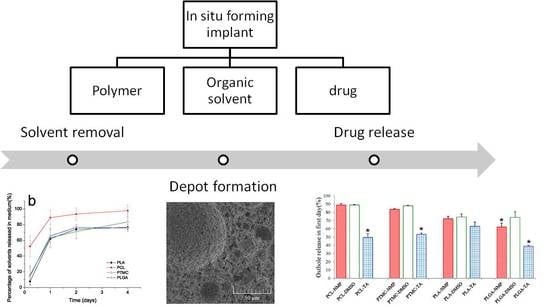
Effect of Polymer Permeability and Solvent Removal Rate on In Situ Forming Implants: Drug Burst Release and Microstructure
Abstract
1. Introduction
2. Materials and Methods
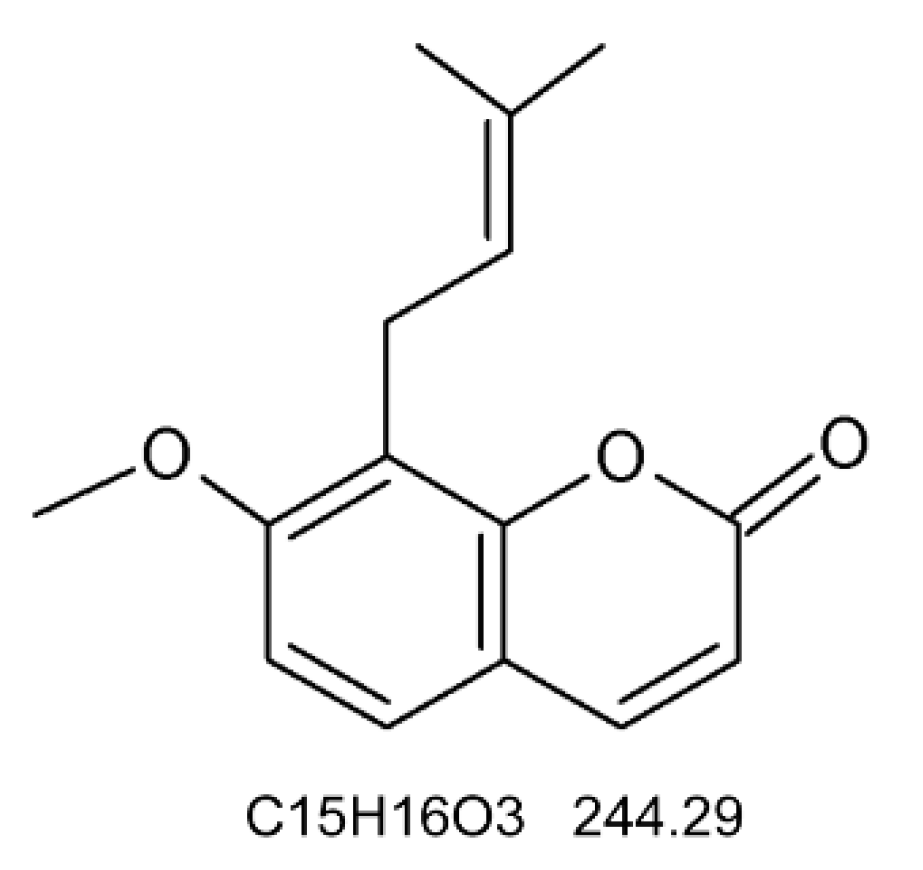
2.1. Materials
2.2. Preparation of Ost-Loaded ISFIs
2.3. In Vitro Drug Release and Polymer Degradation
2.4. In Vitro Solvent Removal Rate from ISFI
2.5. Polymer-Solvent Affinity Evaluation
2.6. Morphological Analysis of ISFI Depots
2.7. In Vivo Drug Release Study
2.8. Statistical Analysis
3. Results
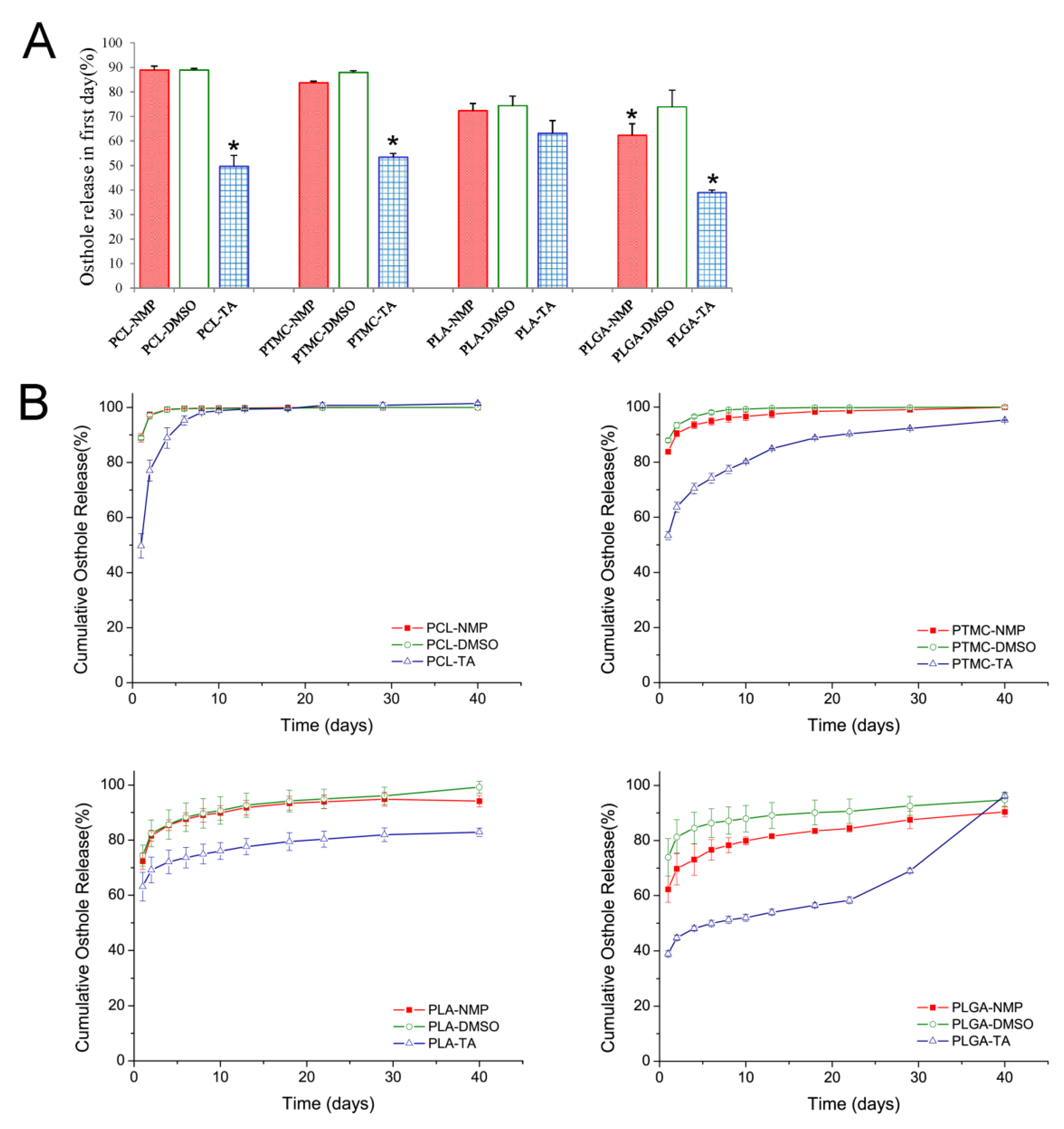
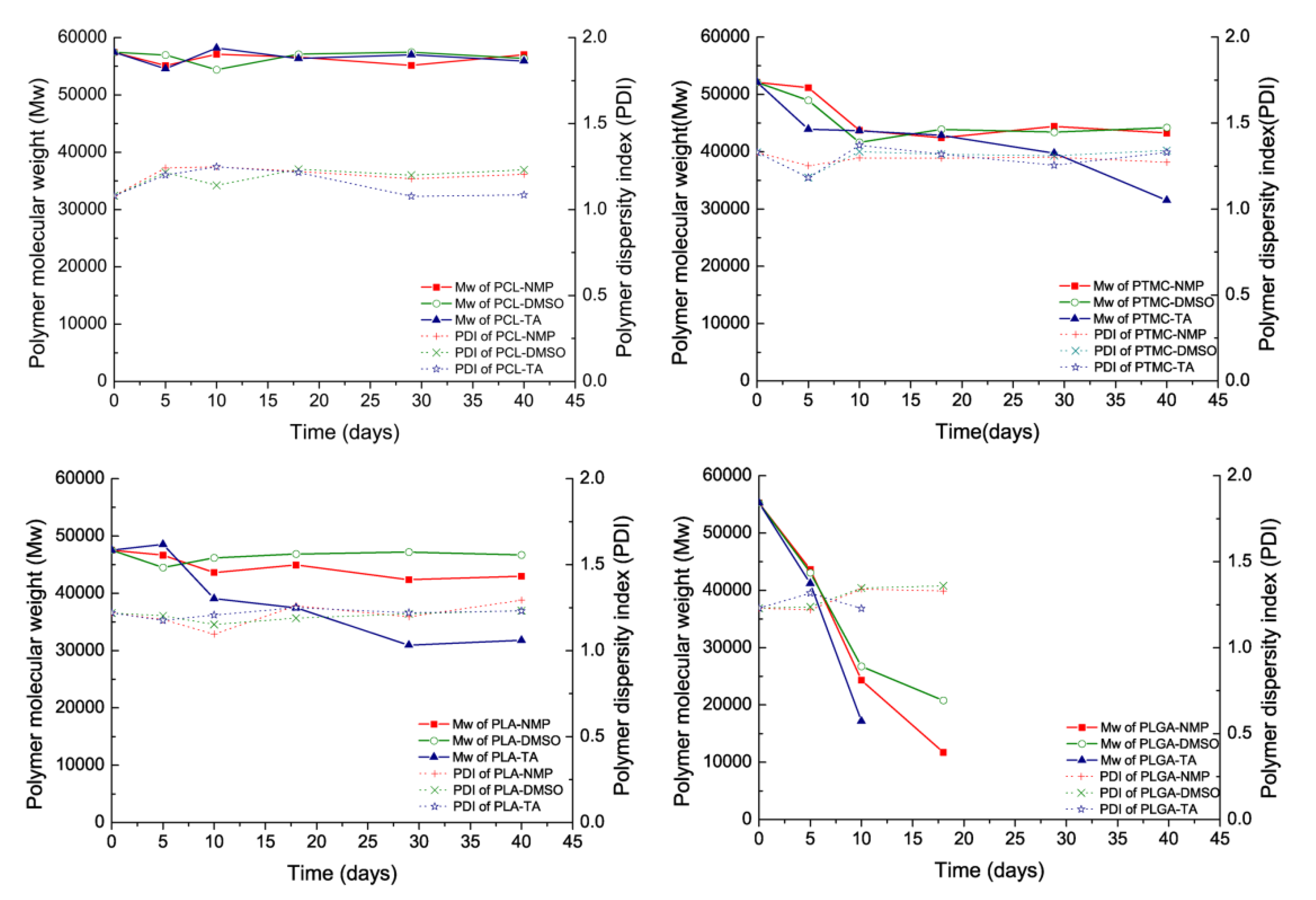
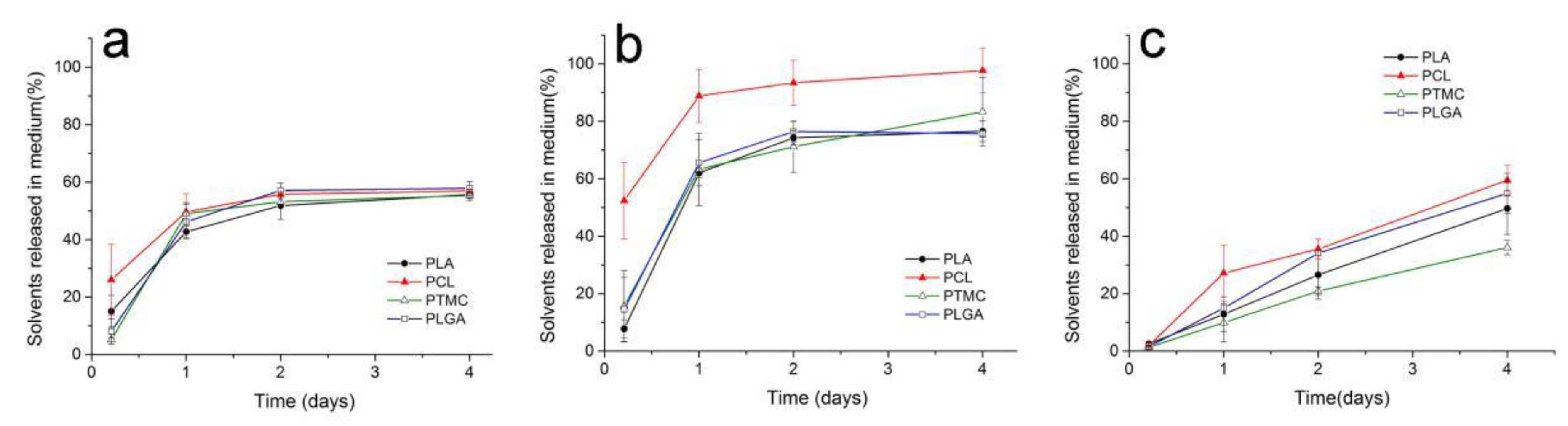
3.1. In Vitro Ost Release and Polymer Degradation
3.2. Solvent Removal Rate from ISFI
3.3. Polymer-Solvent Affinity Evaluation
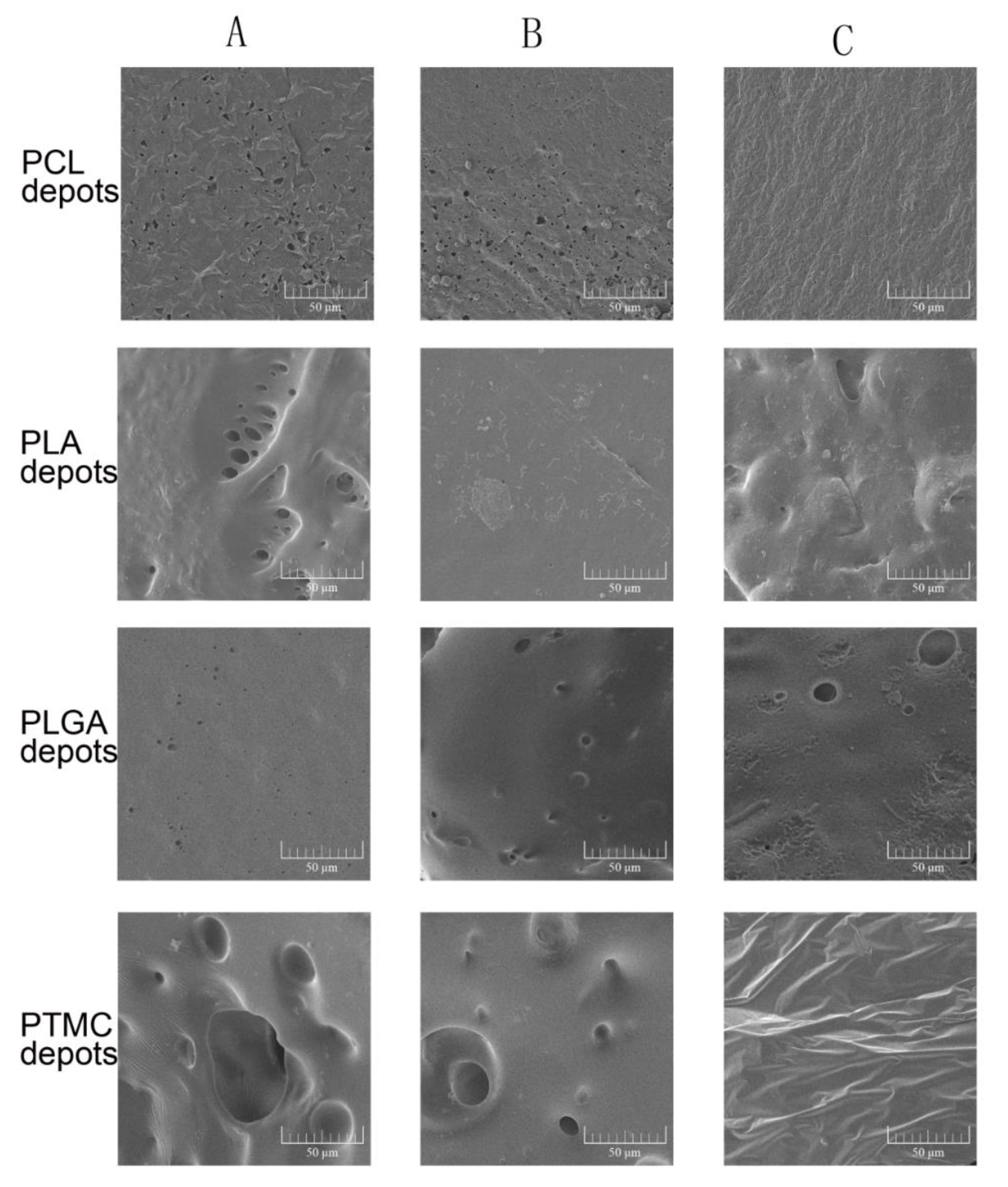
3.4. Morphological Analysis of ISFI Depots
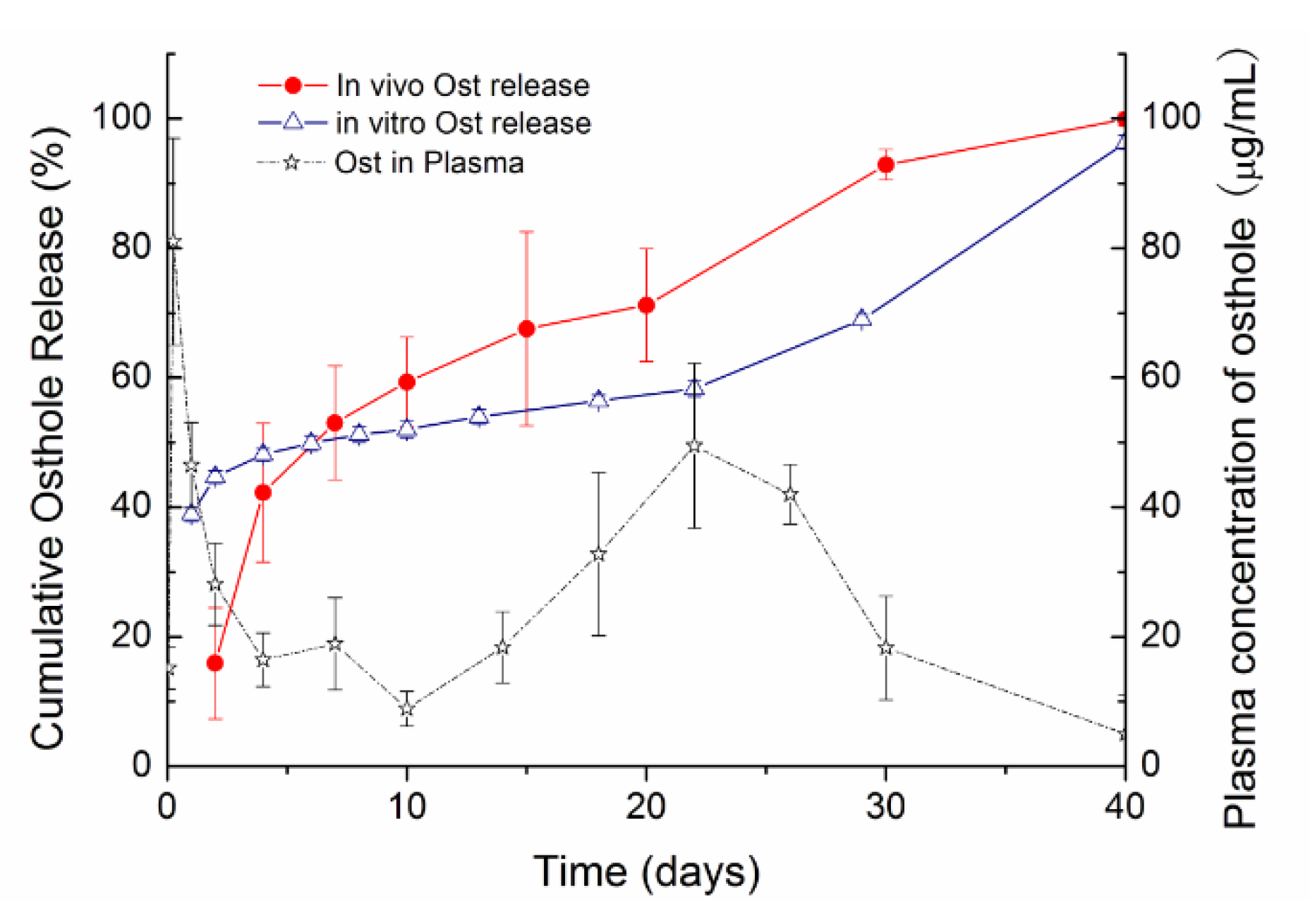
3.5. Drug Release of Ost-ISFI In Vivo
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dunn, R.L.; English, J.P.; Cowsar, D.R.; Vanderbilt, D.P. Biodegradable In-Situ Forming Implants and Methods of Producing the Same. U.S. Patent 5,733,950, 31 March 1998. [Google Scholar]
- Kempe, S.; Mäder, K. In situ forming implants—An attractive formulation principle for parenteral depot formulations. J. Controll. Release 2012, 161, 668–679. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.R.; Stoller, N.H. Rationale for the use of atridox therapy for managing periodontal patients. Compend. Contin. Educ. Dent. 1999, 20, 19. [Google Scholar] [PubMed]
- Mukhatar Ahmed, J.; Vandana, K.L. A comparative evaluation of atrigel delivery system (10% doxycycline hyclate) atridox with scaling and root planing and combination therapy in treatment of periodontitis: A clinical study. J. Indian Soc. Periodontol. 2012, 16, 43–48. [Google Scholar]
