
- Cauda, V.; Schlossbauer, A.; Bein, T. Bio-degradation study of colloidal mesoporous silica nanoparticles: Effect of surface functionalization with organo-silanes and poly(ethylene glycol). Microporous Mesoporous Mater. 2010, 132, 60–71. [Google Scholar] [CrossRef]
- Hao, N.; Liu, H.; Li, L.; Chen, D.; Li, L.; Tang, F. In vitro degradation behavior of silica nanoparticles under physiological conditions. J. Nanosci. Nanotechnol. 2012, 12, 6346–6354. [Google Scholar] [CrossRef] [PubMed]
- Bao, Z.H.; Ernst, E.M.; Yoo, S.; Sandhage, K.H. Syntheses of Porous Self-Supporting Metal-Nanoparticle Assemblies with 3D Morphologies Inherited from Biosilica Templates (Diatom Frustules). Adv. Mater. 2009, 21, 474–478. [Google Scholar] [CrossRef]
- Bao, Z.; Weatherspoon, M.R.; Shian, S.; Cai, Y.; Graham, P.D.; Allan, S.M.; Ahmad, G.; Dickerson, M.B.; Church, B.C.; Kang, Z.; et al. Chemical reduction of three-dimensional silica micro-assemblies into microporous silicon replicas. Nature 2007, 446, 172–175. [Google Scholar] [CrossRef] [PubMed]
- Maher, S.; Alsawat, M.; Kumeria, T.; Fathalla, D.; Fetih, G.; Santos, A.; Habib, F.; Losic, D. Luminescent Silicon Diatom Replicas: Self-Reporting and Degradable Drug Carriers with Biologically Derived Shape for Sustained Delivery of Therapeutics. Adv. Funct. Mater. 2015, 25, 5107–5116. [Google Scholar] [CrossRef]
- Maher, S.; Kumeria, T.; Wang, Y.; Kaur, G.; Fathalla, D.; Fetih, G.; Santos, A.; Habib, F.; Evdokiou, A.; Losic, D. From The Mine to Cancer Therapy: Natural and Biodegradable Theranostic Silicon Nanocarriers from Diatoms for Sustained Delivery of Chemotherapeutics. Adv. Healthc. Mater. 2016, 5, 2667–2678. [Google Scholar] [CrossRef]
- Aw, M.S.; Simovic, S.; Yu, Y.; Addai-Mensah, J.; Losic, D. Porous silica microshells from diatoms as biocarrier for drug delivery applications. Powder Technol. 2012, 223, 52–58. [Google Scholar] [CrossRef]
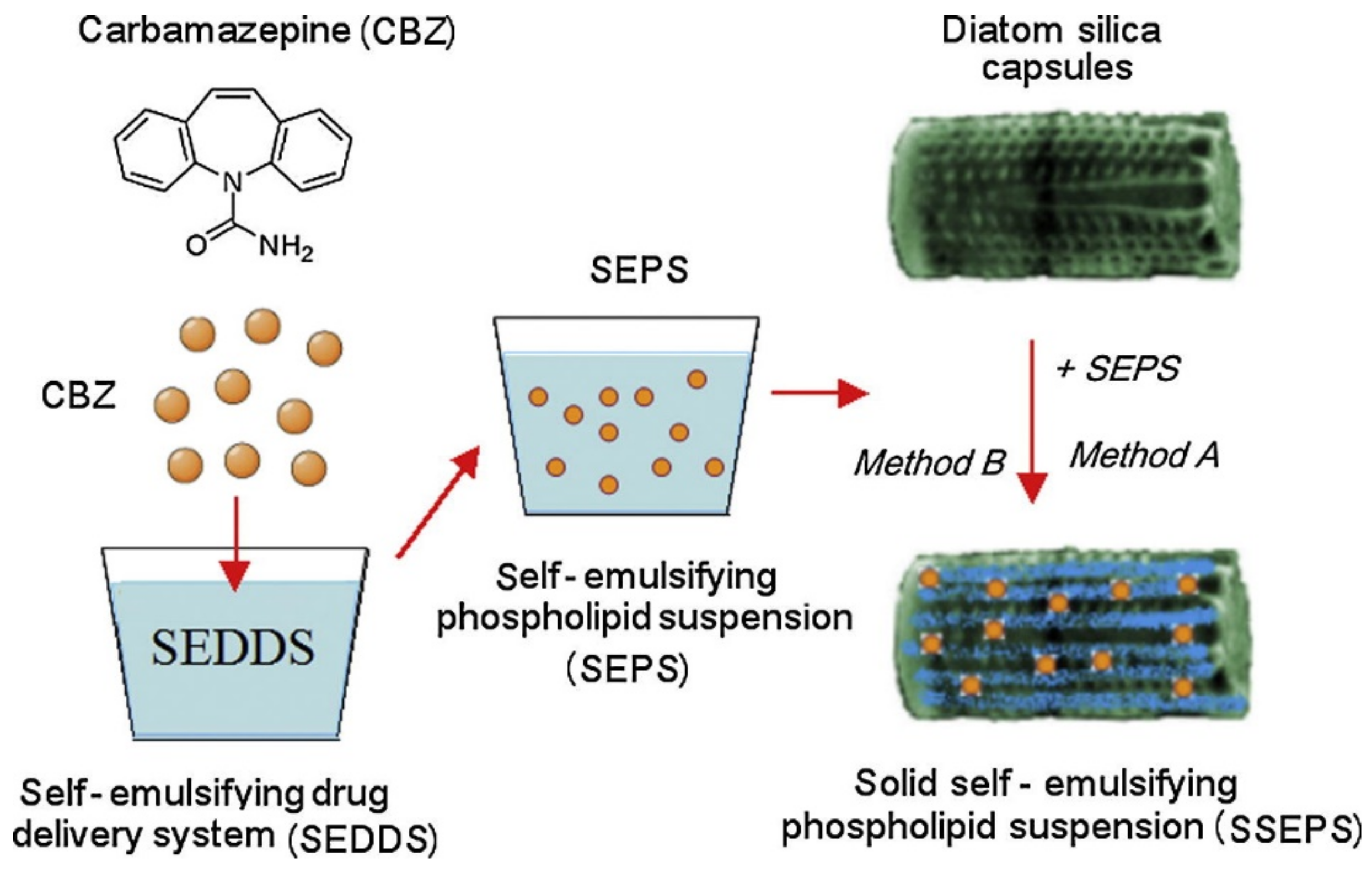
- Milovic, M.; Simovic, S.; Losic, D.; Dashevskiy, A.; Ibric, S. Solid self-emulsifying phospholipid suspension (SSEPS) with diatom as a drug carrier. Eur. J. Pharm. Sci. 2014, 63, 226–232. [Google Scholar] [CrossRef]
- Gnanamoorthy, P.; Anandhan, S.; Prabu, V.A. Natural nanoporous silica frustules from marine diatom as a biocarrier for drug delivery. J. Porous. Mater. 2014, 21, 789–796. [Google Scholar] [CrossRef]
