Enhanced Delivery of 4-Thioureidoiminomethylpyridinium Perchlorate in Tuberculosis Models with IgG Functionalized Poly(Lactic Acid)-Based Particles
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Instruments
2.2. Animals
Animals
2.3. Methods
2.3.1. Preparation of Perchlozone-Loaded Polymeric Micro- and Nanoparticles
2.3.2. Particle Characterization
2.3.3. Camel IgG Fractionation and Attachment to the Particles
2.3.4. Ex Vivo Determination of Particle Phagocytosis by Peritoneal Macrophages
2.3.5. Detection of Particles in Different Organs
2.3.6. Investigation of Perchlozone Distribution
2.3.7. In Vivo Experiments with Tuberculosis Mice
2.3.8. Statistical Analysis
3. Results
3.1. Particle Preparation and PCZ Loading
3.2. In Vitro Particle Degradation and Perchlozone Release
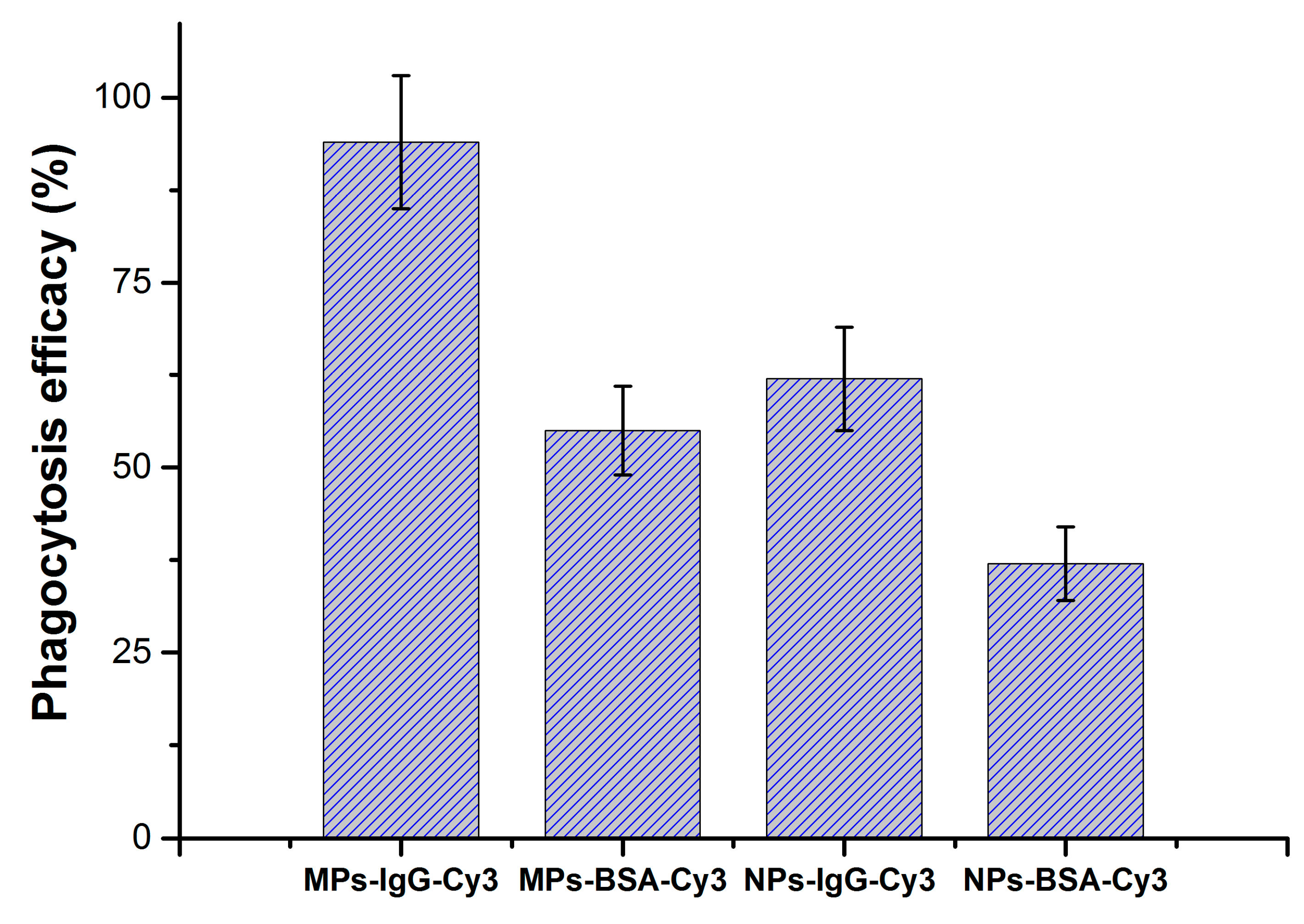
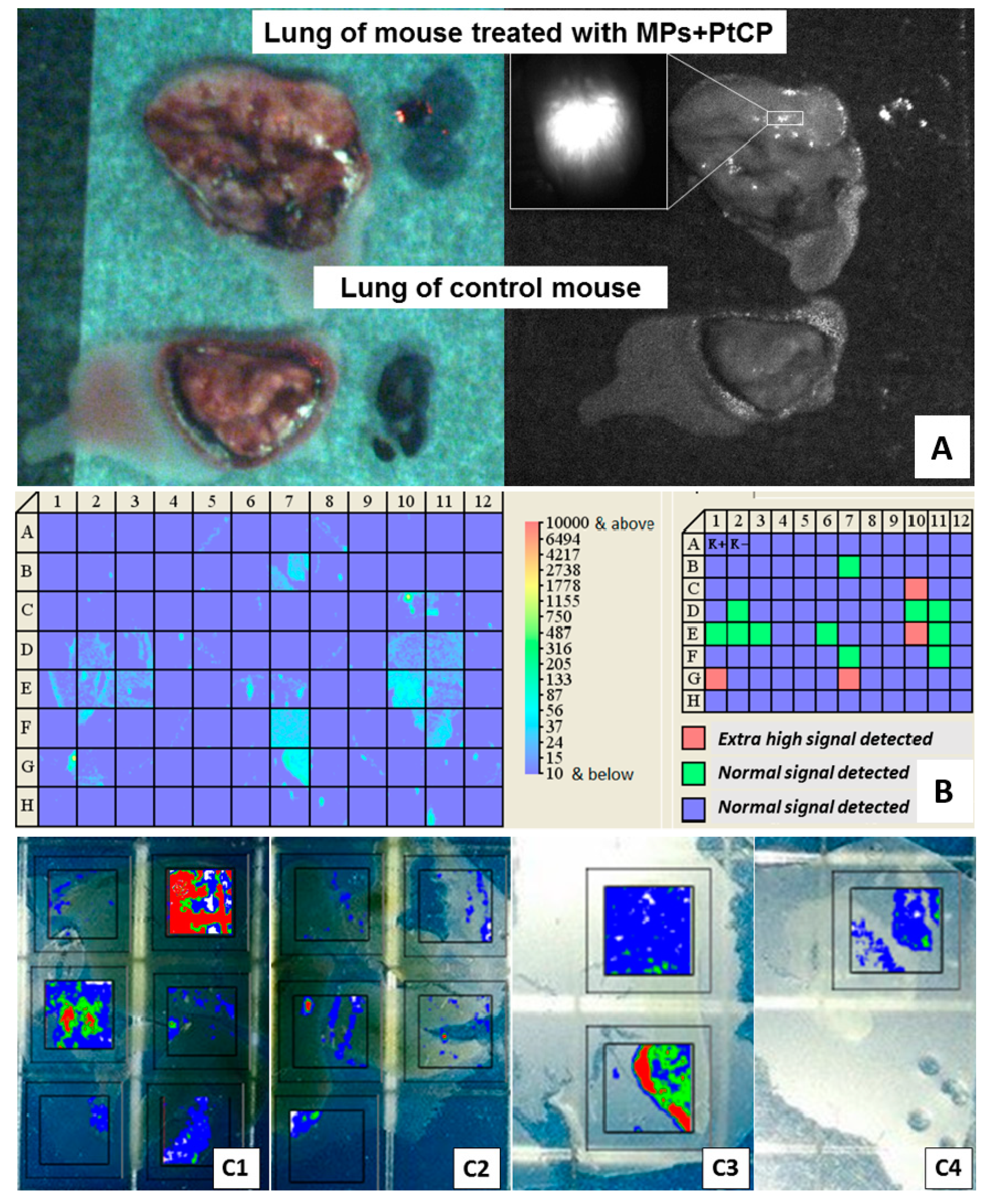
3.3. Particle Phagocytosis In Vivo
3.4. Survival Data and Severity of TB Involvement among Survived Mice
3.5. Concentration of Encapsulated Fluorescent Label and Perchlozone in the Organs of Experimental Mice
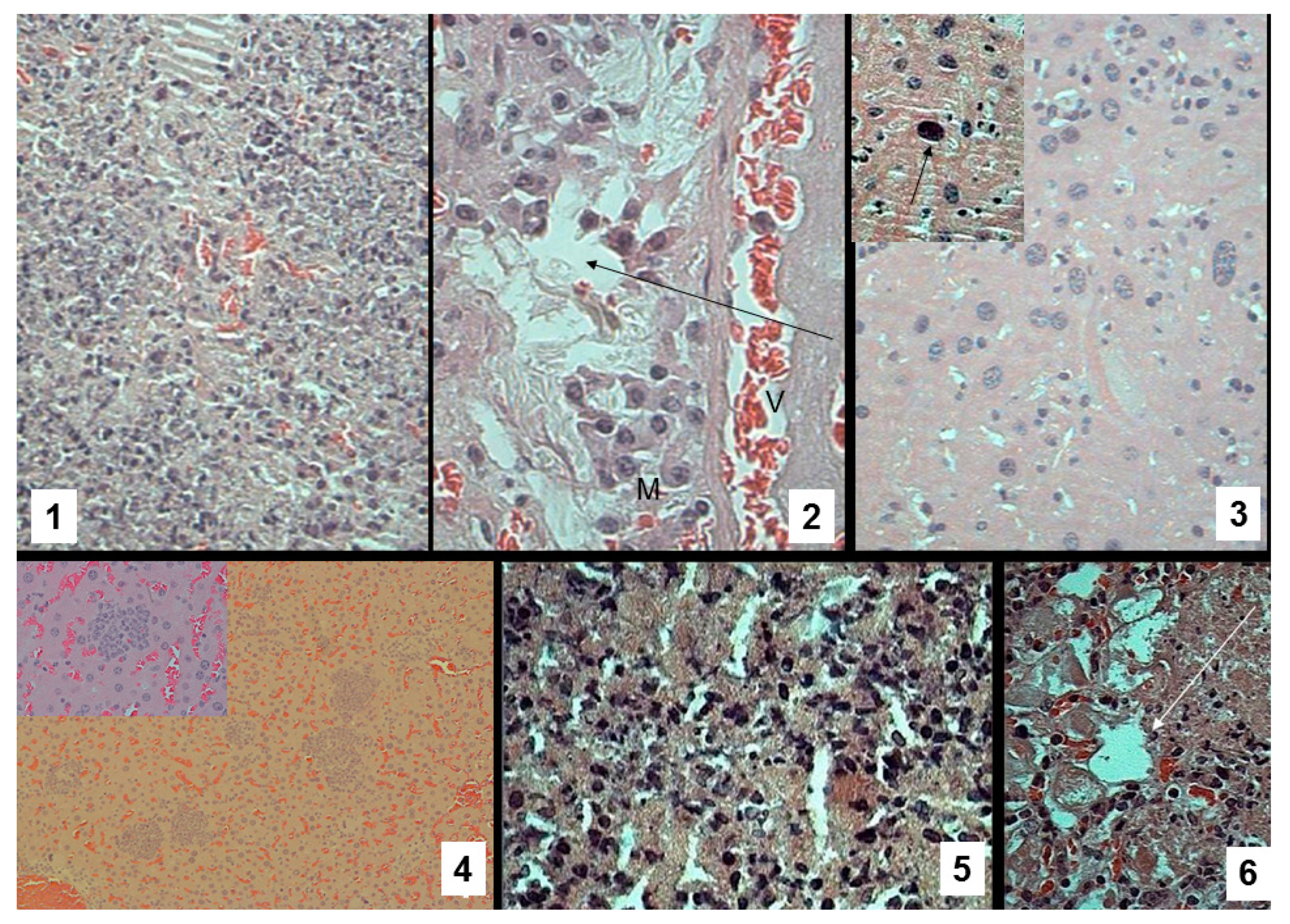
3.6. Pathomorphological Data
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| TB | tuberculosis |
| HIV | human immunodeficiency virus |
| MDR-TB | multiple drug resistant M. tuberculosis |
| PLA | poly(d,l-lactic acid) |
| ICG | indocyanine green |
| IgG | immunoglobulin G |
| PtCP | platinum(II)-coproporphyrin |
| CFU | colony-forming units |
| LC–MS | liquid chromatography/mass spectrometry |
References
- Dye, C. After 2015: Infectious diseases in a new era of health and development. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130426. [Google Scholar] [CrossRef] [PubMed]
- Lienhardt, C.; Raviglione, M.; Spigelman, M.; Hafner, R.; Jaramillo, E.; Hoelscher, M.; Zumla, A.; Gheuens, J. New drugs for the treatment of tuberculosis: Needs, challenges, promise, and prospects for the future. J. Infect. Dis. 2012, 205, S241–S249. [Google Scholar] [CrossRef] [PubMed]
- Trinh, Q.M.; Nguyen, H.L.; Nguyen, V.N.; Nguyen, T.V.A.; Sintchenko, V.; Marais, B.J. Tuberculosis and HIV co-infection-focus on the Asia-Pacific region. Int. J. Infect. Dis. 2015, 32, 170–178. [Google Scholar] [CrossRef] [PubMed]
- WHO. Global Tuberculosis Report 2014; WHO: Geneva, Switzerland, 2014; Volume 2014, ISBN 9789241564809. [Google Scholar]