- Morenofernández, A. Eligard. Tiny implant fights prostate cancer. Nursing 2002, 32, 18. [Google Scholar]
- Sartor, O. Eligard: Leuprolide acetate in a novel sustained-release delivery system. Urology 2003, 61, 25–31. [Google Scholar] [CrossRef]
- Gad, H.A.; El-Nabarawi, M.A.; Abd El-Hady, S.S. Formulation and evaluation of pla and plga in situ implants containing secnidazole and/or doxycycline for treatment of periodontitis. Aaps Pharmscitech 2008, 9, 878–884. [Google Scholar] [CrossRef]
- Camargo, J.A.; Sapin, A.; Nouvel, C.; Daloz, D.; Leonard, M.; Bonneaux, F.; Six, J.L.; Maincent, P. Injectable pla-based in situ forming implants for controlled release of ivermectin a bcs class ii drug: Solvent selection based on physico-chemical characterization. Drug Dev. Ind. Pharm. 2013, 39, 146–155. [Google Scholar] [CrossRef]
- Deadman, C.M.; Kellaway, I.W.; Yasin, M.; Dickinson, P.A.; Murdan, S. An investigation into the influence of drug lipophilicity on the in vivo absorption profiles from subcutaneous microspheres and in situ forming depots. J. Controll. Release 2007, 122, 79–85. [Google Scholar] [CrossRef]
- Berndl, E.; Hysi, E.; Hernandez, C.; Exner, A.; Kolios, M. Using Ultrasound and Photoacoustics to Monitor In Situ Forming Implant Structure and Drug Release; IEEE International Ultrasonics Symposium: Scotland, UK, 2017. [Google Scholar]
- Sun, Y.; Jensen, H.; Petersen, N.J.; Larsen, S.W.; Østergaard, J. Phase separation of in situ forming poly (lactide-co-glycolide acid) implants investigated using a hydrogel-based subcutaneous tissue surrogate and uv-vis imaging. J. Pharm. Biomed. Anal. 2017, 145, 682–691. [Google Scholar] [CrossRef]