- Vona, D.; Leone, G.; Ragni, R.; Palumbo, F.; Evidente, A.; Vurro, M.; Farinola, G.M.; Cicco, S.R. Diatoms Biosilica as Efficient Drug-Delivery System. Mrs Adv. 2016, 1, 3825–3830. [Google Scholar] [CrossRef]
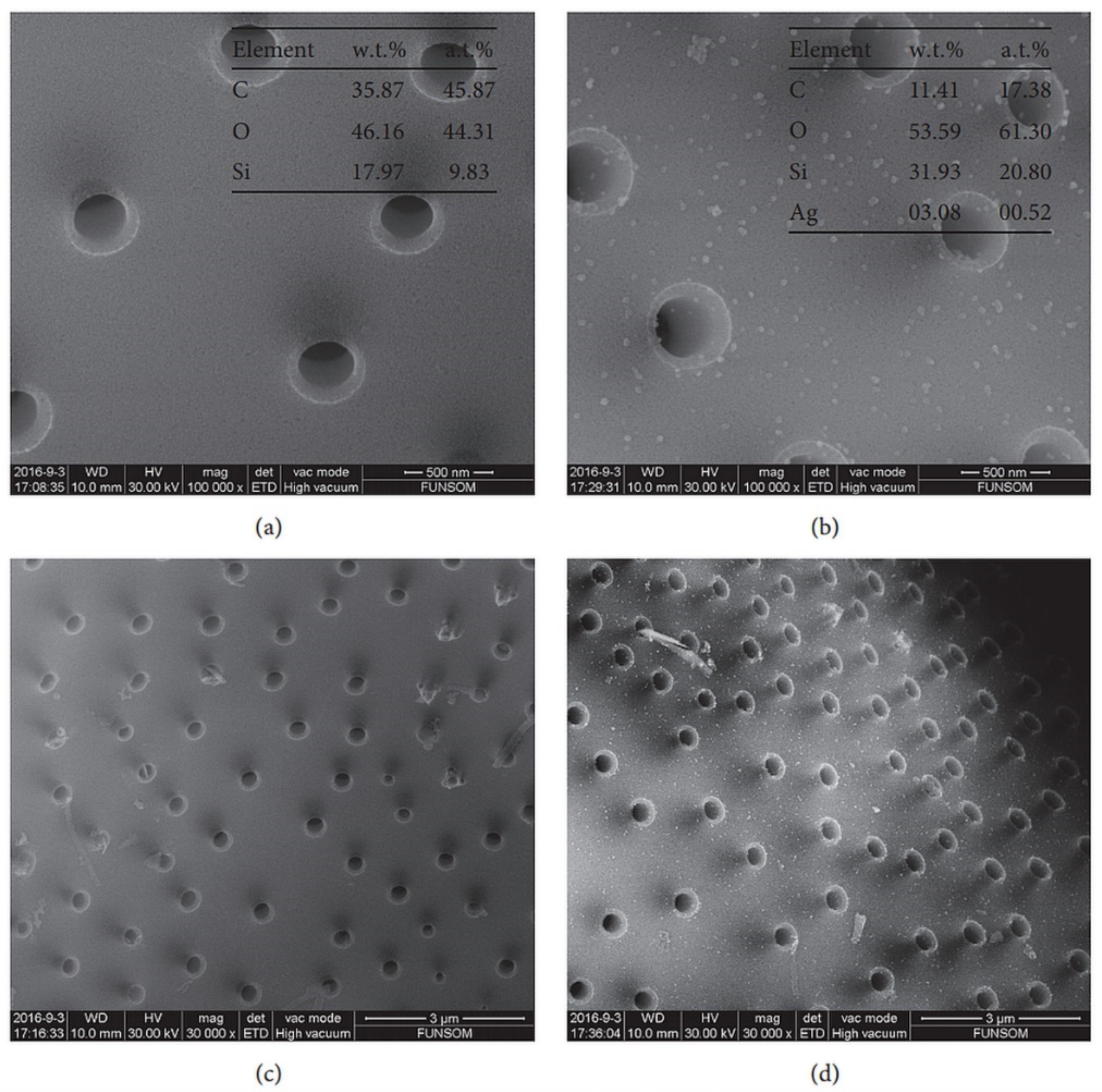
- Sun, H.L.; Wen, X.X.; Zhang, X.; Wei, D.L.; Yang, H.L.; Li, C.D.; Yang, L. Biocompatible Silver Nanoparticle-Modified Natural Diatomite with Anti-Infective Property. J. Nanomater. 2018, 1–8. [Google Scholar] [CrossRef]
- Uthappa, U.T.; Brahmkhatri, V.; Sriram, G.; Jung, H.Y.; Yu, J.; Kurkuri, N.; Aminabhavi, T.M.; Altalhi, T.; Neelgund, G.M.; Kurkuri, M.D. Nature engineered diatom biosilica as drug delivery systems. J. Control. Release 2018, 281, 70–83. [Google Scholar] [CrossRef] [PubMed]
- Losic, D.; Yu, Y.; Aw, M.S.; Simovic, S.; Thierry, B.; Addai-Mensah, J. Surface functionalisation of diatoms with dopamine modified iron-oxide nanoparticles: Toward magnetically guided drug microcarriers with biologically derived morphologies. Chem. Commun. 2010, 46, 6323–6325. [Google Scholar] [CrossRef] [PubMed]
- Bariana, M.; Aw, M.S.; Kurkuri, M.; Losic, D. Tuning drug loading and release properties of diatom silica microparticles by surface modifications. Int. J. Pharm. 2013, 443, 230–241. [Google Scholar] [CrossRef] [PubMed]
- Kumeria, T.; Bariana, M.; Altalhi, T.; Kurkuri, M.; Gibson, C.T.; Yang, W.R.; Losic, D. Graphene oxide decorated diatom silica particles as new nano-hybrids: Towards smart natural drug microcarriers. J. Mater. Chem. B 2013, 1, 6302–6311. [Google Scholar] [CrossRef]
- Ruggiero, I.; Terracciano, M.; Martucci, N.M.; De Stefano, L.; Migliaccio, N.; Tate, R.; Rendina, I.; Arcari, P.; Lamberti, A.; Rea, I. Diatomite silica nanoparticles for drug delivery. Nanoscale Res. Lett. 2014, 9, 329. [Google Scholar] [CrossRef]
- Aw, M.S.; Bariana, M.; Yu, Y.; Addai-Mensah, J.; Losic, D. Surface-functionalized diatom microcapsules for drug delivery of water-insoluble drugs. J. Biomater. Appl. 2013, 28, 163–174. [Google Scholar] [CrossRef]
- Cicco, S.R.; Vona, D.; De Giglio, E.; Cometa, S.; Mattioli-Belmonte, M.; Palumbo, F.; Ragni, R.; Farinola, G.M. Chemically Modified Diatoms Biosilica for Bone Cell Growth with Combined Drug-Delivery and Antioxidant Properties. Chempluschem 2015, 80, 1104–1112. [Google Scholar] [CrossRef]