- Chernokhaeva, I.; Pavlova, M.; Starshinova, A.; Sapozhnikova, N.; Belaeva, E.; Zhuravlev, V.; Archakova, L.; Yablonskii, P.; Starshinova, A. Therapy of pulmonary tuberculosis with multidrug-resistant Mycobacterium tuberculosis using tioureidoiminomethylpyridinium perchlorate (Perchlozon). Int. J. Tech. Res. Appl. 2015, 3, 59–62. [Google Scholar]
- Gopal, P.; Dick, T. The new tuberculosis drug Perchlozone® shows cross-resistance with thiacetazone. Int. J. Antimicrob. Agents 2015, 45, 430–433. [Google Scholar] [CrossRef] [PubMed]
- Pavlova, M.; Vinogradova, T. Confirmation of preclinical studies data on perchlorate (Perchlozon) in clinic. Int. J. Curr. Res. 2015, 7, 14501–14505. [Google Scholar]
- Kalita, S.; Devi, B.; Kandimalla, R.; Sharma, K.K.; Sharma, A.; Kalita, K.; Kataki, A.C.; Kotoky, J. Chloramphenicol encapsulated in poly-ε-caprolactone-pluronic composite: Nanoparticles for treatment of MRSA-infected burn wounds. Int. J. Nanomed. 2015, 10, 2971–2984. [Google Scholar] [CrossRef]
- Lboutounne, H.; Chaulet, J.-F.; Ploton, C.; Falson, F.; Pirot, F. Sustained ex vivo skin antiseptic activity of chlorhexidine in poly(epsilon-caprolactone) nanocapsule encapsulated form and as a digluconate. J. Control. Release 2002, 82, 319–334. [Google Scholar] [CrossRef]
- Rishi, P.; Bhogal, A.; Arora, S.; Pandey, S.K.; Verma, I.; Kaur, I.P. Improved oral therapeutic potential of nanoencapsulated cryptdin formulation against Salmonella infection. Eur. J. Pharm. Sci. 2015, 72, 27–33. [Google Scholar] [CrossRef]
- Pandey, R.; Sharma, A.; Zahoor, A.; Sharma, S.; Khuller, G.K.; Prasad, B. Poly(dl-lactide-co-glycolide) nanoparticle-based inhalable sustained drug delivery system for experimental tuberculosis. J. Antimicrob. Chemother. 2003, 52, 981–986. [Google Scholar] [CrossRef]
- Sharma, R.; Saxena, D.; Dwivedi, A.K.; Misra, A. Inhalable microparticles containing drug combinations to target alveolar macrophages for treatment of pulmonary tuberculosis. Pharm. Res. 2001, 18, 1405–1410. [Google Scholar] [CrossRef] [PubMed]
- Garg, T.; Rath, G.; Goyal, A.K. Inhalable chitosan nanoparticles as antitubercular drug carriers for an effective treatment of tuberculosis. Artif. Cells Nanomed. Biotechnol. 2015, 1–5. [Google Scholar] [CrossRef]