- Hatefi, A.; Amsden, B. Biodegradable injectable in situ forming drug delivery systems. J. Controll. Release 2002, 80, 9–28. [Google Scholar] [CrossRef]
- Yoshioka, T.; Kamada, F.; Kawazoe, N.; Tateishi, T.; Chen, G. Structural changes and biodegradation of plla, pcl, and plga sponges during in vitro incubation. Polym. Eng. Sci. 2010, 50, 1895–1903. [Google Scholar] [CrossRef]
- Zhang, Z.; Kuijer, R.; Bulstra, S.K.; Grijpma, D.W.; Feijen, J. The in vivo and in vitro degradation behavior of poly (trimethylene carbonate). Biomaterials 2006, 27, 1741–1748. [Google Scholar] [CrossRef] [PubMed]
- Kluin, O.S.; Busscher, H.J.; Neut, D.; van der Mei, H.C. Poly (trimethylene carbonate) as a carrier for rifampicin and vancomycin to target therapy-recalcitrant staphylococcal biofilms. J. Orthop. Res. 2016, 34, 1828–1837. [Google Scholar] [CrossRef] [PubMed]
- Thakur, R.R.; McMillan, H.L.; Jones, D.S. Solvent induced phase inversion-based in situ forming controlled release drug delivery implants. J. Controll. Release 2014, 176, 8–23. [Google Scholar] [CrossRef] [PubMed]
- Darestani-Farahani, M.; Vasheghani-Farahani, E.; Mobedi, H.; Ganji, F. The effect of solvent composition on vancomycin hydrochloride and free base vancomycin release fromin situforming implants. Polym. Adv. Technol. 2016, 27, 1653–1663. [Google Scholar] [CrossRef]
- Huang, X.; Brazel, C.S. On the importance and mechanisms of burst release in matrix-controlled drug delivery systems. J. Controll. Release 2001, 73, 121–136. [Google Scholar] [CrossRef]
- Juvekar, S.; Kathpalia, H. Solvent removal precipitation based in situ forming implant for controlled drug delivery in periodontitis. J. Controll. Release 2017, 251, 75–81. [Google Scholar] [CrossRef]