- Vasani, R.B.; Losic, D.; Cavallaro, A.; Voelcker, N.H. Fabrication of stimulus-responsive diatom biosilica microcapsules for antibiotic drug delivery. J. Mater. Chem. B 2015, 3, 4325–4329. [Google Scholar] [CrossRef]
- Delalat, B.; Sheppard, V.C.; Rasi Ghaemi, S.; Rao, S.; Prestidge, C.A.; McPhee, G.; Rogers, M.L.; Donoghue, J.F.; Pillay, V.; Johns, T.G.; et al. Targeted drug delivery using genetically engineered diatom biosilica. Nat. Commun. 2015, 6, 8791. [Google Scholar] [CrossRef]
- Janicijevic, J.; Milic, J.; Calija, B.; Micov, A.; Stepanovic-Petrovic, R.; Tomic, M.; Dakovic, A.; Dobricic, V.; Vasiljevic, B.N.; Krajisnik, D. Potentiation of the ibuprofen antihyperalgesic effect using inorganically functionalized diatomite. J. Mater. Chem. B 2018, 6, 5812–5822. [Google Scholar] [CrossRef]
- Sasirekha, R.; Sheena, T.S.; Sathiya Deepika, M.; Santhanam, P.; Townley, H.E.; Jeganathan, K.; Dinesh Kumar, S.; Premkumar, K. Surface engineered Amphora subtropica frustules using chitosan as a drug delivery platform for anticancer therapy. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 94, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Delasoie, J.; Rossier, J.; Haeni, L.; Rothen-Rutishauser, B.; Zobi, F. Slow-targeted release of a ruthenium anticancer agent from vitamin B12 functionalized marine diatom microalgae. Dalton Trans. 2018, 47, 17221–17232. [Google Scholar] [CrossRef] [PubMed]
- Townley, H.E.; Parker, A.R.; White-Cooper, H. Exploitation of diatom frustules for nanotechnology: Tethering active biomolecules. Adv. Funct. Mater. 2008, 18, 369–374. [Google Scholar] [CrossRef]
- Manago, S.; Migliaccio, N.; Terracciano, M.; Napolitano, M.; Martucci, N.M.; De Stefano, L.; Rendina, I.; De Luca, A.C.; Lamberti, A.; Rea, I. Internalization kinetics and cytoplasmic localization of functionalized diatomite nanoparticles in cancer cells by Raman imaging. J. Biophotonics 2018, 11, e201700207. [Google Scholar] [CrossRef]
- Grommersch, B.M.; Pant, J.; Hopkins, S.P.; Goudie, M.J.; Handa, H. Biotemplated Synthesis and Characterization of Mesoporous Nitric Oxide-Releasing Diatomaceous Earth Silica Particles. ACS Appl. Mater. Interfaces 2018, 10, 2291–2301. [Google Scholar] [CrossRef]