- Parikh, R.; Patel, L.; Dalwadi, S. Microparticles of rifampicin: Comparison of pulmonary route with oral route for drug uptake by alveolar macrophages, phagocytosis activity and toxicity study in albino rats. Drug Deliv. 2013, 7544, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.K.; Gupta, Y.; Ramalingam, L.; Jain, A.; Jain, A.; Khare, P.; Bhargava, D. Lactose-Conjugated PLGA Nanoparticles for Enhanced Delivery of Rifampicin to the Lung for Effective Treatment of Pulmonary Tuberculosis. PDA J. Pharm. Sci. Technol. 2010, 64, 278–287. [Google Scholar] [PubMed]
- Dheda, K.; Schwander, S.K.; Zhu, B.; Van Zyl-Smit, R.N.; Zhang, Y. The immunology of tuberculosis: From bench to bedside. Respirology 2010, 15, 433–450. [Google Scholar] [CrossRef]
- Amarnath Praphakar, R.; Munusamy, M.A.; Sadasivuni, K.K.; Rajan, M. Targeted delivery of rifampicin to tuberculosis-infected macrophages: Design, in-vitro, and in-vivo performance of rifampicin-loaded poly(ester amide)s nanocarriers. Int. J. Pharm. 2016, 513, 628–635. [Google Scholar] [CrossRef]
- Hwang, S.M.; Kim, D.D.; Chung, S.J.; Shim, C.K. Delivery of ofloxacin to the lung and alveolar macrophages via hyaluronan microspheres for the treatment of tuberculosis. J. Control. Release 2008, 129, 100–106. [Google Scholar] [CrossRef]
- Takenaga, M.; Ohta, Y.; Tokura, Y.; Hamaguchi, A.; Igarashi, R.; Disratthakit, A.; Doi, N. Lipid Microsphere Formulation Containing Rifampicin Targets Alveolar Macrophages. Drug Deliv. 2008, 15, 169–175. [Google Scholar] [CrossRef]
- Yoshida, A.; Matumoto, M.; Hshizume, H.; Oba, Y.; Tomishige, T.; Inagawa, H.; Kohchi, C.; Hino, M.; Ito, F.; Tomoda, K.; et al. Selective delivery of rifampicin incorporated into poly(dl-lactic-co-glycolic) acid microspheres after phagocytotic uptake by alveolar macrophages, and the killing effect against intracellular Mycobacterium bovis Calmette–Guérin. Microbes Infect. 2006, 8, 2484–2491. [Google Scholar] [CrossRef]
- Horváti, K.; Bacsa, B.; Szabó, N.; Dávid, S.; Mezo, G.; Grolmusz, V.; Vértessy, B.; Hudecz, F.; Bo’sze, S. Enhanced cellular uptake of a new, in silico identified antitubercular candidate by peptide conjugation. Bioconjug. Chem. 2012, 23, 900–907. [Google Scholar] [CrossRef]