- Gao, Z.; Wen, Q.; Xia, Y.; Yang, J.; Gao, P.; Zhang, N.; Li, H.; Zou, S. Osthole augments therapeutic efficiency of neural stem cells–based therapy in experimental autoimmune encephalomyelitis. J. Pharmacol. Sci. 2014, 124, 54–65. [Google Scholar] [CrossRef]
- Meng, S.; Zhang, C.; Shi, W.; Zhang, X.W.; Liu, D.H.; Wang, P.; Li, J.X.; Jin, Y. Preparation of osthole-loaded nano-vesicles for skin delivery: Characterization, in vitro skin permeation and preliminary in vivo pharmacokinetic studies. Eur. J. Pharm. Sci. 2016, 92, 49–54. [Google Scholar] [CrossRef]
- Zhang, X.N.; Zhang, C.G.; Zhu, Q.L.; Zhou, Y.; Liu, Y.; Chen, W.L.; Yang, S.D.; Zhou, X.F.; Zhu, A.J.; Jin, Y.; et al. N-succinyl-chitosan nanoparticles coupled with low-density lipoprotein for targeted osthole-loaded delivery to low-density lipoprotein receptor-rich tumors. Int. J. Nanomed. 2014, 9, 2919. [Google Scholar] [CrossRef]
- Bakhshi, R.; Vasheghani-Farahani, E.; Mobedi, H.; Jamshidi, A.; Khakpour, M. The effect of additives on naltrexone hydrochloride release and solvent removal rate from an injectablein situ forming plga implant. Polym. Adv. Technol. 2006, 17, 354–359. [Google Scholar] [CrossRef]
- Liu, Y.; Kemmer, A.; Keim, K.; Curdy, C.; Petersen, H.; Kissel, T. Poly (ethylene carbonate) as a surface-eroding biomaterial for in situ forming parenteral drug delivery systems: A feasibility study. Eur. J. Pharm. Biopharm. 2010, 76, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, J.F.; Dawson, P.E. Quantitation of dimethyl sulfoxide in solutions and tissues by high-performance liquid chromatography. Cryobiology 1991, 28, 210–215. [Google Scholar] [CrossRef]