- Ignarro, L.J.; Buga, G.M.; Wood, K.S.; Byrns, R.E.; Chaudhuri, G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc. Natl. Acad. Sci. USA 1987, 84, 9265–9269. [Google Scholar] [CrossRef]
- Liang, H.; Nacharaju, P.; Friedman, A.; Friedman, J.M. Nitric oxide generating/releasing materials. Future Sci. OA 2015, 1. [Google Scholar] [CrossRef]
- Eroy-Reveles, A.A.; Mascharak, P.K. Nitric oxide-donating materials and their potential in pharmacological applications for site-specific nitric oxide delivery. Future Med. Chem. 2009, 1, 1497–1507. [Google Scholar] [CrossRef]
- Al-Sa’doni, H.; Ferro, A. S-Nitrosothiols: A class of nitric oxide-donor drugs. Clin. Sci. 2000, 98, 507–520. [Google Scholar] [CrossRef]
- Todd, T.; Zhen, Z.; Tang, W.; Chen, H.; Wang, G.; Chuang, Y.J.; Deaton, K.; Pan, Z.; Xie, J. Iron oxide nanoparticle encapsulated diatoms for magnetic delivery of small molecules to tumors. Nanoscale 2014, 6, 2073–2076. [Google Scholar] [CrossRef] [PubMed]
- Javalkote, V.S.; Pandey, A.P.; Puranik, P.R.; Deshmukh, P.K. Magnetically responsive siliceous frustules for efficient chemotherapy. Mater. Sci. Eng. C 2015, 50, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Nainiwal, S.; Choudhary, I.; Tewari, H.K.; Verma, L.K. Role of daunorubicin in inhibiting proliferative vitreoretinopathy after retinal detachment surgery. Clin. Exp. Ophthalmol. 2002, 30, 348–351. [Google Scholar] [CrossRef] [PubMed]
- Uthappa, U.T.; Sriram, G.; Brahmkhatri, V.; Kigga, M.; Jung, H.Y.; Altalhi, T.; Neelgund, G.M.; Kurkuri, M.D. Xerogel modified diatomaceous earth microparticles for controlled drug release studies. New J. Chem. 2018, 42, 11964–11971. [Google Scholar] [CrossRef]
- Lopez-Cebral, R.; Peng, G.; Reys, L.L.; Silva, S.S.; Oliveira, J.M.; Chen, J.; Silva, T.H.; Reis, R.L. Dual delivery of hydrophilic and hydrophobic drugs from chitosan/diatomaceous earth composite membranes. J. Mater. Sci. Mater. Med. 2018, 29, 21. [Google Scholar] [CrossRef]

| Diatom sp. | Functionalization | Drug | Ref. | |||
|---|---|---|---|---|---|---|
| Aim | Composition | wt% | Model | Class | ||
| Aulacoseirasp. | Magnetic guide, drug loading and release | DOPA/Fe3O4 | 28 | Indomethacin | NSAID | [63] |