- Horváti, K.; Bacsa, B.; Kiss, É.; Gyulai, G.; Fodor, K.; Balka, G.; Rusvai, M.; Szabó, E.; Hudecz, F.; Bősze, S. Nanoparticle Encapsulated Lipopeptide Conjugate of Antitubercular Drug Isoniazid: In Vitro Intracellular Activity and in Vivo Efficacy in a Guinea Pig Model of Tuberculosis. Bioconjug. Chem. 2014, 25, 2260–2268. [Google Scholar] [CrossRef] [PubMed]
- Shivangi; Meena, L.S. A Novel Approach in Treatment of Tuberculosis by Targeting Drugs to Infected Macrophages Using Biodegradable Nanoparticles. Appl. Biochem. Biotechnol. 2018, 185, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Cheng, H.; Peng, H.; Zhou, H.; Li, P.Y.; Langer, R. Non-genetic engineering of cells for drug delivery and cell-based therapy. Adv. Drug Deliv. Rev. 2015, 91, 125–140. [Google Scholar] [CrossRef] [PubMed]
- Banskota, S.; Yousefpour, P.; Chilkoti, A. Cell-Based Biohybrid Drug Delivery Systems: The Best of the Synthetic and Natural Worlds. Macromol. Biosci. 2017, 17, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Korzhikov, V.; Averianov, I.; Litvinchuk, E.; Tennikova, T.B. Polyester-based microparticles of different hydrophobicity: The patterns of lipophilic drug entrapment and release. J. Microencapsul. 2016, 33, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Cardona, P.J.; Cooper, A.; Luquín, M.; Ariza, A.; Filipo, F.; Orme, I.M.; Ausina, V. The intravenous model of murine tuberculosis is less pathogenic than the aerogenic model owing to a more rapid induction of systemic immunity. Scand. J. Immunol. 1999, 49, 362–366. [Google Scholar] [CrossRef] [PubMed]
- Nuermberger, E. Using Animal Models to Develop New Treatments for Tuberculosis. Semin. Respir. Crit. Care Med. 2008, 29, 542–551. [Google Scholar] [CrossRef]
- Guryanov, I.; Cipriani, S.; Fiorucci, S.; Zashikhina, N.; Marchianò, S.; Scarpelli, P.; Korzhikov-Vlakh, V.; Popova, E.; Korzhikova-Vlakh, E.; Biondi, B.; et al. Nanotraps with Biomimetic Surface as Decoys for Chemokines. Nanomed. Nanotechnol. Biol. Med. 2017. [Google Scholar] [CrossRef]
- Costa, P.; Sousa Lobo, J.M. Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef]
- Salome, A.C.; Godswill, C.O.; Ikechukwu, I.O. Kinetics and mechanisms of drug release from swellable and non swellable matrices: A review. Res. J. Pharm. Biol. Chem. Sci. 2013, 4, 97–103. [Google Scholar] [CrossRef]
- Twair, A.; Al-Okla, S.; Zarkawi, M.; Abbady, A.Q. Characterization of camel nanobodies specific for superfolder GFP fusion proteins. Mol. Biol. Rep. 2014. [Google Scholar] [CrossRef] [PubMed]