- Shea, J.A.; Cook, S.C.; Shamrock, W.F.; Segretario, J. Quantitation of residual n-methylpyrrolidone in losoxantrone hydrochloride by reversed-phase high-performance liquid chromatography. J. Chromatogr. Sci. 1998, 36, 187–190. [Google Scholar] [CrossRef][Green Version]
- Gao, Y.; Li, L.; Zhang, J.; Shu, W.; Gao, L. Simultaneous determination of triacetin, acetic ether, butyl acetate and amorolfine hydrochloride in amorolfine liniment by hplc. Pak. J. Pharm. Sci. 2012, 25, 389. [Google Scholar] [PubMed]
- Hildebrand, J.H.; Scott, R.L. The Solubility of Nonelectrolytes, 3rd ed.; Reinhold Pub. Corporation: New York, NY, USA, 1950; pp. 108–122. [Google Scholar]
- Hansen, C.M. Hansen Solubility Parameters; CRC Press: Boca Raton, FL, USA, 2000; pp. 2–7. [Google Scholar]
- Vayer, M.; Vital, A.; Sinturel, C. New insights into polymer-solvent affinity in thin films. Eur. Polym. J. 2017, 93, 132–139. [Google Scholar] [CrossRef]
- Hansen, C.M. Hansen Solubility Parameters: A User’s Handbook; CRC Press: Boca Raton, FL, USA, 2013; pp. 289–303. [Google Scholar]
- Legrand, P.; Lesieur, S.; Bochot, A.; Gref, R.; Raatjes, W.; Barratt, G.; Vauthier, C. Influence of polymer behaviour in organic solution on the production of polylactide nanoparticles by nanoprecipitation. Int. J. Pharm. 2007, 344, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Bordes, C.; Fréville, V.; Ruffin, E.; Marote, P.; Gauvrit, J.Y.; Briançon, S.; Lantéri, P. Determination of poly(epsilon-caprolactone) solubility parameters: Application to solvent substitution in a microencapsulation process. Int. J. Pharm. 2010, 383, 236. [Google Scholar] [CrossRef]
- Schenderlein, S.; Luck, M.; Muller, B.W. Partial solubility parameters of poly (d,l-lactide-co-glycolide). Int. J. Pharm. 2004, 286, 19–26. [Google Scholar] [CrossRef]