| Aulacoseira sp. | Drug loading and release | APTES | 22 (I) | Indomethacin (I) Gentamicin (G) | NSAID (I) Antibio (G) | [64] |
| 15 (G) | ||||||
| 2-CEPA | 24 (I) | |||||
| 16 (G) | ||||||
| 16-PHA | 14 (I) | |||||
| 22 (G) | ||||||
| OTS | 14 (I) | |||||
| GPTMS | 19 (I) | |||||
| mPEG-silane | 17 (I) | |||||
| Aulacoseira sp. | Drug loading and release | GO | 28.5 | Indomethacin | NSAID | [65] |
| n.i. | Tracker nanosized diatomite Tumor targeting delivery | Rhodamine | - | - | - | [66] |
| Aulacoseira sp. | Drug loading and release | APTES | 19 | Indomethacin | NSAID | [67] |
| AEAPTMS | 24 | |||||
| 2-Phos | 22 | |||||
| 16-Phos | 15 | |||||
| n.i. | Tumor targeting delivery | peptide/siRNA | - | siRNA | Gene silencer | [36,38] |
| Thalassiosira weissflogii | Reactive oxygen species (ROS) scavenger | TEMPO | 2 | Ciprofloxacin | Antibio | [68] |
| Aulacoseira sp. | Temperature-responsive drug release | (O(EG)MA) copolymers | - | Levoflaxin | Antibio | [69] |
| Thalassiosira pseudonana | Tumor targeting delivery | Antibodies | - | Camptothecin and derivatives | Anticancer | [70] |
| n.i. | Tumor targeting delivery | PEG-CPP | 22 | Sorafenib | Anticancer | [37] |
| n.i. | Drug loading and release | Al2(SO4)3 | 20 | Ibuprofen | NSAID | [71] |
| Amphora subtropica | Drug loading and release | Chitosan | - | Doxorubicin | Anticancer | [72] |
| Aulacoseira sp. | Tumor targeting delivery | Vitamin B12 | 6 | Cisplatin | Anticancer | [73] |
| 10 | 5-FU | |||||
| 2 | Ruthenium complex | |||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delasoie, J.; Zobi, F. Natural Diatom Biosilica as Microshuttles in Drug Delivery Systems. Pharmaceutics 2019, 11, 537. https://doi.org/10.3390/pharmaceutics11100537
Delasoie J, Zobi F. Natural Diatom Biosilica as Microshuttles in Drug Delivery Systems. Pharmaceutics. 2019; 11(10):537. https://doi.org/10.3390/pharmaceutics11100537
Chicago/Turabian StyleDelasoie, Joachim, and Fabio Zobi. 2019. "Natural Diatom Biosilica as Microshuttles in Drug Delivery Systems" Pharmaceutics 11, no. 10: 537. https://doi.org/10.3390/pharmaceutics11100537
APA StyleDelasoie, J., & Zobi, F. (2019). Natural Diatom Biosilica as Microshuttles in Drug Delivery Systems. Pharmaceutics, 11(10), 537. https://doi.org/10.3390/pharmaceutics11100537