- Reiner, N.E. (Ed.) Macrophages and Dendritic Cells: Methods and Protocols; Methods in Molecular Biology; Humana Press: New York, NY, USA, 2009; Volume 531, ISBN 978-1-58829-972-7. [Google Scholar]
- Freebern, W.J.; Bigwarfe, T.J.; Price, K.D.; Haggerty, H.G. Methods: Implementation of in vitro and ex vivo phagocytosis and respiratory burst function assessments in safety testing. J. Immunotoxicol. 2013, 10, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Papkovsky, D.B.; O’Riordan, T.C. Emerging applications of phosphorescent metalloporphyrins. J. Fluoresc. 2005, 15, 569–584. [Google Scholar] [CrossRef] [PubMed]
- Almeida, F.M.; Ventura, T.L.B.; Amaral, E.P.; Ribeiro, S.C.M.; Calixto, S.D.; Manhães, M.R.; Rezende, A.L.; Souzal, G.S.; De Carvalho, I.S.; Silva, E.C.; et al. Hypervirulent Mycobacterium tuberculosis strain triggers necrotic lung pathology associated with enhanced recruitment of neutrophils in resistant C57BL/6 mice. PLoS ONE 2017, 12, 1–19. [Google Scholar] [CrossRef]
- Martín-Hernando, M.P.; Höfle, U.; Vicente, J.; Ruiz-Fons, F.; Vidal, D.; Barral, M.; Garrido, J.M.; de la Fuente, J.; Gortazar, C. Lesions associated with Mycobacterium tuberculosis complex infection in the European wild boar. Tuberculosis 2007, 87, 360–367. [Google Scholar] [CrossRef]
- Ordonez, A.A.; Tasneen, R.; Pokkali, S.; Xu, Z.; Converse, P.J.; Klunk, M.H.; Mollura, D.J.; Nuermberger, E.L.; Jain, S.K. Mouse model of pulmonary cavitary tuberculosis and expression of matrix metalloproteinase-9. Dis. Model. Mech. 2016, 9, 779–788. [Google Scholar] [CrossRef] [PubMed]
- Radaeva, T.V.; Kondratieva, E.V.; Sosunov, V.V.; Majorov, K.B.; Apt, A. A human-like TB in genetically susceptible mice followed by the true dormancy in a Cornell-like model. Tuberculosis 2008, 88, 576–585. [Google Scholar] [CrossRef]
- Zabolotnykh, N.V.; Vinogradova, T.I.; Stukova, M.A.; Vasil’eva, S.N.; Vitovskaia, M.L.; Egorov, A.I. The effectiveness of influenza vectors expressing the protective mycobacterial antigen ESAT-6 in the complex therapy of generalized tuberculosis in mice. Probl. Tuberk. Bolezn. Legk. 2008, 12, 30–34. [Google Scholar]
- Lee, E.; Wang, J.; Kleinbaum, D.G.; Collett, D. Statistical Methods for Survival Data Analysis; John Wiley & Sons: Hoboken, NJ, USA, 2003; Volume 36, ISBN 3175723993. [Google Scholar]