- Pearce, E.M. Properties of polymers, their estimation and correlation with chemical structure— (2nd rev. Ed.), d. W. Van krevelen, elsevier, amsterdam—Oxford—New York, 1976, 620 pp. J. Polym. Sci. Part C 1977, 15, 56. [Google Scholar] [CrossRef]
- Pitt, C.G.; Jeffcoat, A.R.; Zweidinger, R.A.; Schindler, A. Sustained drug delivery systems. I. The permeability of poly(epsilon-caprolactone), poly (dl-lactic acid), and their copolymers. J. Biomed. Mater. Res. 1979, 13, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Sinha, V.R.; Bansal, K.; Kaushik, R.; Kumria, R.; Trehan, A. Poly-epsilon-caprolactone microspheres and nanospheres: An overview. Int. J. Pharm. 2004, 278, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.J.; Hendren, R.W.; Jensen, K.; Pitt, C.G. Synthesis, properties, and biodegradation of poly (1,3-trimethylene carbonate). Macromolecules 1991, 24, 1736–1740. [Google Scholar] [CrossRef]
- Makadia, H.K.; Siegel, S.J. Poly lactic-co-glycolic acid (plga) as biodegradable controlled drug delivery carrier. Polymers 2011, 3, 1377. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.S. Synthesis, characterization, biodegradation, and drug delivery application of biodegradable lactic/glycolic acid polymers: Part iii. Drug delivery application. Artif. Cells Blood Substit. Immobil. Biotechnol. 2004, 32, 575. [Google Scholar] [CrossRef] [PubMed]
- Mhlanga, N.; Ray, S.S. Kinetic models for the release of the anticancer drug doxorubicin from biodegradable polylactide/metal oxide-based hybrids. Int. J. Biol. Macromol. 2015, 72, 1301–1307. [Google Scholar] [CrossRef]
- Fang, L.; Singh, R.; Waxman, L.; Zhao, C. Model protein adsorption on polymers explained by hansen solubility parameters. J. Pharm. Sci. 2019, 108, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Siparsky, G.L. Degradation kinetics of poly(hydroxy) acids: Pla and pcl. ACS Symp. Ser. 2001, 764, 230–251. [Google Scholar]