- Ray, K.; Szmacinski, H.; Chowdhury, M.H.; Lakowicz, J.R. Metal-enhanced intrinsic fluorescence of proteins and label-free bioassays. In Proceedings of the Plasmonics in Biology and Medicine VII, San Francisco, CA, USA, 23–28 January 2010; p. 75770K. [Google Scholar]
- Zhao, C.; Fan, T.; Yang, Y.; Wu, M.; Li, L.; Zhou, Z.; Jian, Y.; Zhang, Q.; Huang, Y. Preparation, macrophages targeting delivery and anti-inflammatory study of pentapeptide grafted nanostructured lipid carriers. Int. J. Pharm. 2013, 450, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Cook, D.A.N.; Samarasekara, C.L.; Wagstaff, S.C.; Kinne, J.; Wernery, U.; Harrison, R.A. Analysis of camelid IgG for antivenom development: Immunoreactivity and preclinical neutralisation of venom-induced pathology by IgG subclasses, and the effect of heat treatment. Toxicon 2010, 56, 596–603. [Google Scholar] [CrossRef]
- Shaker, G.H. Evaluation of antidiphtheria toxin nanobodies. Nanotechnol. Sci. Appl. 2010, 3, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Tabata, Y.; Ikada, Y. Effect of the size and surface charge of polymer microspheres on their phagocytosis by macrophage. Biomaterials 1988, 9, 356–362. [Google Scholar] [CrossRef]
- Nikonenko, B.; Sacksteder, K.; Hundert, S.; Einck, L.; Nacy, C. Preclinical Study of New TB Drugs and Drug Combinations in Mouse Models. Recent Pat. Antiinfect. Drug Discov. 2008, 3, 102–116. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Tsai, M.; Lu, D.; Wang, J.; Wientjes, M.G.; Au, J.L.-S. Tumor-Penetrating Microparticles for Intraperitoneal Therapy of Ovarian Cancer. J. Pharmacol. Exp. Ther. 2008, 327, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, A.J.; Regan-Komito, D.; Christou, I.; White, G.E.; McNeill, E.; Kenyon, A.; Taylor, L.; Kapellos, T.S.; Fisher, E.A.; Channon, K.M.; et al. A Real Time Chemotaxis Assay Unveils Unique Migratory Profiles amongst Different Primary Murine Macrophages. PLoS ONE 2013, 8. [Google Scholar] [CrossRef]
- Green, T.D.; Park, J.; Yin, Q.; Fang, S.; Crews, A.L.; Jones, S.L.; Adler, K.B. Directed migration of mouse macrophages in vitro involves myristoylated alanine-rich C-kinase substrate (MARCKS) protein. J. Leukoc. Biol. 2012, 92, 633–639. [Google Scholar] [CrossRef]





| Abbreviation | Description of Animals/Periodicity of Treatment/Type of Particles | Means of Treatment | Suspension Volume/Concentration/Medium | Dose | Number of Animals |
|---|---|---|---|---|---|
| N—non-treated | healthy controls/no treatment/no particles | none | none | none | 15 |
| U—untreated | TB-infected mice/daily/no particles | p/o | 0.2 mL of pure saline | none | 33 |
| PCZ per os—perchlozone per os | TB-infected mice/daily/no particles | p/o | 0.5 mL of drug solution 0.6 mg/mL in saline | single dose = 15 mg/kg total dose for survived mice = 600 mg/kg | 15 |
| MPs i.p.—microparticles introperitoneally | TB-infected mice/once/microparticles | i.p. | 0.5 mL/10 wt.%/saline | none | 11 |
| MPs + PCZ i.p.—microparticles + perchlozone intraperitoneally | TB-infected mice/once/microparticles | i.p. | 0.5 mL/10 wt.%/saline | single dose and total dose = 41 mg/kg | 15 |
| MPs-IgG + PCZ i.p.—microparticles modified with IgG + perchlozone intraperitoneally | TB-infected mice/once/microparticles modified with camel IgG | i.p. | 0.5 mL/10 wt.%/saline | single and total dose drug = 41 mg/kg. IgG = 3 mg/kg. | 11 |
| [NPs-IgG + PCZ] i.v. + [MPs-IgG + PCZ] i.p.—nanoparticles loaded with perchlozone and modified with camel IgG intravenously and microparticles loaded with perchlozone and modified with camel IgG intraperitoneally | TB-infected mice/once intravenously by nanoparticles loaded with perchlozone and modified with camel IgG intravenously and 1 week later microparticles loaded with perchlozone and modified with camel IgG intraperitoneally/nano- and microparticles | i.v. + i.p. | i.v.: 0.2 mL/10 wt.%/saline i.p.: 0.5 mL/10 wt.%/saline | drug dose: i.v. = 3 mg/kg, i.p. = 41 mg/kg. Total dose of drug = 44 mg/kg immobilized IgG doses: i.v. = 2 mg/kg, i.p. = 3 mg/kg Total dose of IgG = 5 mg/kg | 9 |
| Parameter | Group | |
|---|---|---|
| PCZ per os | MPs + PCZ i/p | |
| Lm, g | 0.59 ± 0.06 | 0.41 ± 0.05 |
| Lm % of body mass | 1.97 ± 0.20 | 1.33 ± 0.20 |
| DLD | 3.36 ± 0.27 | 2.50 ± 0.17 |
| lg CFU | 2.08 ± 0.60 | 1.21 ± 0.44 |
| Organ | Group | ||
|---|---|---|---|
| U Mouse, 21st Day of TB, DLD = 4.0 | MPs + PCZ i.p. Mouse, 20th Day of TB, 7th Day after Injection DLD = 3.75 | MPs-IgG + PCZ i.p. Mouse, 21st Day of TB, 8th Day after Injection DLD = 3.75 | |
| Liver | 249 ± 27 | 1314 ± 144 | 2004 ± 220 |
| Lung | 287 ± 32 | 1536 ± 169 | 3865 ± 425 |
| Spleen | 69 ± 8 | 238 ± 26 | 538 ± 59 |