- Yoo, J.Y.; Kim, J.M.; Seo, K.S.; Jeong, Y.K.; Lee, H.B.; Khang, G. Characterization of degradation behavior for plga in various ph condition by simple liquid chromatography method. Bio-Med. Mater. Eng. 2005, 15, 279–288. [Google Scholar]
- Fredenberg, S.; Wahlgren, M.; Reslow, M.; Axelsson, A. The mechanisms of drug release in poly (lactic-co-glycolic acid)-based drug delivery systems—a review. Int. J. Pharm. 2011, 415, 34–52. [Google Scholar] [CrossRef]
- Parent, M.; Nouvel, C.; Sapin, A.; Maincent, P.; Boudier, A. Plga in situ implants formed by phase inversion: Critical physicochemical parameters to modulate drug release. J. Controll. Release 2013, 172, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Limpanuparb, T.; Punyain, K.; Tantirungrotechai, Y. A dft investigation of methanolysis and hydrolysis of triacetin. J. Mol. Struct. 2010, 955, 23–32. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, C.; Zhang, W.; Meng, S.; Liu, D.; Wang, P.; Guo, J.; Li, J.; Guan, Y.; Yang, D. Feasibility of poly (ϵ-caprolactone-co-dl-lactide) as a biodegradable material for in situ forming implants: Evaluation of drug release and in vivo degradation. Drug Dev. Ind. Pharm. 2015, 41, 342. [Google Scholar] [CrossRef] [PubMed]
- Yapar, E.A.; Baykara, T. Effects of solvent combinations on drug release from injectable phase sensitive liquid implants. Turk. J. Pharm. Sci. 2010, 7, 49–56. [Google Scholar]
- Fukushima, K. Poly (trimethylene carbonate)-based polymers engineered for biodegradable functional biomaterials. Biomater. Sci. 2016, 4, 9. [Google Scholar] [CrossRef] [PubMed]