| Brain | 30 ± 3 | 155 ± 17 | 188 ± 21 |
| Organ | Group | ||
|---|---|---|---|
| PCZ Concentration in the Slices of PCZ per os Mouse, 20th Day of TB, 7th Day after Injection (µg/slice) | PCZ Concentration in the Slices of MPs-IgG + PCZ i.p. Mouse, 21st Day of TB, 8th Day after Injection (µg/slice) | PCZ Concentration in the Slices of [NPs-IgG + PCZ] i.v. + [MPs-IgG + PCZ] i.p. Mouse, 21st Day of TB, 8th Day after Injection (µg/slice) | |
| Liver | 335.2 ± 52.8 | 154.5 ± 23.2 | 297.2 ± 44.5 |
| Lung | 132.0 ± 19.8 | 283.0 ± 42.5 | 307.8 ± 46.2 |
| Spleen | 36.9 ± 5.5 | 59.9 ± 9.0 | 55.5 ± 8.3 |
| Brain | 1.2 ± 0.2 | <0.03 | <0.03 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Churilov, L.; Korzhikov-Vlakh, V.; Sinitsyna, E.; Polyakov, D.; Darashkevich, O.; Poida, M.; Platonova, G.; Vinogradova, T.; Utekhin, V.; Zabolotnykh, N.; et al. Enhanced Delivery of 4-Thioureidoiminomethylpyridinium Perchlorate in Tuberculosis Models with IgG Functionalized Poly(Lactic Acid)-Based Particles. Pharmaceutics 2019, 11, 2. https://doi.org/10.3390/pharmaceutics11010002
Churilov L, Korzhikov-Vlakh V, Sinitsyna E, Polyakov D, Darashkevich O, Poida M, Platonova G, Vinogradova T, Utekhin V, Zabolotnykh N, et al. Enhanced Delivery of 4-Thioureidoiminomethylpyridinium Perchlorate in Tuberculosis Models with IgG Functionalized Poly(Lactic Acid)-Based Particles. Pharmaceutics. 2019; 11(1):2. https://doi.org/10.3390/pharmaceutics11010002
Chicago/Turabian StyleChurilov, Leonid, Viktor Korzhikov-Vlakh, Ekaterina Sinitsyna, Dmitry Polyakov, Oleg Darashkevich, Mikhail Poida, Galina Platonova, Tatiana Vinogradova, Vladimir Utekhin, Natalia Zabolotnykh, and et al. 2019. "Enhanced Delivery of 4-Thioureidoiminomethylpyridinium Perchlorate in Tuberculosis Models with IgG Functionalized Poly(Lactic Acid)-Based Particles" Pharmaceutics 11, no. 1: 2. https://doi.org/10.3390/pharmaceutics11010002
APA StyleChurilov, L., Korzhikov-Vlakh, V., Sinitsyna, E., Polyakov, D., Darashkevich, O., Poida, M., Platonova, G., Vinogradova, T., Utekhin, V., Zabolotnykh, N., Zinserling, V., Yablonsky, P., Urtti, A., & Tennikova, T. (2019). Enhanced Delivery of 4-Thioureidoiminomethylpyridinium Perchlorate in Tuberculosis Models with IgG Functionalized Poly(Lactic Acid)-Based Particles. Pharmaceutics, 11(1), 2. https://doi.org/10.3390/pharmaceutics11010002