| Solvents & Polymers | Hildebrand’ (cal/cm3)½ | Hansen’s Solubility Parameters (MPa1/2) | ||
|---|---|---|---|---|
| δ | δd | δp | δh | |
| NMPa | 22.9 | 18.0 | 12.3 | 7.2 |
| DMSOa | 19.4 | 18.4 | 16.4 | 10.2 |
| TAa | 26.7 | 16.5 | 4.5 | 9.1 |
| PLAb | 21.7 | 18.5 | 9.7 | 6.0 |
| PCLc | 19.7 | 17.7 | 6.2 | 7.8 |
| PLGAd | 22.3 | 17.4 | 9.1 | 10.5 |
| PTMCe | 20.2 | 15.3 | 7.4 | 10.8 |
| Watera | 47.8 | 15.5 | 16 | 42.3 |
| Solvents | Δδ Solvent/Polymer (MPa1/2) | |||
|---|---|---|---|---|
| PLA | PCL | PLGA | PTMC | |
| NMP | 2.9 | 6.2 | 4.8 | 8.2 |
| DMSO | 7.7 | 10.6 | 7.6 | 11.0 |
| TA | 7.5 | 3.2 | 5.1 | 4.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Yang, L.; Zhang, C.; Liu, D.; Meng, S.; Zhang, W.; Meng, S. Effect of Polymer Permeability and Solvent Removal Rate on In Situ Forming Implants: Drug Burst Release and Microstructure. Pharmaceutics 2019, 11, 520. https://doi.org/10.3390/pharmaceutics11100520
Zhang X, Yang L, Zhang C, Liu D, Meng S, Zhang W, Meng S. Effect of Polymer Permeability and Solvent Removal Rate on In Situ Forming Implants: Drug Burst Release and Microstructure. Pharmaceutics. 2019; 11(10):520. https://doi.org/10.3390/pharmaceutics11100520
Chicago/Turabian StyleZhang, Xiaowei, Liqun Yang, Chong Zhang, Danhua Liu, Shu Meng, Wei Zhang, and Shengnan Meng. 2019. "Effect of Polymer Permeability and Solvent Removal Rate on In Situ Forming Implants: Drug Burst Release and Microstructure" Pharmaceutics 11, no. 10: 520. https://doi.org/10.3390/pharmaceutics11100520
APA StyleZhang, X., Yang, L., Zhang, C., Liu, D., Meng, S., Zhang, W., & Meng, S. (2019). Effect of Polymer Permeability and Solvent Removal Rate on In Situ Forming Implants: Drug Burst Release and Microstructure. Pharmaceutics, 11(10), 520. https://doi.org/10.3390/pharmaceutics11100520